Abstract
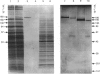
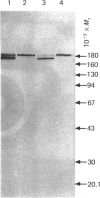
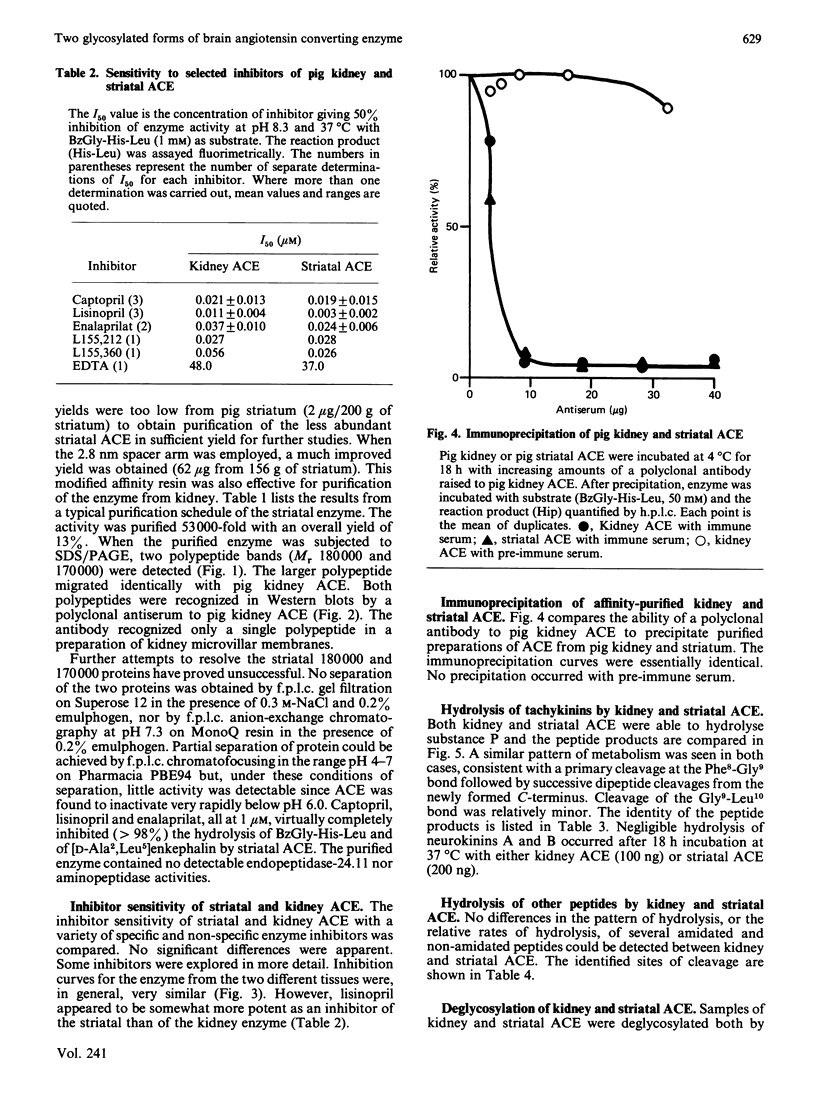
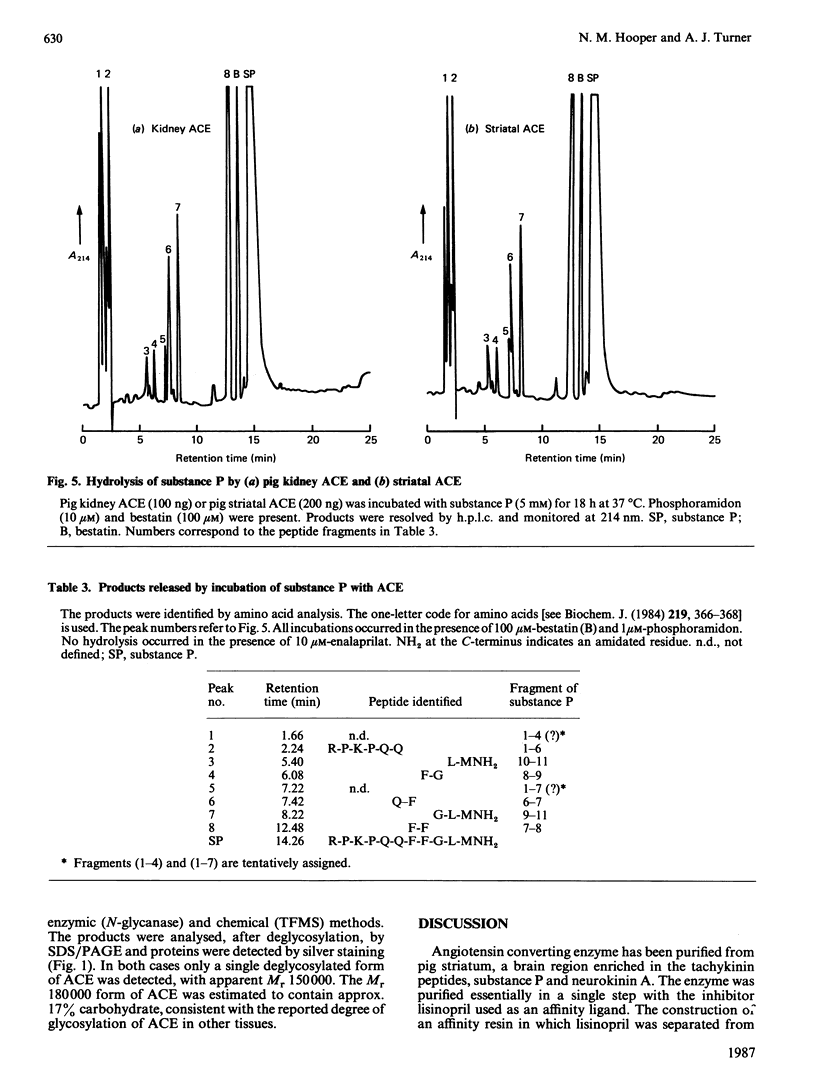
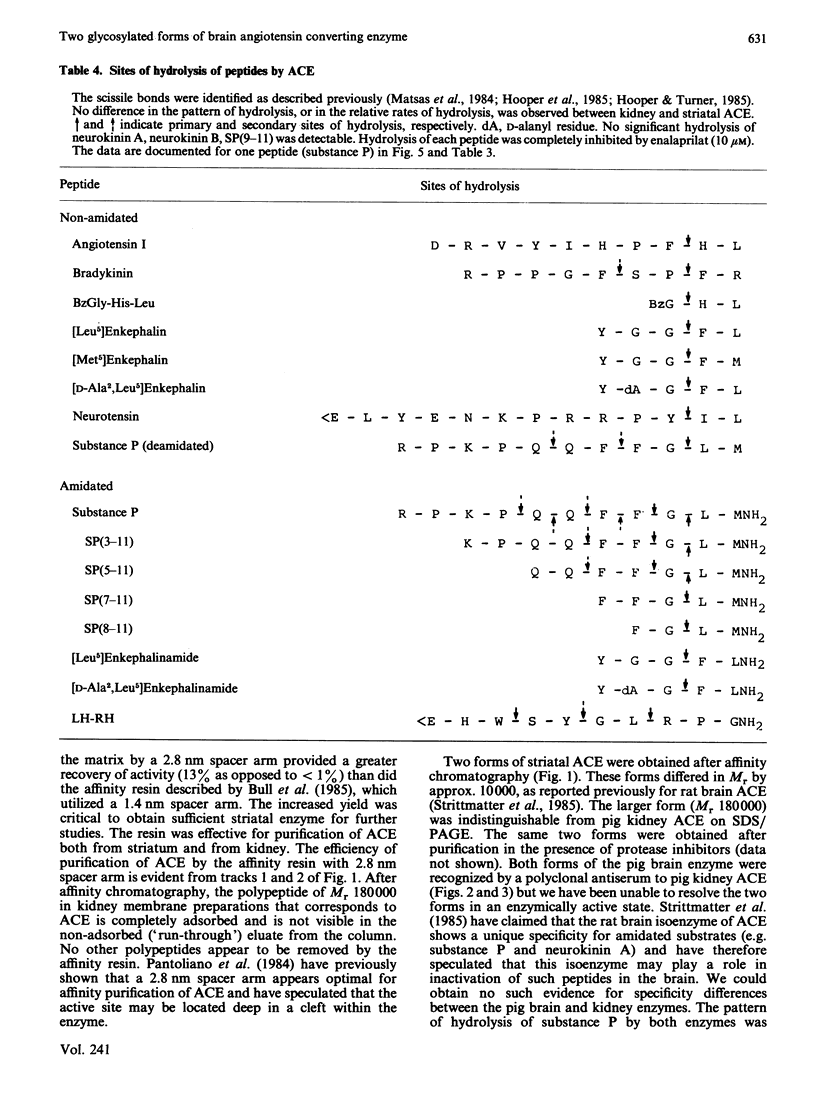
Peptidyl-dipeptidase A (angiotensin converting enzyme; ACE, EC 3.4.15.1), has been purified from pig kidney and striatum by affinity chromatography employing the selective inhibitor lisinopril as ligand. The inclusion of a 2.8 nm spacer arm improved the yield of the enzyme compared with the 1.4 nm spacer arm described in previous work. Two forms of striatal ACE (Mr 180,000 and 170,000), but only a single form of kidney ACE (Mr 180,000), were isolated by this procedure. Both forms of striatal ACE were recognized by a polyclonal antibody to kidney ACE. No significant differences in substrate specificity or inhibitor sensitivity between kidney and striatal ACE could be detected. In particular, the amidated neuropeptide, substance P, was hydrolysed identically by both preparations and no significant hydrolysis of the related tachykinin peptides neurokinin A and neurokinin B could be detected. After chemical or enzymic deglycosylation, kidney and both forms of striatal ACE migrated identically on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis with an apparent Mr of 150,000. We suggest that the two detectable forms of ACE in pig brain are not isoenzymes but are the result of differential glycosylation in different cell types in the brain. It appears that ACE, unlike endopeptidase-24.11, does not have the general capacity to hydrolyse and inactivate the tachykinin peptides at a significant rate in brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams W. B., Davies R. O., Ferguson R. K. Overview: the role of angiotensin-converting enzyme inhibitors in cardiovascular therapy. Fed Proc. 1984 Apr;43(5):1314–1321. [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bull H. G., Thornberry N. A., Cordes E. H. Purification of angiotensin-converting enzyme from rabbit lung and human plasma by affinity chromatography. J Biol Chem. 1985 Mar 10;260(5):2963–2972. [PubMed] [Google Scholar]
- Cascieri M. A., Bull H. G., Mumford R. A., Patchett A. A., Thornberry N. A., Liang T. Carboxyl-terminal tripeptidyl hydrolysis of substance P by purified rabbit lung angiotensin-converting enzyme and the potentiation of substance P activity in vivo by captopril and MK-422. Mol Pharmacol. 1984 Mar;25(2):287–293. [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I. Adsorbents for affinity chromatography. Use of N-hydroxysuccinimide esters of agarose. Biochemistry. 1972 Jun 6;11(12):2291–2299. doi: 10.1021/bi00762a013. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Ondetti M. A. Inhibitors of angiotensin-converting enzyme for treatment of hypertension. Biochem Pharmacol. 1980 Jul 1;29(13):1871–1877. doi: 10.1016/0006-2952(80)90096-9. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Pickett C. B., MacGregor J. S., Soffer R. L. Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4295–4297. doi: 10.1073/pnas.79.14.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Neurokinin A (substance K) is a substrate for endopeptidase-24.11 but not for peptidyl dipeptidase A (angiotensin-converting enzyme). Biochem J. 1985 Oct 15;231(2):357–361. doi: 10.1042/bj2310357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Neurokinin B is hydrolysed by synaptic membranes and by endopeptidase-24.11 (enkephalinase) but not by angiotensin converting enzyme. FEBS Lett. 1985 Oct 7;190(1):133–136. doi: 10.1016/0014-5793(85)80443-9. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Skidgel R. A., Gafford J. T., Erdös E. G. Enzymes in placental microvilli: angiotensin I converting enzyme, angiotensinase A, carboxypeptidase, and neutral endopeptidase ("enkephalinase"). Peptides. 1984 Jul-Aug;5(4):789–796. doi: 10.1016/0196-9781(84)90023-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. An immunohistochemical study of endopeptidase-24.11 ("enkephalinase") in the pig nervous system. Neuroscience. 1986 Aug;18(4):991–1012. doi: 10.1016/0306-4522(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Rattray M., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Endopeptidase-24.11 in human synaptic membrane preparations hydrolyses substance P. Biochem J. 1985 Jun 1;228(2):487–492. doi: 10.1042/bj2280487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Stephenson S. L., Hryszko J., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Phase separation of synaptic membrane preparations with Triton X-114 reveals the presence of aminopeptidase N. Biochem J. 1985 Oct 15;231(2):445–449. doi: 10.1042/bj2310445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoliano M. W., Holmquist B., Riordan J. F. Affinity chromatographic purification of angiotensin converting enzyme. Biochemistry. 1984 Feb 28;23(5):1037–1042. doi: 10.1021/bi00300a036. [DOI] [PubMed] [Google Scholar]
- Parsons W. H., Davidson J. L., Taub D., Aster S. D., Thorsett E. D., Patchett A. A., Ulm E. H., Lamont B. I. Benzolactams. A new class of converting enzyme inhibitors. Biochem Biophys Res Commun. 1983 Nov 30;117(1):108–113. doi: 10.1016/0006-291x(83)91547-4. [DOI] [PubMed] [Google Scholar]
- Relton J. M., Gee N. S., Matsas R., Turner A. J., Kenny A. J. Purification of endopeptidase-24.11 ('enkephalinase') from pig brain by immunoadsorbent chromatography. Biochem J. 1983 Dec 1;215(3):519–523. doi: 10.1042/bj2150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel R. A., Engelbrecht S., Johnson A. R., Erdös E. G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984 Jul-Aug;5(4):769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Erdös E. G. Novel activity of human angiotensin I converting enzyme: release of the NH2- and COOH-terminal tripeptides from the luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1025–1029. doi: 10.1073/pnas.82.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffer R. L. Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu Rev Biochem. 1976;45:73–94. doi: 10.1146/annurev.bi.45.070176.000445. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Chaplin M. F., Kenny A. J. Deglycosylation by trifluoromethanesulphonic acid of endopeptidase-24.11 purified from pig kidney and intestine. Biochem J. 1984 Aug 1;221(3):919–922. doi: 10.1042/bj2210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Lo M. M., Javitch J. A., Snyder S. H. Autoradiographic visualization of angiotensin-converting enzyme in rat brain with [3H]captopril: localization to a striatonigral pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1599–1603. doi: 10.1073/pnas.81.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Thiele E. A., Kapiloff M. S., Snyder S. H. A rat brain isozyme of angiotensin-converting enzyme. Unique specificity for amidated peptide substrates. J Biol Chem. 1985 Aug 15;260(17):9825–9832. [PubMed] [Google Scholar]
- Thiele E. A., Strittmatter S. M., Snyder S. H. Substance K and substance P as possible endogenous substrates of angiotensin converting enzyme in the brain. Biochem Biophys Res Commun. 1985 Apr 16;128(1):317–324. doi: 10.1016/0006-291x(85)91681-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Neff N. H. Distribution and properties of angiotensin converting enzyme of rat brain. J Neurochem. 1972 Oct;19(10):2443–2450. doi: 10.1111/j.1471-4159.1972.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Endo S., Ogura Y., Ishii S. A new feature of angiotensin-converting enzyme in the brain: hydrolysis of substance P. Biochem Biophys Res Commun. 1983 Oct 31;116(2):735–742. doi: 10.1016/0006-291x(83)90586-7. [DOI] [PubMed] [Google Scholar]