Abstract
Background
Previous studies have shown that patients with pre-existing chronic obstructive pulmonary diseases (COPD) were more likely to be infected with coronavirus disease (COVID-19) and lead to more severe lung lesions. However, few studies have explored the severity and prognosis of COVID-19 patients with different phenotypes of COPD.
Purpose
The aim of this study is to investigate the value of the deep learning and radiomics features for the severity evaluation and the nucleic acid turning-negative time prediction in COVID-19 patients with COPD including two phenotypes of chronic bronchitis predominant patients and emphysema predominant patients.
Methods
A total of 281 patients were retrospectively collected from Hohhot First Hospital between October 2022 and January 2023. They were divided to three groups: COVID-19 group of 95 patients, COVID-19 with emphysema group of 94 patients, COVID-19 with chronic bronchitis group of 92 patients. All patients underwent chest computed tomography (CT) scans and recorded clinical data. The U-net model was pretrained to segment the pulmonary involvement area on CT images and the severity of pneumonia were evaluated by the percentage of pulmonary involvement volume to lung volume. The 107 radiomics features were extracted by pyradiomics package. The Spearman method was employed to analyze the correlation of the data and visualize it through a heatmap. Then we establish a deep learning model (model 1) and a fusion model (model 2) combined deep learning with radiomics features to predict nucleic acid turning-negative time.
Results
COVID-19 patients with emphysema was lowest in the lymphocyte count compared to COVID-19 patients and COVID-19 companied with chronic bronchitis, and they have the most extensive range of pulmonary inflammation. The lymphocyte count was significantly correlated with pulmonary involvement and the time for nucleic acid turning negative (r=-0.145, P < 0.05). Importantly, our results demonstrated that model 2 achieved an accuracy of 80.9% in predicting nucleic acid turning-negative time.
Conclusion
The pre-existing emphysema phenotype of COPD severely aggravated the pulmonary involvement of COVID-19 patients. Deep learning and radiomics features may provide more information to accurately predict the nucleic acid turning-negative time, which is expected to play an important role in clinical practice.
Keywords: COVID-19 with COPD, Pulmonary involvement, Nucleic acid turning-negative time, Deep learning method, Radiomics features
Introduction
Coronavirus disease (COVID-19) is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Mudatsir et al. demonstrated that chronic diseases have been associated with the severity and death of COVID-19, such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, hypertension and cardiovascular disease experience [2]. COPD is one of the most common chronic diseases in the world, characterized by chronic respiratory symptoms caused by the airway and/or alveolar abnormalities [3]. Sanchez-Ramirez et al. found that COPD patients with COVID-19 were associated with a four-time higher risk of severe consequences such as intensive care unit admission and death [4]. Increasing evidence suggests that the up-regulation of angiotensin converting enzyme 2 is a significant factor contributing to the susceptibility of COPD patients to SARS-CoV-2 infection [5, 6]. Moreover, COPD patients often have impaired innate and adaptive immune responses, which may delay the clearance of respiratory viruses [7]. Consequently, SARS-CoV-2 may have a higher propensity to spread within the lungs of COPD patients and COPD patients have worse outcomes from COVID-19 [8]. A 2023 bibliometric analysis indicated that, as a key focus of COVID-19 epidemic prevention, COPD combined with COVID-19 still was a hot topic and trend [9].
The diagnostic value of computed tomography (CT) in COVID-19 has been well-established [10]. CT imaging can detect the characteristic features of COVID-19 pneumonia, such as ground-glass opacity, crazy paving pattern, consolidation, and fibrosis [11]. Serial CT scans can track the evolution of lung lesions over time, providing insights into the effectiveness of therapeutic interventions [12]. Additionally, chest CT severity score is a scoring method in the assessment of COVID-19 pulmonary parenchymal involvement, which has demonstrated strong discriminating power for the prediction of disease severity and outcome of COVID-19 patients [13, 14]. These findings could help differentiate COVID-19 pneumonia from other respiratory infections and contribute to early detection and diagnosis of COVID-19 pneumonia. Previous studies revealed that the pre-existing COPD increased the severity and the risk of death of COVID-19 [15, 16]. The classic COPD phenotypes of chronic bronchitis and emphysema have been recognized in Global Initiative for Chronic Obstructive Lung Disease and Global Initiative [17]. However, the differences of pulmonary parenchymal involvement between COVID-19 patients with two COPD phenotypes has not been explored.
Due to the outbreak of COVID-19, global healthcare resources have been facing a tremendous burden. Thus, accumulating studies focus on identification of the patients whose nucleic acid can turn negative in a short time to make rational use of resources. Liu et al. found biochemical indicators such as lactate dehydrogenase, c-reactive protein and Albumin were useful prognostic markers for predicting nucleic acid turning-negative-time [18]. Zhu et al. demonstrated that the value of nutrophil-to-lmphocyte ratio (NLR) and vaccination could predict the negative conversion time of nucleic acid [19]. The time of nucleic acid conversion to negative was closely related to the clinical manifestations and disease progression of COVID-19 patients [20]. With the wide spread of models based on artificial intelligence, the deep learning method combined with lung CT images could provide the tool to the understanding and management of COVID-19 [21]. Zhou et al. used an ensemble deep learning model to realize the rapid detection of novel coronavirus COVID-19 [22]. Xie et al. proposed that the artificial intelligence system based on U-shaped neural network method can quickly provide some quantitative indicators including the radiologist with the position of lesions, dynamic volume changes, ground glass opacity internal organ morphology, the HU value of the lesion and so on, mak it easier for radiologists to identify COVID-19 from other diseases [23]. In addition, the deep learning methods can automatically and more accurately predict the severity of COVID-19 patients, which provide a tool for personalized treatment of patients and significantly reduced mortality rates [24, 25]. Importantly, the deep learning method could assess difficult cases that does not fit in the predefined feature characteristics, still achieving satisfactory results [26]. However, deep learning models typically require more data and have poor interpretability [27]. As another medical imaging analysis method, radiomics could build high performance models out of limited datasets, which has been widely used in quantitative analysis of CT images for screening COVID-19 patients [28, 29], discriminating the severity of pneumonia [30], and prognosis assessment in patients with COVID-19 pneumonia [31]. As of yet, the research on utilizing deep learning and radiomics for predicting the nucleic acid turning-negative-time of COVID-19 patients is still in its early stages and remains relatively limited. Thus, there is an urgent need for intelligent algorithms to accurately and automatically predict the nucleic acid conversion time of patients with COVID-19.
The aims in our study as follows. First, we aim to assess the pulmonary severity and the laboratory variables in COVID-19 with chronic bronchitis patients and COVID-19 with emphysema patients. Second, we aim to evaluate the clinical value of deep learning and radiomics features in predicting the nucleic acid turning-negative-time. Third, we aim to explore influence factors correlated with the nucleic acid turning-negative-time.
Patients and methods
Participants
This retrospective cohort study enrolled 281 COVID-19 patients who had definite clinical outcome (discharge) from October 2022 to January 2023, at Hohhot First Hospital, the designated hospital to treat patients with COVID-19 in Inner Mongolia. According to the guidance for diagnosis and management of COVID-19 released by World Health Organization, COVID-19 patients was diagnosed [32].
Inclusion criteria for this study were: (1) The diagnosis was confirmed by positive result of real-time reverse-transcriptase polymerase-chain-reaction (rRT-PCR) assay for SARS-CoV-2 of throat or nasopharyngeal swab specimens; (2) CT images demonstrated pneumonia; (3) Patients without comorbidities or with only comorbidities of chronic obstructive pulmonary disease. Comorbidities of COPD information was collected based on patients’ self-report on admission and diagnosed by experienced physicians based on Global Initiative for Chronic Obstructive Lung Disease and Global Initiative [17]. Exclusion criteria for this study were: (1) patients with inflammatory diseases such as pancreatitis, prostatitis, and immunological diseases or with severe diseases such as hypertension, diabetes, cerebro-cardiovascular diseases, and chronic kidney disease; (2) patients with missing data; or (3) Patients with uncertain COPD diagnosis.
Among the selected patients, patients with COVID-19 combined with COPD were divided into two groups: chronic bronchitis predominant group and emphysema predominant group. The definitions of chronic bronchitis predominant were chronic sputum for most days, 3 months a year, or no radiological diagnosis of emphysema for at least 2 years. Emphysema predominant was defined as no chronic cough and sputum but having typical clinical and radiological manifestations of emphysema [33]. Clinical data including gender, age, vaccination status, nucleic acid turning-negative-time, and laboratory test results (procalcitonin (PCT) level, C-reactive protein level, white leukocyte count, neutrophil count, lymphocyte count, neutrophil-to-lymphocyte ratio (NLR)) were collected and recorded. The criteria for confirming the patient’s nucleic acid turned negative were twice consecutive negatives, and the interval of tests > 24 h [18]. According to the different time required for nucleic acid to turn negative, the subjects were divided into the following four categories: 0–5 days, 6–10 days, 11–15 days, and ≥ 16 days. The number of people in each category is 22, 122, 94 and 43 respectively. This study was approved by the Ethics Committee of the Hohhot First Hospital (number: IRB2024022).
CT acquisition
Patients underwent chest CT imaging on two 64 detector CT scanners (Discovery CT 750 and u-CT 760). The protocol was as follows: tube voltage: 120 kV; automatic tube current (260 mA for Discovery CT 750, GE and 150 mA for u-CT, United Imaging); reconstruction slice thickness: 1.25 mm; pitch of 1.0; matrix, 512 × 512 and breath hold at full inspiration in a craniocaudal direction from the lung apices to lateral costophrenic sulci. The following windows were used for image display: a mediastinal window with window width of 400 HU and window level of 40 HU and a lung window with a width of 1600 HU and window level of -700 HU. The acquired images were subsequently reconstructed using an iterative reconstruction method.
Segmentation and processing of CT images
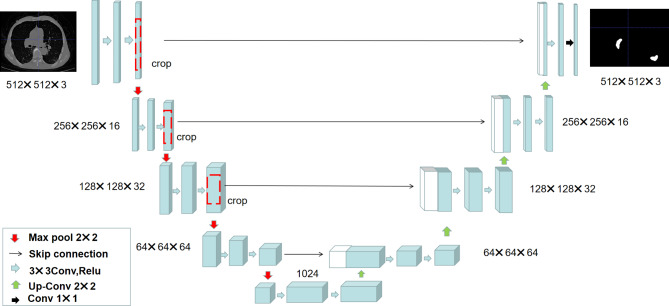
The U-net convolutional neural network was pretrained to segment the pulmonary involvement area based on CT images of 500 pnemonia patients in our study [34]. As seen in Fig. 1, the U-net network mainly consists of three parts: encoder, decoder and skip connection. It has performed four down-sampling and up-sampling operations on CT images respectively. The CT images of were divided into training set, validation set, and test set according to 7:2:1 for U-net model construction. The experiment sets up 100 epoch iterations, the learning rate was set to 0.00001, and the loss function was set to cross entropy combined with the dice function and two radiologists with over 5 years of experience were invited to evaluate the segmentation results of pulmonary involvement area. Then, we quantitatively estimated the pulmonary involvement area of patients [12–14].
Fig. 1.
The architecture diagram of the U-net convolutional network. The network is a traditional end-to-end model, consisting of encoder, decoder and skip connection
Extraction and analysis of radiomics features
Radiomics features were extracted from the pulmonary involvement area of CT images using the PyRadiomics Python tool (v3.11) in our study [35]. Previous study has demonstrated that the 107 standard radiomics features can be used for lung disease research [36]. These features were divided into 14 shape features, 18 first-order statistical features, and 75 textural features, which included the neighborhood gray tone difference matrix (n = 5), gray-level run length matrix (n = 16), gray-level dependence matrix (n = 14), gray-level size zone matrix (n = 16), and gray-level co-occurrence matrix (n = 24). The least absolute shrinkage and selection operator (LASSO) method was used for feature selection, which applied regularization for variable selection to compress the regression coefficients of some unnecessary variables to zero and then eliminated them from the model, achieving the purpose of variable screening [37]. Finally, we selected the 30 features with non-zero coefficients to follow analysis.
Construction of the classification model
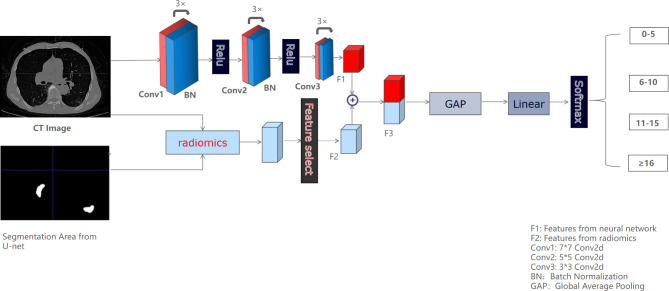
We constructed two models using a universal Pytorch framework (http://pytorch.org) to predict the nucleic acid turning-negative-time, including a deep learning model (model 1) and a fusion model (model 2) combined deep learning with radiomics features. As shown in Fig. 2, the fusion model consisted of the upper branch and lower branch. The upper branch had one input of the original CT image, which consisted of 3 groups of 7 × 7 conv2d, 5 × 5 conv2d and 3 × 3 conv2d plus batch-norm. The rectified linear unit (ReLU) activation function was used to improve the model’s efficiency and the deep learning feature map (F1) was extracted. The lower branch had two inputs of the original CT image and the segmented image of the pneumonia area, which included the extraction and selection of radiomics features (F2). Then, the final features from two branches were concatenated to obtain the F3 feature map. The F3 feature maps were compressed through the global average pooling function and the fully connected layer to obtain the final feature map, and the softmax function was used to achieve the probability output of each target category. In general, the fusion model combined the high-dimensional features by deep learning method and the low-dimensional features by radiomics features to improve the classification results. Deep learning model was the upper branch of fusion model, which was employed as part of ablation experiments to reflect the value of radiomics features in the nucleic acid turning-negative-time prediction by comparing it with the fusion model.
Fig. 2.
The framework of the fusion model for predicting nucleic acid turning-negative-time. The model is divided into two branches, of which the upper branch was used for the deep learning features extraction, and the lower branch was used for the radiomics features extraction and selection.
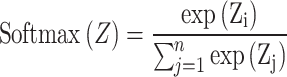 |
1 |
In formula (1), Zi represented the output values of the i-th sample and n represented the number of classes in the classification task.
With respect to the model construction, the backpropagation (BP) algorithm was employed, which updated the network parameters iteratively until an optimal or suboptimal solution was reached. The weight cross entropy loss function was utilized to alleviate the sample imbalance problem according to the weights of different sample numbers.
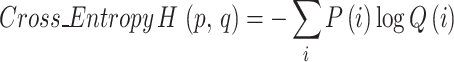 |
2 |
In formula (2), i represented the label or category of a sample, P(i) indicated the probability of the true label, and Q (i) was the probability predicted by the model.
The following metrics were employed to evaluate the performances of each model: accuracy, precision, sensitivity, specificity, and F1-score. Furthermore, radar charts were used to compare the predictive performance between model 1 and model 2 in Classes 1–4 respectively and display the relative relationships between the two models.
Statistical analysis
Categorical variables were presented as frequencies and percentages, which were compared using the chi-square test. Continuous variables were described as means ± standard deviation (SD) or median (interquartile range, IQR) and compared between groups using either a two-sample t-test or Wilcoxon test, depending on the distribution. Spearman correlation was used to assess the correlations between variables. All statistical analyses were performed using IBM SPSS Statistics Software (version 27.0, SPSS Inc., Chicago, IL, USA).
Results
Demographics and baseline clinical characteristics
A total of 281 patients were enrolled including 95 COVID-19 patients, 94 COVID-19 patients with the emphysema‑predominant COPD, and 92 patients with the chronic bronchitis-predominant COPD. As shown in Tables 1 and 62 patients (65.96%) were males in COVID-19 with emphysema group, and more than the other two groups. The laboratory tests including white leukocyte count, neutrophil count, NLR value, and C-reactive protein level increased in COVID-19 patients with COPD than COVID-19 patients. However, the Lymphocyte count was shown to be lower in COVID-19 patients with emphysema compared than other groups and the median was 1.07 × 10^9cells/L. Among the patients with different time required for nucleic acid to turn negative, 51.58% patients during 6–10 days in COVID-19 group, 40.43% patients during 11–15 days of COVID-19 patients with emphysema and 40.22% patients during 11–15 days of COVID-19 patients with chronic bronchitis. A total of 85 patients (89.47%) received at least one dose of inactivated SARS-CoV-2 vaccine in COVID-19 group, in which 56 patients (58.95%) received three doses of inactivated vaccine. Only 6 patients (6.32%) received one dose of the inactivated vaccine, and 23 patients (24.21%) got two doses of the inactivated vaccine. However, 36 patients (38.30%) in COVID-19 with emphysema and 19 patients (20.65%)in COVID-19 with chronic bronchitis have not been vaccinated. There were significant statistical differences in sex, lymphocyte count, neutrophil count, NLR value, C-reactive protein level, nucleic acid turning-negative-time, and vaccination dose between the three groups (P < 0.05).
Table 1.
Comparison of baseline characteristics among the patients
| COVID-19(95) | COVID-19 with emphysema(94) | COVID-19 with chronic bronchitis(92) | P value | |
|---|---|---|---|---|
| Sex (male), N(%) | 36(37.89%) | 62(65.96%) | 35(38.04%) | < 0.001 |
| White leukocyte, per l | 4.58(1.73) | 5.08(2.06) | 5.25(2.04) | 0.076 |
| Lymphocyte, per l | 1.36(0.64) | 1.07(0.57) | 1.37(0.68) | < 0.001 |
| Neutrophil, per l | 2.77(1.31) | 3.27(1.99) | 3.09(1.71) | 0.032 |
| NLR | 2.00(1.43) | 2.90(2.53) | 2.34(1.52) | < 0.001 |
| C-reactive protein, per l | 7.40(8.65) | 13.16(28.87) | 8.70(12.52) | < 0.001 |
| PCT, per ml | 0.05(0.05) | 0.06(0.05) | 0.06(0.08) | 0.191 |
| Nucleic acid turning-negative-time, days | 0.003 | |||
| 0–5 | 7(7.37%) | 13(13.83%) | 5(5.43%) | |
| 6–10 | 49(51.58%) | 26(27.66%) | 30(32.61%) | |
| 11–15 | 33(34.74%) | 38(40.43%) | 37(40.22%) | |
 16 16 |
6(6.32%) | 17(18.09%) | 20(21.74%) | |
| Vaccination dose, N (%) | < 0.001 | |||
| 0 | 10(10.53%) | 36(38.30%) | 19(20.65%) | |
| 1 | 6(6.32%) | 2(2.13%) | 12(13.04%) | |
| 2 | 23(24.21%) | 19(20.21%) | 18(19.57%) | |
| 3 | 56(58.95%) | 37(39.36%) | 43(46.74%) |
The severity of pneumonia analysis
The dice coefficient of U-net model on the test set reaches 0.85 and the pulmonary involvement area were confirmed by two radiologists. As seen in Table 2, the COVID-19 patients with COPD has higher proportion of pulmonary involvement area than those patients with COVID-19 only. The average proportion of pulmonary involvement in COVID-19 with emphysema groups was 3.90% and has the most extensive range of pulmonary inflammation.
Table 2.
The pulmonary involvement degree in the three groups
| COVID-19 | COVID-19 with emphysema | COVID-19 with chronic bronchitis | |
|---|---|---|---|
| Pulmonary involvement | 0.61% | 3.90% | 1.80% |
Correlations between different clinical variables
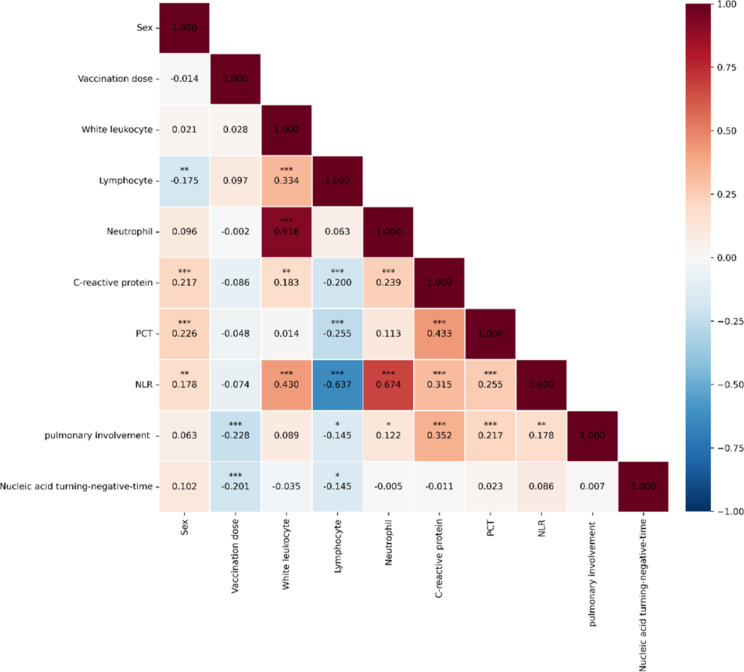
As seen in Fig. 3, in terms of pulmonary involvement indicators, six indicators show significant correlations: vaccination dose (correlation coefficient value (r) = -0.228, P < 0.001), lymphocyte count (r= -0.145, P < 0.05), neutrophil count (r = 0.122, P < 0.05), C-reactive protein level (r = 0.352, P < 0.001), PCT level (r = 0.217, P < 0.001), and NLR (r = 0.178, P < 0.01). Regarding the time to nucleic acid turning negative, two indicators exhibited significant correlations: vaccination dose (r= -0.201, P < 0.001) and lymphocyte count (r = 0.145, P < 0.05). Besides, significant positive correlations were observed between NLR value and white leukocyte count, neutrophil level, C-reactive protein, and PCT (P < 0.001) and significant negative correlations were identified between NLR value and lymphocyte count (P < 0.001).
Fig. 3.
Correlations between different clinical variables. Note: * representing P < 0.05, ** representing P < 0.01, *** representing P < 0.001
Prediction results of nucleic acid turning-negative-time
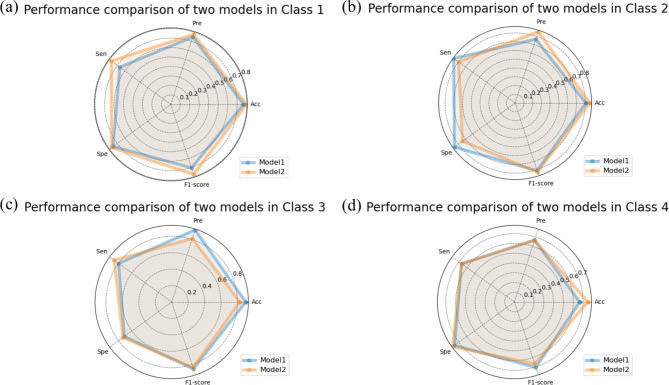
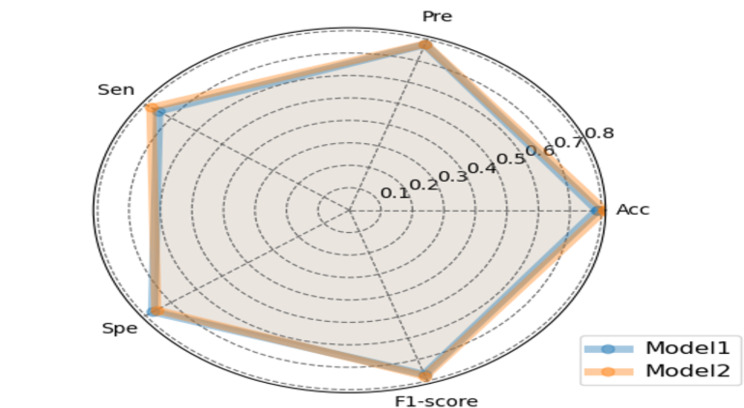
Figure 4 shows the training loss changes of model 1 using deep learning method and Fig. 5 shows the training loss changes of Model 2 using deep learning combined with radiomics features. The model iterated for a total of 120 epochs, with batch size being set to 2, and the learning rate being set to 0.0001, and it can be determined that the both models have effectively converged. The performance of the model 1 on the test set with an overall accuracy of 78.7%, precision of 77.6%, sensitivity of 75.0%, specificity of 77.1% and F1 score of 77.1% (Table 3). The model 2 improved the classification result, achieving an accuracy of 80.9%, precision of 77.9%, sensitivity of 77.8%, specificity of 75.8%, and F1 score of 77.8%. The accuracy of model 1 at classes 1–4 were 77.0%, 80.9%, 90.1%, and 66.7% respectively and the accuracy of model 2 at classes 1–4 were 80.1%, 85.3%, 83.0%, and 75.0% respectively, which illustrated that the accuracy of model 2 for predicting nucleic acid turning-negative-time in classes 1, 2, and 4 were better than model 1. As seen in the radar chart (Figs. 6 and 7), it starts from a central point and extends outward with multiple rays, each representing a specific metrics including accuracy, precision, sensitivity, specificity, and F1-score. The points or line segments on each ray represent the values or scores of this metrics in different models. Specifically, Fig. 6 shows the performance of each classes between model 1 and model 2 respectively and Fig. 7 shows the overall performance of the two models.
Fig. 4.
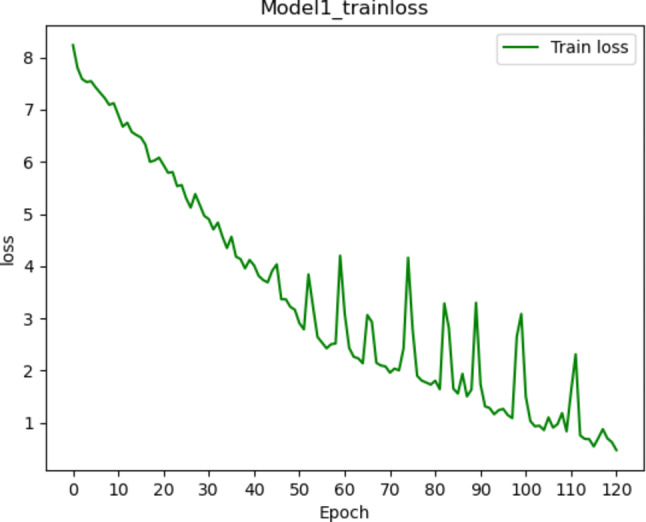
Training loss changes of the model 1 using deep learning method. It shows that the loss value gradually fluctuates, decreases and converges within 120 iterations
Fig. 5.
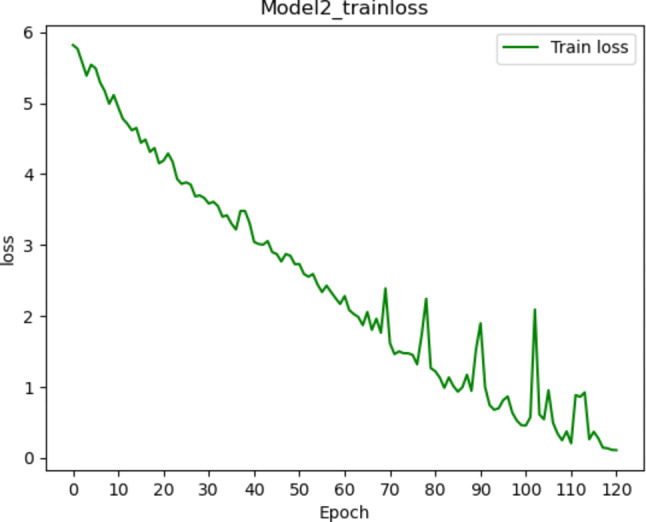
Training loss changes of the model 2 using deep learning combined with radiomics features. It is observed that the loss value gradually fluctuates, decreases and converges within 120 iterations
Table 3.
The classification results by using the two models
| model 1 | Accuracy | Precision | Sensitivity | Specificity | F1-score |
|---|---|---|---|---|---|
| Class 1 (0–5 days) | 77.0% | 75.0% | 66.7% | 75.6% | 70.6% |
| Class 2 (6–10 days) | 80.9% | 76.5% | 86.7% | 85.7% | 81.3% |
| Class 3 (11–15 days) | 90.1% | 92.3% | 80.0% | 71.4% | 85.7% |
Class 4 ( 16 days) 16 days) |
66.7% | 66.7% | 66.7% | 75.6% | 70.6% |
| Overall | 78.7% | 77.6% | 75.0% | 77.1% | 77.1% |
| model 2 | |||||
| Class 1 (0–5 days) | 80.1% | 77.8% | 77.8% | 77.5% | 77.8% |
| Class 2 (6–10 days) | 85.3% | 85.7% | 80.0% | 74.3% | 82.8% |
| Class 3 (11–15 days) | 83.0% | 81.3% | 86.7% | 73.5% | 83.9% |
Class 4 ( 16 days) 16 days) |
75.0% | 66.7% | 66.7% | 78.0% | 66.7% |
| Overall | 80.9% | 77.9% | 77.8% | 75.8% | 77.8% |
Note model 1: The model based on deep learning method; model 2: The model based on deep learning combined with radiomics features
Fig. 6.
Comparisons of each class performance between the model 1 based on deep learning method and the model 2 based on deep learning combined with radiomics features by using the following 5 metrics: Acc (accuracy), Pre (precision), Sen (sensitivity), Spe (specificity), and F1-score
Fig. 7.
Comparisons of overall performance between the model 1 based on deep learning method and the model 2 based on deep learning combined with radiomics features by using the following 5 metrics: Acc (accuracy), Pre (precision), Sen (sensitivity), Spe (specificity), and F1-score
Discussion
In this study, we compared the laboratory variables difference and the severity of pneumonia between COVID-19 patients with emphysema groups and COVID-19 patients with chronic bronchitis. In addition, we constructed two predictive models, i.e. a deep learning model and a fusion model combining deep learning with radiomics to predict the nucleic acid turning-negative-time and explored the influence factors for conversion time from positive to negative nucleic acid test.
Firstly, the study demonstrated that those COVID-19 patients with COPD have higher level of NLR values compared with the COVID-19 patients. The NLR values in COVID-19 with emphysema were highest with a median of 2.90 (Table 1) and the NLR values were positively correlated with the pulmonary involvement (r = 0.178, p < 0.01) (Fig. 3). Such findings were consistent with previous research that the NLR values of severe patients were higher than those that in mild patients [38] and the elevated NLR in COVID-19 patients was associated with poor outcomes [39]. However, the COVID-19 patients with emphysema had the lowest lymphocyte count compared with COVID-19 patients and COVID-19 patients with chronic bronchitis. As a prominent clinical manifestation, the lymphocytes level was shown to be decreased in COVID-19 patients, which related to the body’s immune function and inflammatory status [40]. Especially in severe COVID-19 patients, serum secretion of Interleukin-2 Receptor (IL-2R) were remarkably increased, which was also considered as a sign of lymphopenia [41]. Similarly, COPD patients are always accompanied by immune aging and immune dysfunction, which may lead to a decrease in lymphocyte count. Meanwhile, the aging immune system has a weaker ability to respond to viral infections, which may lead to the loss of lymphocytes as well. On the other hand, the subtypes of emphysema cause structural destruction of lung tissue, including decreased elasticity of lung tissue and increased lung volume. Therefore, when emphysema patients are infected with COVID-19, the lung tissue may release more inflammatory cells and cytokines, such as the highly prionflammatory macrophages abundant in lungs, further leading to the adverse effects on the survival of lymphocytes [42].
Secondly, the present study showed that those COVID-19 patients with COPD had higher pulmonary parenchymal involvement compared with the COVID-19 patients and the COVID-19 with emphysema groups has the most extensive range of pulmonary inflammation, which may be related to the differential expression of angiotensin-converting enzyme 2 (ACE-2) in the three groups [43]. Specifically, we inferred that the COVID-19 with emphysema groups have increased expression of ACE-2 than other groups, which is consistent with the previous research that increased level of ACE-2 expression was strongly associated with viral load and lung injury [44]. In addition, inflammation-associated alveolar damage and vascular injury in COVID-19 patients causes emphysema alterations [45], and the pre-existing emphysema with diffuse lung involvement were more likely to increase the mortality rate of COVID-19 [46]. Thus, more attention should be paid to the COVID-19 with emphysema in the pandemic.
Thirdly, we showed that the nucleic acid turning-negative-time was negatively correlated with vaccination dose (r=-0.201, P < 0.001) in the current study. A previous study demonstrated that the nucleic acid turning-negative-time was related to clinical symptoms of constipation, fever, and expectoration. Specifically, if patients exhibit these symptoms, the nucleic acid turning-negative time is likely to be prolonged [47]. It was shown that the vaccine based on mRNA can stimulate the immune system immediately and produce a remarkable effect through immune cells such as CD8 + T cells [48]. Thus, the patients receiving the vaccine can significantly reduce the incidence of severe symptoms, alleviate the severity of the disease, and lower the risks of hospitalization and death [49–51]. Overall, these research findings corroborate our findings that vaccination can shorten the time to turn negative for nucleic acid, therefore providing evidence for the effectiveness of COVID-19 vaccines in alleviating symptoms.
In our study, we achieved the automatic segmentation of the involvement area to quantify the extent of pulmonary abnormalities in COVID-19 patients and used the radiomics features to accurately and objectively evaluate these abnormal lesions on CT images. We further constructed a fusion model (model 2) to improve the performance for predicting the nucleic acid turning-negative time, which is consistent with the previous research approach of focusing on lesion areas and extracting radiomics features to improve the model’s performance [30, 31]. The result showed that compared with model 1, model 2 has improved accuracy by 2.1%, precision by 0.3%, and sensitivity by 2.8%, and F1 score by 0.7%, and the accuracy of model 2 for predicting nucleic acid turning-negative-time at classes 1, 2, and 4 were better than model 1; these results demonstrated that the radiomics feature could provide complementary information in predicting the nucleic acid turning-negative-time. However, compared to model 1, the improvement of model 2 was not very pronounced and the optimization value of model 2 for each category is also different, indicating that the contribution of radiomics features is limited. Presumably, the following 2 reasons may be utilized to interpret the finding. First, the process of radiomics comprises a series of consecutive steps including image acquisition and pre-processing, segmentation of the desired region of interest, calculation of defined features, feature engineering, and construction of the classification model. However, most of these steps require manual involvement, so the detection results may be subjective. Previous studies also suggested that radiomics methods lack standardized methods including the number of features and feature selection methods [52]. Second, Zorzi et al. extracted radiomics features from the entire lung as a region of interest and developed the models to diagnose the coronavirus disease 2019 (COVID-19), which showed good diagnostic performances in differentiating COVID-19 versus non-COVID-19 pneumonia [53]. However, we only focused the pulmonary involvement area, which may limit the value of radiomics features. Third, the deep learning networks can take whole images as the input, making them independent of region of interest segmentation and allowing for obtaining deeper features as the CNN layers get deeper, which have demonstrated their tremendous potential for image segmentation, reconstruction, recognition, and classification [54].
Importantly, the Protocol for Prevention and Control of COVID-19 (9th edition) points out that one of the goals of the prevention and control of COVID-19 is to control the epidemic situation to the minimum at the lowest cost in the shortest possible time [55]. While nucleic acid detection of SARS CoV-2 virus by rRT-PCR has been considered as the gold standard, it may also produce false negative results and the evaluation of the time to turn negative might be compromised [56]. Our models may provide a tool for negative conversion time of SARS-CoV-2 in COVID-19 prediction, thus reducing the impact of false negative results and promote timely diagnosis. In fact, Ye et al. have demonstrated that the effective prediction of negative conversion time of COVID-19 patients could help optimize the allocation of limited medical resources and prevent the spread of disease during COVID‐19 pandemic [57]. Critically, deep learning model could provide an automatic evaluation method that could reduce the impact of subjective assessments in clinical practice. Specifically, the proposed model achieved the negative conversion time prediction of COVID-19 patients in four time periods of 0–5 days, 5–10 days, 11–15 days, and ≥ 16 days, and stratify the severity of the patients’ condition, which would provide a new perspective on early identification of patients with prolonged viral shedding and facilitate optimal isolation protocols and treatment strategies.
Our study has several limitations that should be acknowledged. First, this is a single-center retrospective study, which may introduce potential biases and limit the generalizability of our findings to other populations or locations. Thus, future multi-center data are needed to confirm results of this study. Second, the amount of data is rather limited and each category is unbalanced, which may restrict the model’s predictive power and/or compromise the performance of the proposed model. Therefore, a data augmentation method may be employed to improve the result and the problem of class imbalance. Third, the U-net model in our study may not be the best and some alternative models, such as V-net and transformer models, could be utilized to improve the segmentation performance, therefore we could use these models for the pneumonia area segmentation in the future research.
Conclusion
In the current study, we revealed that pre-existing emphysema phenotype of COPD severely aggravated the pulmonary involvement in COVID-19 patients. Also, our findings have demonstrated that the lymphocyte number may be the key indicator for predicting severity and nucleic acid turning-negative-time of COVID-19 with COPD. Critically, we have shown that prediction of turning-negative-time could be achieved through deep learning combined with radiomics features, which provides a viable avenue for clinical diagnosis decision-making.
Author contributions
WZ and MS contributed to the conception and design of this research, data analysis, and manuscript writing. YL, LW, JZ, YH and HL were participated in the data acquisition and inspection. XY and XL were participated in the revision and proofreading of the paper. The authors’ order was determined by their contributions. All authors have read and approved the final manuscript.
Funding
This work was supported by the Inner Mongolia Autonomous Region Science and Technology Plan Project (2023YFSH0015).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Hohhot First Hospital (number: IRB2024022).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanhui Liu and Wenxiu Zhang contributed equally to this work.
References
- 1.Guo YR, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Med Res. 2020;7(1):11. 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mudatsir M, et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Research. 2020;9:1107. 10.12688/f1000research.26186.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesan P. GOLD COPD report: 2023 update. The Lancet. Respir Med. 2023;11(1):18. 10.1016/S2213-2600(22)00494-5. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: a systematic review and meta-analysis. Respir Med. 2020;171:106096. 10.1016/j.rmed.2020.106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London England). 2020;395(10224):565–74. 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen MD, et al. Increased SARS-CoV-2 infection, protease, and inflammatory responses in Chronic Obstructive Pulmonary Disease Primary Bronchial epithelial cells defined with single-cell RNA sequencing. Am J Respir Crit Care Med. 2022;206(6):712–29. 10.1164/rccm.202108-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallia P, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–42. 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh D, Mathioudakis AG, Higham A. Chronic obstructive pulmonary disease and COVID-19: interrelationships. Curr Opin Pulm Med. 2022;28(2):76–83. 10.1097/MCP.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, et al. A bibliometric analysis of chronic obstructive pulmonary disease and COVID-19. Med (Baltim). 2023;102(10):e33240. 10.1097/MD.0000000000033240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zu ZY, et al. Coronavirus Disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296(2):E15–25. 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernheim A, et al. Chest CT findings in Coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan F, et al. Time Course of Lung Changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295(3):715–21. 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francone M, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808–17. 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash J, et al. Computed tomography severity score as a predictor of disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. J Med Imaging Radiation Sci. 2023;54(2):364–75. 10.1016/j.jmir.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alqahtani JS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a Rapid systematic review and Meta-analysis. PLoS ONE. 2020;15(5):e0233147. 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941. 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2023. www.goldcopd.org.
- 18.Liu G, et al. LDH, CRP and ALB predict nucleic acid turn negative within 14 days in symptomatic patients with COVID-19. Scot Med J. 2021;66(3):108–14. 10.1177/0036933021994243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu K et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Vaccination for Negative Conversion Time of Nucleic Acid in Nonsevere COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant. International journal of clinical practice. 2023; 2023:9576855. 10.1155/2023/9576855 [DOI] [PMC free article] [PubMed]
- 20.Yang Y, et al. Clinical characteristics of hospitalized mild/moderate COVID-19 patients with a prolonged negative conversion time of SARS-CoV-2 nucleic acid detection. BMC Infect Dis. 2021;21(1):141. 10.1186/s12879-021-05851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Siegel E, Shen D. Deep learning and medical image analysis for COVID-19 diagnosis and prediction. Annu Rev Biomed Eng. 2022;24:179–201. 10.1146/annurev-bioeng-110220-012203. [DOI] [PubMed] [Google Scholar]
- 22.Zhou T, et al. The ensemble deep learning model for novel COVID-19 on CT images. Appl Soft Comput. 2021;98:106885. 10.1016/j.asoc.2020.106885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie H, et al. Helping roles of Artificial Intelligence (AI) in the screening and evaluation of COVID-19 based on the CT images. J Inflamm Res. 2021;14:1165–72. 10.2147/JIR.S301866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farahat IS, et al. An AI-based novel system for predicting respiratory support in COVID-19 patients through CT imaging analysis. Sci Rep. 2024;14(1):851. 10.1038/s41598-023-51053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali AM et al. COVID-19 pneumonia level detection using deep learning algorithm and transfer learning. Evol Intell 2022; Sep 10:1–12. 10.1007/s12065-022-00777-0 [DOI] [PMC free article] [PubMed]
- 26.Chen L et al. Vision Intelligence assisted lung function estimation based on Transformer encoder–Decoder Network with Invertible modeling. IEEE Trans Artif Intell, 5. 10.1109/TAI.2023.3348428
- 27.Dimitsaki S, et al. Benchmarking of Machine Learning classifiers on plasma proteomic for COVID-19 severity prediction through interpretable artificial intelligence. Artif Intell Med. 2023;137:102490. 10.1016/j.artmed.2023.102490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaeijo SM, et al. Screening of COVID-19 based on the extracted radiomics features from chest CT images. J X-Ray Sci Technol. 2021;29(2):229–43. 10.3233/XST-200831. [DOI] [PubMed] [Google Scholar]
- 29.Xiao F, et al. Prediction of potential severe coronavirus disease 2019 patients based on CT radiomics: a retrospective study. Med Phys. 2022;49(9):5886–98. 10.1002/mp.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Z, et al. A novel CT-based radiomics in the distinction of severity of coronavirus disease 2019 (COVID-19) pneumonia. BMC Infect Dis. 2021;21(1):608. 10.1186/s12879-021-06331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, et al. An AI-based radiomics nomogram for disease prognosis in patients with COVID-19 pneumonia using initial CT images and clinical indicators. Int J Med Informatics. 2021;154:104545. 10.1016/j.ijmedinf.2021.104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. Accessed January 30, 2020.
- 33.Büyükşirin M, et al. Does the benefit from pulmonary rehabilitation differ between phenotypes in chronic obstructive pulmonary disease? Eurasian J Pulmonol. 2021;23:32–40. 10.4103/ejop.ejop_26_20. [Google Scholar]
- 34.Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical Image Computing and Computer-Assisted Intervention.Springer, Cham, 2015. 10.1007/978-3-319-24574-4_28
- 35.van Griethuysen JJM, et al. Computational Radiomics System to Decode the Radiographic phenotype. Cancer Res. 2017;77(21):e104–7. 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadoya N, et al. Homology-based radiomic features for prediction of the prognosis of lung cancer based on CT-based radiomics. Med Phys. 2020;47(5):2197–205. 10.1002/mp.14104. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, et al. Early diagnosis of High-Risk Chronic Obstructive Pulmonary Disease based on quantitative high-resolution computed tomography measurements. Int J Chron Obstruct Pulmon Dis. 2023;18:3099–114. 10.2147/COPD.S436803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care (London England). 2020;24(1):647. 10.1186/s13054-020-03374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar S, Khanna P, Singh AK. The impact of Neutrophil-Lymphocyte count ratio in COVID-19: a systematic review and Meta-analysis. J Intensive Care Med. 2022;37(7):857–69. 10.1177/08850666211045626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan L, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Therapy. 2020;5(1):33. 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmoodpoor A, et al. Reduction and exhausted features of T lymphocytes under serological changes, and prognostic factors in COVID-19 progression. Mol Immunol. 2021;138:121–7. 10.1016/j.molimm.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Q, et al. Metabolic modeling of single bronchoalveolar macrophages reveals regulators of hyperinflammation in COVID-19. iScience. 2022;25(11):105319. 10.1016/j.isci.2022.105319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung JM, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688. 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–74. 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celik E, et al. Quantitative determination of pulmonary emphysema in follow-up LD-CTs of patients with COVID-19 infection. PLoS ONE. 2022;17(2):e0263261. 10.1371/journal.pone.0263261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang N, et al. Clinical characteristics and chest CT imaging features of critically ill COVID-19 patients. Eur Radiol. 2020;30(11):6151–60. 10.1007/s00330-020-06955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q et al. Symptoms associated with nucleic acid turning-negative-time in COVID-19 patients? Acupuncture and herbal medicine. 2022; 2(3):207–9. 10.1097/HM9.0000000000000037 [DOI] [PMC free article] [PubMed]
- 48.Liu J, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603(7901):493–6. 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London England). 2021;397(10269):99–111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zang X, et al. The value of early positive nucleic acid test and negative Conversion Time of SARS-CoV-2 RNA in the clinical outcome of COVID-19 patients. Front Med. 2022;9:826900. 10.3389/fmed.2022.826900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu G, et al. Structural and functional radiomics for lung cancer. Eur J Nucl Med Mol Imaging. 2021;48(12):3961–74. 10.1007/s00259-021-05242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zorzi G, et al. Artificial intelligence for differentiating COVID-19 from other viral pneumonias on CT: comparative analysis of different models based on quantitative and radiomic approaches. Eur Radiol Experimental. 2023;7(1):3. 10.1186/s41747-022-00317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avanzo M, et al. Machine and deep learning methods for radiomics. Med Phys. 2020;47(5):e185–202. 10.1002/mp.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Health Commission of the People’s Republic of China. Protocol for prevention and control of COVID-19 (9th Edition). Available online at: http://www.nhc.gov.cn/jkj/s3577/202206/de224e7784fe4007b7189c1f1c9d5e85.shtml [DOI] [PMC free article] [PubMed]
- 56.Li D, et al. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe Acute Respiratory Syndrome Coronavirus 2: role of deep-learning-based CT diagnosis and insights from two cases. Korean J Radiol. 2020;21(4):505–8. 10.3348/kjr.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye J, et al. Predicting the negative conversion time of nonsevere COVID-19 patients using machine learning methods. J Med Virol. 2023;95(4):e28747. 10.1002/jmv.28747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.