Abstract
The constituent polypeptide chains I, II, III and IV of the giant extracellular haemoglobin of the oligochaete Lumbricus terrestris were isolated by mono Q ion-exchange chromatography and C8 reverse-phase chromatography. The N-terminal amino acid sequences of Lumbricus chains I, III and IV were determined and aligned with those of Lumbricus chain II and the four chains of the extracellular haemoglobin of the polychaete Tylorrhynchus heterochaetus. Three invariant amino acid residues, Cys-7, Val-15 and Trp-19, were found to occur in the N-terminal segments (17-22 residues) of the eight chains of Lumbricus and Tylorrhynchus haemoglobins. In addition, it was found that the eight sequences could be separated into two groups: 'A', consisting of Lumbricus chains I and II and Tylorrhynchus chains I and IIA, having invariant Lys-14 and Lys-16, and 'B', consisting of Lumbricus chains III and IV and Tylorrhynchus IIB and IIC, having invariant Cys-6, Ser-8 and Asp-11. This result suggests that there are two strains of globin chain in the annelid extracellular haemoglobins.
Full text
PDF
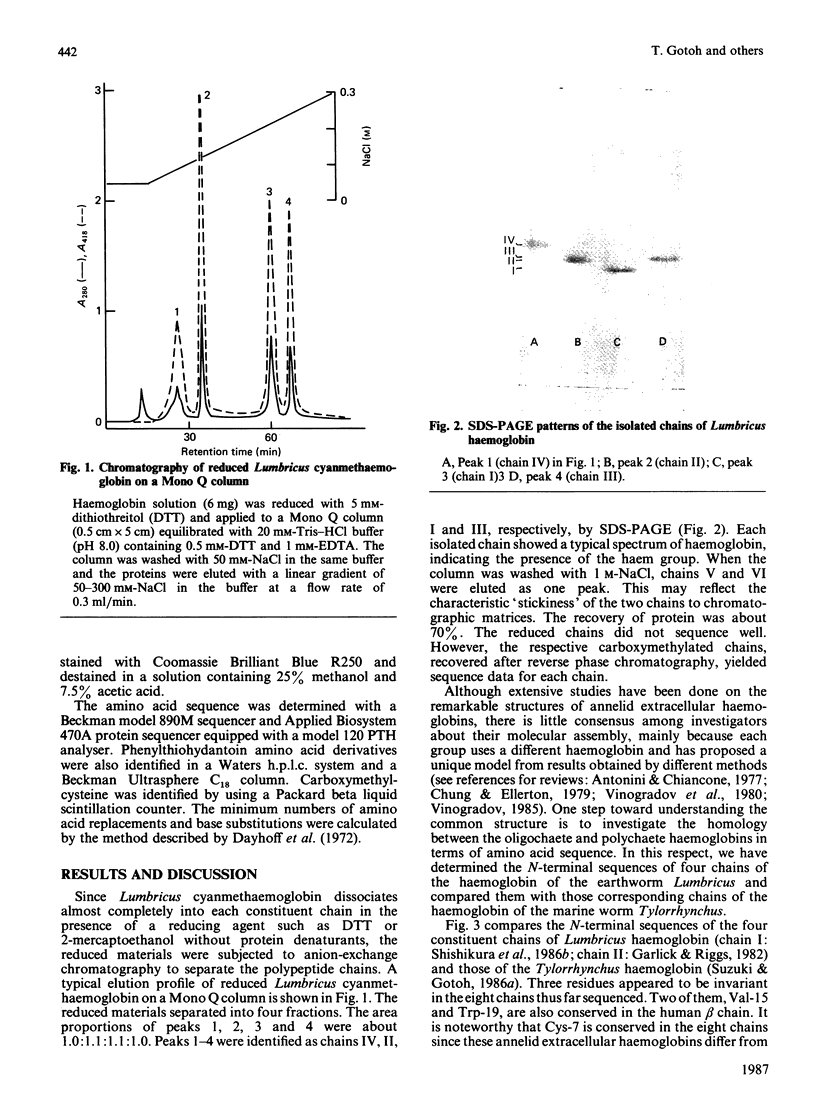


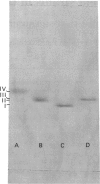
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Chiancone E. Assembly of multisubunit respiratory proteins. Annu Rev Biophys Bioeng. 1977;6:239–271. doi: 10.1146/annurev.bb.06.060177.001323. [DOI] [PubMed] [Google Scholar]
- Chung M. C., Ellerton H. D. The physico-chemical and functional properties of extracellular respiratory haemoglobins and chlorocruorins. Prog Biophys Mol Biol. 1979;35(2):53–102. doi: 10.1016/0079-6107(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Riggs A. F. The amino acid sequence of a major polypeptide chain of earthworm hemoglobin. J Biol Chem. 1982 Aug 10;257(15):9005–9015. [PubMed] [Google Scholar]
- Garlick R. L., Riggs A. Purification and structure of the polypeptide chains of earthworm hemoglobin. Arch Biochem Biophys. 1981 May;208(2):563–575. doi: 10.1016/0003-9861(81)90545-2. [DOI] [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Matsuda G. Darwinian evolution in the genealogy of haemoglobin. Nature. 1975 Feb 20;253(5493):603–608. doi: 10.1038/253603a0. [DOI] [PubMed] [Google Scholar]
- Kapp O. H., Polidori G., Mainwaring M. G., Crewe A. V., Vinogradov S. N. The reassociation of Lumbricus terrestris hemoglobin dissociated at alkaline pH. J Biol Chem. 1984 Jan 10;259(1):628–639. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Shishikura F., Mainwaring M. G., Yurewicz E. C., Lightbody J. J., Walz D. A., Vinogradov S. N. A disulfide-bonded trimer of myoglobin-like chains is the principal subunit of the extracellular hemoglobin of Lumbricus terrestris. Biochim Biophys Acta. 1986 Feb 14;869(3):314–321. doi: 10.1016/0167-4838(86)90071-3. [DOI] [PubMed] [Google Scholar]
- Shlom J. M., Vinogradov S. N. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1973 Nov 25;248(22):7904–7912. [PubMed] [Google Scholar]
- Suzuki T., Furukohri T., Gotoh T. Subunit structure of extracellular hemoglobin from the polychaete Tylorrhynchus heterochaetus and amino acid sequence of the constituent polypeptide chain (IIC). J Biol Chem. 1985 Mar 10;260(5):3145–3154. [PubMed] [Google Scholar]
- Suzuki T., Gotoh T. Subunit assembly of giant haemoglobin from the polychaete Tylorrhynchus heterochaetus. J Mol Biol. 1986 Jul 5;190(1):119–123. doi: 10.1016/0022-2836(86)90081-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Gotoh T. The complete amino acid sequence of giant multisubunit hemoglobin from the polychaete Tylorrhynchus heterochaetus. J Biol Chem. 1986 Jul 15;261(20):9257–9267. [PubMed] [Google Scholar]
- Suzuki T., Yasunaga H., Furukohri T., Nakamura K., Gotoh T. Amino acid sequence of polypeptide chain IIB of extracellular hemoglobin from the polychaete Tylorrhynchus heterochaetus. J Biol Chem. 1985 Sep 25;260(21):11481–11487. [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N., Shlom J. M., Hall B. C., Kapp O. H., Mizukami H. The dissociation of Lumbricus terrestris hemoglobin: a model of its subunit structure. Biochim Biophys Acta. 1977 May 27;492(1):136–155. doi: 10.1016/0005-2795(77)90221-5. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N. The structure of invertebrate extracellular hemoglobins (erythrocruorins and chlorocruorins). Comp Biochem Physiol B. 1985;82(1):1–15. doi: 10.1016/0305-0491(85)90120-8. [DOI] [PubMed] [Google Scholar]