Abstract
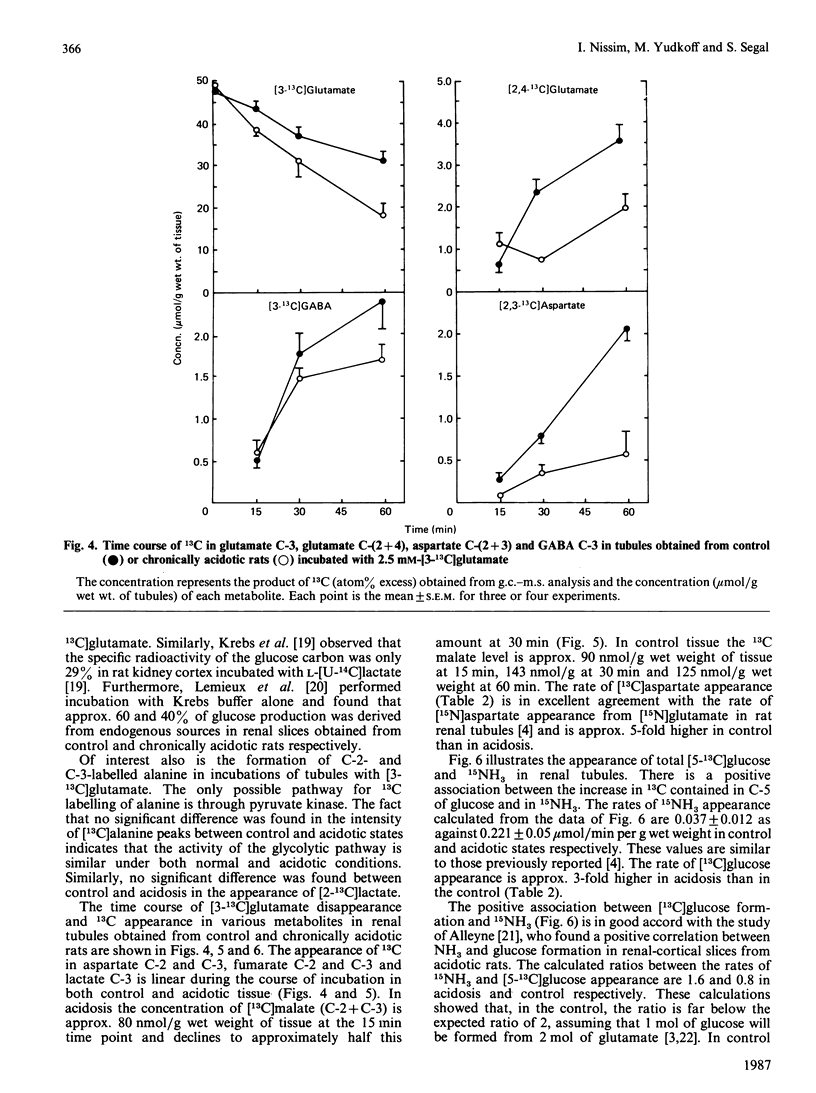
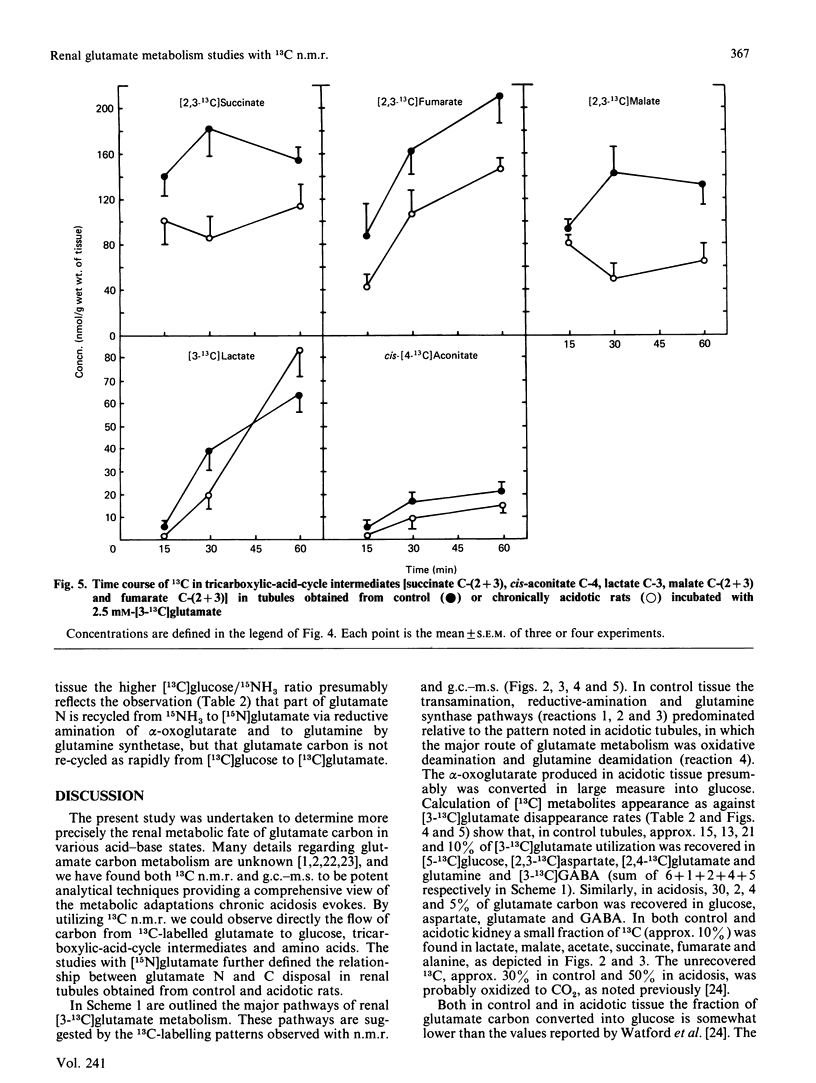
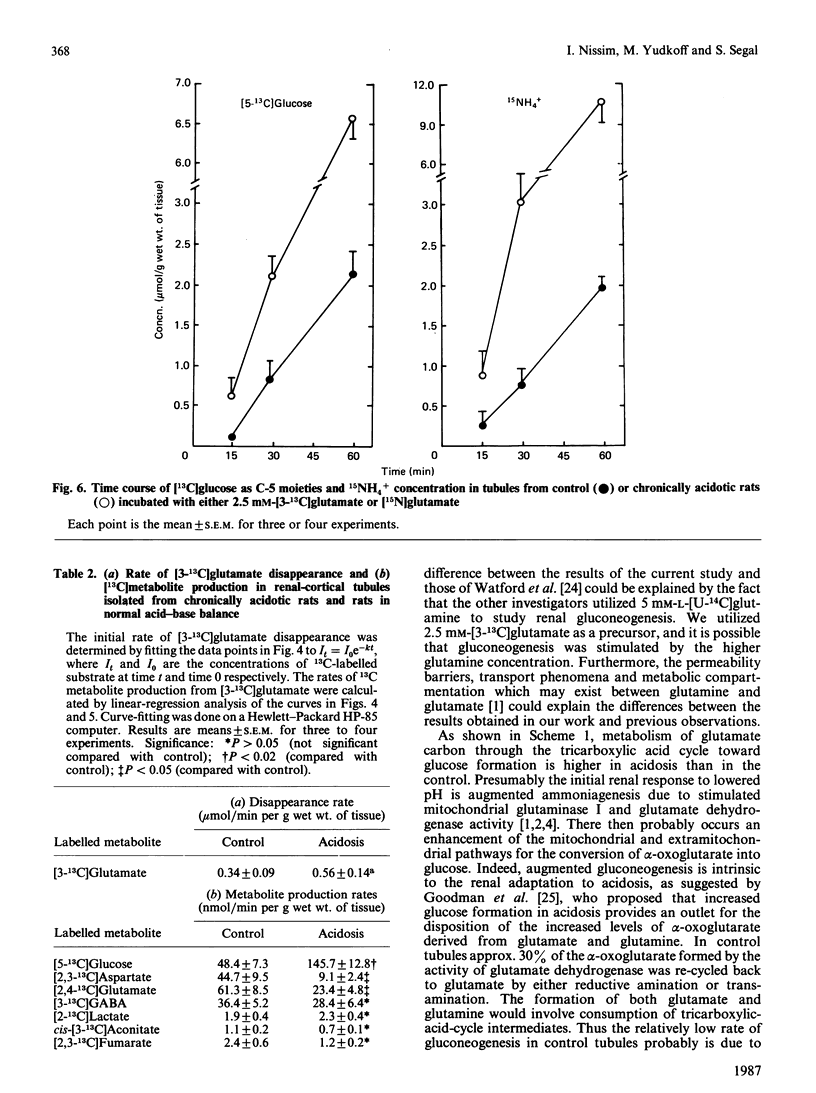
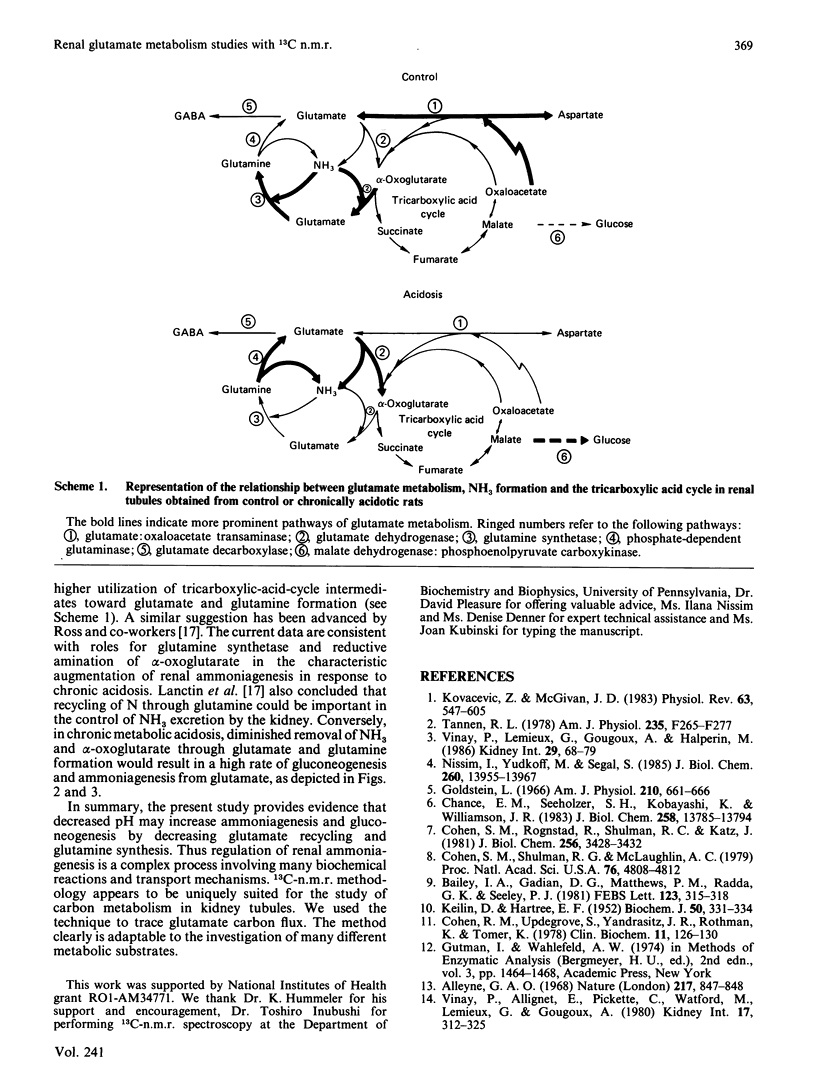
13C-n.m.r. spectroscopy and g.c.-m.s. were used to determine the metabolic fate of glutamate carbon in rat kidney. The main purpose was to characterize the effect of chronic metabolic acidosis on the utilization of glutamate carbon. Renal tubules obtained from normal and chronically acidotic rats were incubated in Krebs buffer, pH 7.4, in the presence of 2.5 mM-[3-13C]glutamate. During the course of incubation the concentrations of total glucose and NH3 were significantly (P less than 0.05) higher in tissue from acidotic rats. The levels of some tricarboxylic-acid-cycle intermediates were higher (P less than 0.05) in control tissue. In control tissue, 13C-n.m.r. spectra demonstrated a significantly higher rate of 13C appearance of aspartate, glutamine and [2,4-13C]glutamate. However, in acidosis the resonances of [13C]glucose carbon atoms were significantly higher. In the control, approx. 15% of glutamate carbon was accounted for by [13C]glucose formation as against 30% in chronic acidosis. However, in control tissue, 44% of glutamate carbon utilization was accounted for by recycling to glutamate and formation of aspartate, glutamine and GABA. In acidosis, only 11% was so recovered. Analysis of 15NH3 formation during the course of incubation with 2.5 mM-[15N]glutamate demonstrated a positive association between the appearance of [13C]glucose and 15NH3 both in the control and in acidosis. The data suggest that the control of gluconeogenesis and ammoniagenesis in acidosis is, in part, referable to a diminution in the rate of the reductive amination of alpha-oxoglutarate, that of the transamination reaction and that of glutamine synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleyne G. A. Concentrations of metabolic intermediates in kidneys of rats with metabolic acidosis. Nature. 1968 Mar 2;217(5131):847–848. doi: 10.1038/217847a0. [DOI] [PubMed] [Google Scholar]
- Alleyne G. A. Renal metabolic response to acid-base changes. II. The early effects of metabolic acidosis on renal metabolism in the rat. J Clin Invest. 1970 May;49(5):943–951. doi: 10.1172/JCI106314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey I. A., Gadian D. G., Matthews P. M., Radda G. K., Seeley P. J. Studies of metabolism in the isolated, perfused rat heart using 13C NMR. FEBS Lett. 1981 Jan 26;123(2):315–318. doi: 10.1016/0014-5793(81)80317-1. [DOI] [PubMed] [Google Scholar]
- Chance E. M., Seeholzer S. H., Kobayashi K., Williamson J. R. Mathematical analysis of isotope labeling in the citric acid cycle with applications to 13C NMR studies in perfused rat hearts. J Biol Chem. 1983 Nov 25;258(22):13785–13794. [PubMed] [Google Scholar]
- Cohen S. M., Rognstad R., Shulman R. G., Katz J. A comparison of 13C nuclear magnetic resonance and 14C tracer studies of hepatic metabolism. J Biol Chem. 1981 Apr 10;256(7):3428–3432. [PubMed] [Google Scholar]
- Cohen S. M., Shulman R. G., McLaughlin A. C. Effects of ethanol on alanine metabolism in perfused mouse liver studied by 13C NMR. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4808–4812. doi: 10.1073/pnas.76.10.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R. M., Updegrove S., Yandrasitz J. R., Rothman R., Tomer K. Evaluation of continuous solvent extraction of organic acids from biological fluids. Clin Biochem. 1978 Jun;11(3):126–130. doi: 10.1016/s0009-9120(78)90168-6. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin M. L., Jungas R. L., Pichette C., Goldstein M. B. A quantitative analysis of renal ammoniagenesis and energy balance: a theoretical approach. Can J Physiol Pharmacol. 1982 Dec;60(12):1431–1435. doi: 10.1139/y82-212. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Specificity of glucose oxidase (notatin). Biochem J. 1952 Jan;50(3):331–341. doi: 10.1042/bj0500331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic Z., McGivan J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983 Apr;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster G., Mohyuddin F., Scriver C. R., Whelan D. T. A -aminobutyrate pathway in mammalian kidney cortex. Biochim Biophys Acta. 1973 Feb 28;297(2):229–240. doi: 10.1016/0304-4165(73)90069-x. [DOI] [PubMed] [Google Scholar]
- Lanctin H., Brosnan J. T., Ross B. D. Glutamine synthesis in the perfused rat kidney and in isolated rat cortical tubules: regulation by pH. Clin Sci (Lond) 1985 Dec;69(6):701–707. doi: 10.1042/cs0690701. [DOI] [PubMed] [Google Scholar]
- Lemieux G., Achkar M., Vinay P., Gougoux A. Characteristics of ammoniagenesis and gluconeogenesis by the diabetic kidney. In vitro studies in the rat. Contrib Nephrol. 1982;31:23–28. doi: 10.1159/000406612. [DOI] [PubMed] [Google Scholar]
- Nissim I., Yudkoff M., Segal S. Metabolism of glutamine and glutamate by rat renal tubules. Study with 15N and gas chromatography-mass spectrometry. J Biol Chem. 1985 Nov 15;260(26):13955–13967. [PubMed] [Google Scholar]
- Rognstad R., Katz J. Gluconeogenesis in the kidney cortex. Quantitative estimation of carbon flow. J Biol Chem. 1972 Oct 10;247(19):6047–6054. [PubMed] [Google Scholar]
- Ross B. D., Espinal J., Silva P. Glucose metabolism in renal tubular function. Kidney Int. 1986 Jan;29(1):54–67. doi: 10.1038/ki.1986.8. [DOI] [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Vinay P., Allignet E., Pichette C., Watford M., Lemieux G., Gougoux A. Changes in renal metabolite profile and ammoniagenesis during acute and chronic metabolic acidosis in dog and rat. Kidney Int. 1980 Mar;17(3):312–325. doi: 10.1038/ki.1980.37. [DOI] [PubMed] [Google Scholar]
- Vinay P., Lemieux G., Gougoux A., Halperin M. Regulation of glutamine metabolism in dog kidney in vivo. Kidney Int. 1986 Jan;29(1):68–79. doi: 10.1038/ki.1986.9. [DOI] [PubMed] [Google Scholar]
- Vinay P., Mapes J. P., Krebs H. A. Fate of glutamine carbon in renal metabolism. Am J Physiol. 1978 Feb;234(2):F123–F129. doi: 10.1152/ajprenal.1978.234.2.F123. [DOI] [PubMed] [Google Scholar]
- Watford M., Vinay P., Lemieux G., Gougoux A. The regulation of glucose and pyruvate formation from glutamine and citric-acid-cycle intermediates in the kidney cortex of rats, dogs, rabbits and guinea pigs. Biochem J. 1980 Jun 15;188(3):741–748. doi: 10.1042/bj1880741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


