Abstract
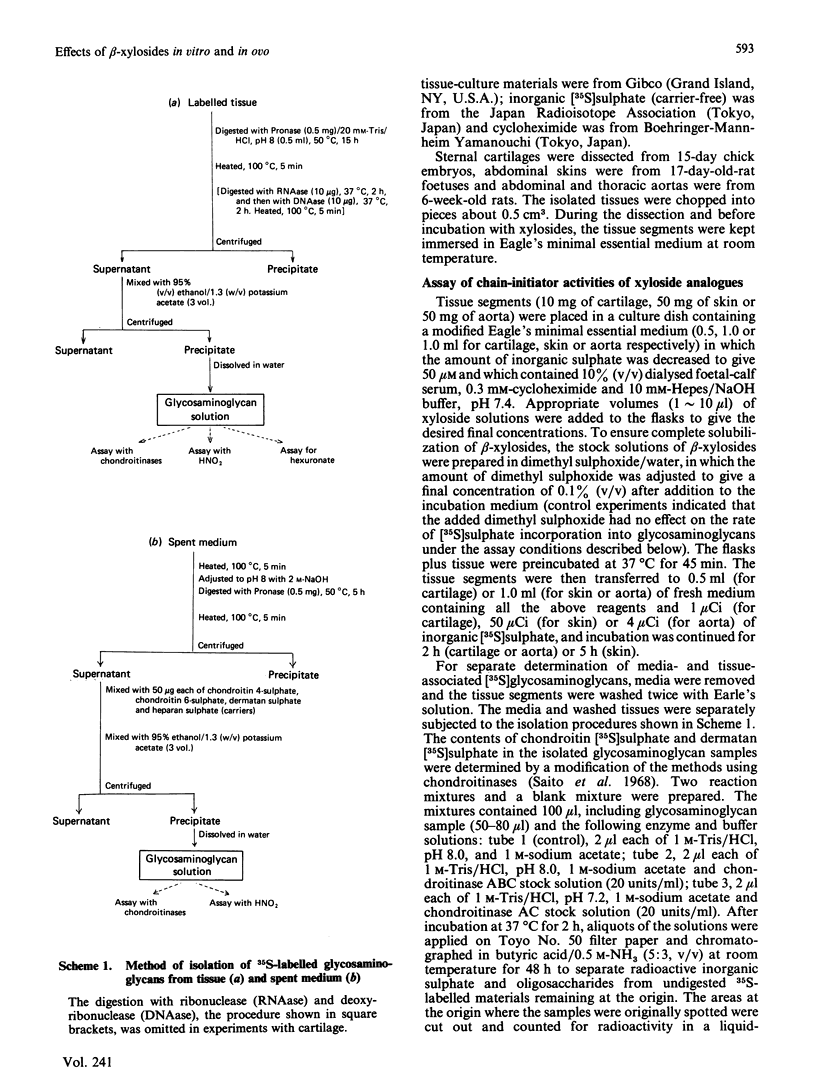
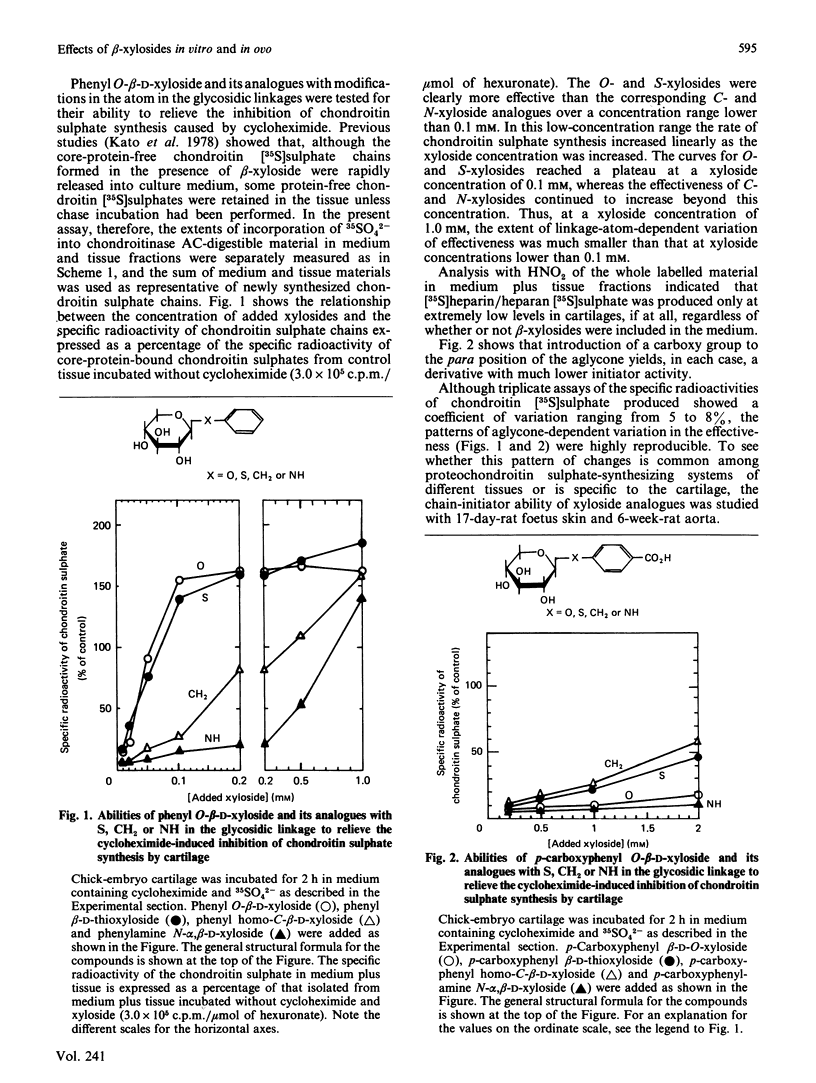
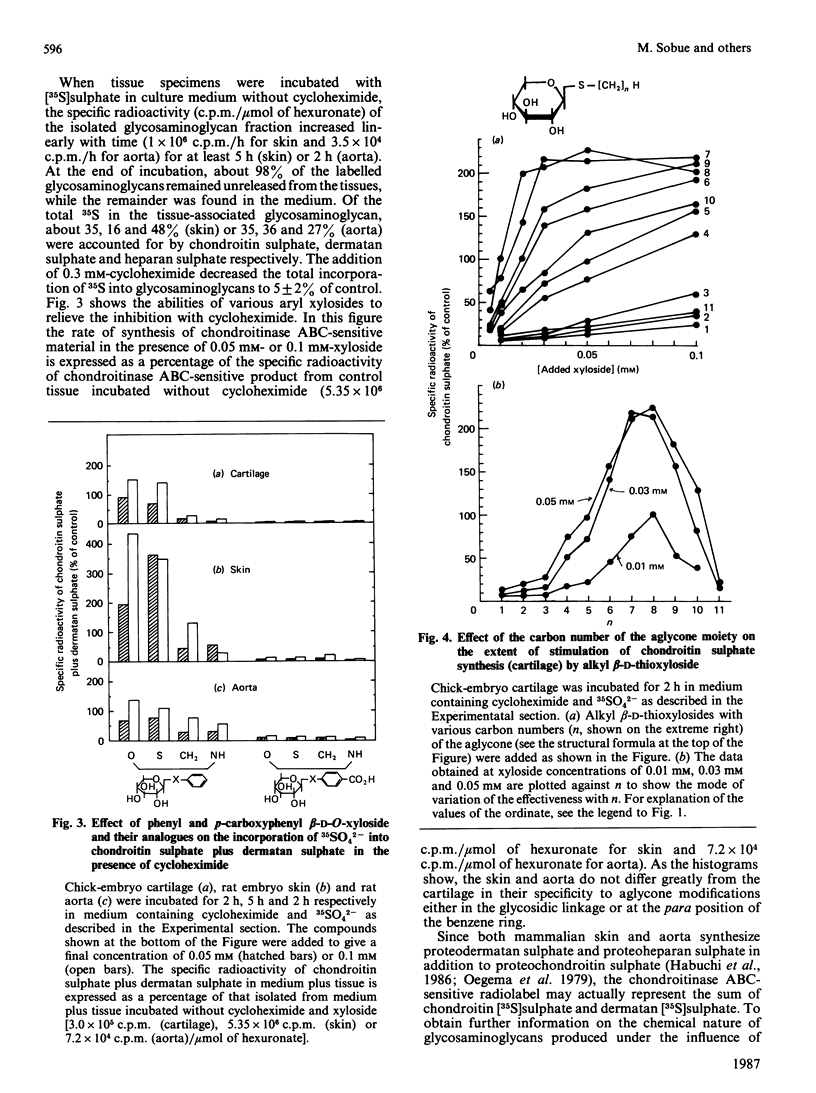
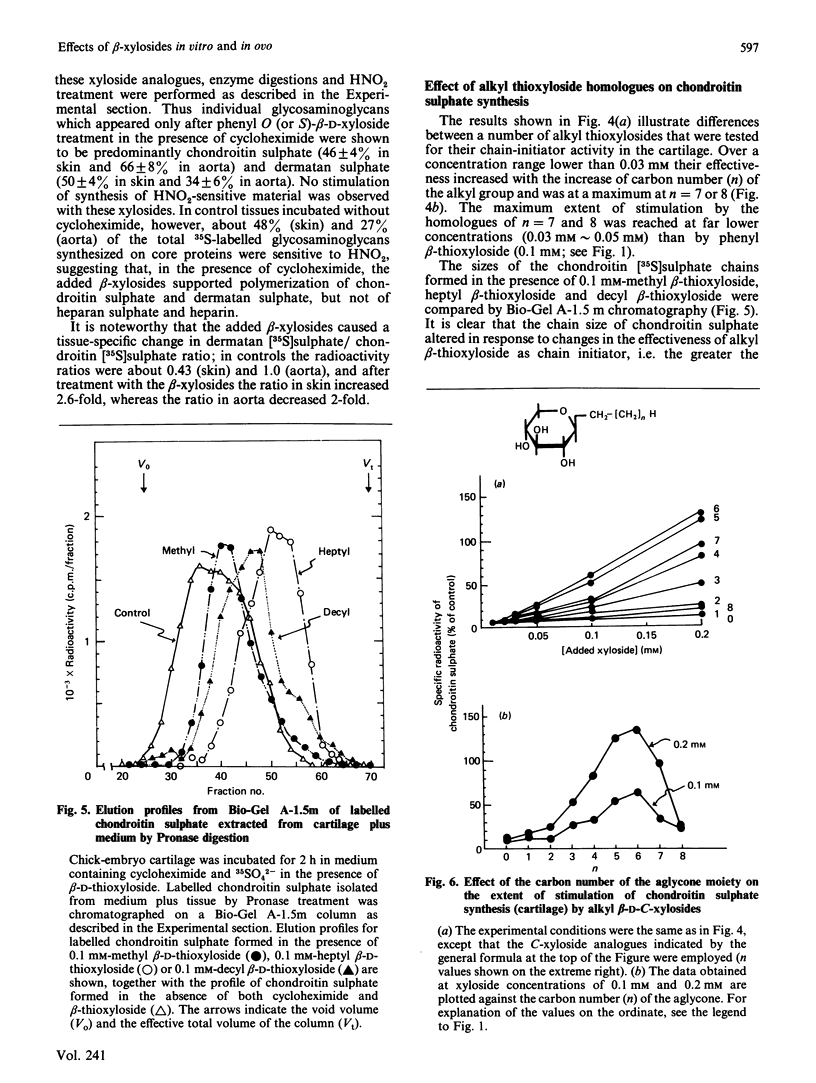
A series of aryl and alkyl O-beta-D-xylosides and their analogues with S, NH or CH2 in the glycosidic linkage were prepared and examined for their ability to act as artificial chain initiators of chondroitin (dermatan) sulphate synthesis in embryonic chick cartilage, foetal rat skin and 6-week-old-rat aorta under conditions where normal protein-core synthesis was inhibited by cycloheximide. For all these tissues in culture, phenyl O-beta-D-xyloside and phenyl beta-D-thioxyloside were clearly more effective than the corresponding N-xyloside and homo-C-xyloside. Introduction of a carboxy group to the para position of their aglycone yielded derivatives with far lower initiator activity. In a concentration range lower than 0.1 mM, the effectiveness of alkyl beta-D-thioxyloside was greatly influenced by the carbon number (n) of the alkyl group and was at a maximum at n = 7 or 8 for the cartilage, at n = 5 for the skin and at n = 4 for the aorta. In the beta-xyloside-treated cartilages, the average length of newly formed chondroitin sulphate chains reflected the chain-initiator activity of added xyloside, i.e. the higher the initiator activity, the shorter the average chain length. In the skin and aorta, none of the drugs could relieve the inhibition of heparan sulphate synthesis caused by cycloheximide. Fertilized hens' eggs were each injected on day 9 with 9.2 mumol of beta-xyloside and the skeletal systems of embryos were examined a week later. The embryos treated with beta-xylosides of relatively high initiator activity showed a 30-40% decrease in the overall growth rate of skeletons, whereas those treated with beta-xylosides of low initiator activity showed little or no decrease in the growth rate. The results are consistent with the notion that the observed change in skeletal morphology results mainly, if not completely, from beta-xyloside-induced synthesis of core-protein-free chondroitin sulphate, and further suggest that a procedure employing a series of beta-xyloside homologues with various initiator activities will furnish an easily applied criterion on which to test the specificity of xyloside action on biological processes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasaka K., Amemiya S., Terayama H. Scanning electron microscopical study of the inside of sea urchin embryos (Pseudocentotus depressus). Effects of Aryl beta-xyloside, tunicamycin and deprivation of sulfate tions. Exp Cell Res. 1980 Sep;129(1):1–13. doi: 10.1016/0014-4827(80)90325-0. [DOI] [PubMed] [Google Scholar]
- Fukunaga Y., Sobue M., Suzuki N., Kushida H., Suzuki S. Synthesis of a fluorogenic mucopolysaccharide by chondrocytes in cell culture with 4-methylumbelliferyl beta-D-xyloside. Biochim Biophys Acta. 1975 Feb 13;381(2):443–447. doi: 10.1016/0304-4165(75)90252-4. [DOI] [PubMed] [Google Scholar]
- Galligani L., Hopwood J., Schwartz N. B., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. J Biol Chem. 1975 Jul 25;250(14):5400–5406. [PubMed] [Google Scholar]
- Gibson K. D., Doller H. J., Hoar R. M. beta-D-xylosides cause abnormalities of growth and development in chick embryos. Nature. 1978 May 11;273(5658):151–154. doi: 10.1038/273151a0. [DOI] [PubMed] [Google Scholar]
- Gibson K. D., Segen B. J., Audhya T. K. The effect of beta-D-xylosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage in the absence of protein synthesis inhibitors. Biochem J. 1977 Feb 15;162(2):217–233. doi: 10.1042/bj1620217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K. D., Segen B. J., Doller H. J., Jr Changes in chemical composition of chick embryos treated with a beta-xyloside and a lathyrogen. Teratology. 1979 Jun;19(3):345–356. doi: 10.1002/tera.1420190311. [DOI] [PubMed] [Google Scholar]
- Habuchi H., Kimata K., Suzuki S. Changes in proteoglycan composition during development of rat skin. The occurrence in fetal skin of a chondroitin sulfate proteoglycan with high turnover rate. J Biol Chem. 1986 Jan 25;261(3):1031–1040. [PubMed] [Google Scholar]
- Johnston L. S., Keller J. M. The effect of beta-xylosides on heparan sulfate synthesis by SV40-transformed Swiss mouse 3T3 cells. J Biol Chem. 1979 Apr 25;254(8):2575–2578. [PubMed] [Google Scholar]
- Kato Y., Kimata K., Ito K., Karasawa K., Suzuki S. Effect of beta-D-xyloside and cycloheximide on the synthesis of two types of proteochondroitin sulfate in chick embryo cartilage. J Biol Chem. 1978 Apr 25;253(8):2784–2789. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lohmander L. S., Hascall V. C., Caplan A. I. Effects of 4-methyl umbelliferyl-beta-D-xylopyranoside on chondrogenesis and proteoglycan synthesis in chick limb bud mesenchymal cell cultures. J Biol Chem. 1979 Oct 25;254(20):10551–10561. [PubMed] [Google Scholar]
- Lohmander S., Madsen K., Hinek A. Secretion of proteoglycans by chondrocytes. Influence of colchicine, cytochalasin B, and beta-D-xyloside. Arch Biochem Biophys. 1979 Jan;192(1):148–157. doi: 10.1016/0003-9861(79)90080-8. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Eisenstein R. Characterization of bovine aorta proteoglycan extracted with guanidine hydrochloride in the presence of protease inhibitors. J Biol Chem. 1979 Feb 25;254(4):1312–1318. [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Robinson H. C. Control of chondroitin sulphate biosynthesis. beta-D-Xylopyranosides as substrates for UDP-galactose: D-xylose transferase from embryonic-chicken cartilage. Biochem J. 1981 Mar 15;194(3):839–846. doi: 10.1042/bj1940839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Gospodarowicz D. Effect of p-nitrophenyl-beta-D-xyloside on proteoglycan synthesis and extracellular matrix formation by bovine corneal endothelial cell cultures. J Biol Chem. 1984 Mar 25;259(6):3818–3824. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schwartz N. B., Galligani L., Ho P. L., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4047–4051. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue M., Takeuchi J., Ito K., Kimata K., Suzuki S. Effect of environmental sulfate concentration on the synthesis of low and high sulfated chondroitin sulfates by chick embryo cartilage. J Biol Chem. 1978 Sep 10;253(17):6190–6196. [PubMed] [Google Scholar]
- Spooncer E., Gallagher J. T., Krizsa F., Dexter T. M. Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J Cell Biol. 1983 Feb;96(2):510–514. doi: 10.1083/jcb.96.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B. S., Bassett K., Stokes B. Sulfated glycosaminoglycan deposition and processing at the basal epithelial surface in branching and beta-D-xyloside-inhibited embryonic salivary glands. Dev Biol. 1985 May;109(1):177–183. doi: 10.1016/0012-1606(85)90358-6. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Effect of p-nitrophenyl-beta-D-xyloside on proteoglycan and glycosaminoglycan biosynthesis in rat serosal mast cell cultures. J Biol Chem. 1982 Jan 10;257(1):253–259. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]



