Abstract
In this study, the nutrients, phytochemicals (including isoflavone and ginsenoside derivatives), and antioxidant activities of cheonggukjang with different ratios (0%, 2.5%, 5%, and 10%) of mountain-cultivated ginseng (MCG) were compared and analyzed using microorganisms isolated from traditional cheonggukjang. The IDCK 30 and IDCK 40 strains were confirmed as Bacillus licheniformis and Bacillus subtilis, respectively, based on morphological, biological, biochemical, and molecular genetic identification, as well as cell wall fatty acid composition. The contents of amino acids and fatty acids showed no significant difference in relation to the ratio of MCG. After fermentation, isoflavone glycoside (such as daidzin, glycitin, and genistin) contents decreased, while aglycone (daidzein, glycitein, and genistein) contents increased. However, total ginsenoside contents were higher according to the ratio of MCG. After fermentation, ginsenoside Rg2, F2, and protopanaxadiol contents of cheonggukjang decreased. Conversely, ginsenoside Rg3 (2.5%: 56.51 → 89.43 μg/g, 5.0%: 65.56 → 94.71 μg/g, and 10%: 96.05 → 166.90 μg/g) and compound K (2.5%: 28.54 → 69.43 μg/g, 5.0%: 41.63 → 150.72 μg/g, and 10%: 96.23 → 231.33 μg/g) increased. The total phenolic and total flavonoid contents were higher with increasing ratios of MCG and fermentation (fermented cheonggukjang with 10% MCG: 13.60 GAE and 1.87 RE mg/g). Additionally, radical scavenging activities and ferric reducing/antioxidant power were significantly increased in fermented cheonggukjang. This study demonstrates that the quality of cheonggukjang improved, and cheonggukjang with MCG as natural antioxidants may be useful in food and pharmaceutical applications.
Keywords: Bacillus, cheonggukjang, cocktail starters, mountain-cultivated ginseng, nutrients, antioxidant
1. Introduction
Ginseng (Panax ginseng C.A Meyer) is classified into artificially cultivated ginseng, mountain wild ginseng, and mountain-cultivated ginseng (MCG) [1]. Recently, interest in MCG has increased alongside growing demand for healthy, functional foods. MCG is less expensive and has higher production rates than wild ginseng while maintaining high pharmacological activity and ginsenoside content, making it a viable alternative to wild ginseng [2]. Tran et al. [3] conducted a comparative study on the anticancer effects of cultivated ginseng, wild ginseng, and MCG, reporting that MCG showed higher effects and contents of physiologically active substances, phenolic components, and free amino acids compared to cultivated ginseng. Known effects of ginseng include antioxidant properties [4], liver toxicity reduction [5], blood lipid improvement [6], angiogenesis promotion [7], anti-inflammatory effects [8], and immune enhancement [9]. MCG contains comparable levels of ginsenosides to regular ginseng. However, there are fewer processing products using MCG compared to ginseng.
Soybean-based foods are generally categorized into processed products (tofu and soymilk) and fermented foods (soybean paste, soy sauce, and cheonggukjang) produced using microorganisms [10]. Cheonggukjang is rich in essential nutrients like protein, carbohydrates, and fat, and contains numerous physiologically active substances, including isoflavones [11]. During fermentation, the proteins, carbohydrates, and fats in cheonggukjang are broken down into easily digestible forms, increasing their absorption rate [12]. Quality indicators such as taste, aroma, texture, color, and functionality of cheonggukjang are influenced by raw materials, fermentation conditions, and fermenting microorganisms. Notably, microorganisms play the most crucial role in developing the taste and aroma of cheonggukjang [13]. Research on cheonggukjang microorganisms has focused on using single or cocktail cultures of Bacillus species, including Bacillus subtilis, Bacillus licheniformis, and Bacillus megaterium. The demand for cheonggukjang is growing due to its various health benefits, such as blood clot dissolution, improved blood pressure and lipid metabolism, anticancer effects, and antioxidant properties [14]. To enhance its functionality and quality, research on the production of cheonggukjang with garlic [15], deodeok [16], red ginseng, Angelica gigas, and Rehmanniae radix [17] has been reported.
Therefore, it was attempted to produce cheonggukjang with added MCG not only to increase its usability, but also to enhance its functionality and quality. First, suitable starters for fermenting MCG-added cheonggukjang were selected, and cheonggukjang with MCG was produced using the selected starter. Finally, the physicochemical properties, nutritional components, phytochemical contents, and antioxidant activities of cheonggukjang containing MCG were analyzed and compared to determine the optimal MCG ratio for cheonggukjang.
2. Materials and Methods
2.1. MCG and Chemicals
The traditional cheonggukjang (Indang cheonggukjang: IDCK) was obtained from the Hamyang-gun Urban Regeneration Center in March 2020. MCG was purchased and used from Ginseng-Bio Co. Ltd. and grown in the Baekjeon-myeon, Hamyang-gun in 2020 (GPS coordinates: 35.575153, 127.613122). Soybeans grown in Hadong-gun in 2022 were stored in the laboratory. Cheonggukjang was produced through natural fermentation using environmental microorganisms following traditional methods. Six standard isoflavone compounds (such as daidzein, genistein, malonylgenistin, malonyldaidzin, daidzein, and genistein) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ginsenoside standards (including compound K, F1, F2, F3, F5, Rb1, Rb2, Rb3, Rc, Rd, Rd2, Re, Rf, Rg1, Rg2, Rg3, Rh1, Rh2, Ro, protopanaxadiol, and protopanaxtriol) were obtained from KOC Biotech (Daejeon, Korea). Folin–Cicalteu phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid), diammonium salt (ABTS), 2,4,5-tri(2-pyridyl)-1,3,5-triazine (TPTZ), thiobarbituric acid (TBA), and trichloroacetic acid (TCA) were purchased from Sigma-Aldrich. Methanol, acetonitrile, and water were obtained from J.T.Baker company (Philipsburg, NJ, USA), while other reagents were of analytical grade.
2.2. Isolation of Bacillus sp.
First, 10 g of IDCK was suspended in 0.85% NaCl solution, and 0.1 mL aliquots were spread on nutrient agar medium (Difco, Becton Dickinson Co., Sparks, MD, USA) and incubated at 37 °C for 24 h. From the numerous colonies that grew, initial selection was based on morphological similarities to Bacillus sp. The selected strains were cultured in tryptic soy broth/agar (TSB/TSA, Difco, Becton Dickinson Co., Sparks, MD, USA) medium, using liquid or solid media as appropriate. Two strains, IDCK 30 and IDCK 40, were ultimately selected. For accurate identification, these strains underwent morphological, biological, biochemical, cell wall fatty acid composition, and molecular genetic analyses.
2.3. Identification of Bacillus sp.
2.3.1. Morphological, Physiological, and Biochemical Characteristics
IDCK30 and IDCK40 strains were cultured on tryptic soy agar (TSA, Difco, Detroit, MI, USA) plates to obtain single colonies. Cell morphology was observed using Gram staining and scanning electron microscopy. Physiological characteristics were assessed by evaluating growth at various temperatures (10 °C to 50 °C), pH levels (3–11), and NaCl concentrations (0%–10%). Biochemical properties, including glycolysis, were analyzed using the API50NE kit (bioMérieux, Marcy-l’Étoile, Auvergne-Rhône-Alpes, France).
2.3.2. Cellular Fatty Acids Analysis
Microbial fatty acid compositions were analyzed using gas chromatography (GC-7890, Agilent Technologies, Santa Clara, CA, USA) following pretreatment according to the MIDI Microbial Identification System protocol (Sherlock MIS, MIDI Inc., Newark, DE, USA). The oven temperature started at 170 °C and increased at a rate of 5 °C/min until reaching 260 °C, then increased at a rate of 4 °C/min until reaching 310 °C, where it was maintained for 1 min. Hydrogen was utilized as the carrier gas at a flow rate of 0.5 mL/min [18].
2.3.3. Molecular and Genetic Characteristics
For 16S rRNA, recA, and gyrB gene sequencing, genomic DNA was extracted using a G-spin Genomic DNA Purification Kit (iNtRON Biotechnology, Suwon, Republic of Korea). The 16S rRNA, recA, and gyrB gene amplification was conducted using the primers indicated in Table S1 [19,20,21]. The polymerase chain reaction (PCR) amplification process consisted of initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 49 °C for 30 s, and extension at 72 °C for 90 s. After a final extension at 95 °C for 5 min, the reaction was terminated by lowering the temperature to 4 °C. PCR products were purified using the MEGA quick-spin Total Fragment DNA Purification Kit (iNtRON Biotechnology, Suwon, Republic of Korea).
2.4. Cheonggukjang Preparation
For single and mixed bacillus fermentation of cheonggukjang, 50 g of Jinyang soybeans were soaked in water for 12–16 h, then steamed and sterilized at 121 °C for 30 min. The steamed soybeans were inoculated with pre-cultured IDCK10, IDCK30, and IDCK40 bacillus strains and fermented at 35 °C for 5 days. For single bacillus fermentation, 3.0% (v/w) culture solution was used; for two-strain mixed fermentation, 1.5% (v/w) of each culture solution was used; and for three-strain mixed fermentation, 1.0% (v/w) each culture was used. Separately, 90, 95, 97.5, and 100 g of water-soaked Jinyang soybeans were mixed with 0% (0 g), 2.5% (2.5 g), 5% (5 g), and 10% (10 g) of dried MCG, respectively, and sterilized at 121 °C for 30 min. These mixtures were inoculated with 2.5% (v/w) of pre-cultured IDCK30 and IDCK40 bacillus strains and fermented at 35 °C for 5 days (Figure S1). The resulting cheonggukjang was dried at 55 °C for 3 days, ground, and stored at −20 °C for experimental use.
2.5. Physicochemical Characteristics and Viable Cell Numbers
The pH was measured using a pH meter (Orion Star A211, Thermo Fisher Scientific Inc., Waltham, MA, USA) after stirring 1 g of cheonggukjang in 49 mL of distilled water. Acidity was expressed as a percentage of lactic acid by neutralizing the moderately diluted sample to pH 8.31 ± 0.01 with 0.1 N NaOH solution. For reducing sugar analysis, 1 g of sample was added to 10 mL distilled water. Then, the sample suspension was stirred at room temperature for 1 h. Next, 1 mL of the 3,5-dinitrosalicylic acid coloring reagent was added to 0.1 mL of stirred sample and followed by 20 min of reaction at 100 °C. After cooling, the absorbance was measured at 570 nm. The reducing sugar content was calculated using a standard calibration curve prepared with glucose.
The viable cell was mixed with 90 mL of sterile distilled water in 10 g of cheonggukjang and diluted step by step to the level of 102 to 1010. Then, 0.1 mL was plated on TSA medium and unfolded, and the collected water produced after 24 h of incubation at 37 °C was measured and represented as log CFU/g.
2.6. Fatty Acid Analysis
The fatty acid content analysis was performed using the modified method of Vargas-Bello-Pérez et al. [22]. Fatty acid pretreatment involved adding 1.5 mL of 0.5 N NaOH (in methanol) and 0.5 mL of triundecanoin (C11:0, 2 mg/mL) to 25 mg of powder sample and heating it at 100 °C for 10 min. Subsequently, 2 mL of boron trifluoride (BF3) was added while stirring, and the mixture was heated again for 30 min to facilitate methyl esterification of fatty acids. After the reaction, 1 mL of isooctane was added, vigorously mixed, and left to settle. The isooctane layer was recovered, dehydrated with anhydrous sodium sulfate, filtered through a 0.45 μm membrane filter (Dismic-25CS, Toyoroshikaisha Ltd., Tokyo, Japan), and analyzed using a GC (Nexis GC-2030, Shimadzu Corp., Kyoto, Japan) equipped with an SP-2560 capillary column (100 m × 0.25 mm i.d., 0.2 μm film thickness, Supelco, St. Louis, MO, USA) and a flame ionization detector (FID). A 1 μL injection volume was used, with the injector set at 225 °C and operating in split mode with a split ratio of 200:1. Helium was utilized as the carrier gas at a flow rate of 0.75 mL/min. The oven temperature started at 100 °C, was held for 4 min, and was then increased at a rate of 3 °C/min until reaching 240 °C, where it was maintained for 15 min. The detector temperature was set at 285 °C. Fatty acid contents in the samples were quantified using a standard mixture (CRM47885, Supelco 37 Component FAME Mix, Sigma Aldrich, St. Louis, MO, USA).
2.7. Amino Acid Analysis
The amino acid content analysis was performed by adding 4 mL of distilled water to 1 g of powder sample. The mixture was hydrolyzed at 60 °C for 1 h, and then cooled to 4 °C for 2 h after adding 10% 5-sulfosalicylic acid to precipitate proteins. The supernatant was obtained by centrifugation for 3 min and filtered through a 0.45 μm membrane filter. The filtrate was then decompressed and concentrated. The resulting dry matter was reconstituted with 2 mL of lithium buffer (pH 2.2) and filtered again through a 0.45 μm membrane filter before analysis using an amino acid automatic analyzer (L-8900, Hitachi High-Technologies Corp., Tokyo, Japan) [23]. Amino acid contents in the samples were quantified using a standard mixture solution (Type H, Wako Pure Chemical Industries Ltd., Osaka, Japan).
2.8. Isoflavone Analysis
Isoflavone content analysis was performed using high-performance liquid chromatography (HPLC) according to the method of Kuligowski et al. [24] with slight modifications. A Lichrophore 100 RP C18 column (4.6 × 250 mm, 5 μm, Merck KGaA, Darmstadt, Germany) was used for the analysis. The mobile phase consisted of HPLC-grade water (solvent A) and acetonitrile (solvent B), both containing 0.2% acetic acid. The mobile phase gradient was as followed: 0 min—100% A, 15 min—90% A, 25 min—80% A, 35 min—75% A, 45 min—65% A, and 50 min—65% A. The sample injection volume was 20 μL, and the flow rate was maintained at 1 mL/min and 30 °C. Isoflavones were detected at UV 254 nm using a diode array detector (DAD). The content of each detected isoflavone was calculated using a standard calibration curve.
2.9. Ginsenoside Analysis
The ginsenoside analysis followed the method of Lee et al. [1] using HPLC (Agilent 1200 system, Agilent Technologies Inc., Waldbronn, Germany). First, 1 g of the sample was extracted twice with 20 mL of 70% methanol in a 70 °C water bath for 1 h each time. The extracts were centrifuged, and the supernatant was filtered through a 0.45 μm membrane filter. The combined filtrate was concentrated under reduced pressure at 60 °C, then reconstituted in 2 mL of HPLC-grade water and filtered again through a 0.45 μm membrane filter before analysis. A TSKgel ODS-100Z column (4.6 × 250 mm, 5 μm, Tosoh Corp., Tokyo, Japan) and a DAD detector were used. The flow rate was 1.0 mL/min, and the injection volume was 10 μL. The mobile phase consisted of HPLC-grade water (solvent A) and acetonitrile (solvent B). Detection was performed at 203 nm using the following gradient elution profile (time in minutes –%B): 10 min—19%, 15 min—20%, 30 min—23%, 42 min—30%, 75 min—35%, 80 min—60%, 90 min—80%, and 100 min—80%.
2.10. Total Phenolic and Total Flavonoid Contents Analysis
Total phenolic contents (TPC) were analyzed using previously described methods with some modifications [25]. A moderately diluted filtrate extract and a 25% Na2CO3 solution (0.5 mL each) were combined in a test tube and vortexed for 3 min. Then, 0.25 mL of 2 N Folin–Ciocalteu phenol reagent was added for 1 h before measuring the absorbance at 750 nm using a spectrophotometer. TPC was quantified using a standard calibration curve prepared with gallic acid and expressed as gallic acid equivalents (GAE) mg/g.
Total flavonoid contents (TFC) were determined using the method described by Kim et al. [25] with some modifications. A mixture of 0.5 mL of appropriately diluted filtrate, 1.0 mL of diethylene glycol, and 0.01 mL of 1 N NaOH was prepared in a test tube. The solution was then incubated at 37 °C for 1 h, and to measure the absorbance, it was measured at 420 nm using a spectrophotometer. TFC was quantified using a standard calibration curve prepared with rutin as the reference compound and expressed as rutin equivalents (RE) in mg/g.
2.11. Radical Scavenging Activity
The antioxidant activities, including DPPH and ABTS radical scavenging activities and ferric-reducing/antioxidant power (FRAP), were performed according to the method described by Lee et al. [1]. DPPH and ABTS were measured at 525 and 734 nm, respectively, after mixing and reacting the DPPH and ABTS radical solutions with sample extracts. The negative control used an extraction solvent instead of a sample. The difference between the experimental and negative controls was expressed as a percentage (%) using Equation (1):
| (1) |
Asample: absorbance of sample, Acontrol: absorbance of negative control
For FRAP measurements, acetate buffer, TPTZ reagent, and FeCl3 solution were preliminarily mixed. Then, 50 μL of extract and 950 μL of FRAP reagent were combined in a test tube and incubated at 37 °C for 15 min. The absorbance was measured at 593 nm using a spectrophotometer (UV-1800 240V, Shimadzu Corp., Kyoto, Japan).
2.12. Statistical Analysis
Values were presented as the mean ± standard deviation of pentaplicate determination. Statistical significance between samples was determined using one-way analysis of variance, followed by Duncan’s multiple range test (p < 0.05). All analysis were performed using the Statistical Analysis System (SAS) software (version 9.4; SAS Institute, Cary, NC, USA).
3. Results and Discussion
3.1. Identification of Fermented Soybean Strains with IDCK 30 and IDCK40
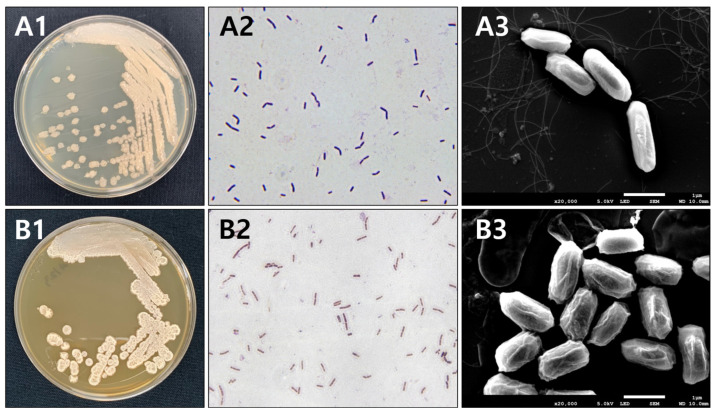
The identification results of the IDCK30 and IDCK40 strains based on morphological and biochemical characteristics are presented in Figure 1 and Table S2. Both the IDCK30 and IDCK40 strains showed bacillus morphology, were gram-positive, and had flagella and spores. Physiologically, IDCK30 hydrolyzed starch, cellulose, and xylan, while IDCK40 hydrolyzed all six tested substrates. IDCK30 used 18 carbon sources, including glycerol, L-arabinose, ribose, D-glucose, and D-fructose, showing 99.9% similarity to Bacillus pumilus. IDCK40 used 17 carbon sources, including L-arabinose, ribose, D-xylose, D-glucose, and D-fructose, demonstrating 99.9% similarity to B. subtilis/amyloliquefaciens. Optical and scanning electron microscopy revealed that IDCK30 colonies had relatively smooth surfaces, while IDCK40 colonies displayed surface irregularities.
Figure 1.
Morphological characteristics of strains with IDCK30 and IDCK40. (A1) Colony shape of strain IDCK30 in TSA media; (A2) optical microscope of gram-stained strain IDCK30; (A3) scanning electron microscopy of strain IDCK30; (B1) colony shape of IDCK40 in TSA media; (B2) optical microscopy of gram-stained strain IDCK40; and (B3) scanning electron microscopy of strain IDCK40.
Since the fatty acid composition of the cell wall is a major indicator for classifying and identifying bacteria, it was analyzed using microbial identification GC. Supplementary Table S3 shows the results of analyzing the fatty acid composition of the IDCK30 and IDCK40 strains. Among branched fatty acids, anteiso-C15:0 showed the highest proportion in both IDCK30 (32.15%) and IDCK40 (47.31%) strains.
The analysis results of 16S rRNA, recA, and gyrB gene sequence similarity in the fermented strains are shown in Table 1 and Figure S2–S7. For accurate identification, the IDCK30 and IDCK40 16S rRNA, recA, and gyrB gene sequences were analyzed. IDCK30 showed 98–99% similarity with B. licheniformis. IDCK40 showed 99% similarity with B. subtilis. Based on morphological, physiological, cell wall fatty acid composition, biochemical, and molecular genetic characteristics, the IDCK30 strain was identified as B. licheniformis and the IDCK40 strain as B. subtilis.
Table 1.
16S rRNA, recA, and gyrB gene sequence similarities of strains IDCK30 and IDCK40.
| Genes | Isolates | Nearest Relatives 1 (Accession No.) | Similarity (%) |
|---|---|---|---|
| 16S rRNA | IDCK30 | Bacillus licheniformis B.licheCEL (LC006127) | 99 |
| IDCK40 | Bacillus subtilis subsp. subtilis 2KL1 (CP032872) | 99 | |
| recA | IDCK30 | Bacillus licheniformis P8_B2 (CP045814) | 99 |
| IDCK40 | Bacillus subtilis P5 (CP045816) | 99 | |
| gyrB | IDCK30 | Bacillus licheniformis P8_B2 (CP045814) | 98 |
| IDCK40 | Bacillus subtilis MB9_B6 (CP045818) | 99 |
1 Accession number of the nearest relative. If more than one sequence had the same similarity value, only the accession number of the first sequence is given.
Previous studies have reported that B. subtilis, B. licheniformis, and Bacillus sp. are known as major fermentation microbial species of cheonggukjang [15,26]. Therefore, it is considered that B. licheniformis IDCK 30 and B. subtilis IDCK 40 strains are appropriate for producing cheonggukjang.
3.2. Characteristics of Cheonggukjang with Single and Cocktail Starters
The analysis results of physicochemical properties, viable cell numbers, free amino acids, isoflavones, physiological activity components, and radical scavenging activities in fermented cheonggukjang with single and complex starters are presented in Table 2. The pH of cheonggukjang with IDCK30 increased, while acidity decreased. Conversely, cheonggukjang with IDCK40 and IDCK30 + IDCK40 showed decreased pH and increased acidity. Reducing sugar content decreased in all cheonggukjang samples with starters after fermentation, and viable cell numbers ranged from 10.20 to 10.57 log CFU/g. Total free amino acid contents increased during fermentation compared to steamed soybeans (4.31 mg/g), with IDCK40 (120.02 mg/g) showing the highest content, followed by IDCK30 + IDCK40 (94.88 mg/g) and IDCK30 (46.64 mg/g). The total contents of six isoflavones (daidzin, glycitin, genistein, daidzein, glycitein, and genistein) were confirmed with 2154.6 (steam), 1405.1 (IDCK30), 2134.7 (IDCK40), and 1575.3 (IDCK30 + IDCK40) μg/g. Aglycone contents were highest (1014.53 μg/g) in cheonggukjang with the IDCK30 + IDCK40 starter. TPC and TFC were highest in cheonggukjang with the IDCK40 starter (13.91 and 1.488 mg/g), and DPPH and ABTS radical scavenging activities followed the same trend (57.57 and 49.63%). Based on a comprehensive comparison of physiologically active substances and antioxidant activities, IDCK30 + IDCK40-fermented cheonggukjang was deemed superior, leading to a further experiment with a cocktail strain.
Table 2.
Characteristics of cheonggukjang according to single and complex starters.
| Index 1 | Steam | Starters | ||
|---|---|---|---|---|
| IDCK30 | IDCK40 | IDCK30 + 40 | ||
| Physicochemical Properties | ||||
| pH | 6.81 ± 0.03b | 7.26 ± 0.02a | 6.20 ± 0.06c | 6.27 ± 0.06c |
| Acidity (%, as lactic acid) | 0.90 ± 0.01c | 0.85 ± 0.01d | 1.06 ± 0.01a | 1.03 ± 0.01b |
| Reducing sugar (mg/g) | 20.71 ± 0.09a | 6.98 ± 0.05d | 11.86 ± 0.11b | 8.10 ± 0.06c |
| Viable cell numbers (log CFU/g) | nd 2 | 10.57 ± 0.08a | 10.27 ± 0.09b | 10.51 ± 0.11a |
| Free amino acid contents (mg/g) | ||||
| Non-essential amino acids | 4.31 ± 0.06d | 24.61 ± 0.27c | 65.06 ± 0.85a | 48.62 ± 0.59b |
| Essential amino acids | 0.73 ± 0.01d | 22.03 ± 0.25c | 54.96 ± 0.65a | 46.26 ± 0.86b |
| Total amino acids | 5.04 ± 0.03d | 46.64 ± 0.54c | 120.02 ± 2.20a | 94.88 ± 1.05b |
| Total isoflavone contents (μg/g) | 2154.6 ± 35.6a | 1405.1 ± 28.7c | 2134.7 ± 40.5a | 1575.3 + 29.2b |
| Daidzin | 709.5 ± 14.2b | 143.5 ± 2.9d | 820.9 ± 14.5a | 175.8 ± 3.2c |
| Glycitin | 303.2 ± 4.1b | 133.9 ± 2.5d | 500.8 ± 10.0a | 240.5 ± 4.1c |
| Genistin | 934.4 ± 13.7a | 155.2 ± 3.3c | 634.3 ± 12.7b | 144.5 ± 2.5d |
| Total glycosides | 1947.1 ± 32.0a | 432.6 ± 8.5c | 1956.0 ± 37.2a | 560.8 ± 9.8b |
| Daidzein | 44.9 ± 0.9d | 660.8 ± 13.9a | 78.1 ± 1.6c | 517.2 ± 10.1bc |
| Glycitein | 117.0 ± 2.1a | 88.1 ± 1.5b | 28.3 ± 0.5c | 86.2 ± 1.5b |
| Genistein | 45.6 ± 0.6d | 223.6 ± 4.8b | 72.3 ± 1.2c | 411.1 ± 7.8a |
| Total aglycones | 207.5 ± 3.6c | 972.5 ± 20.2b | 178.7 ± 3.3d | 1014.5 ± 19.4a |
| Total phenolic contents (mg/g) | 2.58 ± 0.02d | 10.79 ± 0.08c | 13.91 ± 0.11a | 13.31 ± 0.11b |
| Total flavonoid contents (mg/g) | 0.487 ± 0.004d | 0.787 ± 0.009c | 1.488 ± 0.021a | 1.374 ± 0.011b |
| Radical scavenging activity (%) | ||||
| DPPH (1 mg/mL) | 7.81 ± 0.05d | 33.57 ± 0.19c | 57.57 ± 0.65a | 52.35 ± 0.50b |
| ABTS (0.5 mg/mL) | 10.78 ± 0.09d | 33.44 ± 0.23c | 49.63 ± 0.48a | 48.07±0.49b |
1 All values are presented as the mean ± standard deviation of pentaplicate determination. Means with different letters within a row are significantly different between samples for the same index (p < 0.05).2 nd, not detected.
Cho et al. [27] reported that the cheonggukjang with starter B. pumilus HY1 increased the activity of β-glucosidase and esterase, correspondingly reading the TFC, gallic acid, and aglycone contents. Also, the cheonggukjang with B. subtilis CS90 increased phytochemicals (isoflavones, flavanols, phenolic acids) during fermentation according to an increase in β-glucosidase and esterase activity [28]. Shin et al. [29] reported that fermented cheonggukjang by B. subtilis CSY191 increased TPC and aglycone, corresponding to antioxidant activities. In our prior research, it was demonstrated that β-glucosidase and esterase produced by Bacillus strains converted isoflavone glycosides to aglycones and esterified phenolic acid compounds to free phenolic acids, respectively. This conversion resulted in increased TPC, TFC, and antioxidant activity [27,28,29].
3.3. Comparison of Physicochemical Characteristics and Viable Cell Numbers in Cheonggukjang According to MCG Ratio
Table 3 presents the analysis results of the physicochemical characteristics in cheonggukjang according to the MCG addition ratio. In the unfermented sample, pH values were low as the MCG concentration increased from 0% to 10% (pH 6.81, 6.76, 6.26, and 6.20), while the acidity values were correspondingly high. After fermentation, the pH values slightly decreased, except for 10% MCG cheonggukjang (pH 7.38, 6.66, 6.07, and 6.08), and acidity slightly increased, except for 10% MCG cheonggukjang (0.76, 1.05, 1.09, and 1.11%). Reducing sugar notably decreased after fermentation from 28.36–28.24 mg/g (unfermented) to 6.63–10.6 mg/g (fermented). The number of viable cells increased after fermentation from an average level of 5.75 log CFU/g (unfermented) to 8.08–9.20 log CFU/g (fermented). As the MCG ratio increased, the number of viable cells was found to be inversely proportional.
Table 3.
Phytochemical characteristics of cheonggukjang according to the addition ratio of mountain-cultivated ginseng.
| Index 1 | Addition Ratio of Mountain-Cultivated Ginseng (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unfermented Chenoggukjang | Fermented Chenoggukjang | |||||||
| 0 | 2.5 | 5.0 | 10 | 0 | 2.5 | 5.0 | 10 | |
| pH | 6.81 ± 0.08b | 6.76 ± 0.07b | 6.26 ± 0.10d | 6.20 ± 0.07d | 7.38 ± 0.09a | 6.66 ± 0.08bc | 6.07 ± 0.08e | 6.08 ± 0.07e |
| Acidity (%, as lactic acid) | 0.90 ± 0.01d | 0.92 ± 0.01d | 1.05 ± 0.01b | 1.06 ± 0.01b | 0.76 ± 0.01c | 1.05 ± 0.01b | 1.09 ± 0.01a | 1.11 ± 0.01a |
| Reducing sugars (mg/g) | 28.36 ± 0.31b | 32.87 ± 0.43a | 28.38 ± 0.38b | 28.24 ± 0.28b | 6.63 ± 0.07f | 8.94 ± 0.10e | 9.31 ± 0.13d | 10.06 ± 0.10c |
| Viable cell numbers (log CFU/g) | 5.81 ± 0.07f | 5.83 ± 0.05e | 5.76 ± 0.06f | 5.61 ± 0.06g | 9.56 ± 0.10a | 9.33 ± 0.11b | 9.12 ± 0.12c | 8.80 ± 0.12d |
1 All values are presented as the mean ± standard deviation of pentaplicate determination. Means with different letters within a row are significantly different between samples for the same index (p < 0.05).
Recent literature has demonstrated that a decrease in pH and an increase in acidity were observed in ginseng and red ginseng after fermentation. Lee et al. [1] reported that pH was decreased and the acidity values were increased during the processing steps of aging and fermentation. Lee et al. [30] reported that pH decreased during food processing from 5.4 (dry MCG) to 4.44 (fermented red MCG), corresponding to an increase in acidity from 0.21 to 0.51 [30]. Our present results are consistent with these previously reported findings [1,30].
3.4. Comparison of Free Amino Acid Contents of Cheonggukjang According to MCG Ratio
The analysis results of free amino acid contents in cheonggukjang based on MCG addition ratios is presented in Table 4. Amino-type nitrogen, primarily amino acids, is produced from soy proteins via peptides through the action of microbial proteases during cheonggukjang fermentation, contributing to its savory taste [31]. In unfermented cheonggukjang, total free amino acid contents were slightly higher as MCG levels rose from 0% to 5%, except MCG 10% (536.77, 544.84, 589.43, and 551.85 mg/100 g, respectively). In fermented cheonggukjang, free amino acid contents increased more than 20-fold due to various enzymatic actions. Viable cell numbers increased; the highest content was observed in 0% MCG cheonggukjang, along with the highest ammonia content, potentially resulting in a strong, putrid odor. Among the increased free amino acids, glutamic acid (54.68 to 65.90 mg/100 g → 1401.07 to 2720.27 mg/100 g), a non-essential amino acid, showed the most significant increase.
Table 4.
Free amino acid contents of cheonggukjang according to the addition ratio of mountain-cultivated ginseng.
| Indexs 1 (mg/100 g) | Addition Ratio of Mountain-Cultivated Ginseng (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unfermented Chenoggukjang | Fermented Chenoggukjang | |||||||
| 0 | 2.5 | 5.0 | 10 | 0 | 2.5 | 5.0 | 10 | |
| Non-essential amino acids | ||||||||
| Taurine | nd 2 | nd | nd | 2.78 ± 0.03a | nd | nd | nd | nd |
| Proline | nd | nd | nd | nd | 1161.13 ± 25.25a | 895.08 ± 18.14c | 712.64 ± 17.11d | 922.45 ± 20.11b |
| Aspartic acid | 23.47 ± 0.27g | 23.74 ± 0.29f | 24.88 ± 0.25e | 23.77 ± 0.25f | 479.44 ± 7.88a | 310.05 ± 6.45b | 207.11 ± 4.17d | 245.52 ± 6.61c |
| Serine | 8.33 ± 0.13g | 8.35 ± 0.11g | 8.84 ± 0.09e | 8.59 ± 0.10f | 57.54 ± 1.81a | 47.35 ± 0.58b | 34.28 ± 0.74c | 30.00 ± 0.80d |
| Aspartic acid-NH2 | 14.02 ± 0.18e | 14.08 ± 0.18e | 16.66 ± 0.17d | 14.01 ± 0.18e | 42.58 ± 1.03b | 45.02 ± 0.44a | 21.24 ± 0.51c | nd |
| Glutamic acid | 65.90 ± 0.68e | 60.97 ± 0.65f | 61.81 ± 0.60f | 54.68 ± 0.58g | 2720.27 ± 35.11a | 1796.58 ± 28.75c | 1401.07 ± 20.11d | 1960.25 ± 36.10b |
| Aminoadipic acid | 5.12 ± 0.02e | 4.03 ± 0.09f | 4.51 ± 0.05f | 3.15 ± 0.08g | 266.20 ± 4.41d | 421.62 ± 6.12c | 462.98 ± 7.13a | 438.82 ± 9.59b |
| Glycine | 15.42 ± 0.30e | 14.62 ± 0.18f | 14.55 ± 0.16f | 13.51 ± 0.15g | 390.95 ± 4.52a | 327.19 ± 5.72b | 265.37 ± 4.55d | 301.09 ± 7.11c |
| Alanine | 40.21 ± 0.42e | 38.20 ± 0.45g | 39.23 ± 0.40f | 33.78 ± 0.34h | 905.70 ± 11.52d | 1130.12 ± 18.21a | 1020.89 ± 10.21c | 1097.08 ± 21.17b |
| Citrulline | nd | nd | nd | nd | 545.25 ± 8.44a | 306.26 ± 5.22b | 200.63 ± 6.11d | 269.96 ± 5.11c |
| α-aminobutyric aicd | nd | 1.47 ± 0.01e | 1.57 ± 0.02e | nd | 9.49 ± 0.12a | 2.39 ± 0.02c | 1.99 ± 0.05d | 3.30 ± 0.03b |
| Cystine | 21.40 ± 0.35d | 20.54 ± 0.35d | 18.30 ± 0.20e | 17.35 ± 0.20e | 102.34 ± 2.15a | 82.51 ± 0.93b | 64.56 ± 1.15c | 64.25 ± 1.04c |
| Tyrosine | 17.75 ± 0.32e | 16.56 ± 0.19f | 16.49 ± 0.18f | 15.89 ± 0.18g | 868.52 ± 15.95a | 805.91 ± 11.56b | 707.19 ± 15.17d | 734.37 ± 11.14c |
| β-Alanine | 3.19 ± 0.03f | 3.21 ± 0.05f | 3.44 ± 0.03e | 3.43 ± 0.03d | 16.36 ± 0.35c | 20.65 ± 0.21b | 21.98 ± 0.22a | 20.96 ± 0.21b |
| β-aminoisobutyric acid | 1.80 ± 0.01e | 1.43 ± 0.01f | 1.58 ± 0.02g | 1.67 ± 0.02f | 236.09 ± 3.89c | 250.54 ± 5.11a | 228.09 ± 5.88d | 246.77 ± 8.89b |
| γ-aminobutyric acid | 36.11 ± 0.37d | 38.14 ± 0.42c | 41.64 ± 0.48b | 45.71 ± 0.49a | 8.78 ± 0.09h | 15.42 ± 0.24g | 21.32 ± 0.21f | 24.86 ± 0.25e |
| Aminoetahnol | 5.97 ± 0.08d | 3.24 ± 0.03e | 3.03 ± 0.03e | 3.11 ± 0.03e | 10.26 ± 0.15c | 1.56 ± 0.02f | 10.89 ± 0.15b | 14.19 ± 0.25a |
| Hydroxylysine | 9.66 ± 0.11d | 9.51 ± 0.11e | 9.70 ± 0.10d | 9.53 ± 0.10e | 21.65 ± 0.22c | 25.49 ± 0.28a | 23.68 ± 0.31b | 25.16 ± 0.31a |
| Ornithine | nd | nd | nd | nd | 572.91 ± 8.87c | 600.09 ± 6.85b | 606.71 ± 6.57b | 681.77 ± 7.82a |
| Anserine | nd | nd | nd | nd | 39.40 ± 0.91a | nd | nd | nd |
| Arginine | 190.41 ± 1.90d | 206.05 ± 2.26c | 244.14 ± 2.34a | 221.16 ± 2.33b | 14.59 ± 0.18e | 9.12 ± 0.09f | 9.65 ± 0.11f | nd |
| Total | 458.76 ± 4.90h | 464.14 ± 5.38g | 510.37 ± 5.12e | 472.12 ± 5.09f | 8469.45 ± 132.85a | 7092.95 ± 114.94b | 6022.27 ± 100.46d | 7080.8 ± 136.54c |
| Essential amino acids | ||||||||
| Threonine | 6.82 ± 0.11f | 6.99 ± 0.07f | 6.96 ± 0.07f | 7.29 ± 0.07e | 435.24 ± 9.75a | 400.59 ± 7.52b | 367.59 ± 8.18d | 385.84 ± 8.16c |
| Valine | 13.85 ± 0.18f | 14.13 ± 0.17e | 13.94 ± 0.18f | 14.22 ± 0.17e | 1006.66 ± 15.15a | 911.04 ± 15.01b | 788.20 ± 18.11d | 802.19 ± 19.11c |
| Methionine | 5.20 ± 0.08d | 4.82 ± 0.05e | 4.74 ± 0.05e | 4.31 ± 0.04f | 327.18 ± 5.11a | 302.00 ± 7.12b | 268.66 ± 5.87c | 267.97 ± 5.48c |
| Isoleucine | 5.00 ± 0.05f | 5.28 ± 0.05f | 5.08 ± 0.05f | 5.66 ± 0.06e | 864.91 ± 18.88a | 799.15 ± 15.01b | 654.64 ± 11.05c | 635.13 ± 10.55d |
| Leucine | 9.30 ± 0.11f | 9.71 ± 0.10e | 9.53 ± 0.15e | 10.82 ± 0.11d | 1494.15 ± 35.88a | 1406.59 ± 21.71b | 1218.38 ± 17.81c | 1210.99 ± 25.01c |
| Phenylalanine | 23.46 ± 0.29f | 25.10 ± 0.28e | 23.32 ± 0.28f | 23.73 ± 0.30f | 1108.06 ± 29.15a | 1048.15 ± 15.58b | 928.62 ± 10.19d | 945.81 ± 11.06c |
| Lysine | 10.50 ± 0.15f | 10.84 ± 0.11e | 10.00 ± 0.15f | 10.55 ± 0.11f | 1244.72 ± 25.48a | 1102.12 ± 17.02b | 943.42 ± 11.03d | 1028.93 ± 12.09c |
| Histamine | 3.88 ± 0.03f | 3.83 ± 0.04g | 5.49 ± 0.06e | 3.15 ± 0.08g | 380.74 ± 7.74b | 338.81 ± 4.81a | 280.29 ± 3.10d | 308.31 ± 7.11c |
| Total | 78.01 ± 1.00f | 80.7 ± 0.87e | 79.06 ± 0.99e | 79.73 ± 0.94e | 6861.66 ± 147.14a | 6308.45 ± 103.78b | 5449.8 ± 85.34d | 5585.17 ± 98.57c |
| Total amino acids | 536.77 ± 5.90g | 544.84 ± 6.25g | 589.43 ± 6.11e | 551.85 ± 6.03f | 15,331.11 ± 279.99a | 13,401.40 ± 218.72b | 11,472.07 ± 185.80d | 12,665.97 ± 235.11c |
| Ammonia | 27.44 ± 0.25e | 20.76 ± 0.21f | 19.57 ± 0.20g | 20.70 ± 0.21f | 345.94 ± 3.46a | 332.52 ± 3.33b | 322.23 ± 3.22c | 310.01 ± 3.10d |
1 All values are presented as the mean ± standard deviation of pentaplicate determination. Means with different letters within a row are significantly different between samples for the same index (p < 0.05). 2 nd, not detected.
Overall, the amino acid amounts in the cheonggukjang increased notably during fermentation. Most amino acids showed a substantial increase within the first 12 h of fermentation. Notably, glutamic acid, which is strongly associated with the umami taste in cheonggukjang, increased tenfold by the end of fermentation compared to its initial levels [29]. Additionally, various free amino acids increased. Interestingly, ornithine was not detected in unfermented cheonggukjang, but in fermented cheonggukjang, its content increased with higher ratios of MCG, reaching a maximum of 681.77 mg/100 g in cheonggukjang with 10% MCG. Conversely, arginine content decreased in fermented cheonggukjang compared to unfermented cheonggukjang. This decrease in arginine and increase in ornithine was likely due to the action of arginase produced by microorganisms during fermentation. This observation aligns with previous research suggesting the conversion of arginine to ornithine during fermentation [32]. B. subtilis, a major microorganism in fermented foods such as cheonggukjang, produces microbial arginase. The gene encoding arginase was isolated from B. subtilis 168. Furthermore, functional fermented foods such as cheonggukjang have been reported to have enhanced levels of ornithine, and pharmaceutical products have been developed using the key enzyme in arginine degradation and ornithine production [33]. Therefore, compared to the control (0% MCG), fermented cheonggukjang with added MCG is considered to have high potential as a food with such functionality.
3.5. Comparison of Fatty Acid Contents of Cheonggukjang According to MCG Ratio
The analysis results of fatty acid contents in cheonggukjang based on the MCG addition ratio are presented in Table 5. Total fatty acid contents were highest in 2.5% MCG, with unfermented and fermented cheonggukjang containing 1482.34 and 1449.01 mg/100 g, respectively. The major fatty acids in all samples were palmitic acid, oleic acid, linoleic acid, and α-linolenic acid. After fermentation, the contents of palmitic acid and behenic acid among saturated fatty acids, elaidic acid, and eicosadienoic acid among unsaturated fatty acids relatively increased. Other fatty acid contents showed minor increases or decreases. The results indicated no significant difference in fatty acid contents based on the MCG addition ratio.
Table 5.
Fatty acid contents of cheonggukjang according to the addition ratio of mountain-cultivated ginseng.
| Index 1 (mg/100 g) | Addition Ratio of Mountain-Cultivated Ginseng (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unfermented Chenoggukjang | Fermented Chenoggukjang | |||||||
| 0 | 2.5 | 5.0 | 10 | 0 | 2.5 | 5.0 | 10 | |
| Saturated fatty acids | ||||||||
| Myristic acid (C14:0) | 1.04±0.01e | 2.45 ± 0.02b | nd 2 | 1.02 ± 0.01f | 2.44 ± 0.02b | 4.49 ± 0.05a | 2.01 ± 0.03d | 2.14 ± 0.02c |
| Palmitic acid (C16:0) | 171.38 ± 2.51d | 172.92 ± 2.13d | 162.81 ± 3.39f | 129.42 ± 5.07f | 185.97 ± 3.68a | 186.35 ± 4.06a | 180.61 ± 4.01b | 176.84 ± 3.58c |
| Stearic acid (C18:0) | 64.87 ± 0.85c | 63.56 ± 0.81f | 62.52 ± 0.63f | 48.30 ± 0.95e | 67.55 ± 1.08a | 66.54 ± 0.97b | 63.28 ± 0.93f | 64.29 ± 1.14d |
| Arachidic acid (C20:0) | 5.14 ± 0.06d | 5.02 ± 0.05d | 4.99 ± 0.05e | 3.78 ± 0.06f | 5.53 ± 0.06a | 5.49 ± 0.07b | 5.21 ± 0.08c | 5.29 ± 0.10c |
| Behenic acid (C22:0) | 13.26 ± 0.18e | 7.25 ± 0.11f | 6.86 ± 0.10f | 5.07 ± 0.08f | 29.84 ± 0.70a | 19.00 ± 0.19d | 27.41 ± 0.55b | 23.37 ± 0.51c |
| Lignoceric acid (C24:0) | 3.18 ± 0.03a | 2.15 ± 0.02e | 2.21 ± 0.02f | 1.73 ± 0.02f | 2.53 ± 0.03e | 3.12 ± 0.02b | 2.93 ± 0.03d | 3.02 ± 0.03c |
| Total | 258.87 ± 3.64d | 253.35 ± 3.14d | 239.39 ± 4.19f | 189.32 ± 6.19f | 293.86 ± 5.57a | 284.99 ± 5.36b | 281.45 ± 5.63b | 274.9 ± 5.38c |
| Unsaturated fatty acids | ||||||||
| Palmitoleic acid (C16:1) | 1.04 ± 0.01f | 2.96 ± 0.03c | 1.14 ± 0.01f | 0.95 ± 0.01f | 2.07 ± 0.02d | 4.59 ± 0.05a | 1.95 ± 0.03e | 2.45 ± 0.02b |
| Elaidic acid (C18:1t) | 5.75 ± 0.06e | 1.26 ± 0.01f | nd 2 | nd | 19.17 ± 0.38a | 10.86 ± 0.30d | 15.40 ± 0.18b | 14.38 ± 0.18c |
| Oleic acid (C18:1c) | 319.88 ± 7.02c | 325.63 ± 7.86b | 337.74 ± 7.48a | 244.59 ± 6.25 | 297.49 ± 6.01d | 340.89 ± 11.05a | 281.55 ± 5.21f | 290.85 ± 5.41e |
| Linolelaidic acid (18:2t) | 5.51 ± 0.06c | 0.92 ± 0.00d | nd | nd | 0.49 ± 0.00e | nd | 9.52 ± 0.10a | 9.38 ± 0.09b |
| Linoleic acid (C18:2c) | 700.32 ± 12.21c | 754.74 ± 13.35a | 715.75 ± 15.14b | 588.46 ± 15.81f | 603.13 ± 14.03e | 659.78 ± 18.60d | 602.09 ± 15.44e | 608.77 ± 10.01e |
| ɤ-Linolenic acid(C18:3n6) | nd | nd | nd | nd | 1.49 ± 0.01a | 1.21 ± 0.01c | 1.18 ± 0.01b | 1.21 ± 0.01c |
| Eicosenic acid (C20:1) | 2.53 ± 0.03e | 3.21 ± 0.03b | 2.83 ± 0.03c | 2.17 ± 0.02f | 2.77 ± 0.03d | 3.87 ± 0.02a | 2.35 ± 0.03e | 2.53 ± 0.03e |
| α-Linolenic acid (C18:3n3) | 120.81 ± 8.19c | 134.08 ± 3.84a | 125.36 ± 3.59b | 105.04 ± 5.05e | 99.94 ± 2.05e | 112.83 ± 2.93d | 91.92 ± 1.22f | 99.73 ± 1.50e |
| Eicosadienoic acid (C20:2) | 13.21 ± 0.23d | 2.85 ± 0.01e | 1.69 ± 0.02f | 1.03 ± 0.00f | 35.04 ± 0.81a | 23.30 ± 0.35c | 34.57 ± 0.48a | 30.26 ± 0.40b |
| Eicosatrienoic acid (C20:3n6) | nd | 1.48 ± 0.01b | nd | nd | nd | nd | nd | 18.71 ± 0.21a |
| Erucic acid (C22:1n9) | nd | 1.86 ± 0.02b | nd | nd | 0.54 ± 0.00c | 2.66 ± 0.02a | nd | nd |
| Arachidonic acid (C20:4n6) | nd | nd | nd | nd | 0.95 ± 0.01b | 1.42 ± 0.01a | 1.49 ± 0.01a | 1.53 ± 0.02a |
| Eicosapentaenoic acid (C20:5n3) | nd | nd | nd | nd | 0.90 ± 0.01b | 1.37 ± 0.01a | 0.77 ± 0.01c | 0.63 ± 0.01d |
| Nervonic acid (C24:1n9) | 1.03 ± 0.01e | nd | nd | nd | 1.83 ± 0.02a | 1.24 ± 0.01d | 1.79 ± 0.02b | 1.61 ± 0.02c |
| Total | 1171.13 ± 27.76c | 1228.99 ± 25.16a | 1184.51 ± 26.27b | 942.9 ± 22.09f | 1065.81 ± 23.38f | 1164.02 ± 33.36 | 1044.58 ± 22.74g | 1082.04 ± 17.55e |
| Total fatty acids | 1430.0 ± 31.40c | 1482.34 ± 28.30a | 1424.9 ± 30.46d | 1132.22 ± 28.28g | 1359.67 ± 28.95e | 1449.01 ± 38.72b | 1326.03 ± 28.37f | 1356.99 ± 22.93e |
1 All values are presented as the mean ± standard deviation of pentaplicate determination. Means with different letters within a row are significantly different between samples for the same index (p < 0.05). 2 nd, not detected.
Recently, Cho et al. [34] reported that Neulchan soybean cultivars showed palmitic acid (10.33 ± 0.44) and stearic acid (3.61 ± 0.15) after 48 h of fermentation using B. subtilis CSY19. Fatty acid contents varied depending on the soybean cultivars. Unsaturated fatty acids, such as linoleic acid, oleic acid, palmitic acid, and linolenic acid, contributed to approximately 80% of fatty acid content.
The soybean cultivar used in this study was Daewon, and although different from the previous study, it similarly had contents of palmitic acid, stearic acid, and unsaturated fatty acids. Chung et al. [35] reported that mountain-cultivated ginseng roots had higher fatty acid contents than field-cultivated ginseng or wild mountain ginseng. The most abundant unsaturated fatty acids were linoleic acid, palmitic acid, and oleic acid, in that order. However, unlike previous studies, our study used a whole MCG rather than just the roots. Similarly, unsaturated fatty acid contents in ginseng roots were typically 2.5-fold higher than saturated fatty acid contents. These results suggest that the root part of MCG may contribute significantly to the fatty acid content. Although fermentation influenced fatty acid contents, MCG addition did not. Since fatty acid contents did not increase with MCG addition, the amount of MCG added may not be a key factor in fatty acid production.
3.6. Comparison of Isoflavone and Ginsenoside Contents of Cheonggukjang According to MCG Ratio
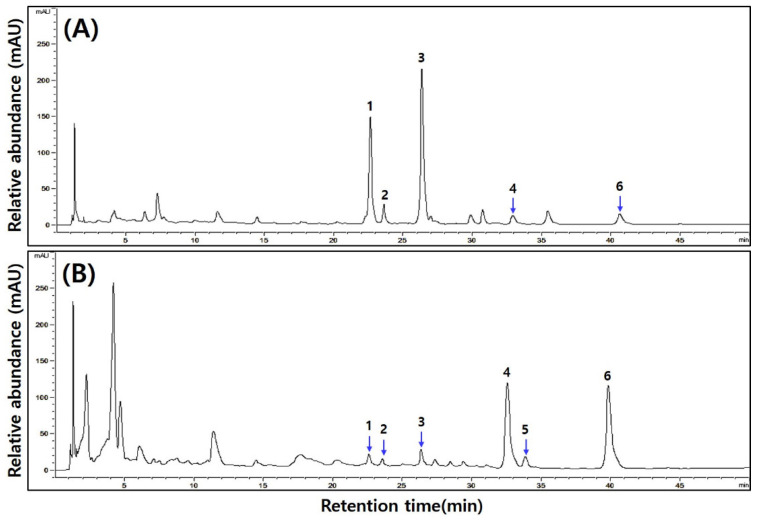
The analysis results of isoflavone contents in cheonggukjang according to the MCG addition ratio are shown in Table 6 and Figure 2. Six compounds were detected: daidzin (peak 1), glycitin (peak 2), genistin (peak 3), daidzein (peak 4), glycitein (peak 5), and genistein (peak 6). The contents of glycosides (daidzin, glycitin, and genistin) before fermentation were generally inversely proportional to the ratio of MCG. After fermentation, as the glycoside form decreased, the content of daidzein in the form of aglycones increased. Fermented cheonggukjang: 0% (57.65 → 612.53 μg/g) > 2.5% (51.26 → 575.99 μg/g) > 5.0% (48.06 → 545.13 μg/g) > 10% (48.34 → 483.17 μg/g). The total isoflavone content was the highest in 0% MCG both before and after fermentation. The increase in daidzein after fermentation occurred due to the enzymatic hydrolysis of daidzin into its aglycone form by β-glucosidase produced by bacteria. Consequently, the daidzein content showed a similar trend corresponding to the daidzin content before fermentation.
Table 6.
Isoflavone and ginsenoside contents of cheonggukjang according to the addition ratio of mountain-cultivated ginseng.
| Contents 1 (μg/g d.w.) | Addition Ratio of Mountain-Cultivated Ginseng (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unfermented Chenoggukjang | Fermented Chenoggukjang | |||||||
| 0 | 2.5 | 5.0 | 10 | 0 | 2.5 | 5.0 | 10 | |
| Isoflavones | ||||||||
| Daidzin | 669.44 ± 13.14a | 659.43 ± 13.19a | 633.50 ± 12.67b | 590.05 ± 10.25c | 138.14 ± 3.45d | 140.44 ± 2.91d | 135.52 ± 2.54d | 121.71 ± 2.44e |
| Glycitin | 309.76 ± 6.21a | 306.36 ± 6.13a | 317.94 ± 6.36a | 285.72 ± 5.71b | 255.14 ± 4.21c | 194.13 ± 3.54d | 194.88 ± 3.88d | 168.94 ± 3.12e |
| Genistin | 847.44 ± 14.25a | 856.8 ± 17.14a | 804.36 ± 14.22b | 739.92 ± 14.80c | 93.68 ± 1.87d | 94.48 ± 1.87d | 85.95 ± 1.68e | 95.83 ± 2.00d |
| Daidzein | 57.65 ± 1.15e | 51.26 ± 1.03f | 48.06 ± 0.96g | 48.34 ± 0.97g | 613.53 ± 12.25a | 575.99 ± 11.52b | 545.13 ± 10.88c | 483.17 ± 9.55d |
| Glycitein | 9.71 ± 0.19d | nd 2 | nd | nd | 87.06 ± 1.74b | 93.24 ± 1.86a | 95.69 ± 2.01a | 82.52 ± 1.70c |
| Genistein | 48.81 ± 0.98d | 45.54 ± 0.91d | 45.39 ± 0.88d | 45.14 ± 0.90d | 354.41 ± 7.09c | 421.7 ± 7.78a | 378.41 ± 7.88b | 343.62 ± 6.54c |
| Total | 1942.81 ± 35.92a | 1919.39 ± 38.39a | 1849.25 ± 35.09b | 1709.17 ± 32.63c | 1540.96 ± 30.61d | 1519.98 ± 29.48e | 1435.58 ± 28.87f | 1295.79 ± 25.35g |
| Ginsenosides | ||||||||
| Ginsenoside Rg1 | nd | nd | nd | 65.64 ± 1.51a | nd | nd | 33.28 ± 0.74c | 45.52 ± 1.01b |
| Ginsenoside Re | nd | nd | 99.35 ± 2.01b | 258.33 ± 6.14a | nd | nd | 33.60 ± 0.77d | 58.85 ± 1.24c |
| Ginsenoside Rf | nd | 19.56 ± 0.39d | 31.64 ± 0.66b | 88.94 ± 1.88a | nd | nd | nd | 22.01 ± 0.38c |
| Ginsenoside Rg2 | nd | 129.51 ± 2.11d | 187.22 ± 3.24c | 360.83 ± 7.54a | nd | nd | nd | 280.23 ± 5.77b |
| Ginsenoside F1 | nd | nd | nd | 54.09 ± 1.25a | nd | nd | nd | 12.15 ± 0.28b |
| Protopanaxtriol | nd | 37.00 ± 0.84c | 36.33 ± 0.87c | 39.93 ± 0.91b | nd | 29.25 ± 0.61d | 30.06 ± 0.65d | 56.79 ± 1.45a |
| Ginsenoside Rb2 | nd | nd | 183.40 ± 3.77b | 294.09 ± 5.99a | nd | nd | nd | 44.57 ± 1.01b |
| Ginsenoside Rd | nd | nd | nd | nd | nd | nd | 18.08 ± 0.37a | nd |
| Ginsenoside Rd2 | nd | nd | nd | 327.40 ± 6.75a | nd | 96.27 ± 2.11c | 94.63 ± 1.99c | 146.20 ± 3.22b |
| Ginsenoside F2 | nd | 66.62 ± 1.64f | 110.48 ± 2.54e | 165.61 ± 3.48d | nd | 200.35 ± 3.58c | 233.68 ± 4.54b | 298.72 ± 6.10a |
| Ginsenoside Rg3 | nd | 56.51 ± 1.03e | 65.56 ± 1.01d | 96.05 ± 2.00b | nd | 89.43 ± 1.87c | 94.71 ± 1.99b | 166.90 ± 3.84a |
| Compound K | nd | 28.54 ± 0.61f | 41.63 ± 0.90e | 96.23 ± 2.15c | nd | 69.43 ± 1.48d | 150.72 ± 3.12b | 231.33 ± 4.88a |
| Ginsenoside Rh2 | nd | nd | nd | 28.60 ± 0.61c | nd | 24.58 ± 0.51d | 33.94 ± 0.71b | 82.32 ± 1.97a |
| Protopanaxdiol | nd | 706.23 ± 15.11d | 811.50 ± 16.55c | 843.07 ± 17.01b | nd | 806.48 ± 16.24c | 840.29 ± 17.11b | 1035.15 ± 21.45a |
| Total | nd | 1043.97 ± 21.73e | 1567.11 ± 31.55c | 2718.81 ± 57.22a | nd | 1315.79 ± 26.40d | 1562.99 ± 31.99c | 2480.74 ± 52.60b |
1 All values are presented as the mean ± standard deviation of pentaplicate determination. Means with different letters within a row are significantly different between samples for the same index (p < 0.05). 2 nd, not detected.
Figure 2.
Isoflavone HPLC chromatography of cheonggukjang according to the addition ratio of mountain-cultivated ginseng (MCG). (A) 10% MCG addition to unfermented cheonggukjang. (B) 10% MCG addition to fermented cheonggukjang. Peak 1, daidzin; peak 2, glycitin; peak 3, genistin; peak 4, daidzein; peak 5, glycitein; peak 6, genistein.
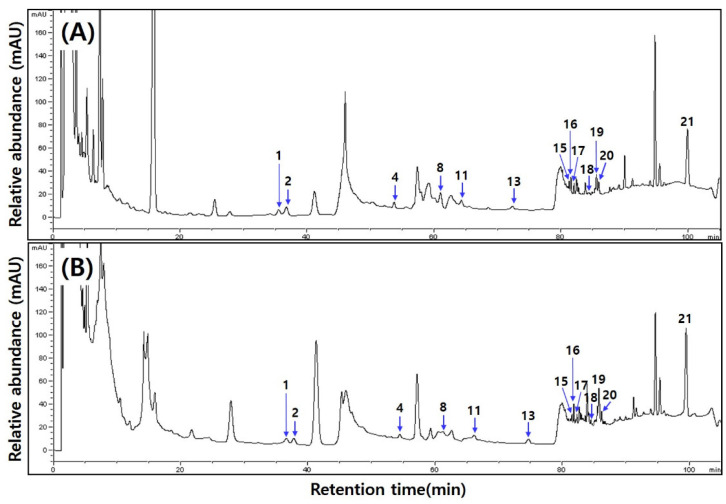
Table 6 and Figure 3 show the analysis results of ginsenoside contents in cheonggukjang based on MCG addition ratios. Ginsenosides were not detected in the 0% MCG sample before or after fermentation. In MCG-added samples, the ginsenoside content increased with higher MCG concentrations and further decreased after fermentation (2.5%: 1043.97 → 1315.79 μg/g, 5.0%: 1567.11 → 1562.99 μg/g, 10%: 2718.81 → 2480.74 μg/g). Rg2, F2, and protopanaxadiol were the major ginsenosides detected in MCG-added cheonggukjang before fermentation. Minor ginsenosides, including Rf, protopanaxtriol, Rg3, and compound K, were also commonly detected. Most of the ginsenosides increased after fermentation, with notable increases in Rg3 (2.5%: 56.51 → 89.43 μg/g, 5.0%: 65.56 → 94.71 μg/g, 10%: 96.05 → 166.90 μg/g) and compound K (2.5%: 28.54 → 69.43 μg/g, 5.0%: 41.63 → 150.72 μg/g, 10%: 96.23 → 231.33 μg/g).
Figure 3.
Ginsenoside HPLC chromatography of cheonggukjang according to the addition ratio of mountain-cultivated ginseng (MCG). (A) 10% MCG addition to unfermented cheonggukjang. (B) 10% MCG addition to fermented cheonggukjang. Peak 1, Rg1; peak 2, Re; peak 3, Ro; peak 4, Rf; peak 5, F5; peak 6, Rb1; peak 7, F3; peak 8, Rg2; peak 9, Rh1; peak 10, Rc; peak 11, Rb2; peak 12, Rb3; peak 13, F1; peak 14, Rd; peak 15, Rd2; peak 16, F2; peak 17, Rg3; peak 18, protopanaxtriol; peak 19, compound K; peak 20, Rh2; peak 21, protopanaxdiol.
The absorption rate in the aglycone form is higher than in the glycoside form, but the amount of aglycones is small in raw soybeans [36,37]. Therefore, to increase the absorption rate of isoflavones in the body, bioconversion process technology and food processing techniques that convert isoflavones into aglycone form are required [38]. The β-glucosidase, essential in biomass conversion, is often isolated from bacteria or fungi by removal of non-reducing terminal glucosyl residues from saccharides and glycosides [39]. Isoflavones exist predominantly as the glycoside forms rather than aglycone forms in soybeans, but are converted from the glycoside to the aglycone forms by the action of β-glucosidase from Bacillus spp. during cheonggukjang fermentation [40]. Previous studies have shown that the proportion of aglycones in total isoflavones is markedly higher in cheonggukjang fractions compared to steamed soybeans [41]. Cho et al. [28] reviewed changes in pH, β-glucosidase, and esterase activities during cheonggukjang fermentation by B. subtilis CS90. They revealed that, among various fermentation times, the crude extract of cheonggukjang, fermented for 36 h, might be most effective for β-glucosidase and esterase activities. In our study, the increase in β-glucosidase activity during fermentation with starter IDCK30 + 40 suggested that the content of daidzein, an aglycone form, increased in fermented cheonggukjang compared to in unfermented cheonggukjang. However, the difference in isoflavone contents was not significant according to the MCG ratio. Therefore, it was considered that MCG did not affect β-glucosidase production.
Ginsenoside Rb1, which accounts for 20% of the total ginsenosides, is commonly used as a precursor to produce minor ginsenosides via β-glucosidases [42]. Specifically, ginsenoside Rb1 is converted to Rd by β-D-glucosidase [43]. Our findings align with previous reports showing that fermentation, high temperature, and aging processes increase the concentration of minor ginsenosides Rg2, Rg3, and compound K, which have higher physiological activity [1]. The primary factors affecting the improvement of ginsenoside pharmacological activities include temperature, heating time, extraction solvent, and stability [44]. Fermentation reduces pH value and increases acidity [1]. By reducing the pH, ginsenoside can be easily deglycosylated and dehydrated at the C-20 position of aglycones when heated under mild acidic conditions rather than neutral and basic conditions. Properties of MCG were changed upon fermentation. To be specific, through acid hydrolysis, formic acid promotes the transformation from ginsenoside Re, Rf, and Rg2 into rare ginsenosides F1, Rh1, Rf2, Rf3, Rg6, and F4 [41]. Compound K (CK), not present in native ginseng, is a degradation product of panaxadiol saponins like ginsenoside Rb1 and Rb2 due to intestinal microflora [45,46]. The rare ginsenoside CK, derived from Panax ginseng, is a panaxadiol saponin that enhances immune function, has anti-inflammatory properties, and resists skin aging, with high bioavailability and absorption in the human body [1,47]. In this study, the Rd2, F2, Rg3, and CK contents in cheonggukjang increased with the proportion of MCG addition, with 10% MCG addition yielding the highest ginsenoside content [48]. Accordingly, it is believed that immune and anti-inflammatory properties can be obtained when consuming cheonggukjang with added MCG compared to 0% MCG cheonggukjang. In our study, cheonggukjang with MCG addition had beneficial effects on the acidity increase, ginsenoside content, and isoflavone levels through the fermentation process. And, through this, the possibility of MCG-added cheonggukjang as a functional food was confirmed.
3.7. Comparison of TPC and TFC of Cheonggukjang According to MCG Ratio
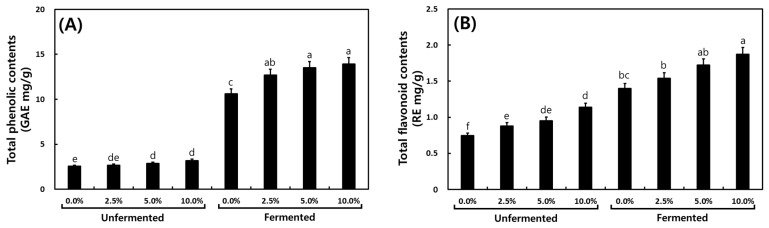
Figure 4 illustrates the analysis results of TPC and TFC in cheonggukjang relative to the MCG addition ratio. In unfermented cheonggukjang, TPC was higher proportionally with the MCG addition (2.55, 2.61, 2.68, and 2.93 GAE mg/g). For fermented cheonggukjang, TPC was observed to be highest in the 10% MCG sample at 13.60 GAE mg/g. The TFC results were similar to those of TPC. TFC increased after fermentation, and the 10% MCG cheonggukjang showed the highest TFC at 1.87 RE mg/g.
Figure 4.
Total phenolic and total flavonoid contents of cheonggukjang according to the addition ratio of mountain-cultivated ginseng. (A) Total phenolic contents. (B) Total flavonoid contents. Different letters above the bars indicate significant difference at p < 0.05 (n = 5).
Phenolic acids like gallic acid and its derivatives exhibit antioxidant, antimutagenic, and anticarcinogenic properties. Daily intake is suggested to offer various health benefits, including reducing disease risk [49,50,51]. While numerous studies have explored increasing ingredients through fermented ginseng, research on TPC and TFC in relation to MCG content remains limited. Fermented aging mountain-cultivated ginseng sprout (FAMCGS) demonstrated the highest average TPC and TFC compared to other processes (MCGS: mountain-cultivated ginseng sprout and AMCGS: aging mountain-cultivated ginseng sprout) [1]. A previous study found that soybeans fermented with B. subtilis had a total phenol content approximately three times higher than unfermented soybeans [52]. Additionally, fermented soybean products with altered isoflavone and phenolic contents showed stronger antioxidant activity than non-fermented ones [53]. Our results align with previous literature reporting that processed soybean products using thermal and fermentative techniques have higher TPC, TFC, and antioxidant capacities than fresh soybean. These changes also occurred due to varying MCG concentrations. These results were similar to the increase in phenol content in cheonggukjang with the addition of garlic [15], red ginseng, Angelica gigas, and Rehmanniae radix [17], and thus, it was confirmed that MCG contributed to the increase in phenol content in cheonggukjang. Consequently, fermenting cheonggukjang with MCG may be recommended as a potent food technique for developing nutraceutical agents and functional materials.
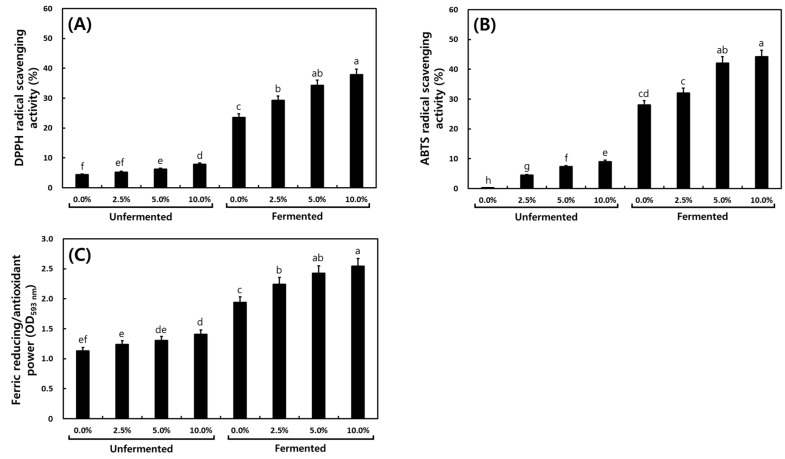
3.8. Comparison of Antioxidant Activity of Cheonggukjang According to MCG Ratio
The antioxidant activities in cheonggukjang according to the MCG addition ratio are shown in Figure 5. DPPH radical scavenging activity increased after fermentation, and the activity increased the MCG ratios correspondingly: 0% MCG (4.39 → 23.59%), 2.5% (5.24 → 29.54%), 5% (6.24 → 34.35%), and 10% (7.90 → 37.86%) (Figure 5A). ABTS radical scavenging activity also increased after fermentation in all samples, from 0.30%, 4.47%, 7.35%, and 9.03% (unfermented) to 28.04%, 32.09%, 42.11%, and 44.22% (fermented), respectively (Figure 5B). FRAP was also increased from 1.23, 1.29, 1.31, and 1.41 OD593nm (unfermented) to 1.94, 2.24, 2.43, and 2.54 OD593nm (fermented), showing a similar trend to radical scavenging activities, but with a slight increase after fermentation compared to DPPH and ABTS radical scavenging activities (Figure 5C). These antioxidant activity results showed similar patterns with the TPC and TFC results.
Figure 5.
Antioxidant activities of cheonggukjang according to the addition ratio of mountain-cultivated ginseng. (A) DPPH radical scavenging activity. (B) ABTS radical scavenging activity. (C) Ferric-reducing/antioxidant power. Different letters above the bars indicate significant differences at p < 0.05 (n = 5).
It is known that the antioxidant effect of MCG depends on the amount added and the processing methods. A similar trend was observed for TPC, indicating that the DPPH radical scavenging activities of MCG and CG could be attributed to their antioxidant and phenolic compounds [54]. Based on the results of antioxidant activities, it is suggested that MCG showed antioxidant properties, especially the effect of fermented 10% MCG cheonggukjang, which was stronger than unfermented 10% MCG. Our results were similar to previously published data showing that antioxidant properties showed high increase rates during fermentation processes. Previous research has shown that changes in DPPH radical-scavenging activity during cheonggukjang fermentation increased from 54.5% to 96.2% by 60 h [15]. Antioxidant activities of foods, including soybeans, can be influenced by the contents of phenolics and aglycones [15]. Furthermore, the transformation of isoflavone glycosides to aglycones, as well as the increase in TPC, TFC, and antioxidant activities during fermentation, improved the functional properties and bioavailability of cheonggukjang. Previous studies on cheonggukjang with added garlic [15] and other ingredients [17] have also reported an increase in antioxidant activity due to an increase in phenolic substances, and it was increased with added garlic, red ginseng, Angelica gigas, and Rehmanniae radix [15,17]. These results also confirmed an increase in antioxidant activity due to the addition of MCG and an increase in the amount added. This suggests that cheonggukjang possesses potential as an additive for functional foods and nutraceuticals to reduce oxidative stress [55].
4. Conclusions
In this study, strains isolated from cheonggukjang in Indang town were selected to improve the preference and functional characteristics of cheonggukjang. The physiologically active substances, antioxidant activity, etc., of cheonggukjang fermented with IDCK 30 and 40, individually or combined, were compared. IDCK 30 + 40 was chosen for fermenting cheonggukjang containing MCG. The nutrients, phytochemical properties, and antioxidant activities of cheonggukjang with different MCG ratios (0%, 2.5%, 5%, and 10%) were analyzed. Fatty acid contents showed no significant difference across MCG ratios. Total isoflavone content was highest before fermentation, while total aglycone content was highest after fermentation. Total ginsenoside content was undetected in MCG-free samples but increased with MCG addition, decreasing after fermentation (2.5%: 1043.97 → 1315.79 μg/g, 5.0%: 1567.11 → 1562.99 μg/g, 10%: 2718.81 → 2480.74 μg/g). But ginsenoside F2, Rg3, and compound K were increased after fermentation. TPC and TFC contents increased with MCG addition and after fermentation (2.61, 2.68, and 2.93 GAE mg/g). DPPH radical scavenging activity increased after fermentation and with MCG addition: 0% MCG (4.39 → 23.59%), 2.5% (5.24 → 29.54%), 5% (6.24 → 34.35%), and 10% (7.90 → 37.86%). ABTS radical scavenging activity (after fermentation: 28.04 < 32.09 < 42.11 < 44.22%) and FRAP (after fermentation: 1.94 < 2.24 < 2.43 < 2.54 OD593nm) also increased. Fermentation significantly enhanced the bioavailability and functional properties of cheonggukjang via conversion from isoflavone glycosides to aglycones. Limited research exists on antioxidant activity related to MCG ratios. This study aimed to improve cheonggukjang quality and provide foundational data for diversification and value addition of cheonggukjang as a natural antioxidant in the food industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13193155/s1, Table S1: Primer for PCR amplification of 16S rRNA, recA, and gyrB; Table S2: Physiological and biochemical characteristics of the fermented soybean strains with IDCK30 and IDCK40; Table S3: The cell wall fatty acid compositions of the fermented soybean strains with IDCK30 and IDCK40; Figure S1: The images of cheonggukjang according to the addition ratio of mountain cultivated ginseng; Figure S2: Nucleotide sequence of 16S rRNA gene from Bacillus licheniformis IDCK30; Figure S3: Nucleotide sequence of 16S rRNA gene from Bacillus subtilis IDCK40; Figure S4: Nucleotide sequence of recA gene from Bacillus licheniformis IDCK30; Figure S5: Nucleotide sequence of recA gene from Bacillus subtilis IDCK40; Figure S6: Nucleotide sequence of gyrB gene from Bacillus licheniformis IDCK30; Figure S7: Nucleotide sequence of gyrB gene from Bacillus subtilis IDCK40.
Author Contributions
Conceptualization, J.H.L. (Jin Hwan Lee) and K.M.C.; methodology, J.H.L. (Jin Hwan Lee) and K.M.C.; validation, J.S., H.Y.L., and K.M.C.; formal analysis, J.S., J.B.J., D.Y.C., D.H.K., J.H.L. (Ji Ho Lee), G.Y.L. and M.Y.J.; investigation, J.S.; data curation, J.S. and K.M.C.; writing—original draft preparation, J.S. and H.Y.L.; writing—review and editing, H.Y.L. and K.M.C.; visualization, D.Y.C. and H.Y.L.; supervision, K.M.C.; project administration, H.Y.L. and K.M.C.; funding acquisition, K.M.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the Development of Forest Life Material Program funded by the Korea Forest Service (Korea Forestry Promotion Institute), grant number 2020187A002022BA01. Also, this research was supported by the Forest science technology commercialization support project funded by the Korea Forestry Promotion Institute, grant number 2023503C10-2323-AB01.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lee J.H., Kim S.C., Lee H.Y., Cho D.Y., Jung J.G., Kang D.W., Kang S.S., Cho K.M. Changes in nutritional compositions of processed mountain-cultivated ginseng sprouts (Panax ginseng) and screening for their antioxidant and anti-inflammatory properties. J. Funct. Food. 2021;86:104668. doi: 10.1016/j.jff.2021.104668. [DOI] [Google Scholar]
- 2.Xu X.F., Cheng X.L., Lin Q.H., Li S.S., Jia Z., Han T., Lin R.C., Wang D., Wei F., Li X.R. Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J. Ginseng Res. 2016;40:344–350. doi: 10.1016/j.jgr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran T.H.M., Puja A.M., Kim H., Kim Y.J. Nanoemulsions prepared from mountain ginseng-mediated gold nanoparticles and silydianin increase the anti-inflammatory effects by regulating NF- κB and MAOK signaling pathways. Biomater. Adv. 2022;137:212814. doi: 10.1016/j.bioadv.2022.212814. [DOI] [PubMed] [Google Scholar]
- 4.Eom S.J., Hwang J.E., Kim H.S., Kim K.T., Paik H.D. Anti-inflammatory and cytotoxic effects of ginseng extract bioconverted by Leuconostoc mesenteroides KCCM 12010P isolated from kimchi. Int. J. Food Sci. Technol. 2018;53:1331–1337. doi: 10.1111/ijfs.13713. [DOI] [Google Scholar]
- 5.Abdelfattah-Hassan A., Shalaby S.I., Khater S.I., El-Shetry E.S., El Fadil H.A., Elsayed S.A. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage. Complement. Ther. Med. 2019;46:95–102. doi: 10.1016/j.ctim.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Park S.H., Chung S., Chung M.Y., Choi H.K., Hwang J.T., Park J.H. Effects of Panax ginseng on hyperglycemia, hypertension, and hyperlipidemia: A systematic review and meta-analysis. J. Ginseng Res. 2022;46:188–205. doi: 10.1016/j.jgr.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.S., Yoo J.M., Park J.E., Kim J., Kim S.G., Seok Y.M., Son J.H., Kim H.J. Anti-angiogenic effect of mountain ginseng in vitro and in vivo: Comparison with farm-cultivated ginseng. Mol. Med. Rep. 2021;24:615. doi: 10.3892/mmr.2021.12254. [DOI] [PubMed] [Google Scholar]
- 8.Kim C.K., Cho D.H., Lee K.S., Lee D.K., Park C.W., Kim W.G., Lee S.J., Ha K.S., Taeg O.G., Kwon Y.G., et al. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid. Based Complement. Altern. Med. 2012;2012:490301. doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park D.H., Han B., Shin M.-S., Hwang G.S. Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide. Polymers. 2020;12:2186. doi: 10.3390/polym12102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Lu Y., Zhao A., Wu Y., Zhang Y., Yang X. Quantitative analysis for several nutrients and volatile components during fermentation of soybean by Bacillus subtilis natto. Food Chem. 2022;374:131725. doi: 10.1016/j.foodchem.2021.131725. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.H., Kim M.J., Ahn J., Lee S.H., Lee H.J., Kim J.H., Park S.H., Jang Y.J., Jung C.H. Nutrikinetics of Isoflavone Metabolites After Fermented Soybean Product (Cheonggukjang) Ingestion in Ovariectomized Mice. Mol. Nutr. Food Res. 2017;61:1700322. doi: 10.1002/mnfr.201700322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.H., Ko J., Kwon D.Y. Jang, Korean fermented soybean product, the result of endeavors of ancients for the best taste of Korean diet. J. Ethn. Food. 2023;10:33. doi: 10.1186/s42779-023-00183-6. [DOI] [Google Scholar]
- 13.Ghosh K., Kang H.S., Hyun W.B., Kim K.P. High prevalence of Bacillus subtilis-infecting bacteriophages in soybean-based fermented foods and its detrimental effects on the process and quality of Cheonggukjang. Food Microbiol. 2018;76:196–203. doi: 10.1016/j.fm.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim I.-S., Hwang C.-W., Yang W.-S., Kim C.-H. Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang. Int. J. Mol. Sci. 2021;22:5746. doi: 10.3390/ijms22115746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.H., Hwang C.E., Lee C.K., Lee J.H., Kim G.M., Jeong S.H., Shin J.H., Kim J.S., Cho K.M. Characteristics and antioxidant effect of garlic in the fermentation of Cheonggukjang by Bacillus amyloliquefaciens MJ1-4. J. Microbiol. Biotechnol. 2014;24:959–968. doi: 10.4014/jmb.1310.10065. [DOI] [PubMed] [Google Scholar]
- 16.Hong S.C., Kwan D.J. Changes in quality characteristics of Cheongkukjang added with Deodeok. Korean J. Food Preserv. 2011;18:171–177. doi: 10.11002/kjfp.2011.18.2.171. [DOI] [Google Scholar]
- 17.Choi E.J., Lee J.S., Chang H.B., Lee M.S., Jang H.D., Kwon Y.I. Changes in the functionality of Cheonggukjang during fermentation supplemented with Angelica gigas, Rehmanniae Radix, and Red ginseng. Microbiol. Biotechnol. Lett. 2010;38:467–474. [Google Scholar]
- 18.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 19.Mahaffee W.F., Kloepper J.W. Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (Cucumis sativus L.) Microb. Ecol. 1997;34:210–223. doi: 10.1007/s002489900050. [DOI] [PubMed] [Google Scholar]
- 20.Seo H.R., Kim J.Y., Kim J.H., Park K.Y. Identification of Bacillus cereus in a Chungkukjang That Showed High Anticancer Effects Against AGS Human Gastric Adenocarcinoma Cells. J. Med. Food. 2009;12:1274–1280. doi: 10.1089/jmf.2009.0081. [DOI] [PubMed] [Google Scholar]
- 21.Goldschmidt-Clermont E., Hochwartner O., Demarta A., Caminada A.P., Fery J. Outbreaks of an ulcerative and haemorrhagic disease in Arctic char Salvelinus alpinus caused by Aeromonas salmonicida subsp. smithia. Dis. Aquat. Org. 2009;86:81–86. doi: 10.3354/dao02110. [DOI] [PubMed] [Google Scholar]
- 22.Vargas-Bello-Pérez E., Pedersen N.C., Khushvakov J., Ye Y., Dhakal R., Hansen H.H., Ahrné L., Khakimov B. Effect of supplementing dairy goat diets with rapeseed oil or sunflower oil on performance, Milk composition, Milk fatty acid profile, and in vitro fermentation kinetics. Front. Vet. Sci. 2022;9:899314. doi: 10.3389/fvets.2022.899314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P.-Y., Shuang F.-F., Yang J.-X., Jv Y.-X., Hu R.-Z., Chen T., Yao X.-H., Zhao W.-G., Liu L., Zhang D.-Y. A rapid and efficient method of microwave-assisted extraction and hydrolysis and automatic amino acid analyzer determination of 17 amino acids from mulberry leaves. Ind. Crops Prod. 2022;186:115271. doi: 10.1016/j.indcrop.2022.115271. [DOI] [Google Scholar]
- 24.Kuligowski M., Pawłowska K., Jasińska-Kuligowska I., Nowak J. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. CyTA-J. Food. 2017;15:27–33. doi: 10.1080/19476337.2016.1197316. [DOI] [Google Scholar]
- 25.Kim J.-H., Shin J.-S., Kim W., Lee H., Baik M.-Y. Effects of Puffing, Acid, and High Hydrostatic Pressure Treatments on Ginsenoside Profile and Antioxidant Capacity of Mountain-Cultivated Panax ginseng. Foods. 2023;12:2174. doi: 10.3390/foods12112174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam Y.D., Yi S.H., Lim S.I. Bacterial diversity of cheonggukjang, a traditional Korean fermented food, analyzed by barcoded pyrosequencing. Food Control. 2012;28:135–142. doi: 10.1016/j.foodcont.2012.04.028. [DOI] [Google Scholar]
- 27.Cho K.M., Hong S.Y., Math R.K., Lee J.H., Kambiranda D.M., Kim J.M., Islam S.M.A., Yun M.G., Cho J.J., Lim W.J., et al. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009;114:413–419. doi: 10.1016/j.foodchem.2008.09.056. [DOI] [Google Scholar]
- 28.Cho K.M., Lee J.H., Yun H.D., Ahn B.Y., Kim H., Seo W.T. Changes in phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011;24:402–410. doi: 10.1016/j.jfca.2010.12.015. [DOI] [Google Scholar]
- 29.Shin E.C., Lee J.H., Hwang C.E., Lee B.W., Kim H.T., Ko J.M., Baek I.Y., Shin J.H., Nam S.H., Seo W.T., et al. Enhancement of total phenolic and isoflavone-aglycone contents and antioxidant activities during Cheonggukjang fermentation of brown soybeans by the potential probiotic Bacillus subtilis CSY191. Food Sci. Biotechnol. 2014;23:531–538. doi: 10.1007/s10068-014-0073-9. [DOI] [Google Scholar]
- 30.Lee H.Y., Lee J.H., Shin E.-C., Cho D.Y., Jung J.G., Kim M.J., Jeong J.B., Kang D., Kang S.S., Cho K.M. Changes in Chemical Compositions and Antioxidant Activities from Fresh to Fermented Red Mountain-Cultivated Ginseng. Molecules. 2022;27:4550. doi: 10.3390/molecules27144550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S.Y., Kim H.E., Kim Y.S. The potentials of Bacillus licheniformis strains for inhibition of B. cereus growth and reduction of biogenic amines in cheonggukjang (Korean fermented unsalted soybean paste) Food Control. 2017;79:87–93. doi: 10.1016/j.foodcont.2017.03.028. [DOI] [Google Scholar]
- 32.Diana M., Rafecas M., Arco C., Quilez J. Free amino acid profile of Spanish artisanal cheeses: Importance of gamma-aminobutyric acid (GABA) and ornithine content. J. Food Compos. Anal. 2014;35:94–100. doi: 10.1016/j.jfca.2014.06.007. [DOI] [Google Scholar]
- 33.Yu J.J., Park K.B., Kim S.G., Oh S.H. Expression, purification, and biochemical properties of arginase from Bacillus subtilis 168. J. Microbiol. 2013;51:222–228. doi: 10.1007/s12275-013-2669-9. [DOI] [PubMed] [Google Scholar]
- 34.Cho K.M., Lim H.J., Kim M.S., Kim D.S., Hwang C.E., Nam S.H., Joo O.S., Lee B.W., Kim J.K., Shin E.C. Time course effects of fermentation on fatty acid and volatile compound profiles of Cheonggukjang using new soybean cultivars. J. Food Drug Anal. 2017;25:637–653. doi: 10.1016/j.jfda.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung I.M., Kim J.K., Yang J.H., Lee J.H., Park S.K., Son N.Y., Kim S.H. Effects of soil type and organic fertilizers on fatty acids and vitamin E in Korean ginseng (Panax ginseng Meyer) Food Res. Int. 2017;102:265–273. doi: 10.1016/j.foodres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Hwang C.E., Kim S.C., Lee J.H., Hong S.Y., Cho K.M. Enhanced biological effect of fermented soy-powder milk with Lactobacillus brevis increasing in γ-aminobutyric acid and isoflavone aglycone contents. J. Appl. Biol. Chem. 2018;61:245–255. doi: 10.3839/jabc.2018.036. [DOI] [Google Scholar]
- 37.Lee M.J., Lee J.M., Kim S., Kim H.J. Simultaneous analysis and measurement of uncertainty estimation of six isoflavones in Cheonggukjang by liquid chromatography-electrospray tandem mass spectrometry. Food Chem. 2019;289:139–144. doi: 10.1016/j.foodchem.2019.02.133. [DOI] [PubMed] [Google Scholar]
- 38.Lu C., Li F., Yan X., Mao S., Zhang T. Effect of pulsed electric field on soybean isoflavone glycosides hydrolysis by β-glucosidase: Investigation on enzyme characteristics and assisted reaction. Food Chem. 2022;378:132032. doi: 10.1016/j.foodchem.2021.132032. [DOI] [PubMed] [Google Scholar]
- 39.Cairns J.R.K., Esen A. β-Glucosidases. Cell Mol. Life Sci. 2010;67:3389–3405. doi: 10.1007/s00018-010-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvia L.H., Celeghini R.M., Chang Y.K. Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chem. 2011;128:640–644. doi: 10.1016/j.foodchem.2011.03.079. [DOI] [Google Scholar]
- 41.Piao Y.Z., Eun J.B. Physicochemical characteristics and isoflavones content during manufacture of short-time fermented soybean product (cheonggukjang) J. Food Sci. Technol. 2020;57:2190–2197. doi: 10.1007/s13197-020-04255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H., Zhang R., Huang Z., Zhou J. Progress in the Conversion of Ginsenoside Rb1 into Minor Ginsenosides Using β-Glucosidases. Foods. 2023;12:397. doi: 10.3390/foods12020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 44.Jang G.Y., Kim M.Y., Lee Y.J., Li M., Shin Y.S., Lee J.S., Jeong H.S. Influence of organic acids and heat treatment on ginsenoside conversion. J. Ginseng Res. 2018;42:532–539. doi: 10.1016/j.jgr.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim K.A., Jung I.H., Park S.H., Ahn Y.T., Huh C.S., Kim D.H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS ONE. 2013;8:e62409. doi: 10.1371/journal.pone.0062409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan Z., Zhu C., Shi J., Fan D., Deng J., Fu R., Huang R., Fan C. High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase. Chin. J. Chem. Eng. 2018;26:1591–1597. doi: 10.1016/j.cjche.2018.02.004. [DOI] [Google Scholar]
- 47.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bekhit A.E.A., Duncan A., Bah C.S.F., Ahmed I.A.M., Al-Juhaimi F.Y., Amin H.F. Impact of fermentation conditions on the physicochemical properties, fatty acid and cholesterol contents in salted-fermented hoki roe. Food Chem. 2018;264:73–80. doi: 10.1016/j.foodchem.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Saraç N., Şen B. Antioxidant, mutagenic, antimutagenic activities, and phenolic compounds of Liquidambar orientalis Mill. var. orientalis. Ind. Crops Prod. 2014;53:60–64. doi: 10.1016/j.indcrop.2013.12.015. [DOI] [Google Scholar]
- 50.Makhafola T.J., Elgorashi E.E., McGaw L.J., Verschaeve L., Eloff J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Altern. Med. 2016;16:490. doi: 10.1186/s12906-016-1437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramadan D.T., Ali M.A.M., Yahya S.M., El-Sayed W.M. Correlation between Antioxidant/Antimutagenic and Antiproliferative Activity of Some Phytochemicals. Anticancer Agents Med. Chem. 2019;19:1481–1490. doi: 10.2174/1871520619666190528091648. [DOI] [PubMed] [Google Scholar]
- 52.Ali M.W., Shahzad R., Bilal S., Adhikari B., Kim I.D., Lee J.D., Lee I.J., Kim B.O., Shin D.H. Comparison of antioxidants potential, metabolites, and nutritional profiles of Korean fermented soybean (Cheonggukjang) with Bacillus subtilis KCTC 13241. J. Food Sci. Technol. 2018;55:2871–2880. doi: 10.1007/s13197-018-3202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chai C., Ju H.K., Kim S.C., Park J.H., Lim J., Kwon S.W., Lee J. Determination of bioactive compounds in fermented soybean products using GC/MS and further investigation of correlation of their bioactivities. J. Chromatogr. B. 2012;880:42–49. doi: 10.1016/j.jchromb.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Zhao B., Wang X., Liu H., Lv C., Lu J. Structural characterization and antioxidant activity of oligosaccharides from Panax ginseng C. A. Meyer. Int. J. Biol. Macromol. 2020;150:737–745. doi: 10.1016/j.ijbiomac.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Sanjukta S., Rai A.K., Muhammed A., Jeyaram K., Talukdar N.C. Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J. Funct. Foods. 2015;14:650–658. doi: 10.1016/j.jff.2015.02.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.