Abstract
We investigated the use of lentivirus vectors for gene transfer to quiescent alveolar epithelial cells. Primary rat alveolar epithelial cells (AEC) grown on plastic or as polarized monolayers on tissue culture-treated polycarbonate semipermeable supports were transduced with a replication-defective human immunodeficiency virus-based lentivirus vector pseudotyped with the vesicular stomatitis virus G (VSV-G) protein and encoding an enhanced green fluorescent protein reporter gene. Transduction efficiency, evaluated by confocal microscopy and quantified by fluorescence-activated cell sorting, was dependent on the dose of vector, ranging from 4% at a multiplicity of infection (MOI) of 0.1 to 99% at an MOI of 50 for AEC grown on plastic. At a comparable titer and MOI, transduction of these cells by a similarly pseudotyped murine leukemia virus vector was ∼30-fold less than by the lentivirus vector. Importantly, comparison of lentivirus-mediated gene transfer from the apical or basolateral surface of confluent AEC monolayers (Rt > 2 kΩ · cm2; MOI = 10) revealed efficient transduction only when VSV-G-pseudotyped lentivirus was applied apically. Furthermore, treatment with EGTA to increase access to the basolateral surface did not increase transduction of apically applied virus, indicating that transduction was primarily via the apical membrane domain. In contrast, differentiated tracheal epithelial cells were transduced by apically applied lentivirus only in the presence of EGTA and at a much lower overall efficiency (∼15-fold) than was observed for AEC. Efficient transduction of AEC from the apical cell surface supports the feasibility of using VSV-G-pseudotyped lentivirus vectors for gene transfer to the alveolar epithelium and suggests that differences exist between upper and lower airways in the polarity of available receptors for the VSV-G protein.
Gene transfer to the alveolar epithelium is an attractive therapeutic approach for a number of acute and chronic acquired lung diseases, including pulmonary inflammation, pulmonary edema, acute lung injury and/or acute respiratory distress syndrome, and pulmonary fibrosis (1, 5, 15, 43). In addition, due to its large surface area and proximity to the vascular endothelium, the alveolar epithelium represents an attractive target for delivery of therapeutic genes encoding secreted proteins. However, in contrast to the large number of studies that have utilized a variety of vectors to achieve gene transfer to tracheal and bronchial epithelium in the upper airways, particularly in the context of gene therapy for cystic fibrosis (54), relatively few studies have examined gene transfer to the alveolar epithelium of the distal respiratory tract.
Nonviral strategies for delivery of exogenous DNA to the lung have been limited by low efficiency of transduction (56). Of the various vectors that have been evaluated for gene therapy thus far, each exhibits characteristic disadvantages, and none has proven effective in achieving efficient, long-term expression in the distal respiratory tract. Due to the relative quiescence of the cells that constitute the alveolar epithelium, viral vectors must be able to efficiently transduce cells that are not actively dividing (37, 46). In this regard, adenovirus vectors have been shown to effectively transduce the alveolar epithelium (15). However, use of these vectors in vivo has been limited by immune responses, which can be especially problematic in lung alveoli due to the potential for inducing serious pulmonary inflammation (55). In any event, use of this nonintegrating vector system would require repeated viral administration to achieve long-term gene expression (12). Adeno-associated virus vectors show episomal expression and eventual integration after cell division and have been used for gene delivery to the distal respiratory tract (16, 17). However, their use has been limited by low packaging capacity and difficulty in obtaining high-titer preparations (20). Repeated administration of adeno-associated virus vectors has been limited by development of neutralizing antibodies (8). Thus, investigation of alternative vectors for gene delivery to the alveolar epithelium is warranted.
Under normal in vivo conditions, the cells that constitute the alveolar epithelium undergo very low rates of proliferation (2, 4, 46, 47). The efficiency of standard murine leukemia virus (MLV)-based retroviruses for gene transfer to the adult alveolar epithelium is therefore predictably inefficient (14, 50). This limitation has been partially overcome by inducing cell proliferation with growth factors (52), but overall transduction efficiency is still quite low. On the other hand, the newer lentivirus-based vectors have recently been shown to transduce several nondividing cell types, including neurons, myocytes, and tracheal epithelial cells, with long-term persistence of transgene expression (21, 23, 34, 40). Pseudotyping of the lentivirus with different fusion proteins has expanded the range of host cells that can be transduced by these vectors and allowed the virus to be easily concentrated to high titers, especially when pseudotyped with the vesicular stomatitis virus G (VSV-G) protein (38). VSV-G-pseudotyped lentivirus vectors would therefore appear to be ideally suited for gene transfer to the relatively quiescent cells of the alveolar epithelium.
To date, studies of lentivirus-based gene transfer in the lung have focused on transduction of the tracheal or bronchial epithelium of the proximal airways, but the efficiency of gene transfer has been much lower than that described in other cell types. In polarized, well-differentiated airway epithelia, only minimal transduction by VSV-G-pseudotyped vectors introduced from the apical surface (the only directly accessible surface in vivo) has been observed (21). The use of lentivirus vectors for transduction of alveolar epithelial cells (AEC) in the distal respiratory tract has not been evaluated to date. In particular, the issue of whether the polarized cells that constitute the alveolar epithelium present a similar barrier to apical transduction by lentivirus has not been explored.
Over a period of 3 to 4 days, isolated rat type II alveolar (AT2) cells maintained in primary culture gradually lose the characteristic hallmarks of type II cells, such as lamellar bodies and production of surfactant-associated proteins, and change morphologically to resemble alveolar type I (AT1) cells, becoming more flattened with expansive cytoplasmic processes (6). Concurrently, they begin to express a number of phenotypic markers specific for alveolar type I cells in situ, suggesting that AT2 cells in culture are undergoing transdifferentiation toward a type I cell-like phenotype (9, 10). AT2-to-AT1 cell transdifferentiation occurs in the absence of appreciable cell division (27). When grown on semipermeable supports, the cells form polarized high-resistance monolayers and exhibit active sodium transport that occurs in a vectorial fashion, similar to that observed in the alveolar epithelium in vivo (7). Previous studies have demonstrated minimal DNA synthesis in AEC cultivated in vitro at high density (>2 × 105 cells/cm2) in the absence of exogenous growth factors, with a nuclear labeling index consistently on the order of 1 to 3% (26, 27, 28, 46, 48). AEC in primary culture therefore constitute a well-characterized in vitro model with which to evaluate characteristics of the polarized and relatively quiescent alveolar epithelium.
In the present report, we have compared the ability of VSV-G-pseudotyped lentivirus and MLV retrovirus vectors to transduce AEC in primary culture. Furthermore, the relative efficiency of transduction of confluent AEC and primary differentiated tracheal epithelial cells after exposure to lentivirus vectors from either the apical or basolateral cell surface was examined.
MATERIALS AND METHODS
Rat AEC isolation and culture.
AT2 cells were isolated from the lungs of adult male Sprague-Dawley rats by disaggregation with elastase (2.0 to 2.5 U/ml) (Worthington Biochemical, Freehold, N.J.), followed by panning on immunoglobulin G-coated bacteriologic plates (3, 11). Enriched AT2 cells were resuspended in defined medium (MDSF) consisting of Dulbecco's modified Eagle medium and Ham's F-12 nutrient mixture in a 1:1 ratio (DME-F12; Sigma-Aldrich Chemical, St. Louis, Mo.), supplemented with 1.25 mg of bovine serum albumin (Collaborative Research, Bedford, Mass.)/ml, 10 mM HEPES, 0.1 mM nonessential amino acids, 2.0 mM glutamine, 100 U of sodium penicillin G/ml, 100 μg of streptomycin/ml, and 10% newborn bovine serum (Omega Scientific, Tarzana, Calif.). Cells were seeded onto plastic, chamber slides or tissue culture-treated polycarbonate (Nuclepore) filter cups (0.4-μm pore size; 1.1 cm2; Transwell; Corning-Costar, Cambridge, Mass.) at a density of 1.0 × 106 cells/cm2 and grown to confluence. Media were changed on the second day after plating and every other day thereafter. Cultures were maintained in a humidified 5% CO2 incubator at 37°C. AT2 cell purity (>90%) was assessed by staining freshly isolated cells for lamellar bodies with tannic acid (29). Cell viability (>90%) was measured by trypan blue dye exclusion.
RTEC isolation and culture.
Rat tracheal epithelial cells (RTEC) were isolated from adult male Sprague-Dawley rats by incubation of tracheas overnight at 4°C in 0.15% pronase (Roche Molecular Biochemicals, Indianapolis, Ind.). Tracheas were agitated to release cells that were then disaggregated in DNase I (0.5 mg/ml, 5 min on ice). Cells were adhered (2 h) to remove fibroblasts; nonadherent cells were counted, and viability (>90%) was determined by trypan blue dye exclusion. RTEC were resuspended in defined medium consisting of DME-F12 supplemented with l-glutamine (6.5 mM), NaHCO3 (25 mM), insulin (10 μg/ml), hydrocortisone (0.1 μg/ml), transferrin (5 μg/ml), phosphoethanolamine (50 μM), ethanolamine (80 μM), cholera toxin (0.1 μg/ml), bovine pituitary extract (0.03 mg/ml), epidermal growth factor (10 ng/ml; Becton Dickinson Labware, Bedford, Mass.), HEPES (30 mM), bovine serum albumin (0.5 mg/ml), retinoic acid (0.05 μM), penicillin (50 U/ml), streptomycin (50 μg/ml), and amphotericin B (0.25 μg/ml) as described by others (22, 35). Cells were seeded (5 × 105 cells/cm2) onto filter cups (0.4-μm pore size; 0.33 cm2; Transwell Clear; Corning-Costar) coated with rat tail collagen (50 μg/ml; Becton Dickinson Labware) and maintained in a humidified 5% CO2 incubator at 37°C. Nuserum (10%; Becton Dickinson Labware) was added during the first 2 days. Cells were maintained with medium in the apical and basolateral chambers until transmembrane resistance was >300 Ω · cm2. Medium was then removed from the apical chamber (typically at days 2 to 4), and cells were maintained at air-liquid interface (ALI) by daily supplying fresh medium to the basolateral chamber only. Differentiated, multilayered epithelial cell populations were assessed by histology of paraffin-embedded sections of RTEC cultures.
Measurement of bioelectric properties.
The transepithelial resistance (Rt) and the spontaneous potential difference (SPD) of AEC and RTEC grown on filters were measured by using a rapid screening device (Millicell-ERS; Millipore, Bedford, Mass.) as previously described (24). Short-circuit current (ISC) was calculated from the relationship ISC = SPD/Rt. Cell culture media and all other chemicals were purchased from Sigma-Aldrich Chemical unless otherwise noted and were of the highest commercial purity available.
Assessment of alveolar epithelial cell proliferation.
On day 4, AEC grown on plastic in MDSF plus 10% newborn bovine serum were labeled with bromodeoxyuridine (BrdU; 10 μM), a thymidine analog that is incorporated into newly synthesized DNA, for 6 h. Cells were washed twice with phosphate-buffered saline (PBS), released by incubation for 10 min with 5 μM EDTA, and washed again with PBS. Cells were fixed, permeabilized, refixed, and treated with DNase by using the BrdU Flow Kit protocol (BD Pharmingen, San Diego, Calif.). Samples were stained with a fluorescein isothiocyanate-conjugated anti-BrdU antibody and analyzed by fluorescence-activated cell sorting (FACS).
Vector production and virus preparation
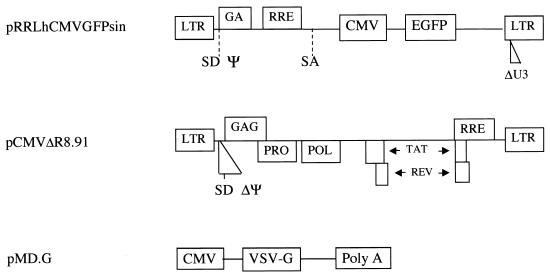
Recombinant lentivirus vector and packaging constructs (generously provided by L. Naldini, University of Turin, Turin, Italy) were produced as previously described (34), by using the constructs shown in Fig. 1. The vector construct, pRRLhCMVGFPsin, consists of a human immunodeficiency virus (HIV)-based self-inactivating (SIN) replication-defective lentivirus transfer vector expressing an enhanced green fluorescent protein (EGFP) reporter gene driven by the cytomegalovirus (CMV) immediate-early promoter (57). Human 293T cells (80 to 90% confluence) were cotransfected by calcium phosphate precipitation with 12 μg of pRRLhCMVGFPsin, 10 μg of pCMVΔR8.91 for viral packaging, and 8 μg of pMD.G for VSV-G pseudotyping (58). Virus was isolated and for some experiments was concentrated through a centrifugal concentrator (Macrosep; Pall Gelman Laboratory, Ann Arbor, Mich.) with a 300-kDa molecular mass cutoff and stored at −80°C. Titers of vector stocks were determined on HeLa cells by FACS with a Becton-Dickinson FACScan equipped with a 488-nm argon laser and were in the range of 106 to 108 transducing units (TU)/ml. MLV-based vectors were generated by using a similar protocol (44) and similarly pseudotyped with VSV-G.
FIG. 1.
Vectors used for generation of lentivirus. SIN lentivirus vectors (pRRLhCMVGFPsin) were used to produce infectious particles by cotransfection of gag-pol (pCMVΔR8.91) and pMD.G (for VSV-G envelope) into 293T cells.
Viral transduction of AEC.
AEC grown on plastic or chamber slides were transduced with lentivirus (multiplicity of infection [MOI] of 0.1 to 50) on day 4 to ascertain a dose-response relationship. Medium was aspirated, and cells were incubated with virus for 1 h in the presence of Polybrene (8 μg/ml). Cells were trypsinized 72 h after transduction, washed, and resuspended in PBS. The efficiency of cell transduction was analyzed by FACS. To confirm that GFP expression was not the result of pseudotransduction, control experiments were performed in the presence of zidovudine (AZT; 200 μM) to inhibit viral reverse transcriptase. The efficiency of transduction of AEC by VSV-G-pseudotyped lentivirus or MLV-based retrovirus of comparable titers were compared and analyzed in a similar fashion. Live gating of viable cells was performed by using forward-scatter and side-scatter parameters, and the percentage of cells exhibiting EGFP fluorescence was quantified on fluorescence channel 1 (FL1).
To assess the polarity of lentivirus entry into AEC, confluent polarized AEC monolayers (Rt > 2 kΩ · cm2) grown on tissue culture-treated polycarbonate filters were incubated on day 4 with concentrated lentivirus applied either to the apical or the basolateral surface for 1 h at 37°C. Rt was measured 30 min before and 30 min and 3 days after infection. For infection from the apical side, medium was aspirated and cells were incubated with 100 μl of virus. For basolateral infection, medium was aspirated and the filter was inverted before the addition of 40 μl of concentrated virus. In some experiments, EGTA was first added at a final concentration of 4.5 mM to disrupt tight junctions, followed by the addition of virus to the apical side as described above. Cells on filters at 72 h postinfection were rinsed with PBS, trypsinized, washed, and resuspended in PBS. To ensure cell recovery, filters were also flushed once with PBS. Transduction efficiency after infection from either apical or basolateral surface was assessed by FACS analysis as described above.
Southern analysis of transduced AEC.
Genomic DNA was harvested from AEC 72 h posttransduction by proteinase K digestion and phenol-chloroform extraction. A 10-μg portion was digested overnight with NotI, which yields a 2.2-kb internal fragment that encompasses EGFP, and then separated by agarose gel electrophoresis. After alkali denaturation, gels were transferred to Pall Biodyne B nylon membranes (Pall Biosupport Division, Port Washington, N.Y.) in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer. A 1.1-kb DNA probe specific for EGFP was labeled by using a random primer DNA biotinylation kit (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) and hybridized to the membranes in formamide hybridization buffer. Membranes were washed at high stringency, followed by incubation with alkaline phosphatase-streptavidin conjugate. After the washing, signal was detected by incubation with CDP-Star chemiluminescent substrate (Tropix, Inc., Bedford, Mass.) and exposure to X-ray film.
Viral transduction of RTEC.
Cells grown on membranes maintained at ALI for 2 to 4 weeks were transduced by incubation with lentivirus (MOI = 5) applied to the apical surface for 2 h at 37°C. Infection was carried out as described above. To disrupt tight junctions, cells were pretreated in some experiments with EGTA in hypotonic solution (6 mM in 10 mM HEPES [pH 7.4]) as previously described (53). Transduction efficiency after infection from the apical surface was assayed by FACS analysis.
Confocal microscopy.
AEC grown on chamber slides (Lab-Tek II; Nalge Nunc, Rochester, N.Y.) were infected with lentivirus on day 4. At 72 h after transduction, cells were rinsed, fixed in 2% paraformaldehyde, and mounted (ProLong Antifade Kit; Molecular Probes, Eugene, Oreg.). Cells were viewed with a PCM 2000 confocal microscopy system (Nikon USA, Melville, N.Y.).
Statistical analysis.
Data are presented as mean ± the standard error of the mean. Significance (P < 0.05) of differences between two experimental conditions was assessed by use of unpaired Student t tests.
RESULTS
Lentivirus vectors achieve efficient transduction of AEC.
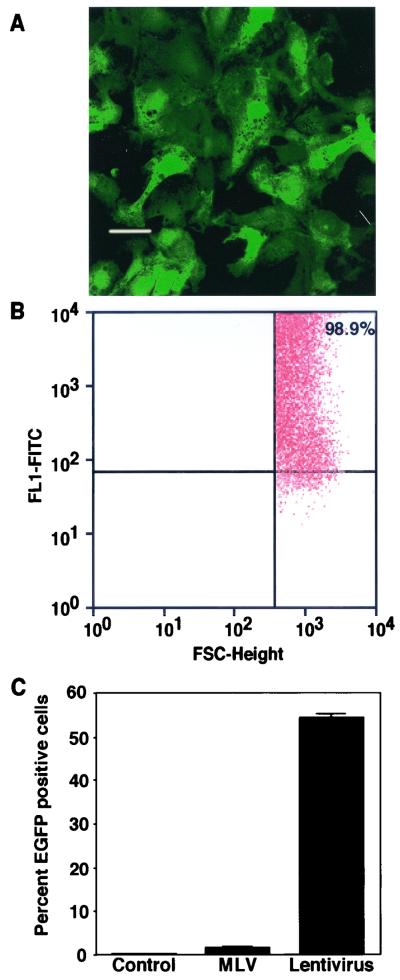
A SIN lentivirus transfer vector encoding the EGFP marker gene was packaged by a standard three-plasmid cotransfection procedure (33) to produce vectors pseudotyped with the VSV-G protein (Fig. 1). The titers of unconcentrated vector supernatants generated by this procedure are typically on the order of 5 × 106 TU/ml when titers were determined on 293T or HeLa cells (data not shown). After concentration, titers on the order of 108 TU/ml were achieved. These vectors were used to transduce rat AEC in primary culture on day 4 postisolation, after the AT2 cells grown on tissue culture-treated plastic surfaces had progressed toward an AT1 cell-like phenotype. Consistent with previous reports that AEC in culture are largely quiescent, FACS analysis demonstrated that only 1.0% ± 0.1% of cells were labeled with BrdU. Nevertheless, confocal microscopy of AEC grown on chamber slides demonstrates strong expression of EGFP at 3 days posttransduction in a majority of cells (Fig. 2A). Representative FACS analysis at 3 days posttransduction shows a shifted population of cells exhibiting higher fluorescence intensity specifically in the EGFP wavelength (FL1 channel), with 99% GFP-positive cells, further demonstrating that AEC are efficiently transduced by this vector (Fig. 2B). Changing the plating density of AEC did not affect the percentage of GFP-positive cells after incubation with the same preparation of lentivirus, indicating that cell density has little effect on transduction efficiency (data not shown). Comparison with VSV-G-pseudotyped standard MLV-based retrovirus vectors of comparable titer demonstrates ∼30-fold greater transduction by the lentivirus vectors (Fig. 2C). A reduction in the number of GFP-expressing cells was observed after transduction by lentivirus vectors in the presence of AZT (∼75% inhibition at 200 μM AZT), confirming that GFP expression is not due to pseudotransduction. Furthermore, Southern analysis demonstrates a 2.2-kb band of predicted size after NotI digestion and hybridization with a GFP-specific probe, confirming vector integration (data not shown).
FIG. 2.
Transduction of AEC with VSV-G-pseudotyped lentivirus vectors. (A) AEC in primary culture on chamber slides were transduced with VSV-G-pseudotyped lentivirus vectors at an MOI of 10. Confocal microscopy from a representative experiment at 3 days posttransduction demonstrates strong expression of EGFP in the majority of cells. Bar, 20 μm. (B) AEC grown on plastic tissue culture dishes were transduced with VSV-G-pseudotyped lentivirus vectors. Representative FACS analysis at 72 h after infection demonstrates 99% EGFP-positive cells at an MOI of 50. (C) Transduction of AEC grown on plastic is ∼30-fold greater for VSV-G-pseudotyped lentivirus vectors than VSV-G-pseudotyped MLV-based vectors at a comparable titer.
Transduction of AEC by lentivirus vectors is dose dependent.
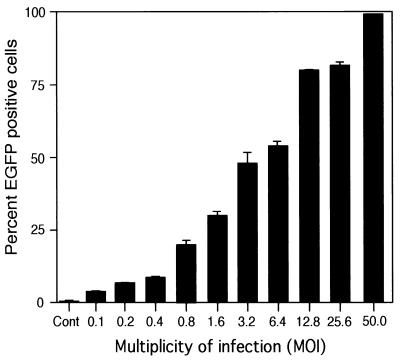
To determine whether varying the MOI would alter the efficiency of gene delivery by the lentivirus vectors, fixed numbers of AEC in primary culture were plated, and replicate plates were subsequently challenged with increasing dosages of the vectors. For this experiment, lentivirus vector supernatants were first concentrated to produce a vector preparation in the range of 2 × 108 TU/ml (as determined by FACS analysis of transduced HeLa cells). A dose-dependent increase in transduction efficiency of AEC was observed with increasing MOI, with the EGFP-positive population ranging from 4 to 99% after infection at an MOI from 0.1 to 50 relative to the titers determined on HeLa cells (Fig. 3). Although transduction of AEC by VSV-G-pseudotyped lentivirus vectors is thus somewhat less efficient than transduction of HeLa cells, almost complete transduction could be achieved by increasing the MOI. No toxicity was noted at a higher MOI as reflected by lack of change in morphology or cell number and the absence of a subpopulation of dead cells observed by FACS analysis.
FIG. 3.
Effect of various MOIs on the efficiency of transduction of AEC with VSV-G-pseudotyped lentivirus vectors. AEC grown on plastic were transduced with VSV-G-pseudotyped lentivirus vectors previously titered on 293T cells (1 × 108 to 2 × 108 IU/ml). The transduction efficiency ranged from 4 to 99% EGFP-positive cells with MOIs of from 0.1 to 50, respectively.
Polarity of AEC monolayer transduction by VSV-G-pseudotyped lentivirus vectors.
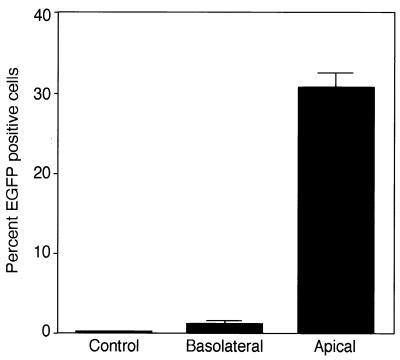
AEC monolayers were infected with VSV-G-pseudotyped lentivirus from either the apical or basolateral side and analyzed by FACS at 3 days posttransduction (Fig. 4). VSV-G-pseudotyped lentivirus vectors were somewhat unexpectedly preferentially transduced from the apical surface with an efficiency of 31% ± 2% at an MOI of 10 (Fig. 4). Transduction from the apical side was ∼25-fold higher than when the virus was introduced from the basolateral surface. As shown in Table 1, Rt measurements taken before and after infection confirmed that the integrity of the tight junctions was maintained, suggesting that leakage to the basolateral side did not account for transduction when the virus was introduced from the apical side.
FIG. 4.
Polarized transduction of AEC by lentivirus vectors. AEC monolayers on polycarbonate filters were infected with VSV-G-pseudotyped lentivirus vectors (MOI = 10) from either the apical or basolateral surface and analyzed by FACS at 3 days posttransduction. VSV-G-pseudotyped lentivirus vectors infected polarized AEC monolayers more efficiently from the apical surface (apical/basolateral, 25:1).
TABLE 1.
Transepithelial resistance of AEC monolayers before and after exposure to apical or basolateral lentivirus vectors (MOI = 10)
| Timea | Mean Rt (kΩ · cm2) ± SEMb
|
||
|---|---|---|---|
| Control | Apical vector | Basolateral vector | |
| 30 min (pre) | 3.71 ± 0.22 | 3.78 ± 0.24 | 3.81 ± 0.19 |
| 30 min (post) | 2.75 ± 0.92 | 2.07 ± 0.46 | 1.64 ± 0.55 |
| 72 h (post) | 2.66 ± 0.24 | 2.66 ± 0.34 | 2.47 ± 0.33 |
pre and post refer to before and after exposure, respectively.
Control = uninfected monolayers.
We then investigated whether increasing access to the basolateral surface by disrupting tight junctions in the polarized AEC monolayer would facilitate entry of apically applied lentivirus. Disruption of tight junctions was achieved by addition of the Ca2+-chelating agent EGTA at a final concentration of 4.5 mM, prior to addition of virus to the apical side as described above (49, 53). Despite a reduction in Rt to <0.25 kΩ · cm2 after addition of EGTA, there was no increase in transduction by apically introduced virus, indicating that vector leakage to the basolateral surface does not contribute significantly to the observed level of transduction by VSV-G-pseudotyped lentivirus vectors from the apical side.
RTEC transduction by VSV-G-pseudotyped lentivirus vectors.
Differentiated RTEC maintained at ALI for 2 to 4 weeks had mean Rt of 848 ± 16 Ω · cm2. Proximal human airway epithelial cells have previously been shown to be quiescent (19, 50). In contrast to AEC, apical transduction of RTEC resulted in only rare (<1%) GFP-expressing cells (Fig. 5). This finding is consistent with previous reports that human tracheobronchial cells are preferentially transduced from the basolateral surface (19, 21). RTEC were also treated with EGTA, causing the Rt to fall to <15 Ω · cm2. EGFP-expressing cells were significantly increased (>15-fold) compared to the percentage of cells transduced after apical application in the absence of EGTA.
FIG. 5.
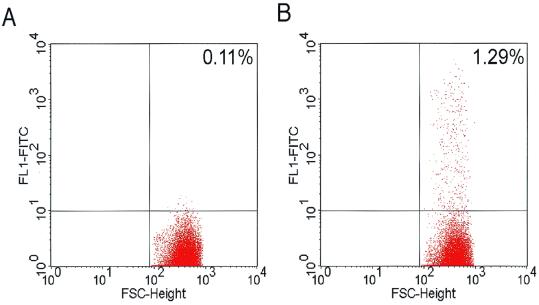
Transduction of RTEC by lentivirus vector expressing EGFP. Representative analyses of EGFP expression as determined by FACS are shown. Differentiated RTEC were infected with VSV-G-pseudotyped lentivirus vectors from the apical surface without (A) or with (B) EGTA and analyzed 3 days posttransduction. Delivery of lentivirus vector alone to the apical surface resulted in only rare transduced cells (0.14% ± 0.02%, n = 9). Apical delivery of lentivirus vectors in the presence of EGTA in hypotonic HEPES to facilitate access to the basolateral surface significantly (P < 0.05) enhanced transduction (2.11% ± 0.45%, n = 11).
DISCUSSION
The alveolar epithelium is an attractive target for gene therapy for the treatment of a number of acute and chronic pulmonary diseases and, by virtue of its large surface area, for systemic delivery of therapeutic proteins. In addition, the development of techniques for high-efficiency transduction of primary AEC would facilitate investigations of the biological characteristics of these cells in vitro. In this study, we have demonstrated that VSV-G-pseudotyped lentivirus vectors efficiently transduce quiescent AEC in primary culture, with transduction being favored by virus application from the apical side in polarized monolayers. With their ability to integrate into the host genome, these findings suggest that lentivirus vectors may be advantageous for efficient transfer and long-term expression of transgenes in the distal respiratory tract.
Analysis by flow cytometry on day 4 demonstrated that ∼1% of cells are labeled with BrdU, confirming the relatively quiescent state of AEC in vitro. Consistent with previous observations in other quiescent cell types (e.g., neurons) (34), we demonstrate that transduction of AEC with a VSV-G-pseudotyped lentivirus vector is highly efficient, with more than 90% of infected cells expressing GFP as determined by FACS analysis at the highest MOI. The reduction in GFP expression after transduction in the presence of AZT, together with the results of Southern analysis, make it likely that the observed GFP fluorescence reflects true transduction by the lentivirus vectors. Transduction of AEC by lentivirus vectors greatly exceeded that achieved with similarly pseudotyped MLV vectors. Despite concentration to relatively high titers facilitated by VSV-G pseudotyping, only low-level transduction of AEC by MLV-based vectors was observed in the current study. The low level of MLV vector transduction that we observed in AEC is consistent with previous reports of inefficient gene transfer by amphotropic MLV vectors in quiescent airway epithelial cells from trachea and bronchi, even after disruption of epithelial tight junctions to allow basolateral access (19, 21, 31). Together, these results demonstrate the superiority of VSV-G-pseudotyped lentivirus vectors for transduction of AEC relative to similarly pseudotyped MLV-based vectors at a comparable titer.
One of the important observations in gene transfer over the past decade has been that transduction is typically polarized. The apparent basolateral preference in airway epithelial cells has precluded the use of retrovirus and, additionally, has limited apical adenovirus gene transfer due, at least in part, to a preferential basolateral distribution of the adenovirus fiber protein receptors (36, 49). Although lentivirus-mediated gene transfer has the dual advantages of long-term expression by virtue of integration into the genome and transduction of quiescent cells, the vector has also been viewed as inefficient for pulmonary gene delivery due to reports of limited transduction by apically applied VSV-G-pseudotyped lentivirus vectors in the upper respiratory tract (19, 21). Therefore, assessment of the polarity of transduction was central to our investigation of lentivirus transduction of AEC. To address this, we utilized a well-characterized model in which AEC form tight monolayers with high transepithelial resistance and exhibit polarized distribution of a variety of membrane-associated proteins (e.g., Na channel and Na pump subunits) (7, 24, 39). Concurrent with the development of resistance, the cells undergo transition toward a type I cell-like phenotype, closely mimicking the properties of the alveolar epithelium in vivo (6). We found that polarized monolayers of primary AEC exhibiting high transepithelial resistance could be efficiently transduced when VSV-G-pseudotyped lentivirus vectors were introduced from the apical cell surface. In contrast, transduction of VSV-G-pseudotyped lentivirus vectors applied to the basolateral surface of AEC was markedly lower. Transduction efficiency of apically applied virus was also assessed after the addition of EGTA in order to facilitate access of the virus to the basolateral cell surface. Lack of an increase in transduction in the presence of EGTA, despite a marked reduction in Rt, suggests that AEC uptake of VSV-G-pseudotyped lentivirus occurs preferentially from the apical surface. By comparison, transduction of polarized RTEC by VSV-G-pseudotyped lentivirus was far lower from the apical surface and was significantly increased after disruption of tight junctions, a finding consistent with more efficient transduction from the basolateral cell surface in RTEC.
These observations in RTEC are similar to previous studies in which human bronchial or nasal airway epithelial cells in culture were 30-fold more efficiently transduced from the basolateral relative to the apical side by VSV-G-pseudotyped lentivirus, although the overall efficiency of transduction was not stated (21). In that study, enhanced in vivo gene transfer efficiency to the nasal and tracheal epithelium of rodents of a VSV-G-pseudotyped human lentivirus vector was observed after sulfur dioxide injury, presumably by increasing access of vector to the basolateral cell surface. Similarly, Goldman et al. (19) demonstrated that lentivirus vectors pseudotyped with VSV-G were able to transduce undifferentiated airway epithelia but failed to transduce the well-differentiated pseudostratified columnar epithelium in human bronchial xenografts, whereas Wang et al. (51) reported that feline immunodeficiency viral vectors pseudotyped with VSV-G could transduce differentiated airway epithelium in rabbits only in the presence of EGTA. Kobinger et al. (25), in a recent comparative analysis of the effects of pseudotyping on transduction, demonstrated similar low levels of apical transduction by VSV-G-pseudotyped lentivirus vectors in well-differentiated human airway epithelial cells.
Polarized gene transfer to epithelia can be mediated by a number of mechanisms, including differential distribution of viral receptors on apical and basolateral cell surfaces, inactivation or inaccessibility of viral receptors, or inactivation or inhibition of virus after entry. High-resistance monolayers of MDCK cells have previously been shown to be infected more efficiently from the basolateral cell surface by wild-type VSV (18). This has led to the presumption that the putative receptor for wild-type VSV (suggested to be a phospholipid such as phosphatidylserine) (41) is located on the basolateral surface of all polarized epithelia. It has similarly been hypothesized that a predominant basolateral distribution of amphotropic MLV retrovirus receptors in mature airway epithelia may account for the limited viral entry observed from the apical side for amphotropic vectors (30, 50). However, the precise distribution and/or function of cellular VSV-G receptors in airway or alveolar epithelia has not been characterized and could conceivably be different among various cell types. Consistent with this notion, more efficient apical transduction by VSV-G-pseudotyped lentivirus vectors has recently been demonstrated in intestinal epithelial cells (42), suggesting the presence of receptors for VSV-G on the apical cell surface in specific cell types.
In addition to the possibility of reversed polarity of VSV-G receptors in AEC compared with airway epithelium or other cell types, differential intracellular trafficking or processing of virus after infection from either surface could also account for the observed differences in transduction in polarized cells. Duan et al. (13) recently observed that, in human airway epithelia, preferential basolateral transduction of adeno-associated virus could be attributed to differences in endosomal processing of apically or basolaterally internalized virions. This difference in intracellular processing led to degradation and low levels of gene transfer of adeno-associated virus applied to the apical surface of polarized airway epithelia. Inhibition of this pathway led to an increase in apical gene delivery by more than 200-fold. The possibility that differences in the postendocytotic processing pathways between airway and alveolar epithelia may account for the differences in apical or basolateral transduction at these two sites requires further investigation.
The adaptation of lentiviruses such as HIV for use as gene transfer vectors, while achieving a high transduction efficiency in primary cells compared to standard murine retrovirus vectors, has raised concerns regarding inadvertent production of replication-competent virus. However, recent advances in lentivirus vector design have substantially reduced such concerns. The lentivirus vectors used in this study include only a very small fraction of the wild-type HIV genome (57). An added safety feature introduced in the SIN transfer vector is the deletion of U3 sequences in the 3′ long terminal repeat (LTR), which is then also deleted in the 5′ LTR during reverse transcription and eliminates production of full-length vector RNA (32, 57). This change further minimizes the likelihood of producing replication-competent lentivirus. Finally, since these replication-defective vectors do not themselves express any lentivirus proteins, they have not yet been noted to trigger an immune response against transduced cells in animal models (45).
In summary, we have demonstrated that VSV-G-pseudotyped lentivirus vectors efficiently transduce AEC in primary culture, with transduction being favored by virus application from the apical side. In contrast, transduction of RTEC by apically applied lentivirus was negligible. Transduction efficiency in AEC increased with increasing MOI and greatly exceeded that achieved with a similarly pseudotyped MLV retrovirus vector. The ability of these vectors to efficiently integrate into the quiescent cells of the alveolar epithelium in vitro suggests that, in contrast to the experience with airway epithelium, lentivirus vectors may be advantageous for achieving efficient gene transfer and long-term gene expression in the distal respiratory tract in vivo.
ACKNOWLEDGMENTS
Z.B. and J. E.H.-B. contributed equally to this study.
We note with appreciation the expert technical support of Martha Jean Foster and Suzie Parra.
This work was supported in part by the American Heart Association, the National Institutes of Health (grants HL38578, HL38621, HL 38658, HL62576, HL63988, HL 64365, and AR46366), the Baxter Foundation, and the Hastings Foundation. E. D. Crandall is Hastings Professor of Medicine and Kenneth T. Norris, Jr., Chair of Medicine.
REFERENCES
- 1.Albelda S M, Wiewrodt R, Zuckerman J B. Gene therapy for lung disease: hype or hope? Ann Intern Med. 2000;132:649–660. doi: 10.7326/0003-4819-132-8-200004180-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bertalanffy F D. Respiratory tissue: structure, histophysiology, cytodynamics. II. New approaches and interpretations. Int Rev Cytol. 1964;17:213–297. doi: 10.1016/s0074-7696(08)60408-8. [DOI] [PubMed] [Google Scholar]
- 3.Borok Z, Danto S I, Zabski S M, Crandall E D. Defined medium for primary culture de novo of adult rat alveolar epithelial cells. In Vitro Cell Dev Biol Anim. 1994;30A:99–104. doi: 10.1007/BF02631400. [DOI] [PubMed] [Google Scholar]
- 4.Bowden D H, Davies E, Wyatt J P. Cytodynamics of pulmonary alveolar cells in the mouse. Arch Pathol. 1968;86:667–670. [PubMed] [Google Scholar]
- 5.Brigham K L, Stecenko A A. Gene therapy for acute lung injury. Intensive Care Med. 2000;26:S119–S123. doi: 10.1007/s001340051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheek J M, Evans M J, Crandall E D. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp Cell Res. 1989;184:375–387. doi: 10.1016/0014-4827(89)90337-6. [DOI] [PubMed] [Google Scholar]
- 7.Cheek J M, Kim K J, Crandall E D. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am J Physiol. 1989;256:C688–C693. doi: 10.1152/ajpcell.1989.256.3.C688. [DOI] [PubMed] [Google Scholar]
- 8.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 9.Christensen P J, Kim S, Simon R H, Toews G B, Paine R D. Differentiation-related expression of ICAM-1 by rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993;8:9–15. doi: 10.1165/ajrcmb/8.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Danto S I, Zabski S M, Crandall E D. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol. 1992;6:296–306. doi: 10.1165/ajrcmb/6.3.296. [DOI] [PubMed] [Google Scholar]
- 11.Dobbs L G, Gonzalez R, Williams M C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 12.Dong J Y, Wang D, Van Ginkel F W, Pascual D W, Frizzell R A. Systematic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum Gene Ther. 1996;7:319–331. doi: 10.1089/hum.1996.7.3-319. [DOI] [PubMed] [Google Scholar]
- 13.Duan D, Yue Y, Yan Z, Yang J, Engelhardt J F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Investig. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhardt J F, Yankaskas J R, Wilson J M. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Investig. 1992;90:2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe H A, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder J I. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase beta1 subunit gene. J Clin Investig. 1998;102:1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flotte T R, Afione S A, Zeitlin P L. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 18.Fuller S, von Bonsdorff C H, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- 19.Goldman M J, Lee P S, Yang J S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 20.Grimm D, Kleinschmidt J A. Progress in adeno-associated virus type 2 vector production: promises and prospects for clinical use. Hum Gene Ther. 1999;10:2445–2450. doi: 10.1089/10430349950016799. [DOI] [PubMed] [Google Scholar]
- 21.Johnson L G, Olsen J C, Naldini L, Boucher R C. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- 22.Kaartinen L, Nettesheim P, Adler K B, Randell S H. Rat tracheal epithelial cell differentiation in vitro. In Vitro Cell Dev Biol Anim. 1993;29A:481–492. [PubMed] [Google Scholar]
- 23.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 24.Kim K J, Cheek J M, Crandall E D. Contribution of active Na+ and Cl− fluxes to net ion transport by alveolar epithelium. Respir Physiol. 1991;85:245–256. doi: 10.1016/0034-5687(91)90065-q. [DOI] [PubMed] [Google Scholar]
- 25.Kobinger G P, Weiner D J, Yu Q C, Wilson J M. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- 26.Leslie C C, McCormick-Shannon K, Cook J L, Mason R J. Macrophages stimulate DNA synthesis in rat alveolar type II cells. Am Rev Respir Dis. 1985;132:1246–1252. doi: 10.1164/arrd.1985.132.6.1246. [DOI] [PubMed] [Google Scholar]
- 27.Leslie C C, McCormick-Shannon K, Robinson P C, Mason R J. Stimulation of DNA synthesis in cultured rat alveolar type II cells. Exp Lung Res. 1985;8:53–66. doi: 10.3109/01902148509069679. [DOI] [PubMed] [Google Scholar]
- 28.Leslie C C, McCormick-Shannon K, Shannon J M, Garrick B, Damm D, Abraham J A, Mason R J. Heparin-binding EGF-like growth factor is a mitogen for rat alveolar type II cells. Am J Respir Cell Mol Biol. 1997;16:379–387. doi: 10.1165/ajrcmb.16.4.9115748. [DOI] [PubMed] [Google Scholar]
- 29.Mason R J, Walker S R, Shields B A, Henson J E, Williams M C. Identification of rat alveolar type II epithelial cells with a tannic acid and polychrome stain. Am Rev Respir Dis. 1985;131:786–788. doi: 10.1164/arrd.1985.131.5.786. [DOI] [PubMed] [Google Scholar]
- 30.Miller A D. Cell-surface receptors for retroviruses and implications for gene transfer. Proc Natl Acad Sci USA. 1996;93:11407–11413. doi: 10.1073/pnas.93.21.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyoshi H, Blomer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998;9:457–463. doi: 10.1016/s0958-1669(98)80029-3. [DOI] [PubMed] [Google Scholar]
- 34.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 35.Ostrowski L E, Randell S H, Clark A B, Gray T E, Nettesheim P. Ciliogenesis of rat tracheal epithelial cells in vitro. Methods Cell Biol. 1995;47:57–63. doi: 10.1016/s0091-679x(08)60791-8. [DOI] [PubMed] [Google Scholar]
- 36.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polonovsky V, Bitterman P B. Regulation of cell population size. In: West J B, Crystal R G, editors. The lung: scientific foundations. 2nd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 133–144. [Google Scholar]
- 38.Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- 39.Russo R M, Lubman R L, Crandall E D. Evidence for amiloride-sensitive sodium channels in alveolar epithelial cells. Am J Physiol. 1992;262:L405–L411. doi: 10.1152/ajplung.1992.262.4.L405. [DOI] [PubMed] [Google Scholar]
- 40.Sakoda T, Kasahara N, Hamamori Y, Kedes L. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol. 1999;31:2037–2047. doi: 10.1006/jmcc.1999.1035. [DOI] [PubMed] [Google Scholar]
- 41.Schlegel R, Tralka T S, Willingham M C, Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983;32:639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- 42.Seppen J, Barry S C, Klinkspoor J H, Katen L J, Lee S P, Garcia J V, Osborne W R. Apical gene transfer into quiescent human and canine polarized intestinal epithelial cells by lentivirus vectors. J Virol. 2000;74:7642–7645. doi: 10.1128/jvi.74.16.7642-7645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisson T H, Hattori N, Xu Y, Simon R H. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Ther. 1999;10:2315–2323. doi: 10.1089/10430349950016960. [DOI] [PubMed] [Google Scholar]
- 44.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trono D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 2000;7:20–23. doi: 10.1038/sj.gt.3301105. [DOI] [PubMed] [Google Scholar]
- 46.Uhal B D. Cell cycle kinetics in the alveolar epithelium. Am J Physiol. 1997;272:L1031–L1045. doi: 10.1152/ajplung.1997.272.6.L1031. [DOI] [PubMed] [Google Scholar]
- 47.Uhal B D, Etter M D. Type II pneumocyte hypertrophy without activation of surfactant biosynthesis after partial pneumonectomy. Am J Physiol. 1993;264:L153–L159. doi: 10.1152/ajplung.1993.264.2.L153. [DOI] [PubMed] [Google Scholar]
- 48.Uhal B D, Rannels D E. DNA distribution analysis of type II pneumocytes by laser flow cytometry: technical considerations. Am J Physiol. 1991;261:L296–L306. doi: 10.1152/ajplung.1991.261.4.L296. [DOI] [PubMed] [Google Scholar]
- 49.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Davidson B L, Melchert P, Slepushkin V A, van Es H H, Bodner M, Jolly D J, McCray P B. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, Slepushkin V, Zabner J, Keshavjee S, Johnston J C, Sauter S L, Jolly D J, Dubensky T W, Jr, Davidson B L, McCray P B., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Investig. 1999;104:R55–R62. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang G, Slepushkin V A, Bodner M, Zabner J, van Es H H, Thomas P, Jolly D J, Davidson B L, McCray P B. Keratinocyte growth factor induced epithelial proliferation facilitates retroviral-mediated gene transfer to distal lung epithelia in vivo. J Gene Med. 1999;1:22–30. doi: 10.1002/(sici)1521-2254(199901/02)1:1<22::aid-jgm1>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 53.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly D J, Davidson B L, McCray P B., Jr Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol. 2000;22:129–138. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- 54.Welsh M J. Gene transfer for cystic fibrosis. J Clin Investig. 1999;104:1165–1166. doi: 10.1172/JCI8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zabner J. Cationic lipids used in gene transfer. Adv Drug Deliv Rev. 1997;27:17–28. doi: 10.1016/s0169-409x(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 57.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]