Abstract
We investigated fasting hypertriglyceridemia as predictors of all‐cause, cardiovascular, and non‐cardiovascular mortality in an elderly male Chinese population, while accounting for various conventional cardiovascular risk factors. Our participants were elderly men recruited from residents living in a suburban town of Shanghai (≥60 years of age, n = 1583). Hypertriglyceridemia was defined as a fasting serum triglycerides concentration ≥1.70 mmol/L. Subgroup analyses were performed according to current smoking (yes vs. no), alcohol intake (yes vs. no), and the presence and absence of hypertension and hyperglycemia. During a median of 7.9 years follow‐up, all‐cause, cardiovascular, and non‐cardiovascular deaths occurred in 279, 112, and 167 participants, respectively. After adjustment for confounding factors, fasting hypertriglyceridemia was not significantly (p ≥ .33) associated with the risk of all‐cause, cardiovascular, and non‐cardiovascular mortality. However, there was significant (p = .03) interaction between hypertriglyceridemia and the presence and absence of hypertension in relation to all‐cause mortality. In normotensive, but not hypertensive individuals, hypertriglyceridemia was significantly associated with a higher risk of all‐cause mortality (hazard ratio 1.57, 95% confidence interval 1.06–2.31). In further non‐parametric analyses in normotensive individuals, the age‐standardized rate for all‐cause mortality increased from 18.9 in quartile 1 to 20.0, to 24.7, and to 39.9 per 1000 person‐years in quartiles 2, 3, and 4 of serum triglycerides concentration, respectively (p trend = .0004). Similar results were observed for cardiovascular mortality. Our study in elderly male Chinese showed that fasting hypertriglyceridemia was associated with a higher risk of all‐cause and cardiovascular mortality in patients with normotension but not those with hypertension.
Keywords: fasting hypertriglyceridemia, hypertension, mortality, normotension, serum triglycerides
1. INTRODUCTION
In a recent study in an elderly Chinese population, we found that serum triglycerides concentration tended to be associated with a higher risk of total and cardiovascular mortality in men but not women. 1 In fact, although there is growing interest in the role of hypertriglyceridemia in the development and prevention of cardiovascular disease, 2 , 3 it is still a matter of debate and an unresolved and insufficiently addressed research question. Indeed, the prevalence of fasting hypertriglyceridemia is high, being 15.0% in the general population and 43.7% in patients with diabetes mellitus (DM). 4 , 5 Some drugs are efficacious in reducing serum triglycerides, 6 and some of them also show significant benefit in preventing cardiovascular events. 7 , 8 , 9 It is therefore clinically relevant to investigate the risks of hypertriglyceridemia.
Why there was possible risk in elderly men but not women as observed in our previous study is not entirely understood. Our hypothesis is that there is often an aggregation of cardiovascular risk factors in men but not women, 10 , 11 , 12 , 13 such as cigarette smoking and alcohol intake, and hypertriglyceridemia probably interacts with these risk factors to increase the risk of cardiovascular diseases and mortality. In the present study, we therefore investigated the association between fasting hypertriglyceridemia and the risk of all‐cause, cardiovascular, and non‐cardiovascular mortality in an elderly male Chinese population, with a particular focus on its interaction with conventional cardiovascular risk factors, such as cigarette smoking, alcohol intake, hypertension, and hyperglycemia.
2. METHODS
2.1. Study population
Our study was conducted in the framework of the Chronic Disease Detection and Management in the Elderly (≥60 years of age) Program supported by the municipal government of Shanghai. 14 , 15 , 16 , 17 In a newly urbanized suburban town 30 km from the city center, we invited all residents of at least 60 years of age to participate in comprehensive examinations of cardiovascular disease and risk. The Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, approved the study protocol. All participants provided written informed consent.
Our analysis was restricted to 1785 men, who were enrolled in the period from 2006 to 2011 and followed up for vital status and cause of death till June 2015. We excluded 202 participants because of extreme (≥10.0 mmol/L, n = 10) or missing values of serum triglycerides concentration (n = 192). The number of participants included in the present analysis was therefore 1583.
2.2. Blood biochemistry
Venous blood samples were drawn after overnight fasting for the measurement of serum triglycerides, serum total cholesterol, and plasma glucose. According to the 2023 Chinese guidelines for the management of dyslipidemia in adults, 18 fasting hypertriglyceridemia was defined as a serum triglycerides concentration ≥1.70 mmol/L. DM was defined as a plasma glucose level of at least 7.0 mmol/L fasting or 11.1 mmol/L at any time, or as the use of antidiabetic agents. Hyperglycemia included DM and fasting plasma glucose in the range from 6.1 to 6.9 mmol/L.
2.3. Blood pressure, questionnaire, and anthropometry
One experienced physician measured each participant's blood pressure three times consecutively on the nondominant arm using a validated Omron 7051 oscillometric blood pressure monitor (Omron Healthcare, Kyoto, Japan), after the participants had rested for at least 5 min in the sitting position. These three blood pressure readings were averaged for analysis. Hypertension was defined as a sitting blood pressure of at least 140 mmHg systolic or 90 mmHg diastolic, or as the use of antihypertensive drugs.
The same physician administered a standardized questionnaire to collect information on medical history, smoking habits, alcohol consumption, and the use of medications. A trained technician performed anthropometric measurements, including body height and body weight. Body mass index was calculated as the body weight in kilograms divided by the body height in meters squared.
2.4. Follow‐up
Information on vital status and the cause of death was obtained from the official death certificate, with further confirmation by the local Community Health Center and family members of the deceased people. The International Classification of Diseases Ninth Revision (ICD‐9) was used to classify the cause of death. Cardiovascular mortality included deaths attributable to stroke, myocardial infarction, and other cardiovascular diseases (ICD‐9, 390.0‐459.9). Deaths other than cardiovascular reasons were considered as non‐cardiovascular mortality.
2.5. Statistical methods
Statistical analysis was performed using the SAS software 9.4 (SAS Institute, Cary, North Carolina, USA). All statistical tests were two‐sided at the .05 significance level. Means and proportions were compared by the analysis of variance (ANOVA) and Chi‐square test, respectively. The log‐rank test was used to compare the cumulative incidence of mortality between groups with the Kaplan‐Meier survival function to show the time to death. Cox regression analysis was performed to compute hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the association between serum triglycerides and mortality. Proportional hazards assumption was checked by assessing the Schoenfeld residuals. Subgroup analyses were performed according to the status of current smoking, alcohol intake, and the presence and absence of hypertension and hyperglycemia. Statistical interaction was tested by including an interaction term (the subgroup variable times the dummy variable for hypertriglyceridemia) in a multivariable Cox regression model. The age‐standardized mortality rate was calculated using the direct method. p values for trend were computed by the analysis of Cochran‐Armitage test. The receiver‐operating characteristic (ROC) curve was used to assess the predictive ability of serum triglycerides concentration for mortality.
3. RESULTS
3.1. Characteristics of the study participants
The 1583 male study participants had a mean age (± SD) of 68.2 (± 7.0) years and included 442 (27.9%) patients with fasting hypertriglyceridemia (≥1.70 mmol/L). The study participants significantly differed (p < .05) according to the presence and absence of hypertriglyceridemia in most of the baseline characteristics, except for the proportion of current smoking, systolic blood pressure, and pulse rate (p≥.31, Table 1).
TABLE 1.
Characteristics of the study participants by serum triglycerides.
| Serum triglycerides concentration (mmol/L) | |||
|---|---|---|---|
| Characteristic | < 1.7 (No. = 1141) | ≥ 1.7 (No. = 442) | p |
| Age (years) | 68.5 ±7.2 | 67.4 ±6.5 | .01 |
| Body mass index (kg/m2) | 23.2 ± 3.3 | 24.4 ±3.7 | <.0001 |
| Current smoking, no. (%) | 617 (54.1) | 254 (57.5) | .22 |
| Alcohol intake, no. (%) | 395 (34.6) | 184 (41.6) | .01 |
| Systolic blood pressure (mmHg) | 137.9 ± 19.5 | 139.3 ± 19.8 | .20 |
| Diastolic blood pressure (mmHg) | 81.7 ± 10.9 | 82.5 ± 10.6 | <.0001 |
| Pulse rate (beats/min) | 74.5 ± 11.8 | 75.2 ± 12.2 | .31 |
| Hypertension, no. (%) | 492 (43.1) | 221 (50.0) | .01 |
| Use of antihypertensive drugs, no. (%) | 402 (35.2) | 198 (44.8) | .0004 |
| Diabetes mellitus, no. (%) | 41 (3.6) | 28 (6.3) | .02 |
| Hyperglycemia, no. (%) | 154 (13.5) | 84 (19.0) | .01 |
| Use of antidiabetic drugs, no. (%) | 14 (1.2) | 13 (2.9) | .02 |
| Fasting plasma glucose (mmol/L) | 5.2 ± 1.0 | 5.4 ± 1.2 | .001 |
| Serum total cholesterol (mmol/L) | 5.4 ± 1.5 | 5.8 ± 1.3 | <.0001 |
Values are mean ± standard deviation, or number of participants (% of column total).
Patients with hypertriglyceridemia, compared with those with normal serum triglycerides concentration, were slightly younger (−1.1 years, p = .01), had a greater body mass index (24.4 vs. 23.2 kg/m2, p < .0001), higher diastolic blood pressure (82.5 vs. 81.7 mmHg, p < .0001), fasting plasma glucose (5.4 vs. 5.2 mmol/L, p = .001), and serum total cholesterol (5.8 vs. 5.4 mmol/L, p < .0001), and had a higher proportion of alcohol intake (41.6% vs. 34.6%, p = .01), and a high prevalence of hypertension (50.0% vs. 43.1%, p = .01), DM (6.3% vs. 3.6%, p = .02), and hyperglycemia (19.0% vs. 13.5%, p = .01).
3.2. Association between hypertriglyceridemia and mortality
During a median of 7.9 years follow‐up (interquartile range, 6.8‐8.8 years), the cumulated number of person‐years was 11 277, and all‐cause, cardiovascular and non‐cardiovascular deaths occurred in 279, 112, and 167 participants, respectively. The corresponding incidence rates were 24.7, 9.9 and 14.8 per 1000 person‐years, respectively.
Kaplan‐Meier survival analyses did not show any statistically significant difference in the risk of all‐cause, cardiovascular, and non‐cardiovascular mortality between participants with hypertriglyceridemia and those with normal serum triglycerides concentration (log‐rank test, p≥.29, Figure S1). After adjustment for age at baseline and further adjustment for body mass index, the prevalence of hypertension and DM, current smoking, alcohol intake, serum total cholesterol, and fasting plasma glucose, none of the associations between hypertriglyceridemia and all‐cause, cardiovascular, and non‐cardiovascular mortality were statistically significant (p≥.33, Table 2).
TABLE 2.
Unadjusted and age‐ and fully‐adjusted analyses on the hazard associated with hypertriglyceridemia (≥1.7 mmol/L).
| Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Number of deaths | Rate per 1000 person‐years | Unadjusted | Age‐adjusted | Fully adjusted | p | |
| All‐cause mortality | 279 | 22.1 | 0.93 (0.71–1.21) | 1.11 (0.85–1.45) | 1.13 (0.86–1.48) | .40 |
| Cardiovascular mortality | 112 | 9.9 | 0.79 (0.51–1.22) | 1.01 (0.65–1.56) | 1.00 (0.64–1.56) | .99 |
| Non‐cardiovascular mortality | 167 | 14.8 | 1.03 (0.74–1.43) | 1.17 (0.84–1.64) | 1.19 (0.84–1.68) | .33 |
p value for fully adjusted analyses.
Abbreviation: CI, confidence interval.
Fully adjusted models included as covariates age, body mass index, current smoking, alcohol intake, serum total cholesterol, fasting plasma glucose and the presence of hypertension and diabetes mellitus at baseline.
3.3. Subgroup analyses
Subgroup analyses were performed according to current smoking (yes vs. no), alcohol intake (yes vs. no), and the presence and absence of hypertension and hyperglycemia. There was significant interaction between hypertriglyceridemia and the presence and absence of hypertension in relation to all‐cause mortality (p = .03, Table 3). Similar trends were observed for cardiovascular and non‐cardiovascular mortality (p = .10–.11) and in Kaplan‐Meier survival analyses, although the log‐rank test did not show any statistically significant difference between participants with hypertriglyceridemia and those with normal serum triglycerides concentration in the presence or absence of hypertension (p≥.14, Figure S2). After adjustment for covariates, in the presence of normotension (n = 870), but not hypertension (n = 713), patients with hypertriglyceridemia had a significantly higher risk of all‐cause mortality than participants with normal serum triglycerides concentration (HR 1.57, 95% CI 1.06–2.31, p = .02).
TABLE 3.
Fully adjusted subgroup analyses on the hazard associated with hypertriglyceridemia (≥1.7 mmol/L).
| Number of deaths/participants | Rate per 1000 person‐years | Hazard ratio (95% CI) | p for interaction | |
|---|---|---|---|---|
| All‐cause mortality | ||||
| Current smoking | .31 | |||
| Yes | 132/871 | 20.9 | 1.29 (0.89–1.87) | |
| No | 147/712 | 29.6 | 0.94 (0.63–1.42) | |
| Alcohol intake | .21 | |||
| Yes | 84/579 | 19.8 | 1.34 (0.83–2.16) | |
| No | 195/1004 | 27.7 | 1.03 (0.73–1.44) | |
| Hyperglycemia | .66 | |||
| Yes | 45/238 | 26.1 | 1.25 (0.66–2.38) | |
| No | 234/1345 | 24.5 | 1.10 (0.81–1.48) | |
| Hypertension | .03 | |||
| Yes | 138/713 | 27.5 | 0.85 (0.58–1.26) | |
| No | 141/870 | 22.5 | 1.57 (1.06–2.31) | |
| Cardiovascular mortality | ||||
| Current smoking | .29 | |||
| Yes | 48/871 | 7.6 | 1.32 (0.71–2.46) | |
| No | 64/712 | 12.9 | 0.73 (0.37–1.43) | |
| Alcohol intake | .72 | |||
| Yes | 33/579 | 7.8 | 0.87 (0.37–2.08) | |
| No | 79/1004 | 11.2 | 1.04 (0.62–1.75) | |
| Hyperglycemia | .52 | |||
| Yes | 14/238 | 8.1 | 1.33 (0.40–4.42) | |
| No | 98/1345 | 10.3 | 0.93 (0.57–1.52) | |
| Hypertension | .10 | |||
| Yes | 59/713 | 11.8 | 0.77 (0.42–1.41) | |
| No | 53/870 | 8.5 | 1.49 (0.76–2.90) | |
| Non‐cardiovascular mortality | ||||
| Current smoking | .65 | |||
| Yes | 84/871 | 13.3 | 1.26 (0.79–2.02) | |
| No | 83/712 | 16.7 | 1.07 (0.64–1.78) | |
| Alcohol intake | .07 | |||
| Yes | 51/579 | 27.4 | 1.65 (0.92–2.96) | |
| No | 116/1004 | 16.5 | 0.98 (0.63–1.52) | |
| Hyperglycemia | .95 | |||
| Yes | 31/238 | 18.0 | 1.27 (0.59–2.72) | |
| No | 136/1345 | 14.2 | 1.19 (0.81–1.76) | |
| Hypertension | .11 | |||
| Yes | 79/713 | 12.6 | 0.89 (0.54–1.47) | |
| No | 88/870 | 15.7 | 1.61 (1.00–2.59) |
Abbreviation: CI, confidence interval.
Fully adjusted models included as covariates age, body mass index, serum total cholesterol, fasting plasma glucose, and the presence of hypertension and diabetes mellitus at baseline.
In further non‐parametric analyses, the age‐standardized rate for all‐cause mortality increased from 18.9 per 1000 person‐years in quartile 1 to 20.0, to 24.7, and to 39.9 per 1000 person‐years, respectively, in quartiles 2, 3, and 4 of serum triglycerides concentration in participants with normotension (p trend = .0004, Figure 1). Similar results were observed for cardiovascular mortality. The corresponding age‐standardized rate was 7.0, 8.7, 11.1, and 26.6 per 1000 person‐years, respectively (p trend < .0001).
FIGURE 1.
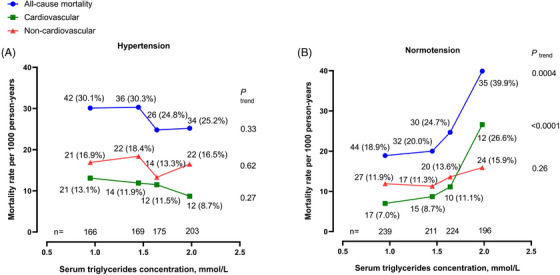
Age‐standardized mortality rate per 1000 person‐years according to the quartile distributions of fasting serum triglycerides concentration in patients with hypertension (A) and those with normotension (B). The number of participants is given for each quartile at the bottom of the figure. The number of deaths (rate per 1000 person‐years) is given for each quartile alongside the symbols.
After exclusion of patients who were taking antihypertensive medication, further analyses showed significant interaction between hypertriglyceridemia and systolic blood pressure in relation to all‐cause mortality (p = .0051, Table S2). In untreated patients with a systolic blood pressure less than 140 mmHg, the age‐standardized rate for all‐cause mortality significantly increased 17.6 per 1000 person‐years in quartile 1 to 22.7, to 22.5, and to 44.8 per 1000 person‐years, respectively, in quartiles 2, 3, and 4 of serum triglycerides concentration (p trend = .0002, Figure 2). The corresponding age‐standardized rate for cardiovascular mortality was 7.4, 8.2, 9.3, and 26.0 per 1000 person‐years, respectively (p trend = .0002).
FIGURE 2.
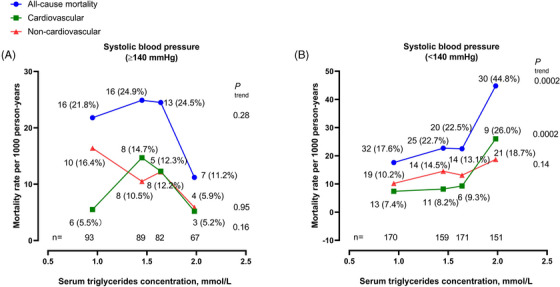
Age‐standardized mortality rate per 1000 person‐years according to the quartile distributions of fasting serum triglycerides concentration in untreated patients with a systolic blood pressure of ≥140 mmHg (A) or < 140 mmHg (B). The number of participants is given for each quartile at the bottom of the figure. The number of deaths (rate per 1000 person‐years) is given for each quartile alongside the symbols.
3.4. Serum triglycerides concentration for the prediction of mortality
The predictive performance of serum triglycerides concentration for mortality was evaluated using receiver operating characteristic (ROC) curves (Figure S3). In all participants, the cut‐off point was 1.49, 1.49, and 1.39, respectively, for all‐cause, cardiovascular, and non‐cardiovascular mortality (Figure S3, panel A). In participants with hypertension, the cut‐off point was 1.57 for all three mortality endpoints (Figure S3, panel B). In participants with normotension, the cut‐off points were 1.04, 1.49, and 1.68, respectively (Figure S3, panel C).
4. DISCUSSION
Our new finding was that hypertriglyceridemia was significantly associated with all‐cause and cardiovascular mortality in participants with normotension, especially those untreated individuals with a systolic blood pressure below 140 mmHg. The finding was to some extent in contradiction to our hypothesis that hypertriglyceridemia might be associated with a higher cardiovascular risk in the presence of other cardiovascular risk factors.
Our observation on the mortality risk associated with hypertriglyceridemia in normotension but not hypertension is not entirely understood. There are several possible explanations. First, the mortality risk associated with hypertriglyceridemia is relatively mild, and hence can only be shown when the background risk is low, such as those participants with normotension. In our present study, the adjusted HR for hypertension versus normotension was 1.57 and 1.49 for all‐cause and cardiovascular mortality, respectively. Second, serum triglycerides concentration is positively associated with plasma and whole blood viscosity. 19 , 20 A speculative and hypothetical explanation might be that the risk associated with hypertriglyceridemia is to some extent via increased viscosity, which may have been enhanced by the low blood pressure, especially during systole. Third, awareness may also be a matter. Patients with diagnosed hypertension may have a higher awareness of hypertriglyceridemia than those with normotension, which may lead to differences in the management of hypertriglyceridemia and hence in the risk associated with the disease. Indeed, in a cross‐sectional study in 4052 participants, the awareness, treatment, and control rates of dyslipidemia (including 43.9% hypertriglyceridemia) were 14.4%, 33.9%, and 19.9%, respectively. The awareness of dyslipidemia was significantly higher in 2335 participants with hypertension (odds ratio 1.75, 95% CI 1.3–2.3) than those with normotension. 21 Finally, hypertension often presents with other comorbid chronic diseases, such as DM. In a 2015 nationwide registry of outpatients with hypertension (n = 1291), the prevalence of type 2 diabetes was 32.9%. 22 These patients might have been treated for comorbidities while receiving treatment for hypertension, which may also help reduce the risk of death.
Whether a serum triglycerides concentration of ≥1.70 mmol/L is appropriate for the diagnosis of hypertriglyceridemia is to some extent questionable, because it is the threshold for the definition of the metabolic syndrome but not conventional hypertriglyceridemia. 23 The latter has been a serum triglycerides concentration of 2.30 and 1.70 mmol/L, respectively, as definite and borderline hypertriglyceridemia.. 18 However, if we scrutinize the figure of the age‐standardized mortality rate of our study, it visually appears that 1.70 mmol/L is almost right at the turning point, from which the mortality rate started to increase for both all‐cause and cardiovascular mortality.
Our study should be interpreted within the context of its limitations. First, our study had a relatively small sample size. The possibility of a chance finding for both the null finding in general and the significant finding in normotensive individuals in particular cannot be entirely excluded. Second, we did not collect sufficient information on non‐fatal cardiovascular events and other risk factors for death. Third, we did not perform measurements of postprandial or non‐fasting serum triglycerides. There is some evidence that it is the non‐fasting but not fasting serum triglycerides concentration, that is, associated with a higher cardiovascular risk. 24 , 25 , 26
In conclusion, our study in elderly male Chinese showed that hypertriglyceridemia was associated with a higher risk of all‐cause and cardiovascular mortality in participants with normotension but not those with hypertension. The finding, though not entirely understood, highlights the necessity in both clinical and fundamental research on the health consequence of hypertriglyceridemia, which is highly prevalent in almost all populations.
AUTHOR CONTRIBUTIONS
Ji‐Guang Wang and Yan Li contributed to the conception and design of the work. Qi‐Fang Huang, Chang‐Sheng Sheng, Wei Zhang, Wen‐Yuan‐Yue Wang, and Xiao‐Fei Ye participated in the data collection. Xin‐Yu Wang performed data analysis and prepared the first draft of the manuscript together with Ji‐Guang Wang. All authors critically revised the manuscript and gave the final approval.
CONFLICT OF INTEREST STATEMENT
Ji‐Guang Wang reports receiving consulting and lecture fees from Novartis, Omron, Servier, and Viatris. Yan Li reports receiving research grants from A&D, Bayer, Omron, Salubris, and Shyndec and lecture fees from A&D, Novartis, Omron, Servier, Salubri,s and Shyndec. The other authors declared no conflicts of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors gratefully acknowledge the voluntary participation of the study participants from Zhaoxiang Community, and the technical assistance of the physicians, nurses, technicians, and master and PhD students from Zhaoxiang Community Health Centre (Qingpu District, Shanghai) and the Shanghai Institute of Hypertension (Huangpu District, Shanghai). The study investigators were financially supported by grants from the National Natural Science Foundation of China (grants 82070432, 82070435, 82270469, and 82370426), and Ministry of Science and Technology (grants 2018YFC1704902 and 2022YFC3601302), Beijing, China, and the Shanghai Commissions of Science and Technology (19DZ2340200) and Health (“Three‐year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System” GWV‐10.1‐XK05 and special grant for “leading academics” 2022LJ022), Shanghai, China.
Wang X‐Y, Ye X‐F, Wang W‐Y‐Y, et al. Fasting hypertriglyceridemia in relation to mortality in an elderly male Chinese population. J Clin Hypertens. 2024;26:1163–1170. 10.1111/jch.14887
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
REFERENCES
- 1. Miao CY, Ye XF, Zhang W, et al. Serum triglycerides concentration in relation to total and cardiovascular mortality in an elderly Chinese population. J Geriatr Cardiol. 2022;19:603‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arca M, Veronesi C, Derasmo L, et al. Association of hypertriglyceridemia with all‐cause mortality and atherosclerotic cardiovascular events in a low‐risk Italian population: the TG‐REAL retrospective cohort analysis. J Am Heart Assoc. 2020;9:e015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626‐635. [DOI] [PubMed] [Google Scholar]
- 4. Song PK, Man QQ, Li H, et al. Trends in lipids level and dyslipidemia among Chinese adults, 2002–2015. Biomed Environ Sci. 2019;32:559‐570. [DOI] [PubMed] [Google Scholar]
- 5. Zhao S, Wang Y, Mu Y, et al. Prevalence of dyslipidaemia in patients treated with lipid‐lowering agents in China: results of the DYSlipidemia International Study (DYSIS). Atherosclerosis. 2014;235:463‐469. [DOI] [PubMed] [Google Scholar]
- 6. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111‐188. [DOI] [PubMed] [Google Scholar]
- 7. Scott R, O'Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ACCORD Study Group ; Ginsberg HN, Elam MB, Lovato LC, et al, ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keech A, Simes RJ, Barter P, et al. Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849‐1861. [DOI] [PubMed] [Google Scholar]
- 10. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Millwood IY, Li L, Smith M, et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio‐demographic and health‐related correlates. Int J Epidemiol. 2013;42:816‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park H, Kim K. Relationship between alcohol consumption and serum lipid levels in elderly Korean men. Arch Gerontol Geriatr. 2012;55:226‐230. [DOI] [PubMed] [Google Scholar]
- 13. Wakabayashi I. Associations of alcohol drinking and cigarette smoking with serum lipid levels in healthy middle‐aged men. Alcohol. 2008;43:274‐280. [DOI] [PubMed] [Google Scholar]
- 14. Sheng CS, Li Y, Huang QF, et al. Pulse waves in the lower extremities as a diagnostic tool of peripheral arterial disease and predictor of mortality in elderly Chinese. Hypertension. 2016;67:527‐534. [DOI] [PubMed] [Google Scholar]
- 15. Sheng CS, Li Y, Li LH, et al. Brachial‐ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 16. Sheng CS, Liu M, Kang YY, et al. Prevalence, awareness, treatment and control of hypertension in elderly Chinese. Hypertens Res. 2013;36:824‐828. [DOI] [PubMed] [Google Scholar]
- 17. Sheng CS, Liu M, Zeng WF, et al. Four‐limb blood pressure as predictors of mortality in elderly Chinese. Hypertension. 2013;61:1155‐1160. [DOI] [PubMed] [Google Scholar]
- 18. Wang ZW, LIU J, JJ LI, et al. Chinese blood lipid management guidelines (2023). Chin Circ J. 2023;38:297. [Google Scholar]
- 19. Rosenson RS, Shott S, Lu L, et al. Hypertriglyceridemia and other factors associated with plasma viscosity. Am J Med. 2001;110:488‐492. [DOI] [PubMed] [Google Scholar]
- 20. Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: triglycerides and blood viscosity. Atherosclerosis. 2002;161:433‐439. [DOI] [PubMed] [Google Scholar]
- 21. Zhang FL, Xing YQ, Wu YH, et al. The prevalence, awareness, treatment, and control of dyslipidemia in northeast China: a population‐based cross‐sectional survey. Lipids Health Dis. 2017;16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song J, Sheng CS, Huang QF, et al. Management of hypertension and diabetes mellitus by cardiovascular and endocrine physicians: a China registry. J Hypertens. 2016;34:1648‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415‐1428. [DOI] [PubMed] [Google Scholar]
- 24. Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309‐316. [DOI] [PubMed] [Google Scholar]
- 25. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299‐308. [DOI] [PubMed] [Google Scholar]
- 26. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.


