Abstract
In recent years, cell therapy research and commercialization have significantly accelerated, especially after the US FDA approved CAR-T therapy. While cell therapy now leads immuno-oncology in clinical trials, challenges such as redundant R&D, target clustering, and unmet clinical need remain. Since 2017, China has established a dual-track regulatory framework, facilitating rapid growth in its cell therapy pipeline, making it the second largest in the world. Despite this progress, China faces similar global challenges. Our study covers 2,794 registered cell therapy clinical trials in China, including 2,045 for immune cell, 683 for stem cell, and 66 for other somatic cell. It compares cell therapy products approved in China, the US, EU, and Japan, analyzes the evolving clinical trials landscape, and highlights the characteristics of investigator-initiated trials (IITs) and industry-sponsored trials (ISTs) in China. Our findings indicate that despite the high disease burden and unmet clinical needs for solid tumors in China, over 38% of trials between 2021 and 2023 focused on hematologic malignancies with established targets like CD19 and BCMA. Over 90% of trials are IITs, which show notable clinical differences from ISTs. We recommend that Chinese regulators establish specific guidelines to promote clinical-value-driven research. Stricter regulatory standards should also be implemented to minimize redundant R&D. Additionally, a value-based reimbursement system for within-class targeted cell therapy products may further reduce duplicated R&D efforts. Given the prevalence of IITs, specifying requirements for IITs could create a new pathway to accelerate product development and better address unmet clinical needs in China.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01616-8.
Keywords: Cell therapy, Dual-track regulatory framework, Investigator-initiated trials, Industry-sponsored trials
To the Editor
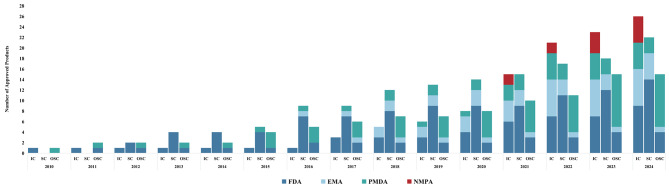
In recent years, cell therapy research and commercialization have significantly accelerated [1]. Recognizing the complexity and higher risks, the US, EU, and Japan have established specific regulatory frameworks for cell therapy (eTable1). By August 1, 2024, 27, 13 and 18 cell therapy products were approved in the US, EU and Japan, respectively (Fig. 1, eFigure2). Since 2017, China has established a dual-track regulatory framework for cell therapy, clarifying the requirements for cell therapy and facilitating the growth of both investigator-initiated trials (IITs) and industry-sponsored trials (ISTs) [2]. Consequently, China’s cell therapy pipeline has grown rapidly to the world’s second largest. However, challenges such as redundant R&D, target clustering, and unmet clinical needs persist both in China and globally [3]. This study examines China’s evolving clinical trial landscape, comparing it to global trends, while highlighting differences between IITs and ISTs, and proposing potential solutions for unmet clinical needs.
Fig. 1.
Cumulative Number of Cell Therapy Products Approved for Marketing by the NMPA, FDA, EMA and PMDA (2010–2024). Abbreviations: FDA, Food and Drug Administration; EMA, European Medicines Agency; PMDA, Pharmaceuticals and Medical Devices Agency; NMPA, National Medical Products Administration. IC, Immune Cell; SC, Stem Cell; OSC, Other Somatic Cell. Note: The cut-off date was August 1, 2024
Overview of cell therapy clinical trials in China: types and phases
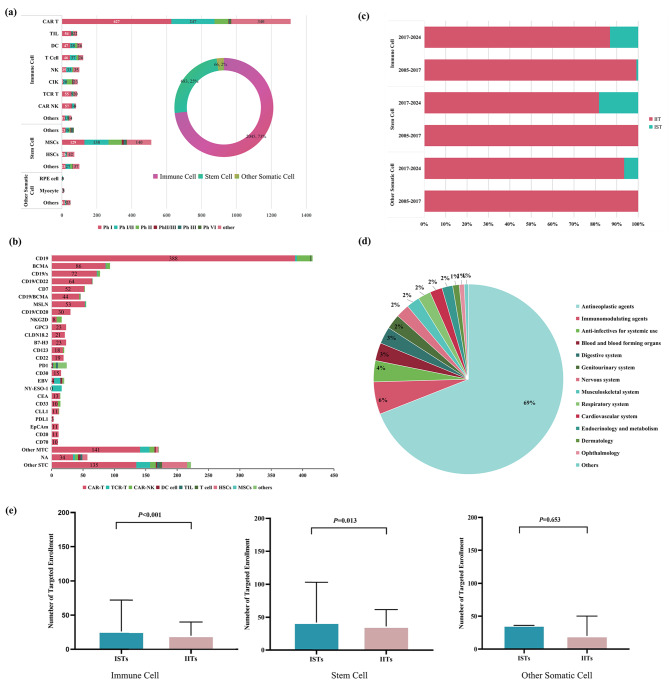
This study analyzed cell therapy clinical trials from January 1, 2005 to August 1, 2024, with classifications consistent with previous research (eAppendix, eFigure 1) [2, 4]. It encompassed 2,794 registered trials: 2,045 for immune cell (73.2%), 683 for stem cell (24.4%), and 66 for other somatic cell (2.4%). The most common immune cell therapies were CAR-T cells (46.9%). For stem cells, mesenchymal stem cells (18.3%) dominated. Other somatic cells mainly included cardiomyocytes (0.4%). Over half of the trials were in Phase I (40.5%) or Phase I/II (21.2%) (Fig. 2a, eFigure 4, eTable 3).
Fig. 2.
Scope and Distribution of Clinical Trials for Cell Therapy in China. (a) Cell therapy across various therapy types by different development phases in China from 2005 to 2024. (b) Molecular target distribution of clinical trials for cell therapy in China from 2005 to 2024. (c) Comparison of ISTs and IITs of all three categories of cell therapies initiated between 2000–2017 and 2017–2024. (d) Distribution of indications in clinical trials for cell therapy from 2005 to 2024. (e) A comparison of the targeted enrollment numbers between IITs and ISTs from 2005 to 2024 of all three categories of cell therapy. Abbreviations: ISTs, industry-sponsored trials; IITs, investigator-initiated trials. Note: The cut-off date was August 1, 2024
Characteristics of indications and targets
The majority of cell therapy clinical trials in China target cancer, comprising 69.0% of all trials (Fig. 2d, eTable 4). From 2005 to 2024, over 37.5% of the trials focused on hematologic malignancies. Beyond oncology, clinical trials increasingly focus on other areas, including infections (4.0%) and immune disorders (5.5%) (Fig. 2d). The most prominent targets were hematological malignancies, with CD19 leading at 416 trials (26.7%), of which 388 (93.3%) being CAR-T (Fig. 2b and d, eTable 5, eFigure 7, eFigure 8). Notably, between 2021 and 2023, there was an increase in trials for less common targets in solid tumors, such as CLDN18.2, HER2 and MSLN.
Characteristics of IITs and ISTs
As of August 1, 2024, IITs (n = 2,519, 90.2%) far exceeds ISTs (n = 275, 9.8%). Since the 2017 National Medical Products Administration (NMPA) guidelines clarified regulatory requirements for cell therapy products, ISTs in stem cell and somatic cell therapy increased significantly from zero. In immune cell therapy, ISTs generally had higher target enrollments than IITs (26 vs. 20, P < 0.001). Stem cells had more target enrollment in IITs than in ISTs (36 vs. 27, P = 0.013), with no significant difference in somatic cells (Fig. 2e, eTable6, eTable7, eTable8).
Outlook
Advancements in cellular technology have diversified cell therapy products [5]. Currently, nine solid tumors products have approved globally; but in China, the scope of approved products remains limited and relatively homogeneous, despite solid tumors making up over 90% of cancer cases (eTable 2) [6]. To address unmet clinical needs, we recommend that the NMPA establish specific guidelines to promote clinical-value-driven research [7].
Limited R&D resources in China hinder the exploration of new targets in cell therapy trials, leading to redundant R&D and target clustering. While global trials investigate new targets like CLDN18 (+ 400%), KRAS (+ 125%), Chinese trials remain focused on established targets, with over 38.0% targeting CD19 and BCMA, compared to 36.2% globally.3 This trend implies a preference for proven targets to reduce costs and shorten development cycles. The FDA recently issued guidance on CAR T-cell therapy, encouraging concurrent companion diagnostic tool development to improve trial accuracy [8]. We thereby recommend stricter regulatory standards to minimize redundant R&D. Furthermore, as specific cell therapy indications are included in China’s reimbursement list, establishing a value-based reimbursement system could help lower costs for within-class targeted cell therapy products and reduce market clustering [9, 10].
IITs offer more flexibility in trial design and generate substantial early exploratory data. Although NMPA guidelines since 2017 allow IIT data to support IND applications, challenges persist in research quality and cell production management [11]. We recommend that the NMPA specify regulatory and technical requirements for using IIT data for IND applications to establish a new pathway that accelerates cell therapy research and address unmet clinical needs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- IITs
Investigator-initiated trials
- ISTs
Industry-sponsored trials
- US
United States
- EU
European Union
- R&D
Research and development
- IND
Investigational New Drug
- NMPA
National Medical Products Administration
- CAR
Chimeric antigen receptor
- DC
Dendritic cell
- NK
Natural killer
- NKT
Natural killer T cell
- MSC
Mesenchymal stem cell
- HSC
Hematopoietic stem cell
- RPE
Retinal pigment epithelium
- TCR
T cell receptor
- TIL
Tumor-infiltrating lymphocyte
- CD
Cluster of differentiation (such as CD19)
- BCMA
B-cell maturation antigen
- MSLN
Mesothelin
- PD1
Programmed cell death protein 1
- B7-H3
B7 Homolog 3
- GPC3
Glypican 3
- CLDN18.2
Claudin 18.2
- EBV
Epstein-Barr Virus
- NY-ESO-1
New York esophageal squamous cell carcinoma 1
- NKG2D
Natural killer group 2 member D
- CEA
Carcinoembryonic antigen
- CLL1
C-type lectin-like molecule-1
- EpCAM
Epithelial cell adhesion molecule
- PDL1
Programmed death ligand 1.
Author contributions
XD and XXL were involved in the conception and design of the study. XD contributed to framework planning, data analysis, interpretation, and draft writing. XXL contributed to framework planning and data interpretation. LL was responsible for framework planning and data analysis. YC handled data cleansing and processing. YZ and YZ led the overall framework planning and provided guidance for data interpretation. All authors read and approved the final manuscript.
Funding
This work was supported by the Research Fund, Vanke School of Public Health, Tsinghua University and National Natural Science Foundation of China (72374115).
Data availability
Cell therapy clinical trial data were retrieved from the China Drug Trials Registry established by the National Medical Products Administration (www.chinadrugtrials.org.cn), Chinese Clinical Trial Registry (www.chictr.org.cn/), and ClinicalTrials. gov (clinicaltrials.gov) established by the U.S. Clinical trials conducted or registered in China up until August 1, 2024 were retrieved and analyzed. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yajuan Zhang, Email: zhangyajuan@mail.tsinghua.edu.cn.
Yi Zhang, Email: yi_zhang@mail.tsinghua.edu.cn.
References
- 1.Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM. Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov. 2020;19:583–4. [DOI] [PubMed] [Google Scholar]
- 2.Yin C, Gao J, Li G, Hu H, Zhou L, Lu S, Chen X. Gene and cell therapies in China: booming landscape under dual-track regulation. J Hematol Oncol. 2022;15(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saez-Ibañez AR, Upadhaya S, Partridge T, Winkelman D, Correa D, Campbell J. The changing landscape of cancer cell therapies: clinical trials and real-world data. Nat Rev Drug Discov. 2024 May 31. [DOI] [PubMed]
- 4.El-Kadiry AE, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med (Lausanne). 2021;8:756029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Zhong H, Hu HX, Kong FP, Liang WN, Li GQ. Chinese innovative drug R&D trends in 2024. Nat Rev Drug Discov. 2024. 10.1038/d41573-024-00120-5. [Google Scholar]
- 6.Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.China Center for Drug Evaluation, National Medical Products Administration. Guiding Principles for Clinical Research and Development of Anti-tumor Drugs Guided by Clinical Value.https://www.cde.org.cn/main/news/viewInfoCommon/ef7bfde96c769308ad080bb7ab2f538e (2021). Accessed September 5, 2024 (in Chinese).
- 8.U.S. Food and Drug Administration. Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products (2021). Accessed September 5, 2024.
- 9.Beaver JA, Pazdur R. The wild west of checkpoint inhibitor development. N Engl J Med. 2022;386(14):1297–301. [DOI] [PubMed] [Google Scholar]
- 10.Luo X, Du X, Huang L, Guo Q, Tan R, Zhou Y, Li Z, Xue X, Li T, Le K, Qian F, Chow SC, Yang Y. The price, efficacy, and safety of within-class targeted anticancer medicines between domestic and imported drugs in China: a comparative analysis. Lancet Reg Health West Pac. 2022;32:100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.China Center for Drug Evaluation, National Medical Products Administration. Guidelines for clinical trials of human stem cells and derived cell therapy products (for Trial Implementation). https://www.cde.org.cn/main/news/viewInfoCommon/f82a0fee1e625a1a3834a93cee3836c7 (2023). Accessed 5 Sept 2024 (in Chinese).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cell therapy clinical trial data were retrieved from the China Drug Trials Registry established by the National Medical Products Administration (www.chinadrugtrials.org.cn), Chinese Clinical Trial Registry (www.chictr.org.cn/), and ClinicalTrials. gov (clinicaltrials.gov) established by the U.S. Clinical trials conducted or registered in China up until August 1, 2024 were retrieved and analyzed. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.