Abstract
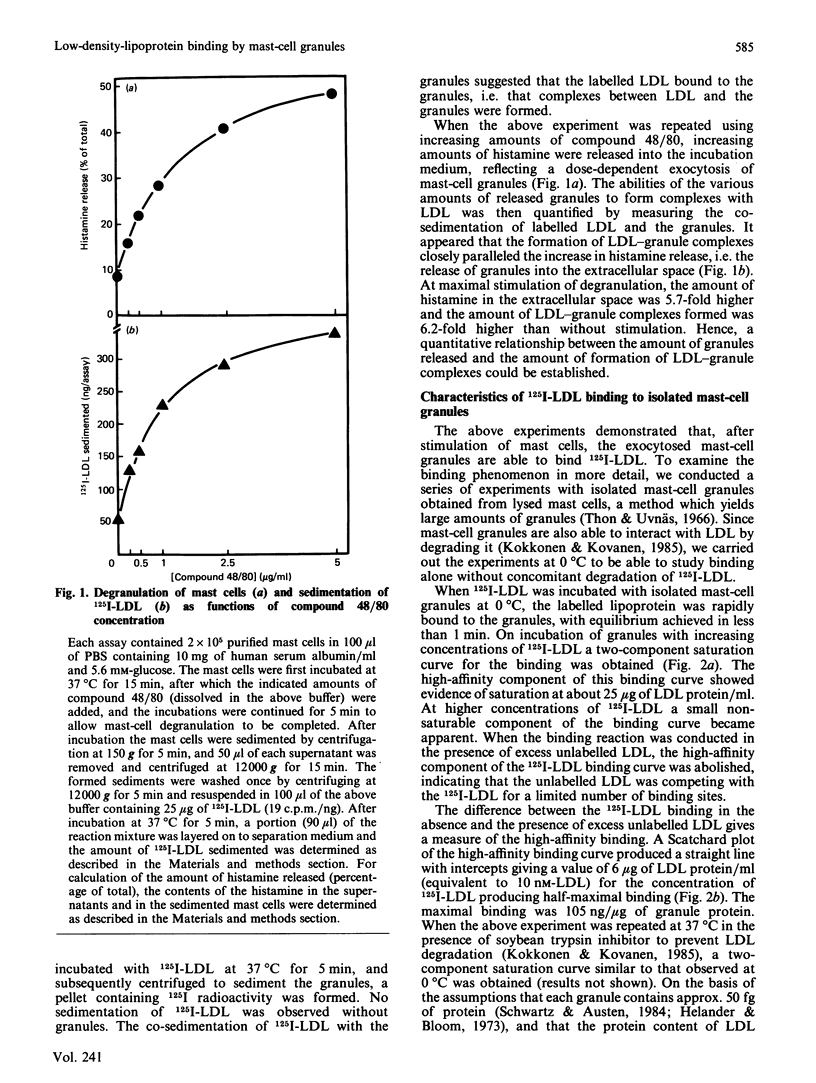
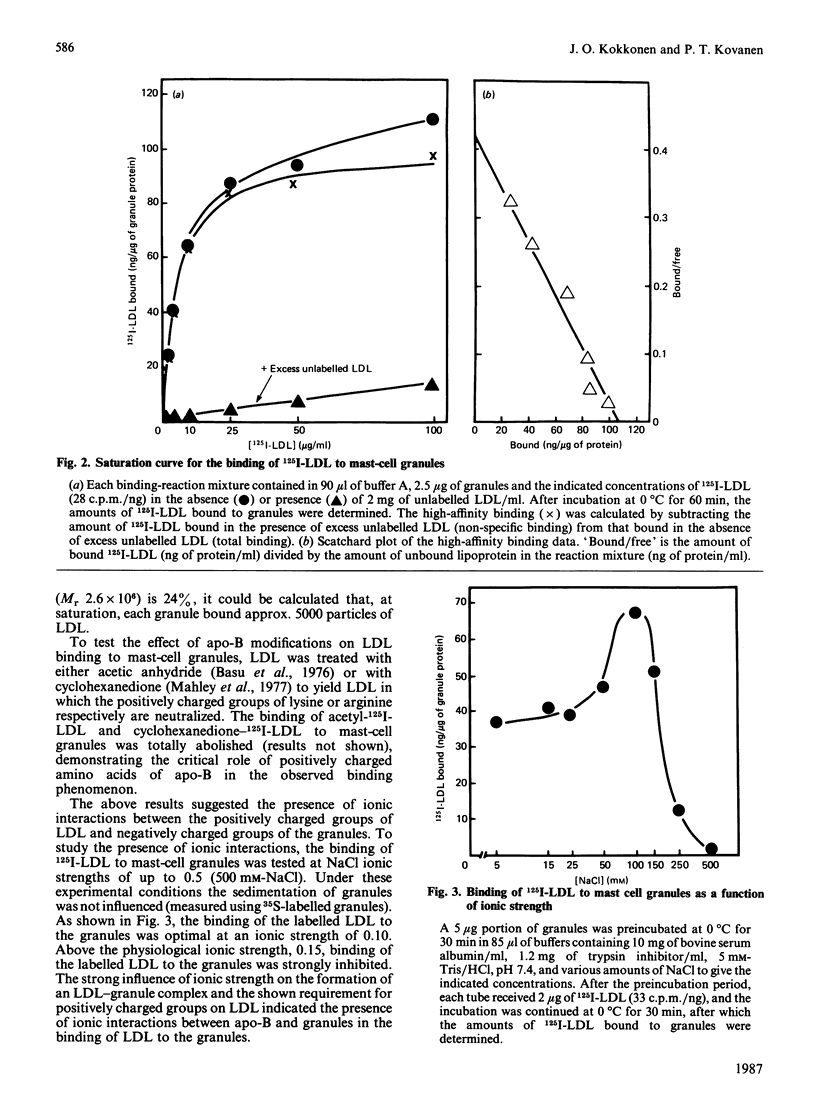
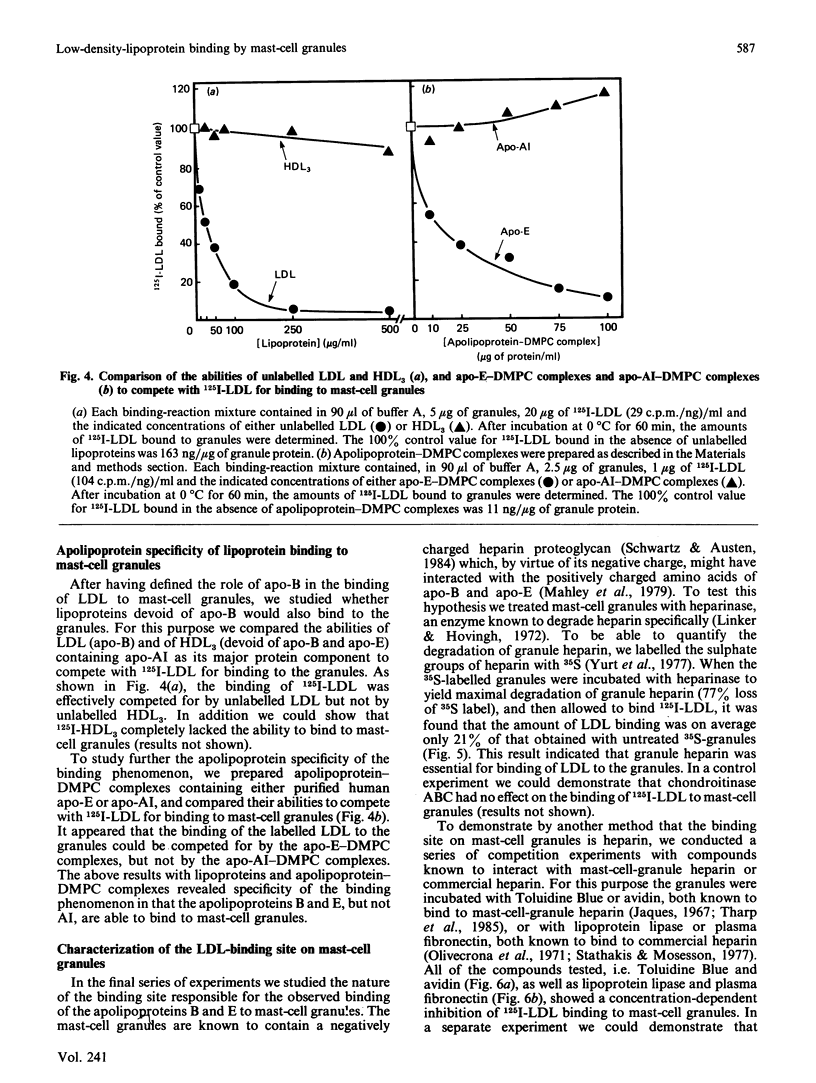
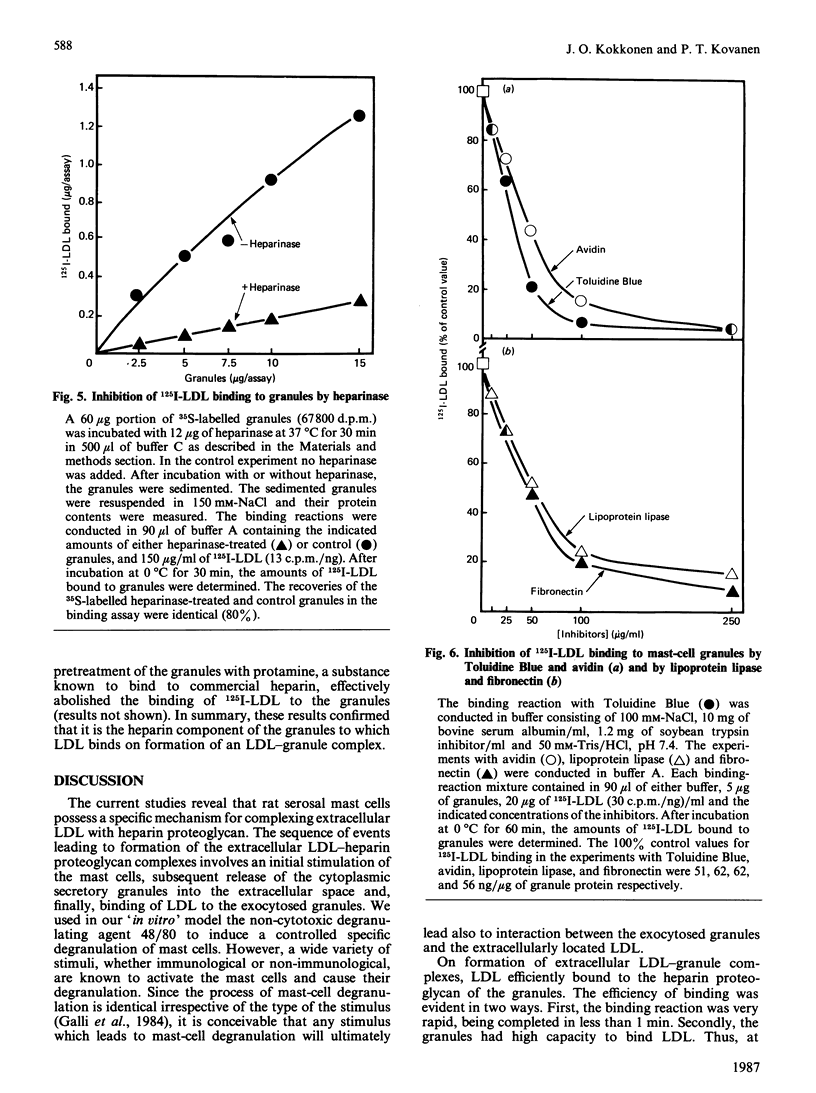
To study the interaction between low-density lipoprotein (LDL) and granules from rat serosal mast cells in vitro, mast cells were stimulated with the degranulating agent 48/80 to induce exocytosis of the secretory granules. Subsequent incubation of the exocytosed granules with 125I-LDL resulted in binding of the labelled LDL to the granules. When increasing amounts of agent 48/80 were added to mast-cell suspensions, a dose-dependent release of granules was observed and a parallel increase in the amount of 125I-LDL bound to granules resulted. 125I-LDL bound to a single class of high-affinity binding sites on the granules. At saturation, 105 ng of LDL were bound per microgram of granule protein. The lipoprotein binding to mast-cell granules was apolipoprotein(apo)-B + E-specific. Thus 125I-LDL binding to the granules was effectively compared for by LDL (apo-B) or by dimyristoyl phosphatidylcholine vesicles containing apo-E, but not by high-density lipoprotein (HDL3) containing apo-AI as their major protein component. Neutralization by acetylation of the positively charged amino groups of apo-B of LDL or presence of a high ionic strength in the incubation medium prevented LDL from binding to the granules, indicating the presence of ionic interactions between the positively charged amino acids of LDL and negatively charged groups of the granules. It could be demonstrated that LDL bound to the negatively charged heparin proteoglycan of the granules. Thus treatment of granules with heparinase resulted in loss of their ability to bind LDL, and substances known to bind to heparin, such as Toluidine Blue, avidin, lipoprotein lipase, fibronectin and protamine, all effectively competed with LDL for binding to the granules. The results show that LDL is efficiently bound to the heparin proteoglycan component of mast-cell granules once the mast cells are stimulated to release their granules into the extracellular space.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins F. M., Friedman M. M., Metcalfe D. D. Biochemical and microscopic evidence for the internalization and degradation of heparin-containing mast cell granules by bovine endothelial cells. Lab Invest. 1985 Mar;52(3):278–286. [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Srinivasan S. R., Vijayagopal P., Dalferes E. R., Jr, Sharma C. Recent advances in molecular pathology. Carbohydrate-protein macromolecules and arterial wall integrity--a role in atherogenesis. Exp Mol Pathol. 1984 Oct;41(2):267–287. doi: 10.1016/0014-4800(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Dvorak H. F. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander H. F., Bloom G. D. Quantitative analysis of mast cell structure. J Microsc. 1974 Apr;100(3):315–321. doi: 10.1111/j.1365-2818.1974.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Jaques L. B. The pharmacology of heparin and heparinoids. Prog Med Chem. 1967;5:139–198. doi: 10.1016/s0079-6468(08)70443-0. [DOI] [PubMed] [Google Scholar]
- Kokkonen J. O., Kovanen P. T. Low density lipoprotein degradation by rat mast cells. Demonstration of extracellular proteolysis caused by mast cell granules. J Biol Chem. 1985 Nov 25;260(27):14756–14763. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl U., Pertoft H., Seljelid R. Uptake and degradation of mast-cell granules by mouse peritoneal macrophages. Biochem J. 1979 Jul 15;182(1):189–193. doi: 10.1042/bj1820189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L. Interaction of plasma lipoproteins containing apolipoproteins B and E with heparin and cell surface receptors. Biochim Biophys Acta. 1979 Oct 26;575(1):81–91. doi: 10.1016/0005-2760(79)90133-4. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Egelrud T., Iverius P. H., Lindahl U. Evidence for an ionic binding of lipoprotein lipase to heparin. Biochem Biophys Res Commun. 1971 May 7;43(3):524–529. doi: 10.1016/0006-291x(71)90645-0. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., STEINBERG D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960 Oct;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. I., Jackson R. L., Pownall H. J., Gotto A. M., Jr Interaction of plasma "arginine-rich" apolipoprotein with dimyristoylphosphatidylcholine. Biochemistry. 1977 Nov 15;16(23):5030–5036. doi: 10.1021/bi00642a014. [DOI] [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba Rao P. V., Friedman M. M., Atkins F. M., Metcalfe D. D. Phagocytosis of mast cell granules by cultured fibroblasts. J Immunol. 1983 Jan;130(1):341–349. [PubMed] [Google Scholar]
- Tharp M. D., Seelig L. L., Jr, Tigelaar R. E., Bergstresser P. R. Conjugated avidin binds to mast cell granules. J Histochem Cytochem. 1985 Jan;33(1):27–32. doi: 10.1177/33.1.2578142. [DOI] [PubMed] [Google Scholar]
- Thon I. L., Uvnäs B. Mode of storage of histamine in mast cells. Acta Physiol Scand. 1966 Jul-Aug;67(3):455–470. doi: 10.1111/j.1748-1716.1966.tb03332.x. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]


