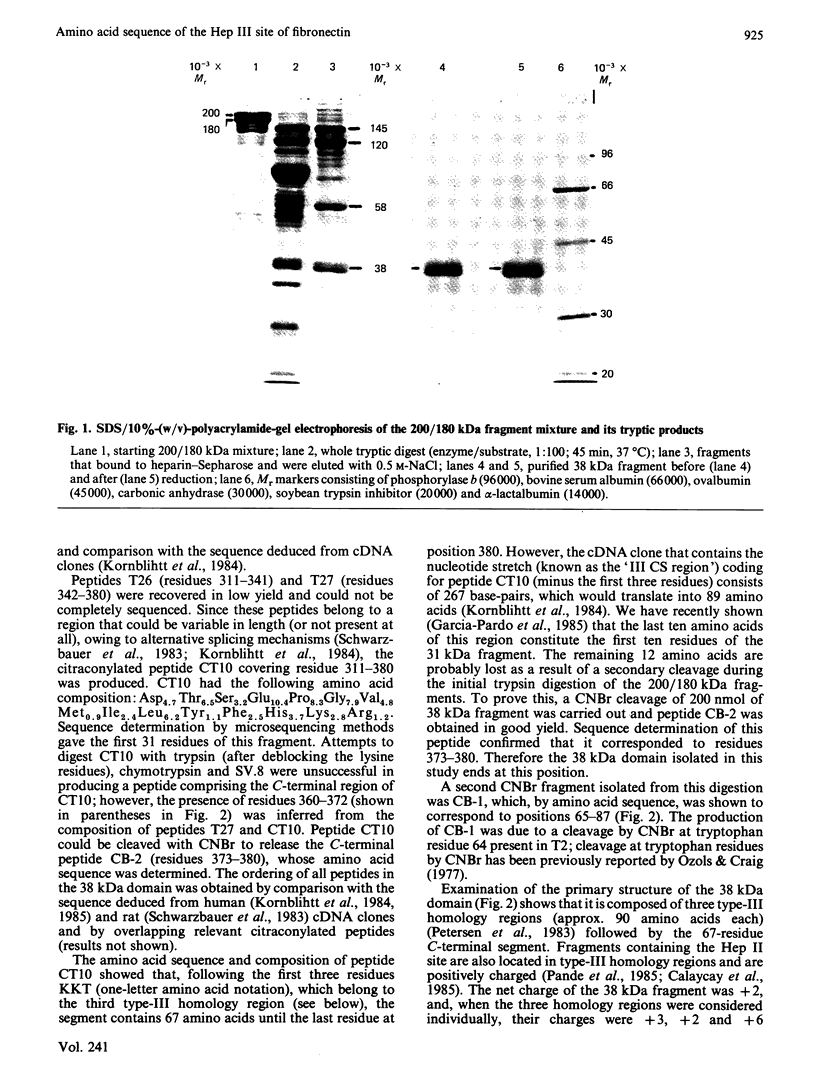
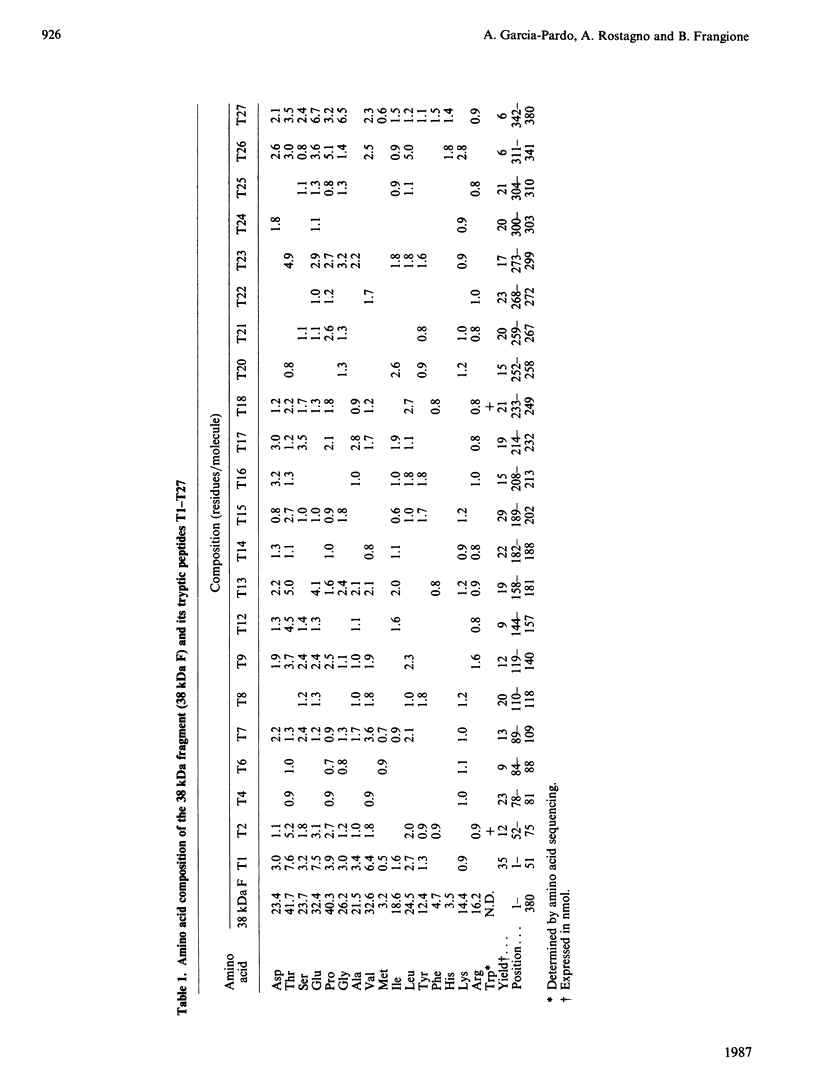
Abstract
The primary structure of a 38 kDa heparin-binding domain from human plasma fibronectin has been determined. This domain contains 380 residues arranged in three type-III homology regions of approx. 90 residues each, and a 67-amino-acid C-terminal segment. This segment has been shown to be encoded by certain mRNA species only, due to alternative splicing [Kornblihtt, Vibe-Pedersen & Baralle (1984) Nucleic Acids Research 12, 5853-5868], and therefore represents a region of heterogeneity in fibronectin. Our data indicate that at least one of the constituent polypeptide chains contains this region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calaycay J., Pande H., Lee T., Borsi L., Siri A., Shively J. E., Zardi L. Primary structure of a DNA- and heparin-binding domain (Domain III) in human plasma fibronectin. J Biol Chem. 1985 Oct 5;260(22):12136–12141. [PubMed] [Google Scholar]
- Click E. M., Balian G. Domain structure of human plasma and cellular fibronectin. Use of a monoclonal antibody and heparin affinity to identify three different subunit chains. Biochemistry. 1985 Nov 5;24(23):6685–6696. doi: 10.1021/bi00344a058. [DOI] [PubMed] [Google Scholar]
- Garcia-Pardo A., Pearlstein E., Frangione B. Primary structure of human plasma fibronectin--characterization of the 6,000 dalton C-terminal fragment containing the interchain disulfide bridges. Biochem Biophys Res Commun. 1984 May 16;120(3):1015–1021. doi: 10.1016/s0006-291x(84)80208-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Pardo A., Pearlstein E., Frangione B. Primary structure of human plasma fibronectin. Characterization of a 31,000-dalton fragment from the COOH-terminal region containing a free sulfhydryl group and a fibrin-binding site. J Biol Chem. 1985 Aug 25;260(18):10320–10325. [PubMed] [Google Scholar]
- Garcia-Pardo A., Pearlstein E., Frangione B. Primary structure of human plasma fibronectin. The 29,000-dalton NH2-terminal domain. J Biol Chem. 1983 Oct 25;258(20):12670–12674. [PubMed] [Google Scholar]
- Gold L. I., Frangione B., Pearlstein E. Biochemical and immunological characterization of three binding sites on human plasma fibronectin with different affinities for heparin. Biochemistry. 1983 Aug 16;22(17):4113–4119. doi: 10.1021/bi00286a019. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Divalent cation modulation of fibronectin binding to heparin and to DNA. J Biol Chem. 1982 May 10;257(9):5263–5267. [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983 Mar 10;258(5):3332–3340. [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: cell specific alternative mRNA splicing generates polypeptide chains differing in the number of internal repeats. Nucleic Acids Res. 1984 Jul 25;12(14):5853–5868. doi: 10.1093/nar/12.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Cleavage of tryptophanyl peptide bonds in cytochrome b5 by cyanogen bromide. J Biol Chem. 1977 Sep 10;252(17):5986–5989. [PubMed] [Google Scholar]
- Pande H., Calaycay J., Hawke D., Ben-Avram C. M., Shively J. E. Primary structure of a glycosylated DNA-binding domain in human plasma fibronectin. J Biol Chem. 1985 Feb 25;260(4):2301–2306. [PubMed] [Google Scholar]
- Pande H., Shively J. E. NH2-terminal sequences of DNA-, heparin-, and gelatin-binding tryptic fragments from human plasma fibronectin. Arch Biochem Biophys. 1982 Jan;213(1):258–265. doi: 10.1016/0003-9861(82)90460-x. [DOI] [PubMed] [Google Scholar]
- Pardo A. G., Rosenwasser E. S., Frangione B. The primary structure of a human immunoglobulin G2 (IgG2) pFc' fragment. J Immunol. 1978 Sep;121(3):1040–1044. [PubMed] [Google Scholar]
- Pearlstein E., Gold L. I., Garcia-Pardo A. Fibronectin: a review of its structure and biological activity. Mol Cell Biochem. 1980 Feb 8;29(2):103–128. doi: 10.1007/BF00220304. [DOI] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Domain structure of human plasma fibronectin. Differences and similarities between human and hamster fibronectins. J Biol Chem. 1983 Mar 25;258(6):3967–3973. [PubMed] [Google Scholar]
- Skorstengaard K., Jensen M. S., Petersen T. E., Magnusson S. Purification and complete primary structures of the heparin-, cell-, and DNA-binding domains of bovine plasma fibronectin. Eur J Biochem. 1986 Jan 2;154(1):15–29. doi: 10.1111/j.1432-1033.1986.tb09353.x. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Furcht L. T. Localization of two unique heparin binding domains of human plasma fibronectin with monoclonal antibodies. J Biol Chem. 1982 Jun 10;257(11):6518–6523. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]