Abstract
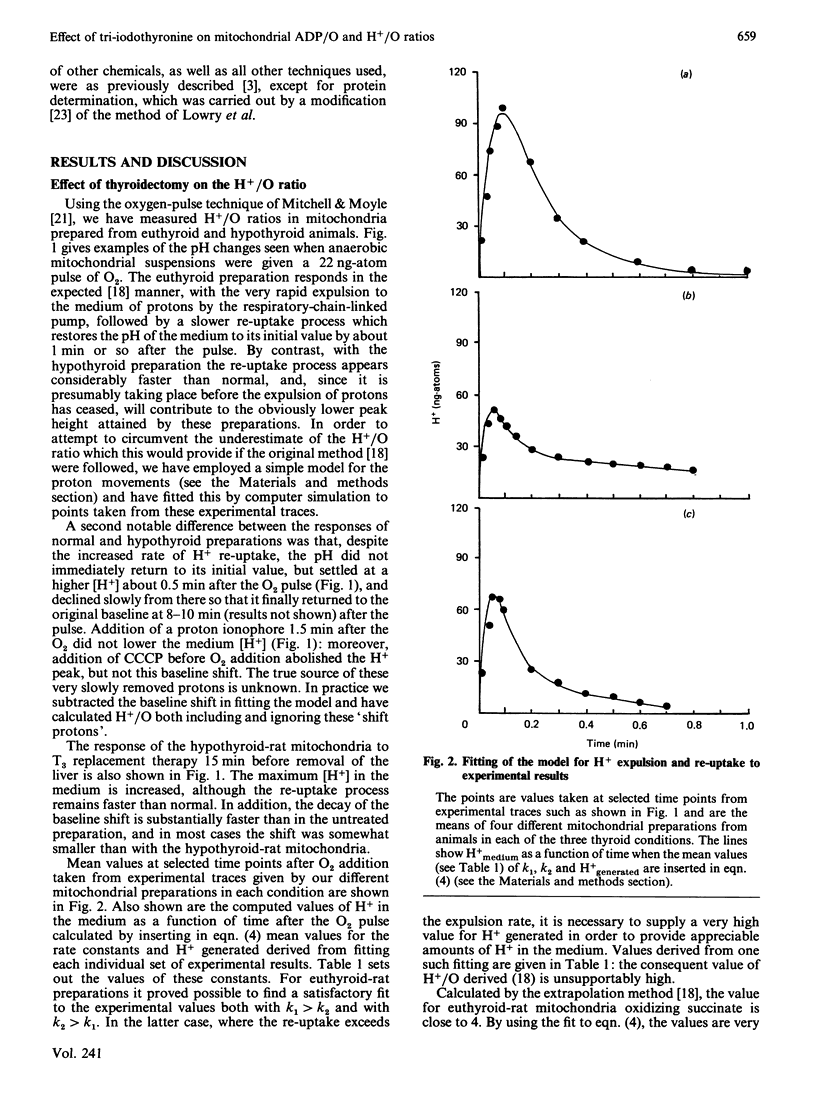
Mitochondria from the livers of thyroidectomized rats have a lowered ADP/O ratio, which can be restored to normal within 15 min after intravenous injection of a near-physiological dose of tri-iodothyronine. Thyroidectomy lowered the measured delta pH, which appears to be compensated by a rise (not statistically significant) in the membrane potential, so that the protonmotive force is unaltered. A simple simulation technique is described for use in estimating H+/O ratios by the oxygen-pulse technique, which circumvents the problem that this ratio can be seriously underestimated because of re-uptake of protons from the bulk phase by the mitochondria before their expulsion is complete. By this procedure the H+/O ratio of hypothyroid mitochondria is shown to be lowered by the same factor as the ADP/O ratio, and both these ratios are very rapidly restored in parallel by hormone administration. Although these findings could be consistent with a proposal that tri-iodothyronine rapidly modulates by some mechanism the efficiency of the respiratory-chain-linked proton pumps, the kinetic properties of the proton exchange suggest that the bulk-phase protons measured may not reflect faithfully those that drive the ATP synthetase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzone G. F., Pozzan T., Massari S. Proton electrochemical gradient and phosphate potential in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):307–316. doi: 10.1016/0005-2728(78)90036-1. [DOI] [PubMed] [Google Scholar]
- Corrigall J., Tselentis B. S., Mowbray J. The efficiency of oxidative phosphorylation and the rapid control by thyroid hormone of nicotinamide nucleotide reduction and transhydrogenation in intact rat liver mitochondria. Eur J Biochem. 1984 Jun 1;141(2):435–440. doi: 10.1111/j.1432-1033.1984.tb08210.x. [DOI] [PubMed] [Google Scholar]
- Goglia F., Torresani J., Bugli P., Barletta A., Liverini G. In vitro binding of triiodothyronine to rat liver mitochondria. Pflugers Arch. 1981 May;390(2):120–124. doi: 10.1007/BF00590193. [DOI] [PubMed] [Google Scholar]
- Hashizume K., Ichikawa K. Localization of 3,5,3'-L-triiodothyronine receptor in rat kidney mitochondrial membranes. Biochem Biophys Res Commun. 1982 Jun 15;106(3):920–926. doi: 10.1016/0006-291x(82)91798-3. [DOI] [PubMed] [Google Scholar]
- Herd P. A. Thyroid hormone-divalent cation interactions. Effect of thyroid hormone on mitochondrial calcium metabolism. Arch Biochem Biophys. 1978 May;188(1):220–225. doi: 10.1016/0003-9861(78)90375-2. [DOI] [PubMed] [Google Scholar]
- Hitchens G. D., Kell D. B. On the extent of localization of the energized membrane state in chromatophores from Rhodopseudomonas capsulata N22. Biochem J. 1982 Aug 15;206(2):351–357. doi: 10.1042/bj2060351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch F. L. Early action of injected L-thyroxine on mitochondrial oxidative phosphorylation. Proc Natl Acad Sci U S A. 1967 Aug;58(2):506–512. doi: 10.1073/pnas.58.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M., Crespo-Armas A., Mowbray J. The influence of thyroid hormone on the degree of control of oxidative phosphorylation exerted by the adenine nucleotide translocator. FEBS Lett. 1984 Nov 19;177(2):231–235. doi: 10.1016/0014-5793(84)81289-2. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Proton translocation coupled to mitochondrial electron transport. Biochem Soc Trans. 1984 Jun;12(3):386–388. doi: 10.1042/bst0120386. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J., Mitchell R. Measurement of translocation of H+/O in mitochondria and submitochondrial vesicles. Methods Enzymol. 1979;55:627–640. doi: 10.1016/0076-6879(79)55071-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray J., Corrigall J. Short-term control of mitochondrial adenine nucleotide translocator by thyroid hormone. Eur J Biochem. 1984 Feb 15;139(1):95–99. doi: 10.1111/j.1432-1033.1984.tb07981.x. [DOI] [PubMed] [Google Scholar]
- Müller M. J., Seitz H. J. Dose dependent stimulation of hepatic oxygen consumption and alanine conversion to CO2 and glucose by 3,5,3'-triiodo-L-thyronine (T3) in the isolated perfused liver of hypothyroid rats. Life Sci. 1981 May 18;28(20):2243–2249. doi: 10.1016/0024-3205(81)90576-2. [DOI] [PubMed] [Google Scholar]
- Müller M. J., Seitz H. J. Rapid and direct stimulation of hepatic gluconeogenesis by L-triiodothyronine (T3) in the isolated-perfused rat liver. Life Sci. 1980 Sep 8;27(10):827–835. doi: 10.1016/0024-3205(80)90076-4. [DOI] [PubMed] [Google Scholar]
- Palacios-Romero R., Mowbray J. Evidence for the rapid direct control both in vivo and in vitro of the efficiency of oxidative phosphorylation by 3,5,3'-tri-iodo-L-thyronine in rats. Biochem J. 1979 Dec 15;184(3):527–538. doi: 10.1042/bj1840527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Romero R., Mowbray J. The rapid transient stimulation of both cytoplasmic and mitochondrial ornithine decarboxylases in the liver of thyroidectomized rats by 3,5,3'-triiodo-L-thyronine. Biosci Rep. 1981 Jan;1(1):71–77. doi: 10.1007/BF01115151. [DOI] [PubMed] [Google Scholar]
- Petronilli V., Pietrobon D., Zoratti M., Azzone G. F. Free energy coupling between H+-generating and H+-consuming pumps. Ratio between output and input forces. Eur J Biochem. 1986 Mar 3;155(2):423–431. doi: 10.1111/j.1432-1033.1986.tb09508.x. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Bronk J. R. The influence of thyroxine administered in vivo on the transmembrane protonic electrochemical potential difference in rat liver mitochondria. Biochem J. 1979 Feb 15;178(2):505–507. doi: 10.1042/bj1780505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Branca D., Ferguson S. J. The rate of ATP synthesis by submitochondrial particles can be independent of the magnitude of the protonmotive force. Biochem J. 1980 Jun 15;188(3):945–948. doi: 10.1042/bj1880945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Sakurada T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science. 1980 Oct 17;210(4467):340–342. doi: 10.1126/science.7423197. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O., Brenner M. A., Lazarus J. H. Thyroid hormone action: the mitochondrial pathway. Science. 1977 Sep 2;197(4307):996–999. doi: 10.1126/science.196334. [DOI] [PubMed] [Google Scholar]
- Sterling K. Thyroid hormone action at the cell level (second of two parts). N Engl J Med. 1979 Jan 25;300(4):173–177. doi: 10.1056/NEJM197901253000405. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Simonetti A. L., Van Dam K. The hypothesis of localized chemiosmosis is unsatisfactory. Biochem J. 1981 Nov 15;200(2):193–202. doi: 10.1042/bj2000193. [DOI] [PMC free article] [PubMed] [Google Scholar]


