Abstract
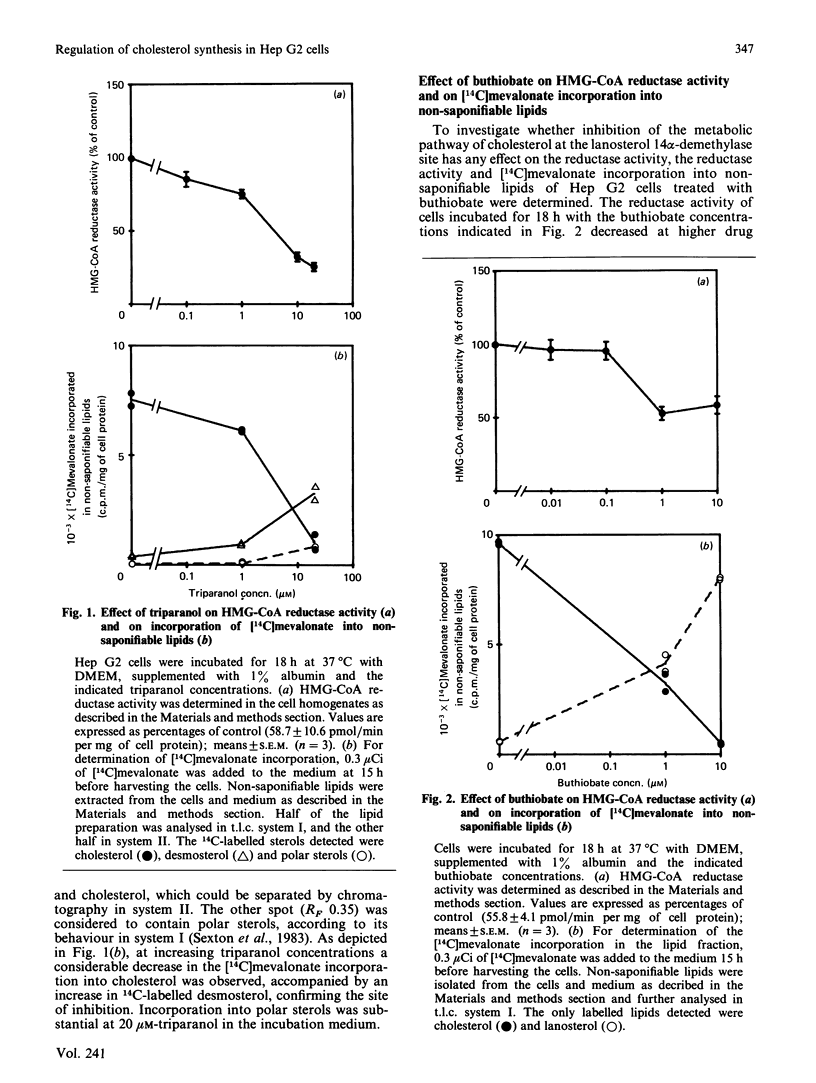
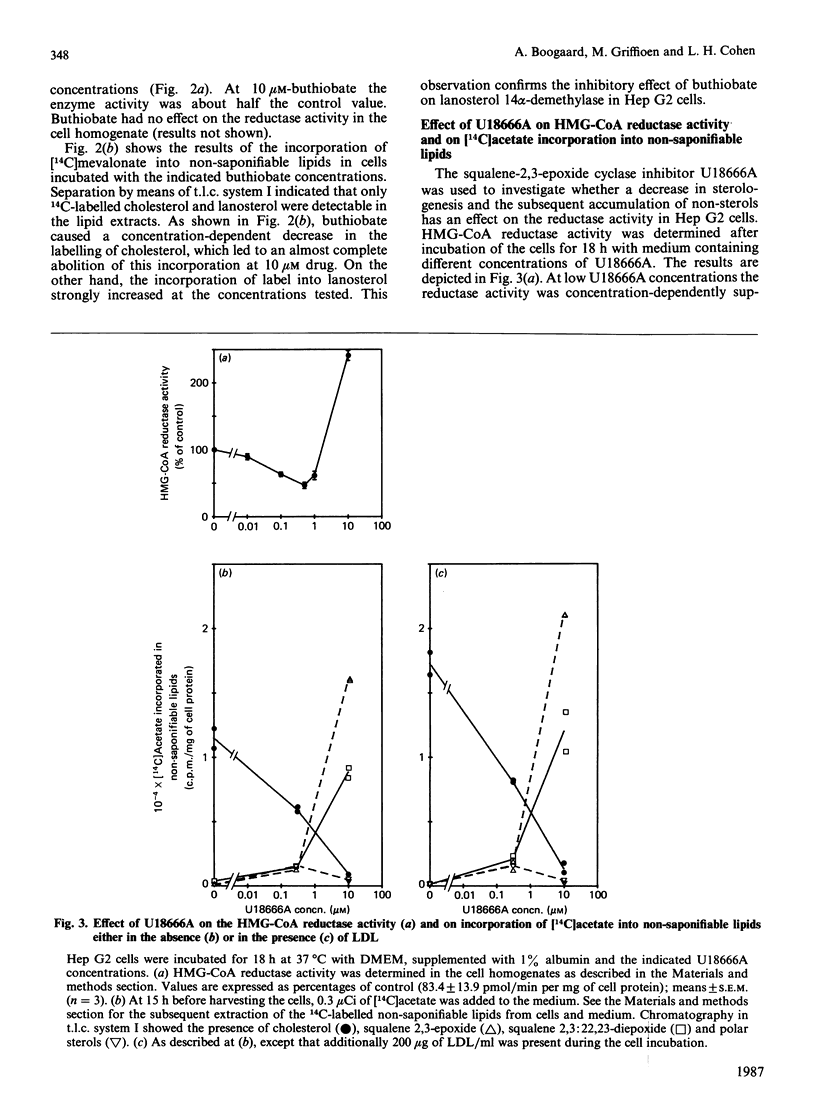
Incubating Hep G2 cells for 18 h with triparanol, buthiobate and low concentrations (less than 0.5 microM) of U18666A, inhibitors of desmosterol delta 24-reductase, of lanosterol 14 alpha-demethylase and of squalene-2,3-epoxide cyclase (EC 5.4.99.7) respectively, resulted in a decrease of the HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase activity. However, U18666A at concentrations higher than 3 microM increased the HMG-CoA reductase activity in a concentration-dependent manner. None of these inhibitors influenced directly the reductase activity in Hep G2 cell homogenates. Analysis by t.l.c. of 14C-labelled non-saponifiable lipids formed from either [14C]acetate or [14C]mevalonate during the cell incubations confirmed the sites of action of the drugs used. Beside the 14C-labelled substrates of the blocked enzymes and 14C-labelled cholesterol, another non-saponifiable lipid fraction was observed, which behaves as polar sterols on t.l.c. This was the case with triparanol and at those concentrations of U18666A that decreased the reductase activity, suggesting that polar sterols may play a role in suppressing the reductase activity. In the presence of 30 microM-U18666A (sterol formation blocked) the increase produced by simultaneously added compactin could be prevented by addition of mevalonate. This indicates the existence of a non-sterol mevalonate-derived effector in addition to a sterol-dependent regulation. LDL (low-density lipoprotein), which was shown to be able to decrease the compactin-induced increase in reductase activity, could not prevent the U18666A-induced increase. On the contrary, LDL enhanced the U18666A effect, showing that the LDL regulation is not merely the result of introducing cholesterol to the cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J., STEINBERG D., VROMAN H. E., THOMPSON M. J., MOSETTIG E. Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. J Biol Chem. 1960 Nov;235:3123–3126. [PubMed] [Google Scholar]
- Aoyama Y., Yoshida Y., Hata S., Nishino T., Katsuki H. Buthiobate: a potent inhibitor for yeast cytochrome P-450 catalyzing 14 alpha-demethylation of lanosterol. Biochem Biophys Res Commun. 1983 Sep 15;115(2):642–647. doi: 10.1016/s0006-291x(83)80192-2. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Chang T. Y., Schiavoni E. S., Jr, McCrae K. R., Nelson J. A., Spencer T. A. Inhibition of cholesterol biosynthesis in Chinese hamster ovary cells by 4,4,10 beta-trimethyl-trans-decal-3 beta-ol. A specific 2,3-oxidosqualene cyclase inhibitor. J Biol Chem. 1979 Nov 25;254(22):11258–11263. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cohen D. C., Massoglia S. L., Gospodarowicz D. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in vascular endothelial cells. Separate sterol and non-sterol components. J Biol Chem. 1982 Sep 25;257(18):11106–11112. [PubMed] [Google Scholar]
- Cohen L. H., Griffioen M., Havekes L., Schouten D., van Hinsbergh V., Kempen H. J. Effects of compactin, mevalonate and low-density lipoprotein on 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity and low-density-lipoprotein-receptor activity in the human hepatoma cell line Hep G2. Biochem J. 1984 Aug 15;222(1):35–39. doi: 10.1042/bj2220035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Measurement of the absolute rates of cholesterol biosynthesis in isolated rat liver cells. Biochem J. 1977 Feb 15;162(2):321–330. doi: 10.1042/bj1620321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Sexton R. C., Rudney H. Modulation of regulatory oxysterol formation and low density lipoprotein suppression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity by ketoconazole. A role for cytochrome P-450 in the regulation of HMG-CoA reductase in rat intestinal epithelial cells. J Biol Chem. 1986 Jun 25;261(18):8348–8356. [PubMed] [Google Scholar]
- Havekes L., van Hinsbergh V., Kempen H. J., Emeis J. The metabolism in vitro of human low-density lipoprotein by the human hepatoma cell line Hep G2. Biochem J. 1983 Sep 15;214(3):951–958. doi: 10.1042/bj2140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel C., Hansbury E., Scallen T. J., Watson J. A. Regulation of cholesterol synthesis in primary rat hepatocyte culture cells. Possible regulatory site at sterol demethylation. J Biol Chem. 1979 Oct 10;254(19):9573–9582. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Panini S. R., Sexton R. C., Rudney H. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by oxysterol by-products of cholesterol biosynthesis. Possible mediators of low density lipoprotein action. J Biol Chem. 1984 Jun 25;259(12):7767–7771. [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. The relationship between the rate of hepatic sterol synthesis and the incorporation of [3H]water. J Lipid Res. 1983 Oct;24(10):1321–1328. [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Schroepfer G. J., Jr Sterol biosynthesis. Annu Rev Biochem. 1981;50:585–621. doi: 10.1146/annurev.bi.50.070181.003101. [DOI] [PubMed] [Google Scholar]
- Sexton R. C., Panini S. R., Azran F., Rudney H. Effects of 3 beta-[2-(diethylamino)ethoxy]androst-5-en-17-one on the synthesis of cholesterol and ubiquinone in rat intestinal epithelial cell cultures. Biochemistry. 1983 Dec 6;22(25):5687–5692. doi: 10.1021/bi00294a001. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Hoak J. C. An improved method for the addition of long-chain free fatty acid to protein solutions. Anal Biochem. 1969 Nov;32(2):297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Aoyama Y. Effects of buthiobate, a fungicide, on cytochrome P-450 of rat liver microsomes. J Pharmacobiodyn. 1985 Jun;8(6):432–439. doi: 10.1248/bpb1978.8.432. [DOI] [PubMed] [Google Scholar]


