Abstract
Simple Summary
This research explores non-invasive methods for detecting Human Papillomavirus (HPV) in patients with oral squamous cell carcinoma (OSCC). The study compares two self-collection techniques, oral rinse vs. salivary sponge, to determine their effectiveness in identifying HPV DNA. Results from 26 patients indicated that the salivary sponge method was more accurate and sensitive compared to the oral rinse. This finding suggests that a salivary sponge may be a superior option, especially for patients with functional limitations caused by OSCC. Our research could lead to improved non-invasive diagnostic tools for HPV in clinical settings.
Abstract
Background/Objectives: Human Papillomavirus (HPV) is a significant etiological factor in the development of oropharyngeal carcinogenesis. The detection of HPV in oral squamous cell carcinoma (OSCC) could be also crucial for diagnosis, prognosis, and treatment planning. This study compares the efficacy and accuracy of two non-invasive sampling methods, oral rinse, and oral sponge, in detecting HPV DNA in patients with OSCC. Methods: Twenty-six patients with histologically confirmed OSCCs were recruited (M/F = 15/11; mean age 68.6). From each patient, two self-collected oral specimens, in the form of an oral rinse and a salivary sponge (i.e., LolliSponge), were collected, and subsequently processed, utilizing INNO-LiPA HPV Genotyping Extra II for HPV DNA detection; Results: Oral sponge detection showed high specificity (100%), sensitivity (85.7%), and accuracy (96.2%) compared to the oral rinse sampling, also demonstrating an area AUC for its diagnostic performance significantly greater than 0.5 (0.93 vs. 0.5, p < 0.0001). Conclusions: This study supports that oral sponge sampling offers valuable non-invasive alternatives for HPV detection in patients with OSCC, with the potentiality to facilitate saliva sampling in patients that may exhibit functional deficit due to OSCC. Further research is recommended to validate these findings in larger cohorts and to explore the integration of these methods into routine clinical practice for the management of HPV-related OSCC.
Keywords: human papillomavirus, oral squamous cell carcinoma, polymerase chain reaction, salivary diagnostics, human papillomavirus DNA test, HPV DNA
1. Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common types of head and neck cancers, accounting for over 90% of malignancies in the oral cavity [1]. Traditionally, OSCC has been strongly associated with risk factors such as tobacco use, alcohol consumption, and betel nut chewing [2]. However, over the past few decades, a growing body of epidemiological evidence has highlighted the significant role of Human Papillomavirus (HPV) in the etiology of head-neck SCC, particularly in the oropharyngeal district [3,4,5].
HPV is the most common sexually transmitted pathogen worldwide, with an estimated 80% of sexually active people contracting the infection at least once in their lifetime [6]. High-risk HPVs (hrHPV) are classically involved in the genesis of anogenital malignancies (i.e., cervical cancer) [7,8], however, the recent major changes witnessed in sexual behaviors have made oral sexual practices the most likely way of exposing the oral cavity to HPV infection and HPV-related carcinogenesis phenomena [9,10,11,12,13,14,15,16].
There has been a marked increase in the incidence of HPV-positive oropharyngeal squamous cell carcinoma (OPSCC), particularly in younger and non-smoker populations, data that contrasts with the traditional demographic of oropharyngeal cancer patients who are typically older and have a history of significant tobacco and alcohol use [17,18,19,20]. This shift suggests distinct biological behaviors and risk profiles between HPV-positive and HPV-negative oropharyngeal carcinogenesis [21,22].
There are no FDA-approved tests to detect HPV DNA or RNA in saliva; however, salivary rinse samples are usually used in research settings to assess oral HPV infection among both cancer patients and healthy people [23,24,25,26].
Oral rinse for HPV-DNA detection offers significant advantages, including non-invasiveness, ease of repeated testing, and cost-effectiveness. Particularly for patients with suspected/confirmed OSCC or oral potentially malignant disorders (OPMD), who may already be experiencing discomfort due to these oral suspected lesions or procedures for their diagnosis, oral rinse collection is particularly well accepted due to ease of use and less invasive nature. Moreover, oral rinse samples cells from the entire oral cavity, may increase the likelihood of detecting HPV if it is present, which could be important for identifying widespread or non-localized infections [27].
Challenges such as variable sample quality, potential contamination, and the need for advanced laboratory processing must be addressed to ensure accurate and reliable results. Variable sample quality could be attributed to inconsistent collection methods and the dilution effect of the liquid used for rinsing. Improper sample collection may vary depending on patient adherence to instructions and thoroughness during the rinsing process; and the dilution of saliva during rinsing can reduce the concentration of HPV DNA, potentially leading to false negatives, especially in cases of low viral load. Moreover, oral rinse samples can be contaminated by extraneous DNA from the environment, the container, or the patient’s hands, which may result in false positives [28]. The proximity between the oral cavity and oropharynx also exposes a risk of altered oral HPV detection, given the lack of site-specificity of sampling and the different susceptibility to infection known between the two districts. This is very important for appreciating the role of HPV in strictly oral carcinogenesis [14].
In this context, ensuring standardized collection protocols and performing other saliva sampling methods that can enhance diagnostic accuracy, especially for certain HPV-related cancers, such as OSCC, should be supposed.
The LolliSponge (Copan Italia S.p.A., Brescia, Italy) is a novel device designed for saliva collection, commonly used for self-salivary sampling in active COVID-19 surveillance programs, known for its greater ease of use compared to oral rinse. The useful features of this saliva-direct device are that it is non-invasive, less expensive, and validated for use with reagents and instruments from multiple vendors and for several viral detections [29]. Its extreme versatility is associated with the greater adequacy of the sampling, due to the presence of a sponge which guarantees the scraping of the mucosa. This could translate into a more robust sample with reduced collection time and greater acceptance by the oncological patient, often with oral function already compromised by the cancer.
The main aim of this pilot study is to report the preliminary performance results of the LolliSponge sampling, compared a standard technique such as the oral rinse, in identifying oral HPV status, in a cohort of patients with strictly OSCC.
2. Materials and Methods
2.1. Preliminary Accuracy Report
The study protocol adhered to the ethical principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments or equivalent ethical standards. It was approved by the institutional review board of the University Hospital “Policlinico Paolo Giaccone” in Palermo, Italy (approval numbers #03/2013 and #04/2024). Prior to sample collection, all participants provided written informed consent.
2.2. Entry Criteria
Patient recruitment commenced on 1 January 2024 and concluded in June 2024. Participants were enrolled from the Oral Medicine Unit at the University Hospital “Policlinico Paolo Giaccone” in Palermo, Italy.
The inclusion criteria were as follows:
-
(i)
Age 18 years or older;
-
(ii)
Ability to provide informed consent;
-
(iii)
Presence of suspected OSCC strictly within the oral cavity, categorized according to the 2024 NIH/SEER ICD-0-3.2 topographical classification codes;
-
(iv)
No prior cancer diagnosis or treatment in the head and neck regions.
2.3. Data Collection and Clinical Examination
Patients suspected of having OSCC were interviewed using a structured questionnaire to collect socio-demographic and medical history data. Regarding smoking and alcohol use, participants were categorized as never, current, or former smokers (those who had quit at least one year before the study) [2]. Alcohol consumption was classified as non-drinkers, moderate drinkers (fewer than 16 units per week), or heavy drinkers (16 or more units per week).
Lesions were categorized based on the 2024 NIH/SEER ICD-0-3.2 topographical codes used in the eligibility criteria [14], and grouped into the following categories:
-
-
Mobile tongue (including ventral/lateral tongue) (C020-C021-C022-C023, C028, C029);
-
-
Gum (including upper/lower gum and retromolar area) (C030, C031, C039, C062)
-
-
Hard palate (C050);
-
-
Buccal mucosa (C060, C061);
-
-
Floor of the mouth (C040, C041, C048-C049).
Local mechanical risk factors such as sharp cusps or incongruent prostheses were recorded, and details of overlapping lesion sites/codes were noted.
2.4. Saliva Samples Collection for DNA Extraction and HPV DNA Detection
Each recruited patient provided two collected oral specimens, in the form of an oral rinse and a salivary sponge, both sent to the Microbiology and Virology Unit of the University Hospital “Policlinico Paolo Giaccone” in Palermo for HPV detection. Each patient was instructed to abstain from food, drink, and oral hygiene products for at least 1 h before sampling.
The oral rinse was performed by rinsing the oral cavity with 10 mL of Original Mint Scope mouthwash (Procter & Gamble, Cincinnati, OH, USA), which is considered the most suitable buccal cell collection medium for obtaining DNA for clinical and research applications [30]. Each sample was collected into a sterile 50 mL Falcon tube and centrifuged at 1600 rpm for 10′ to isolate the cellular component. The pellet was then resuspended in 1 to 4 mL of phosphate-buffered saline (PBS) and centrifuged again at 13,000 rpm for 5 min. After removing the supernatant, samples were stored at −20 °C or processed immediately.
The second sampling consisted of collecting saliva through the absorbent sponge of the LolliSponge device (Copan Italia S.p.A., Brescia, Italy). The sample was then centrifuged at 1800 rpm for 1 min to extract the saliva from the sponge and stored at −20 °C or processed immediately.
PBS resuspended oral pellet (200 uL each) and LolliSponge collected saliva underwent DNA extraction using the QIAamp Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. HPV-DNA was then detected by INNO-LiPA HPV Genotyping Extra II (Fujirebio, Tokyo, Japan), a reverse hybridization assay which identifies 20 hrHPV genotypes (HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV67, HPV68, HPV26, HPV53, HPV66, HPV70, HPV73, HPV82) and 12 lrHPV genotypes (HPV6, HPV11, HPV40, HPV42, HPV43, HPV44, HPV54, HPV61, HPV62, HPV81, HPV83, HPV89).
Samples that were positive but could not be genotyped using the primary diagnostic kit underwent a nested PCR assay for enhanced sensitivity. This involved initial amplification with the PGMY09/11 primers, followed by a secondary amplification using GP05+/GP06+ primers, as described in previous studies [31]. Genotypes were determined through Sanger sequencing of the PCR products, followed by sequence alignment using the Basic Local Alignment Search Tool (BLAST).
The entire HPV sampling and detecting protocols, by the two oral devices, are described in Table 1. All saliva sampling using the LolliSponge device was conducted first, followed by the oral rinse with Scope mouthwash, immediately before the diagnostic biopsy for OSCC. This sequence was followed to minimize any potential interference between the two sampling methods, with all procedures taking place on the same day during patient preparation.
Table 1.
Oral HPV sampling and detecting protocols by Scope Oral Rinse and LolliSponge.
| Step | Description | Procedures |
|---|---|---|
| 1 | Sample collection | Scope Oral Rinse: (i) dispense 10 mL of Original Mint Scope mouthwash into a sterile 50 mL Falcon tube; (ii) rinse orally with the mouthwash for 60 s, carefully reaching all parts of the mouth, avoiding gargling; then, spitting back into the Falcon tube. LolliSponge: (i) open the LolliSponge test tube holding the device by the cap and insert it in the mouth; (ii) gently move it around the mouth and scrape into suspected lesions, for 60 s, so that the sponges are well moistened; (iii) place the cap on the LolliSponge test tube, screw it on and securely close the test tube. |
| 2 | Sample process | Scope Oral Rinse: (i) centrifuge at 1600 rpm for 10′ to isolate the cellular component; (ii) resuspended the pellet in 1 to 4 mL of phosphate-buffered saline (PBS) and centrifuged again at 13,000 rpm for 5 min; (iii) after removing the supernatant, store the samples at −20 °C or process immediately. LolliSponge: (i) centrifuge at 1800 rpm for 10′ to isolate saliva form the absorbent sponge by centrifugating the device at 1800 rpm for 1 min; (ii) after removing the supernatant, store the samples at −20 °C or process immediately. |
| 3 | HPV-DNA extraction | Scope Oral Rinse and LolliSponge Use QIAamp Mini Kit (Qiagen, Hilden, Germany) to extract DNA following manufacturers’ protocol. |
| 4 | HPV detection and genotype | Scope Oral Rinse and LolliSponge Use INNO-LiPA HPV Genotyping Extra II (Fujirebio, Tokyo, Japan) and Basic Local Alignment Search Tool (BLAST) to identify HPV genotypes. Perform Nested PCR, followed by Sanger sequencing and Basic Local Alignment Search Tool (BLAST) alignment, for samples positive to Inno-lipa HPV controls, but not genotyping by the diagnostic kit. |
2.5. Tissue Sample for Histological Examination
All patients underwent incisional biopsies of selected areas of suspected carcinoma, performed using a scalpel punch under local anesthesia. Biopsy samples were fixed in formalin and processed in the Pathology Laboratory of the University Hospital “Policlinico Paolo Giaccone” in Palermo. Formalin-fixed, paraffin-embedded (FFPE) tissue sections (5 µm thick) were stained with hematoxylin and eosin for histopathological examination to confirm OSCC diagnosis. Carcinomas were graded according to the WHO classification, and only OSCC coded as 807*/* by the ICD-0-3 SEER site/histology validation list were included.
2.6. Statistical Analysis
Categorical variables were presented as counts and percentages, while continuous variables were expressed as mean ± standard deviation (SD) unless otherwise indicated. The diagnostic performance of the LolliSponge device for HPV-positive patients was assessed using sensitivity, specificity, and the Receiver Operating Characteristic (ROC) curve, which plots the true positive rate against the false positive rate across various cut-off points. The area under the ROC curve (AUC) was calculated with standard error and 95% confidence intervals. Comparisons between two AUCs were performed using the z-test. Statistical significance was set at a p-value (p) < 0.05. All analyses were conducted using MATLAB analytical toolbox version 2008 (MathWorks, Natick, MA, USA) for Windows at 32 bits.
3. Results
Twenty-six patients with OSCC histological diagnoses were definitively recruited. A majority of the patients were males (15/26, 57.7%), with a mean age of 68.6 ± 11.7 years (range 44–89 years). In Table 2, demographic/clinical and tobacco/alcohol data are reported.
Table 2.
Information regarding age, sex, oral lesions site, local trauma, and smoking/alcohol consumption.
| OSCC (Total No. 26) | % (No.) | |
|---|---|---|
| Gender | Male | 57.7% (15) |
| Female | 42.3% (11) | |
| Age Groups | ≤50 | 7.7% (2) |
| 51–60 | 19.2% (5) | |
| 61–70 | 30.8% (8) | |
| >70 | 42.3% (11) | |
| Mean ± SD | 67.5 ± 11.7 | |
| Median (IQR) | 68.5 (60, 76) | |
| Site | Mobil tongue (C021, C022, C023) | 57.7% (15) * |
| Gum (C030, C031, C062) | 38.7% (10) * | |
| Buccal mucosa (C060) | 15.4% (4) * | |
| Hard palate (C050) | 3.8% (1) * | |
| Floor of mouth (C041) | 7.6% (2) * | |
| HPV status | Positive | 26.9% (7) |
| Negative | 73.1% (19) | |
| Local Trauma | No trauma | 92.3% (24) |
| Presence of trauma | 7.7% (2) | |
| Smoking Status | Never | 69.3% (18) |
| Current | 11.5% (3) | |
| Former | 19.2% (5) | |
| Alcohol Consumption | Non-drinker | 96.2% (25) |
| Moderate | 3.8% (1) | |
* some OSCC presented at multiple anatomical sites simultaneously (see Table 2 for details).
Regarding oral lesions sites, the tongue was the most affected site (15/26; 57.7%), followed by the gums (10/26; 38.7%), and the buccal mucosa (4/26; 15.4%). Some OSCC cases involved multiple anatomical sites simultaneously.
Up to a quarter of the lesions were HPV-positive (7/26, 26.9%).
Regarding the seven HPV+ lesions, most of the lesions occurred on the gum (4/7), in detail, two on the mandibular gingiva (C031), and two on the retromolar trigone area (C062). The remaining three lesions were located on the tongue (3/7), two on the border of the tongue (C021), and one on the anterior 2/3 of the tongue (C023) (Table 2).
As presented in Table 3, the oral rinse with Scope Oral Rinse and LolliSponge investigations highlighted the presence of different HPV genotypes among the HPV-positive OSCCs. Specifically, the investigation showed the presence of the same HPV genotype in the HPV-positive OSCCs, with only two exceptions. On one OSCC (#5) on the anterior 2/3 of the tongue (C023), the Scope Oral Rinse revealed the presence of genotype 38, while the LolliSponge showed the presence of genotype 17, even if the latter was 38-related. One OSCC (#12), on the border of the tongue (C021), was associated with the 66 HPV genotype by the Scope Oral Rinse, while the LolliSponge did not reveal any HPV in the sample.
Table 3.
Comprehensive HPV diagnostic results by anatomical site of OSCC and risk factors from the oral rinse with Scope Oral Rinse and LolliSponge. The site codes are categorized using NIH/SEER ICD-0-3.2 topographical classification codes; ‘-‘ indicates a negative result.
| No. Case | Sex | Site | Scope | LolliSponge | Local Trauma | Smoking Status | Alcohol Consumption |
|---|---|---|---|---|---|---|---|
| #1 | F | Upper gums–Lower gums | - | - | No | Never | Non-drinker |
| #2 | M | Buccal mucosa |
- | - | No | Current | Non-drinker |
| #3 | F | Buccal mucosa–Hard palate |
- | - | No | Never | Non-drinker |
| #4 | M | Buccal mucosa |
- | - | No | Former | Non-drinker |
| #5 | M | Anterior 2/3 of tongue | 38 | 17 (38 related) | No | Former | Non-drinker |
| #6 | M | Retromolar area | 16 | 16 | No | Never | Non-drinker |
| #7 | M | Retromolar area | 56 62 66 68 | 56 62 66 68 | No | Current | Moderate |
| #8 | M | Ventral surface of tongue–Lateral floor of mouth | - | - | No | Never | Non-drinker |
| #9 | F | Border of tongue | - | - | No | Never | Non-drinker |
| #10 | F | Border of tongue | - | - | No | Never | Non-drinker |
| #11 | F | Border of tongue | - | - | No | Never | Non-drinker |
| #12 | M | Border of tongue | 66 | - | No | Former | Non-drinker |
| #13 | F | Border of tongue | beta-HPV | beta-HPV | No | Never | Non-drinker |
| #14 | M | Border of tongue | - | - | No | Former | Non-drinker |
| #15 | M | Border of tongue | - | - | No | Former | Non-drinker |
| #16 | M | Border of tongue– Ventral surface of tongue |
- | - | Presence of Trauma | Never | Non-drinker |
| #17 | F | Buccal mucosa |
- | - | Presence of Trauma | Never | Non-drinker |
| #18 | F | Lower gums–Lateral floor of mouth | - | - | No | Never | Non-drinker |
| #19 | F | Lower gums | 120 | 120 | No | Never | Non-drinker |
| #20 | M | Dorsal surface of tongue | - | - | No | Never | Non-drinker |
| #21 | M | Border of tongue | - | - | No | Current | Non-drinker |
| #22 | M | Ventral surface of tongue–Anterior 2/3 of tongue | - | - | No | Never | Non-drinker |
| #23 | F | Upper gums | - | - | No | Never | Non-drinker |
| #24 | F | Lower gums | K1/K2 | K1/K2 | No | Never | Non-drinker |
| #25 | M | Lower gums | - | - | No | Never | Non-drinker |
| #26 | M | Lower gums | - | - | No | Never | Non-drinker |
Moreover, in two cases (#13, #19), by both oral samples tested, beta-HPV genotypes were identified, and only in one case, even if the HPV control generic band resulted positive by INNO-LiPA, genotyping it was not possible due to the low amount of amplified viral DNA.
Other risk factors, such as smoking status, alcohol consumption, and local trauma were detected in a minimum proportion of overall OSCC cases (11.5, 3.8%, and 7.7%, respectively). In HPV-positive OSCCs, no local trauma was identified, and only in one case of multiple HPV detection (#7), current smoking and moderate alcohol consumption were registered.
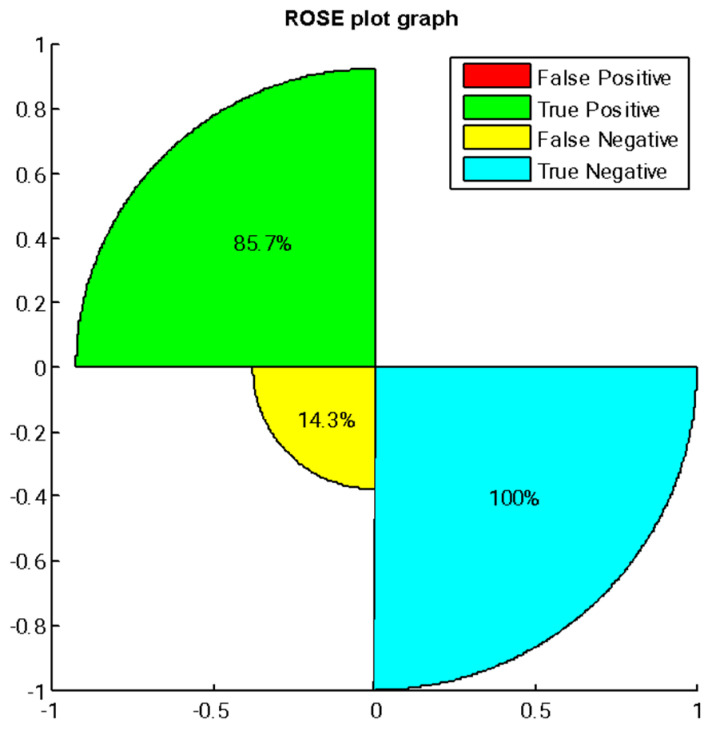
Table 4 presents the performance of the LolliSponge diagnostic to individualize patients HPV positive, through statistical indices such as sensitivity, specificity, and accuracy, considered as the gold standard of the Scope Oral Rinse diagnostic. LolliSponge detection shows high specificity (100%) sensitivity (85.7%), and accuracy (96.2%) considering oral rinse diagnostic results.
Table 4.
LolliSponge diagnostic test performance parameters about HPV-positive patients.
| Sensitivity (CI at 95%) |
Specificity (CI at 95%) |
Accuracy (CI at 95%) |
|
|---|---|---|---|
| LolliSponge | 85.7% (65.5%, 96.7%) |
100% (84%, 100%) |
96.2% (78.4%, 100%) |
Figure 1 represents a rose-plot graph, where we illustrated the percentages of true negative (100%), true positive (85.7%), false negative (14.3%), and false positive (0.0%). For the rose-plot, we used the area of segments of the circle to convey amounts, where the angle is constant i.e., divided 360 by the number of parameters considered thus, the square root of the radius is proportional to percentages.
Figure 1.
Rose-plot graph about the percentages of correct and incorrect cases diagnosed by LolliSponge diagnostic.
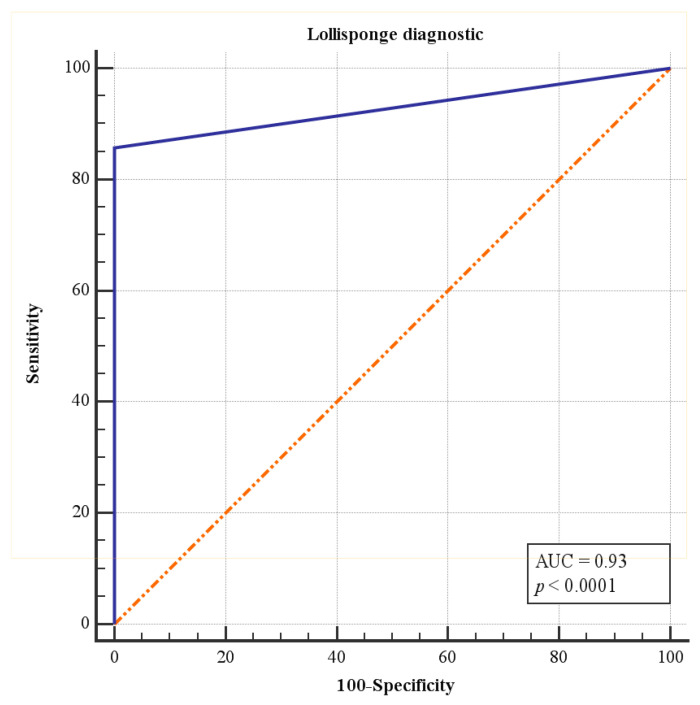
To obtain a complete sensitivity/specificity report, the Operating Characteristic (ROC) curve analysis was applied. Particularly, the graph reported the area under the ROC curve (AUC) and compared to the area under the red line equal to the value 0.5. Figure 2 presents the area AUC associated with the LolliSponge diagnostic performance which was significantly greater than 0.5 (0.93 vs. 0.5, p < 0.0001).
Figure 2.
ROC curve analysis for the LolliSponge diagnostic performance for HPV positive patients. AUC under the red line is equal to 0.5.
4. Discussion
The prevalence of HPV in OSCC varies widely across the world. Globally, it is estimated that approximately 25–30% of OSCC cases are HPV-positive, though this can range from as low as 6% in some countries to as high as 60% in others [5,32,33,34].
This discrepancy is attributed to the variability of oral HPV sampling and detection methods, as well as the lack of detail of the sites of lesions studied, not strictly including just the oral region but bordering the oropharyngeal region, which is known to be more susceptible to HPV infection [14]. Consequently, the use of HPV sampling techniques must be as site-specific as possible, such as fresh tissue of suspected lesion from biopsy, which would be preferred in research settings. Nevertheless, this procedure involves high professional experience and collaboration and is difficult to perform in conventional clinical practice, where more accessible approaches with the same diagnostic accuracy are required [26,35].
Non-invasive methods for collecting buccal epithelial cells include cotton swabs, cytology brushes, and oral rinse/mouthwashes. Oral rinse is a valuable sampling tool for detecting oral HPV due to its non-invasive nature, ease of use, and cost-effectiveness. It is widely adopted in clinical and research settings to investigate oral HPV status, both as a tool for screening in healthy people and as an adjunct test in patients with OSCC and potentially malignant oral lesions, to investigate the possible role of HPV in etiological prognosis and recurrence process of oral cancer [23,27,36].
However, it does have limitations regarding sample quality and sensitivity. Oral rinse samples, independent from carrier fluids used, might contain lower viral loads when compared to tissue biopsies, potentially leading to false negatives in individuals with low-level infections. Moreover, oral rinses might not effectively capture HPV infections localized in specific areas of the oral cavity, limiting their site-diagnostic accuracy [37,38]. Furthermore, an aspect that is not well valued is the potential difficulty of making a proper oral rinse according to standard protocol, by patients with muscle-function joint impairment by the presence of cancer (e.g., OSCC of the tongue or floor of the mouth).
The Copan LolliSponge is a novel saliva specimen collection system that can be particularly useful for diagnostic purposes, including DNA viral detection. It has been validated by employing a molecular test for the search for SARS-CoV-2 confirming it as an extremely robust collection sample (https://www.copangroup.com/product-ranges/lollisponge/, accessed on 20 August 2024).
This device simplifies the process by allowing individuals to collect their saliva samples using a sponge on a stick, which is held in the mouth for a few minutes. This method minimizes contamination risks and does not require rinse/gargle or spitting, making it suitable for self-and/or supported collection, without specific professional assistance. Moreover, the sample site specificity in HPV detection could be greatly improved by directing the sponge specifically on the lesion.
In our study, we compare the LolliSponge device with the oral rinse procedure using the most suitable medium for buccal cells/DNA collection tested in clinical and research testing (Scope Mouthwash) [30] to detect HPV status in a cohort of 26 patients with strictly oral OSCC. To the best of our knowledge, it is the first time that this device is proposed in the literature for oral HPV sampling and detection.
The overall practical advantages vs. disadvantages detected by the two salivary sample devices are detailed in Table 5.
Table 5.
Advantages and disadvantages of Scope Oral Rinse and LolliSponge applications.
| Application | Scope Oral Rinse | LolliSponge | ||
|---|---|---|---|---|
| Sample Collection | Non-invasive, painless, easy to perform; requires patient adherence to instructions. |

|
Non-invasive, painless, and less expensive; greater ease of use also without patient’s compliance, reducing sample collection time. |

|
| Patient Acceptance | Acceptance due to ease of use and less invasive nature. | Particularly beneficial for patients already experiencing oral discomfort. | ||
| Sample Adequacy | Samples cells from the entire oral cavity; increases likelihood of detecting non-localized HPV infections. | Ensures mucosal scraping, potentially leading to more adequate samples. | ||
| Potential Contamination | Risk of contamination from extraneous DNA, leading to false positives. | Reduced contamination risk compared to oral rinse. | ||
Regarding the performance of oral accuracy HPV diagnostic, between the two salivary samples, our results show a high overlapping diagnostic accuracy of LolliSponge compared to Scope Oral Rinse scores.
In one case of OSCC of the gum (#7), both the Scope Oral Rinse and LolliSponge demonstrated equal suitability to identify all hrHPV genotypes of multiple infections, likely supported by the presence of other risk factors (i.e., smoking and alcohol habits) and/or the susceptibility of the site to plaque-related inflammatory (i.e., gingivitis and/or periodontitis), supporting a hypothesis of a potential complex interplay between local periodontal diseases and oral HPV infections [39].
Only in one case the LolliSponge device was not able to confirm the same HPV positivity as detected by oral rinse. We believe that this discrepancy may be attributable to the full mouth sampling done with Scope Oral Rinse, possibly detecting the presence of HPV in other location than the OSCC site, where instead the LolliSponge had not identified any HPV-DNA.
To endorse this hypothesis, it would be useful to validate the HPV detection results by these two salivary diagnostics methods with those arising from an adjunctive site-specific sample (i.e., fresh tissue fragment lesion), as done previously [14]. This will be the future aim of our research group, together with the validation of salivary diagnostics methods’ accuracy in a larger sample.
This study has some limitations and potential biases. First, the sample size is relatively small, though it is considered appropriate given the preliminary nature of the study. For the same reason, other potential confounders, such as metastatic cancer status and disease stage, have not been analyzed at this stage of the investigation. Secondly, while non-invasive sampling methods such as oral rinse and sponge are practical, they may result in lower viral load detection when compared to tissue biopsies, potentially leading to false negatives. Additionally, although the LolliSponge device showed promising results, its use for HPV detection in oral cavity lesions has not yet been extensively validated in larger, more diverse populations. Finally, operator variability in sample collection and handling could introduce inconsistencies. Addressing these limitations in future research would enhance the reliability and applicability of the findings.
5. Conclusions
In conclusion, in this preliminary study, the LolliSponge diagnostic device, used for the first time to individualize HPV status in OSCC patients, showed excellent performance compared to the oral rinse gold standard. This potential, to be validated in the future, could open new horizons for the non-invasive study of HPV prevalence strictly in the oral cavity.
Author Contributions
Conceptualization, V.P., G.C. (Giuseppina Capra) and G.C. (Giuseppina Campisi); methodology, V.P., G.C. (Giuseppina Campisi) and G.C. (Giuseppina Capra); validation, V.P., G.C. (Giuseppina Campisi), G.G. and R.M.; formal analysis, N.S.; investigation, G.C. (Giuseppina Campisi), G.C. (Giuseppina Capra), M.B., F.B., G.P. and R.M; resources, V.P., G.C. (Giuseppina Campisi), A.F. and G.P.; data curation, V.P., G.C. (Giuseppina Campisi), M.B. and F.B.; writing—original draft preparation, V.P., M.B., A.F. and F.B.; writing, review, and editing, V.P., F.B., G.C. (Giuseppina Campisi), G.C. (Giuseppina Capra), G.P., G.G. and R.M.; visualization, V.P. and R.M.; supervision, V.P. and G.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University Hospital “Policlinico Paolo Giaccone” in Palermo, Italy (approval numbers #03/2013 and #04/2024).
Informed Consent Statement
All patients signed written informed consent prior to specimen collection.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Badwelan M., Muaddi H., Ahmed A., Lee K.T., Tran S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023;30:3721–3734. doi: 10.3390/curroncol30040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nokovitch L., Maquet C., Crampon F., Taihi I., Roussel L.-M., Obongo R., Virard F., Fervers B., Deneuve S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023;12:3264. doi: 10.3390/jcm12093264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faraji F., Rettig E.M., Tsai H., El Asmar M., Fung N., Eisele D.W., Fakhry C. The Prevalence of Human Papillomavirus in Oropharyngeal Cancer Is Increasing Regardless of Sex or Race, and the Influence of Sex and Race on Survival Is Modified by Human Papillomavirus Tumor Status. Cancer. 2019;125:761–769. doi: 10.1002/cncr.31841. [DOI] [PubMed] [Google Scholar]
- 4.Ko Y.C.K., Liu K.Y.P., Chen E., Zhu S.Y., Poh C.F. P53-Abnormal Oral Epithelial Dysplasias Are Associated with High Risks of Progression and Local Recurrence—A Retrospective Study in a Longitudinal Cohort. Mod. Pathol. 2024. in press . [DOI] [PubMed]
- 5.Timbang M.R., Sim M.W., Bewley A.F., Farwell D.G., Mantravadi A., Moore M.G. HPV-Related Oropharyngeal Cancer: A Review on Burden of the Disease and Opportunities for Prevention and Early Detection. Hum. Vaccines Immunother. 2019;15:1920–1928. doi: 10.1080/21645515.2019.1600985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesson H.W., Dunne E.F., Hariri S., Markowitz L.E. The Estimated Lifetime Probability of Acquiring Human Papillomavirus in the United States. Sex. Transm. Dis. 2014;41:660–664. doi: 10.1097/OLQ.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans. Biological Agents: Volume 100B, Human Papillomaviruses. International Agency for Research on Cancer; Geneva, Switzerland: 2012. pp. 255–295. [Google Scholar]
- 8.Kombe Kombe A.J., Li B., Zahid A., Mengist H.M., Bounda G.-A., Zhou Y., Jin T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health. 2021;8:552028. doi: 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monti E., Barbara G., Libutti G., Boero V., Parazzini F., Ciavattini A., Bogani G., Pignataro L., Magni B., Merli C.E.M., et al. A Clinician’s Dilemma: What Should Be Communicated to Women with Oncogenic Genital HPV and Their Partners Regarding the Risk of Oral Viral Transmission? BMC Womens Health. 2022;22:379. doi: 10.1186/s12905-022-01965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballini A., Cantore S., Fatone L., Montenegro V., De Vito D., Pettini F., Crincoli V., Antelmi A., Romita P., Rapone B., et al. Transmission of Nonviral Sexually Transmitted Infections and Oral Sex. J. Sex. Med. 2012;9:372–384. doi: 10.1111/j.1743-6109.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- 11.Queirós C., Costa J.B. da Oral Transmission of Sexually Transmissable Infections: A Narrative Review. Acta Med. Port. 2019;32:776–781. doi: 10.20344/amp.12191. [DOI] [PubMed] [Google Scholar]
- 12.Caltabiano M., Castiglioni M., De-Rose A. Changes in the Sexual Behaviour of Young People: Introduction. Genus. 2020;76:38. doi: 10.1186/s41118-020-00107-1. [DOI] [Google Scholar]
- 13.Bruno M.T., Boemi S., Caruso G., Sgalambro F., Ferlito S., Cavallaro A., Sudano M.C., Palumbo M. Oral HPV Infection in Women with HPV-Positive Cervix Is Closely Related to Oral Sex. Diagnostics. 2023;13:2096. doi: 10.3390/diagnostics13122096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panzarella V., Campisi G., Giardina Y., Maniscalco L., Capra G., Rodolico V., Di Fede O., Mauceri R. Low Frequency of Human Papillomavirus in Strictly Site-Coded Oral Squamous Cell Carcinomas, Using the Latest NHI/SEER-ICD Systems: A Pilot Observational Study and Critical Review. Cancers. 2021;13:4595. doi: 10.3390/cancers13184595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannovo N., Bianchini E., Gironacci L., Garbati E., Di Prospero F., Cingolani M., Scendoni R., Fedeli P. Sexually Transmitted Infections in Adolescents and Young Adults: A Cross Section of Public Health. Int. J. Env. Res. Public Health. 2024;21:501. doi: 10.3390/ijerph21040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazra A., Collison M.W., Davis A.M. CDC Sexually Transmitted Infections Treatment Guidelines, 2021. JAMA. 2022;327:870. doi: 10.1001/jama.2022.1246. [DOI] [PubMed] [Google Scholar]
- 17.Wierzbicka M., San Giorgi M.R.M., Dikkers F.G. Transmission and Clearance of Human Papillomavirus Infection in the Oral Cavity and Its Role in Oropharyngeal Carcinoma—A Review. Rev. Med. Virol. 2023;33:e2337. doi: 10.1002/rmv.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimi S. HPV-Related Squamous Cell Carcinoma of Oropharynx: A Review. J. Clin. Pathol. 2020;73:624–629. doi: 10.1136/jclinpath-2020-206686. [DOI] [PubMed] [Google Scholar]
- 19.Berman T.A., Schiller J.T. Human Papillomavirus in Cervical Cancer and Oropharyngeal Cancer: One Cause, Two Diseases. Cancer. 2017;123:2219–2229. doi: 10.1002/cncr.30588. [DOI] [PubMed] [Google Scholar]
- 20.CDC: Centers for Disease Control and Prevention Cancers Linked with HPV Each Year. [(accessed on 19 August 2024)]; Available online: https://www.cdc.gov/cancer/hpv/cases.html?CDC_AAref_Val=https://www.cdc.gov/
- 21.Lechner M., Liu J., Masterson L., Fenton T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022;19:306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeggblom L., Ramqvist T., Tommasino M., Dalianis T., Näsman A. Time to Change Perspectives on HPV in Oropharyngeal Cancer. A Systematic Review of HPV Prevalence per Oropharyngeal Sub-Site the Last 3 Years. Papillomavirus Res. 2017;4:1–11. doi: 10.1016/j.pvr.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gipson B.J., Robbins H.A., Fakhry C., D’Souza G. Sensitivity and Specificity of Oral HPV Detection for HPV-Positive Head and Neck Cancer. Oral Oncol. 2018;77:52–56. doi: 10.1016/j.oraloncology.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buttà M., Serra N., Panzarella V., Fasciana T.M.A., Campisi G., Capra G. Orogenital Human Papillomavirus Infection and Vaccines: A Survey of High- and Low-Risk Genotypes Not Included in Vaccines. Vaccines. 2023;11:1466. doi: 10.3390/vaccines11091466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauceri R., Coppini M., Vacca D., Bertolazzi G., Panzarella V., Di Fede O., Tripodo C., Campisi G. Salivary Microbiota Composition in Patients with Oral Squamous Cell Carcinoma: A Systematic Review. Cancers. 2022;14:5441. doi: 10.3390/cancers14215441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung R.S., Wong S., Lin D., Kokot N.C., Sinha U.K., Han A.Y. Mechanisms of Crosstalk between the Oropharyngeal Microbiome and Human Papillomavirus in Oropharyngeal Carcinogenesis: A Mini Review. Front. Oncol. 2024;14:1425545. doi: 10.3389/fonc.2024.1425545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loermann G., Kolb M., Prascevic D., Siemert J., Wiegand S., Zebralla V., Pirlich M., Stöhr M., Dietz A., Wald T., et al. High-Risk Human Papillomavirus (HR-HPV) DNA Detection in Mouthwashes for Diagnosis of HPV-Driven Oropharynx Cancer and Its Curative Therapy—A Feasibility Study. J. Clin. Med. 2022;11:5509. doi: 10.3390/jcm11195509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castañeda-Avila M.A., Pérez C.M., Vivaldi-Oliver J.A., Díaz-Toro E.C., Ortiz A.P. Comparison of Oral Human Papilloma Virus Detection Methods among Hispanic Adults. Clin. Exp. Dent. Res. 2022;8:169–175. doi: 10.1002/cre2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogels C.B.F., Watkins A.E., Harden C.A., Brackney D.E., Shafer J., Wang J., Caraballo C., Kalinich C.C., Ott I.M., Fauver J.R., et al. SalivaDirect: A Simplified and Flexible Platform to Enhance SARS-CoV-2 Testing Capacity. Med. 2021;2:263–280.e6. doi: 10.1016/j.medj.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heath E.M., Morken N.W., Campbell K.A., Tkach D., Boyd E.A., Strom D.A. Use of Buccal Cells Collected in Mouthwash as a Source of DNA for Clinical Testing. Arch. Pathol. Lab. Med. 2001;125:127–133. doi: 10.5858/2001-125-0127-UOBCCI. [DOI] [PubMed] [Google Scholar]
- 31.Fuessel Haws A.L., He Q., Rady P.L., Zhang L., Grady J., Hughes T.K., Stisser K., Konig R., Tyring S.K. Nested PCR with the PGMY09/11 and GP5+/6+ Primer Sets Improves Detection of HPV DNA in Cervical Samples. J. Virol. Methods. 2004;122:87–93. doi: 10.1016/j.jviromet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Katirachi S.K., Grønlund M.P., Jakobsen K.K., Grønhøj C., von Buchwald C. The Prevalence of HPV in Oral Cavity Squamous Cell Carcinoma. Viruses. 2023;15:451. doi: 10.3390/v15020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buttà M., Serra N., Mannino E., Panzarella V., Cabibi D., Campisi G., Pistoia D., Capra G. Evaluation of the Prevalence and Potential Impact of HPV Vaccines in Patients with and without Oral Diseases: A Ten-Year Retrospective Study. Arch. Med. Res. 2024;55:103059. doi: 10.1016/j.arcmed.2024.103059. [DOI] [PubMed] [Google Scholar]
- 34.Syrjänen S., Lodi G., von Bültzingslöwen I., Aliko A., Arduino P., Campisi G., Challacombe S., Ficarra G., Flaitz C., Zhou H., et al. Human Papillomaviruses in Oral Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Oral Dis. 2011;17:58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 35.Verma G., Aggarwal N., Chhakara S., Tyagi A., Vishnoi K., Jadli M., Singh T., Goel A., Pandey D., Sharma A., et al. Detection of Human Papillomavirus Infection in Oral Cancers Reported at Dental Facility: Assessing the Utility of FFPE Tissues. Med. Oncol. 2022;39:13. doi: 10.1007/s12032-021-01608-5. [DOI] [PubMed] [Google Scholar]
- 36.Mauceri R., Coppini M., Vacca D., Bertolazzi G., Cancila V., Tripodo C., Campisi G. No Clear Clustering Dysbiosis from Salivary Microbiota Analysis by Long Sequencing Reads in Patients Affected by Oral Squamous Cell Carcinoma: A Single Center Study. Cancers. 2023;15:4211. doi: 10.3390/cancers15174211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinau M., Reddy D., Sumbry A., Reznik D., Gunthel C.J., del Rio C., Lennox J.L., Unger E.R., Nguyen M.L.T. Oral Sampling and Human Papillomavirus Genotyping in HIV-infected Patients. J. Oral Pathol. Med. 2012;41:288–291. doi: 10.1111/j.1600-0714.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 38.Sahebjamee M., Boorghani M., Ghaffari S.-R., AtarbashiMoghadam F., Keyhani A. Human Papillomavirus in Saliva of Patients with Oral Squamous Cell Carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2009;14:e525–e528. doi: 10.4317/medoral.14.e525. [DOI] [PubMed] [Google Scholar]
- 39.Aldhubaiei H., Alzuabi M.M., Marafi Y., Alzalzalah F., Aljalahmah M., Natto Z.S. Association of Periodontitis and Various Genotypes of Human Papillomavirus in Oral Rinse Specimens. Cureus. 2024;16:e60190. doi: 10.7759/cureus.60190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.