Abstract
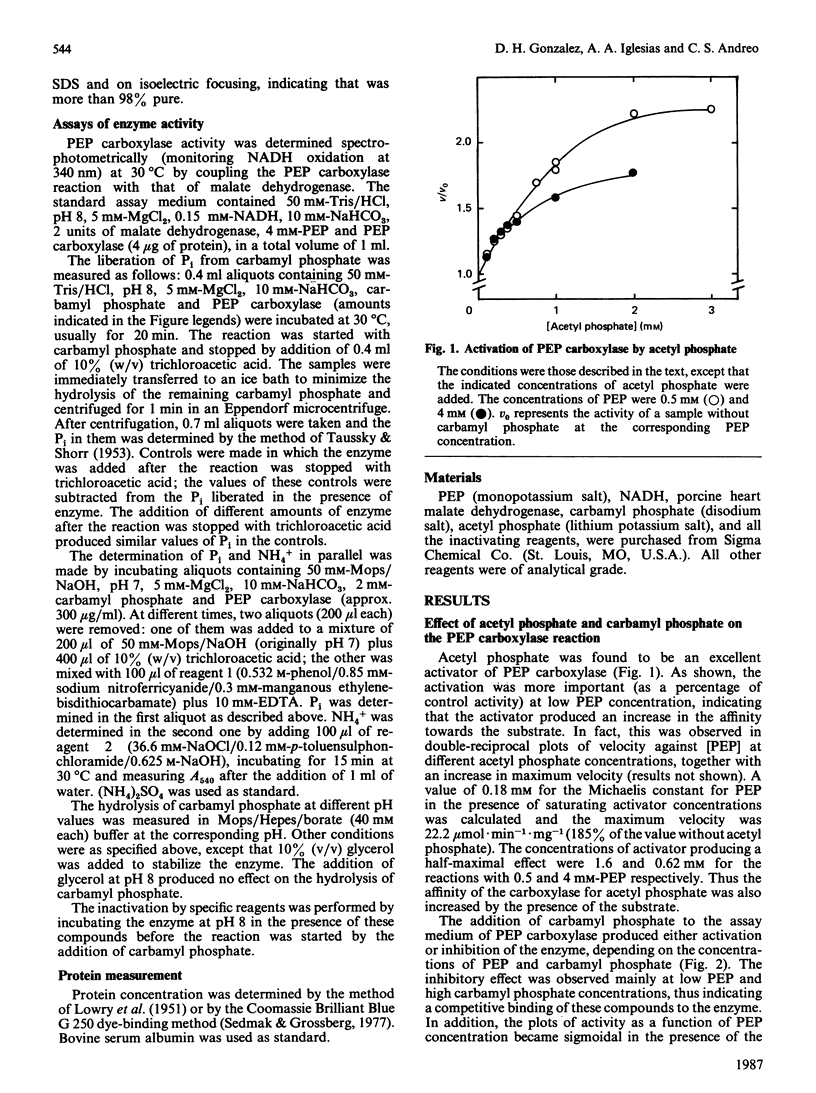
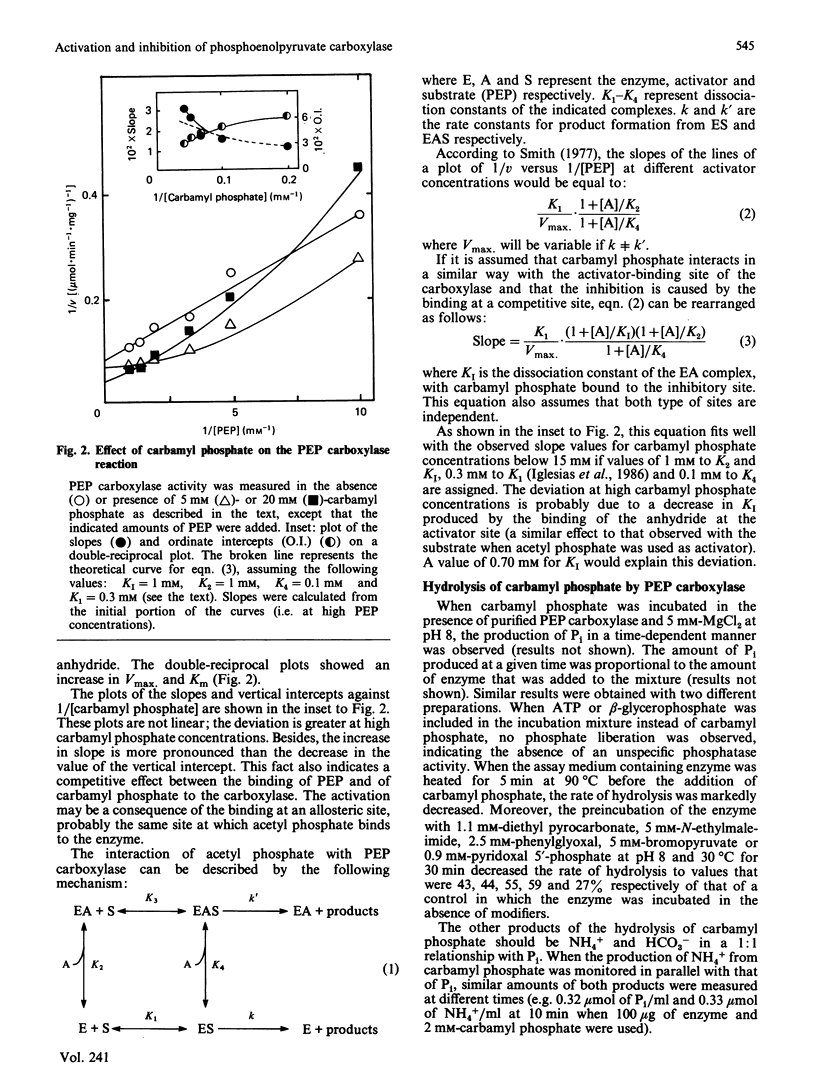
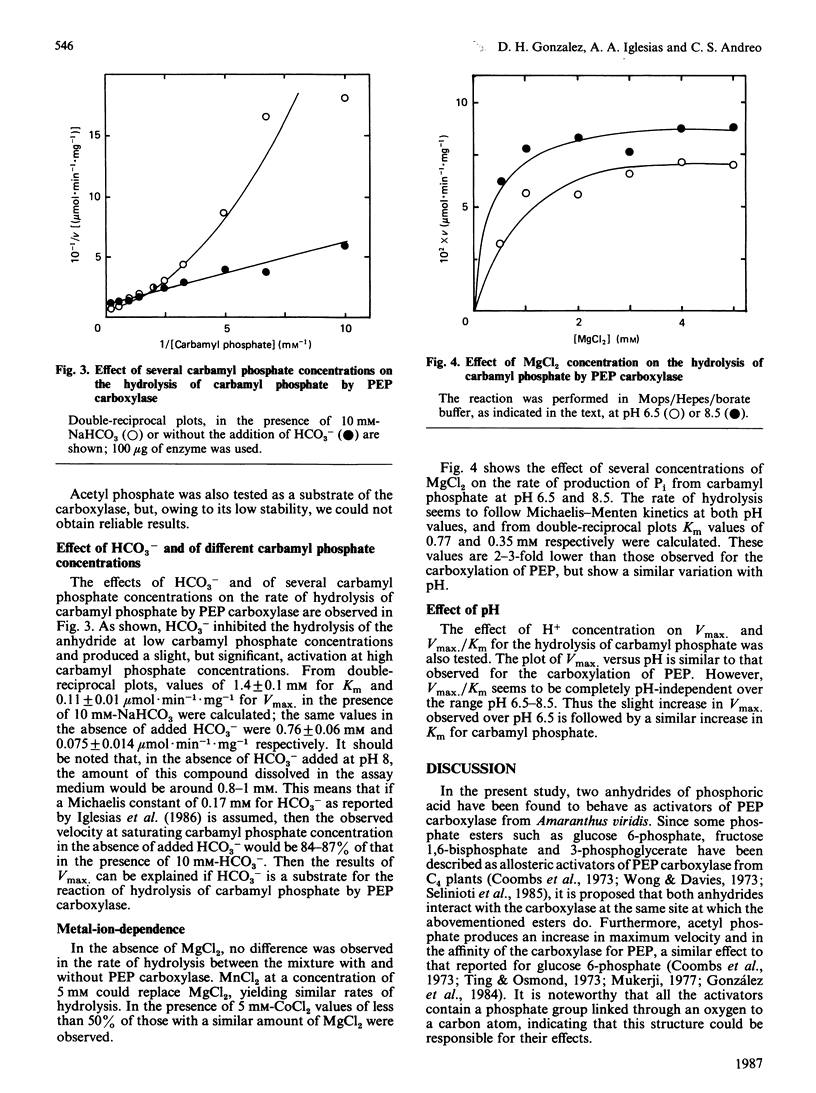
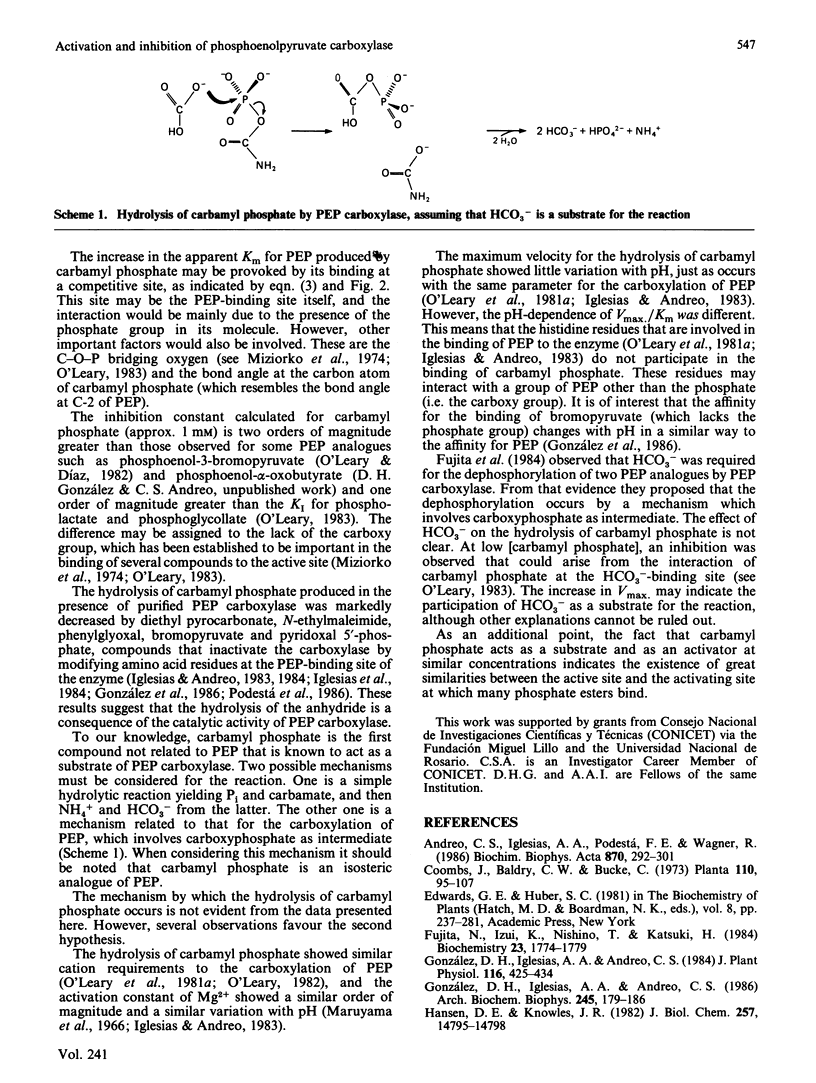
Acetyl phosphate produced an increase in the maximum velocity (Vmax. for the carboxylation of phosphoenolpyruvate catalysed by phosphoenolpyruvate carboxylase. The limiting Vmax. was 22.2 mumol X min-1 X mg-1 (185% of the value without acetyl phosphate). This compound also decreased the Km for phosphoenolpyruvate to 0.18 mM. The apparent activation constants for acetyl phosphate were 1.6 mM and 0.62 mM in the presence of 0.5 and 4 mM-phosphoenolpyruvate respectively. Carbamyl phosphate produced an increase in Vmax. and Km for phosphoenolpyruvate. The variation of Vmax./Km with carbamyl phosphate concentration could be described by a model in which this compound interacts with the carboxylase at two different types of sites: an allosteric activator site(s) and the substrate-binding site(s). Carbamyl phosphate was hydrolysed by the action of phosphoenolpyruvate carboxylase. The hydrolysis produced Pi and NH4+ in a 1:1 relationship. Values of Vmax. and Km were 0.11 +/- 0.01 mumol of Pi X min-1 X mg-1 and 1.4 +/- 0.1 mM, respectively, in the presence of 10 mM-NaHCO3. If HCO3- was not added, these values were 0.075 +/- 0.014 mumol of Pi X min-1 X mg-1 and 0.76 +/- 0.06 mM. Vmax./Km showed no variation between pH 6.5 and 8.5. The reaction required Mg2+; the activation constants were 0.77 and 0.31 mM at pH 6.5 and 8.5 respectively. Presumably, carbamyl phosphate is hydrolysed by phosphoenolpyruvate carboxylase by a reaction the mechanism of which is related to that of the carboxylation of phosphoenolpyruvate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujita N., Izui K., Nishino T., Katsuki H. Reaction mechanism of phosphoenolpyruvate carboxylase. Bicarbonate-dependent dephosphorylation of phosphoenol-alpha-ketobutyrate. Biochemistry. 1984 Apr 10;23(8):1774–1779. doi: 10.1021/bi00303a029. [DOI] [PubMed] [Google Scholar]
- Gonzalez D. H., Iglesias A. A., Andreo C. S. Active-site-directed inhibition of phosphoenolpyruvate carboxylase from maize leaves by bromopyruvate. Arch Biochem Biophys. 1986 Feb 15;245(1):179–186. doi: 10.1016/0003-9861(86)90203-1. [DOI] [PubMed] [Google Scholar]
- Hansen D. E., Knowles J. R. The stereochemical course at phosphorus of the reaction catalyzed by phosphoenolpyruvate carboxylase. J Biol Chem. 1982 Dec 25;257(24):14795–14798. [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maruyama H., Easterday R. L., Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. I. Purification and properties of phosphoenolpyruvate carboxylase. J Biol Chem. 1966 May 25;241(10):2405–2412. [PubMed] [Google Scholar]
- Miziorko H. M., Nowak T., Mildvan A. S. Spinach leaf phosphoenolpyruvate carboxylase: purification, properties, and kinetic studies. Arch Biochem Biophys. 1974 Jul;163(1):378–389. doi: 10.1016/0003-9861(74)90489-5. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. Inhibition of 14CO2 fixation by SO3(2-) and activation by glucose 6-phosphate. Arch Biochem Biophys. 1977 Jul;182(1):360–365. doi: 10.1016/0003-9861(77)90317-4. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., DeGooyer W. J., Dougherty T. M., Anderson V. 1-Hydroxycyclopropane carboxylic acid phosphate: a potent inhibitor of enzymes metabolizing phosphoenolpyruvate. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1320–1325. doi: 10.1016/0006-291x(81)91968-9. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Diaz E. Phosphoenol-3-bromopyruvate. A mechanism-based inhibitor of phosphoenolpyruvate carboxylase from maize. J Biol Chem. 1982 Dec 25;257(24):14603–14605. [PubMed] [Google Scholar]
- Podesta F. E., Iglesias A. A., Andreo C. S. Modification of an essential amino group of phosphoenolpyruvate carboxylase from maize leaves by pyridoxal phosphate and by pyridoxal phosphate-sensitized photooxidation. Arch Biochem Biophys. 1986 May 1;246(2):546–553. doi: 10.1016/0003-9861(86)90309-7. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase: studies on the mechanism of multiple allosteric interactions. Arch Biochem Biophys. 1977 Oct;183(2):538–552. doi: 10.1016/0003-9861(77)90389-7. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsching P., O'Leary M. H. (E)-3-Cyanophosphoenolpyruvate, a new inhibitor of phosphoenolpyruvate-dependent enzymes. Biochemistry. 1985 Dec 17;24(26):7602–7606. doi: 10.1021/bi00347a015. [DOI] [PubMed] [Google Scholar]
- Wong K. F., Davies D. D. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J. 1973 Mar;131(3):451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]


