Abstract
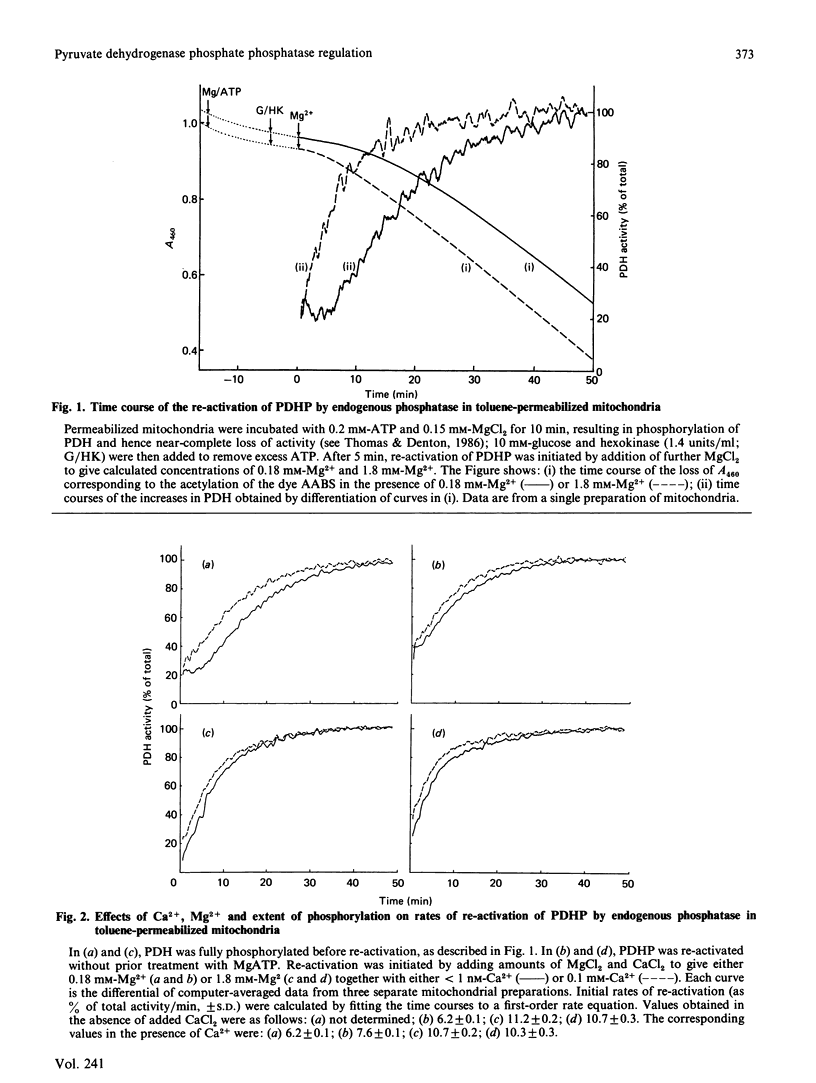
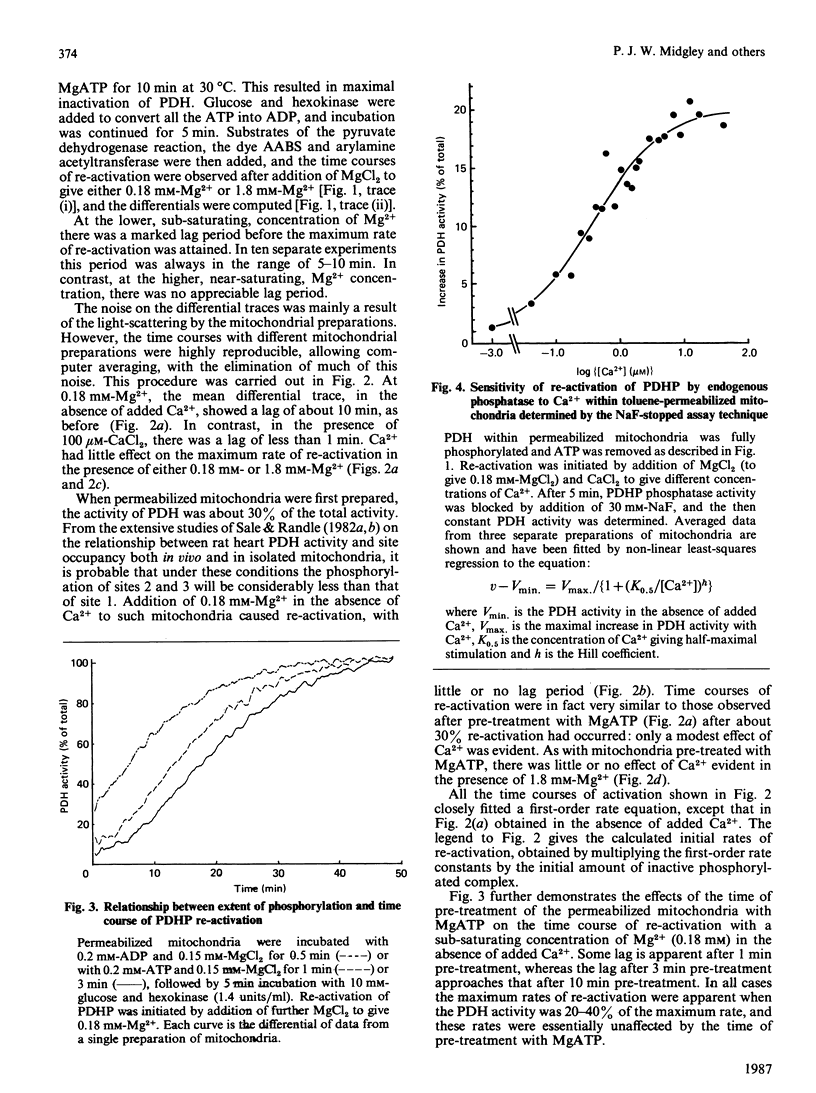
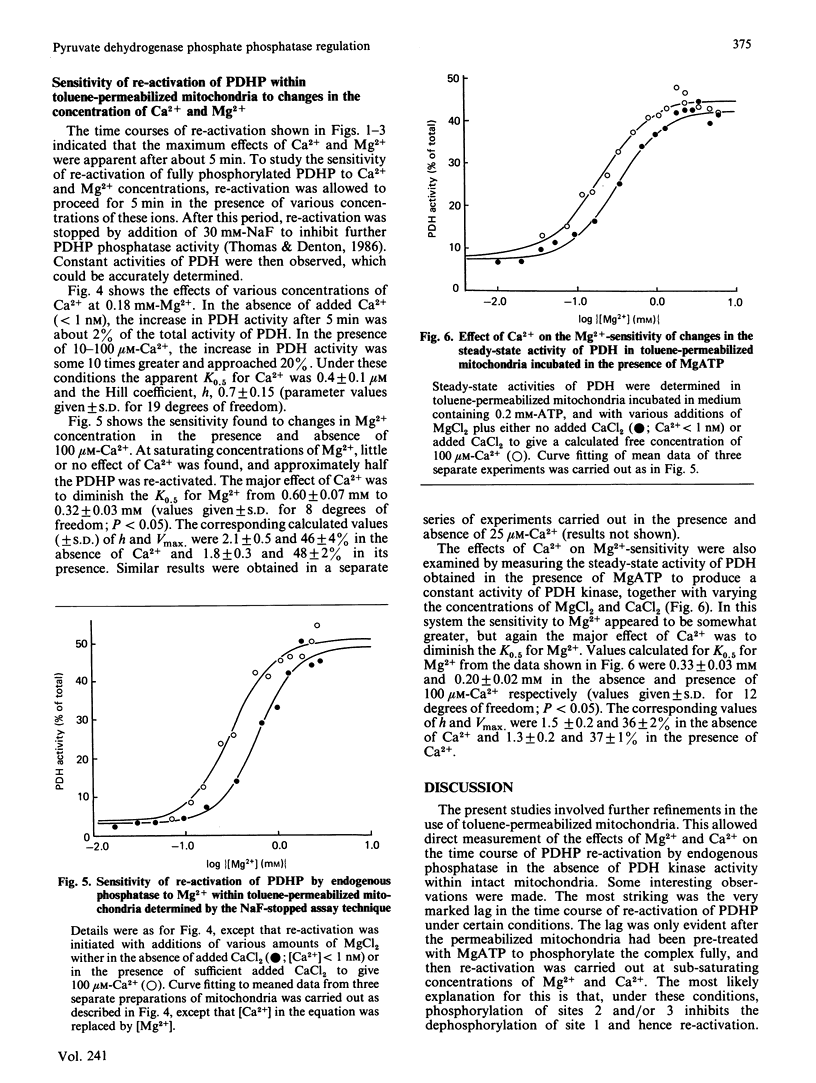
Mitochondria from rat epididymal white adipose tissue were made permeable to small molecules by toluene treatment and were used to investigate the effects of Mg2+ and Ca2+ on the re-activation of pyruvate dehydrogenase phosphate by endogenous phosphatase. Re-activation of fully phosphorylated enzyme after addition of 0.18 mM-Mg2+ showed a marked lag of 5-10 min before a maximum rate of reactivation was achieved. Increasing the Mg2+ concentration to 1.8 mM (near saturating) or the addition of 100 microM-Ca2+ resulted in loss of the lag phase, which was also greatly diminished if pyruvate dehydrogenase was not fully phosphorylated. It is concluded that, within intact mitochondria, phosphatase activity is highly sensitive to the degree of phosphorylation of pyruvate dehydrogenase and that the major effect of Ca2+ may be to overcome the inhibitory effects of sites 2 and 3 on the dephosphorylation of site 1. Apparent K0.5 values for Mg2+ and Ca2+ were determined from the increases in pyruvate dehydrogenase activity observed after 5 min. The K0.5 for Mg2+ was diminished from 0.60 mM at less than 1 nM-Ca2+ to 0.32 mM at 100 microM-Ca2+; at 0.18 mM-Mg2+, the K0.5 for Ca2+ was 0.40 microM. Ca2+ had little or no effect at saturating Mg2+ concentrations. Since effects of Ca2+ are readily observed in intact coupled mitochondria, it follows that Mg2+ concentrations within mitochondria are sub-saturating for pyruvate dehydrogenase phosphate phosphatase and hence less than 0.5 mM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Corkey B. E., Duszynski J., Rich T. L., Matschinsky B., Williamson J. R. Regulation of free and bound magnesium in rat hepatocytes and isolated mitochondria. J Biol Chem. 1986 Feb 25;261(6):2567–2574. [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Marshall S. E. Persistence of the effect of insulin on pyruvate dehydrogenase activity in rat white and brown adipose tissue during the preparation and subsequent incubation of mitochondria. Biochem J. 1984 Jan 15;217(2):441–452. doi: 10.1042/bj2170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C. A survey of readily available chelators for buffering calcium ion concentrations in physiological solutions. Cell Calcium. 1983 Feb;4(1):33–46. doi: 10.1016/0143-4160(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978 Nov;191(1):65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Studies on inactivation of pyruvate dehydrogenase by palmitoylcarnitine oxidation in isolated rat heart mitochondria. J Biol Chem. 1977 Mar 10;252(5):1552–1560. [PubMed] [Google Scholar]
- Hansford R. G. Studies on the effects of coenzyme A-SH: acetyl coenzyme A, nicotinamide adenine dinucleotide: reduced nicotinamide adenine dinucleotide, and adenosine diphosphate: adenosine triphosphate ratios on the interconversion of active and inactive pyruvate dehydrogenase in isolated rat heart mitochondria. J Biol Chem. 1976 Sep 25;251(18):5483–5489. [PubMed] [Google Scholar]
- Hughes W. A., Brownsey R. W., Denton R. M. Studies on the incorporation of [32P]phosphate into pyruvate dehydrogenase in intact rat fat-cells. Effects of insulin. Biochem J. 1980 Nov 15;192(2):469–481. doi: 10.1042/bj1920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Kearns A. Dephosphorylation of pig heart pyruvate dehydrogenase phosphate complexes by pig heart pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1981 Apr 1;195(1):51–59. doi: 10.1042/bj1950051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J. Role of multi-site phosphorylation in regulation of pig heart pyruvate dehydrogenase phosphatase. FEBS Lett. 1979 Dec 15;108(2):485–488. doi: 10.1016/0014-5793(79)80594-3. [DOI] [PubMed] [Google Scholar]
- Marshall S. E., McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat epididymal adipose tissue. Evidence against a role for Ca2+ in the activation of pyruvate dehydrogenase by insulin. Biochem J. 1984 Feb 15;218(1):249–260. doi: 10.1042/bj2180249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M., Pask H. T., Randle P. J. Mechanisms regulating adipose-tissue pyruvate dehydrogenase. Biochem J. 1972 Sep;129(3):763–773. doi: 10.1042/bj1290763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlib M. A., Shannon W. A., Jr, Srere P. A. Measurement of matrix enzyme activity in isolated mitochondria made permeable with toluene. Arch Biochem Biophys. 1977 Jan 30;178(2):396–407. doi: 10.1016/0003-9861(77)90209-0. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem J. 1985 Nov 1;231(3):581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem J. 1980 Jul 15;190(1):95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Pratt M. L., Roche T. E. Mechanism of pyruvate inhibition of kidney pyruvate dehydrogenasea kinase and synergistic inhibition by pyruvate and ADP. J Biol Chem. 1979 Aug 10;254(15):7191–7196. [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Analysis of site occupancies in [32P]phosphorylated pyruvate dehydrogenase complexes by aspartyl-prolyl cleavage of tryptic phosphopeptides. Eur J Biochem. 1981 Dec;120(3):535–540. doi: 10.1111/j.1432-1033.1981.tb05733.x. [DOI] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Occupancy of phosphorylation sites in pyruvate dehydrogenase phosphate complex in rat heart in vivo. Relation to proportion of inactive complex and rate of re-activation by phosphatase. Biochem J. 1982 Aug 15;206(2):221–229. doi: 10.1042/bj2060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Occupancy of sites of phosphorylation in inactive rat heart pyruvate dehydrogenase phosphate in vivo. Biochem J. 1981 Mar 1;193(3):935–946. doi: 10.1042/bj1930935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Role of individual phosphorylation sites in inactivation of pyruvate dehydrogenase complex in rat heart mitochondria. Biochem J. 1982 Apr 1;203(1):99–108. doi: 10.1042/bj2030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A. Measurements of cation transport with metallochromic indicators. Methods Enzymol. 1979;56:301–338. doi: 10.1016/0076-6879(79)56030-3. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Hutson N. J., Kerbey A. L., Randle P. J. Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1978 Feb 1;169(2):433–435. doi: 10.1042/bj1690433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Kerbey A. L., Randle P. J., Waller C. A., Reid K. B. Amino acid sequences around the sites of phosphorylation in the pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):419–426. doi: 10.1042/bj1810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Simister N. E. Role of multisite phosphorylation in the regulation of ox kidney pyruvate dehydrogenase complex. FEBS Lett. 1980 Mar 10;111(2):299–302. doi: 10.1016/0014-5793(80)80814-3. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Denton R. M. Use of toluene-permeabilized mitochondria to study the regulation of adipose tissue pyruvate dehydrogenase in situ. Further evidence that insulin acts through stimulation of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1986 Aug 15;238(1):93–101. doi: 10.1042/bj2380093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. P., Diggle T. A., Denton R. M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J. 1986 Aug 15;238(1):83–91. doi: 10.1042/bj2380083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


