Abstract
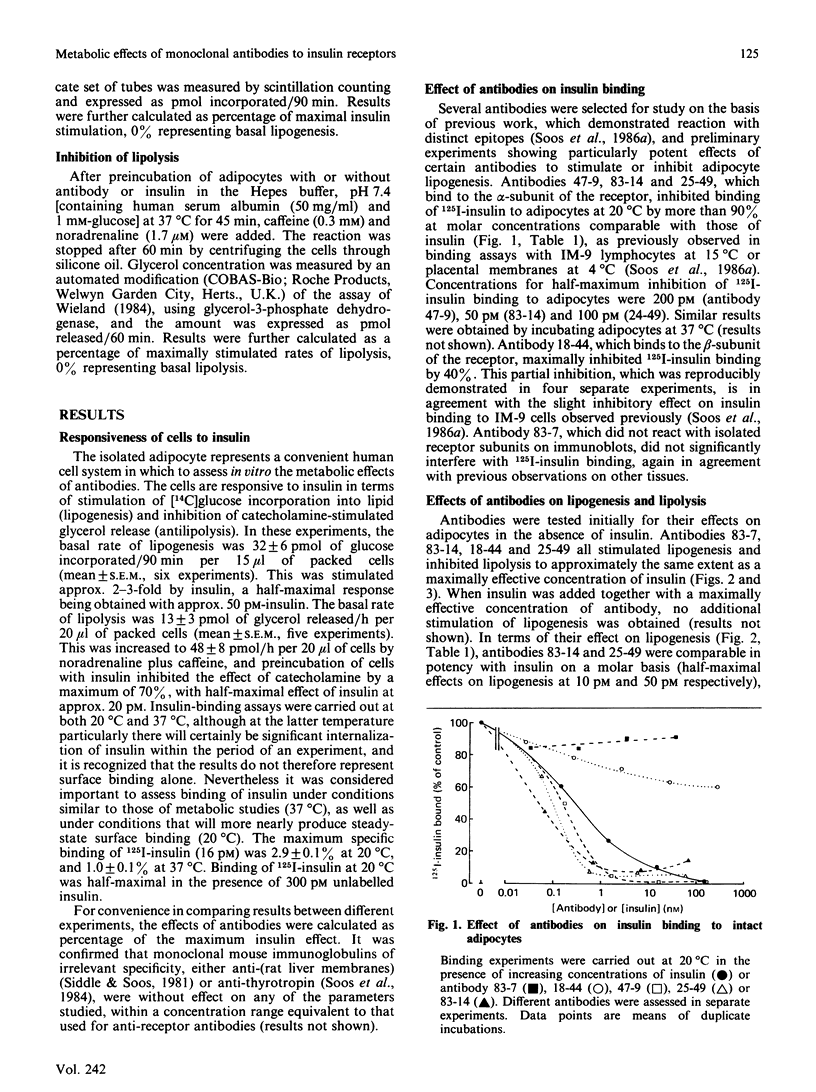
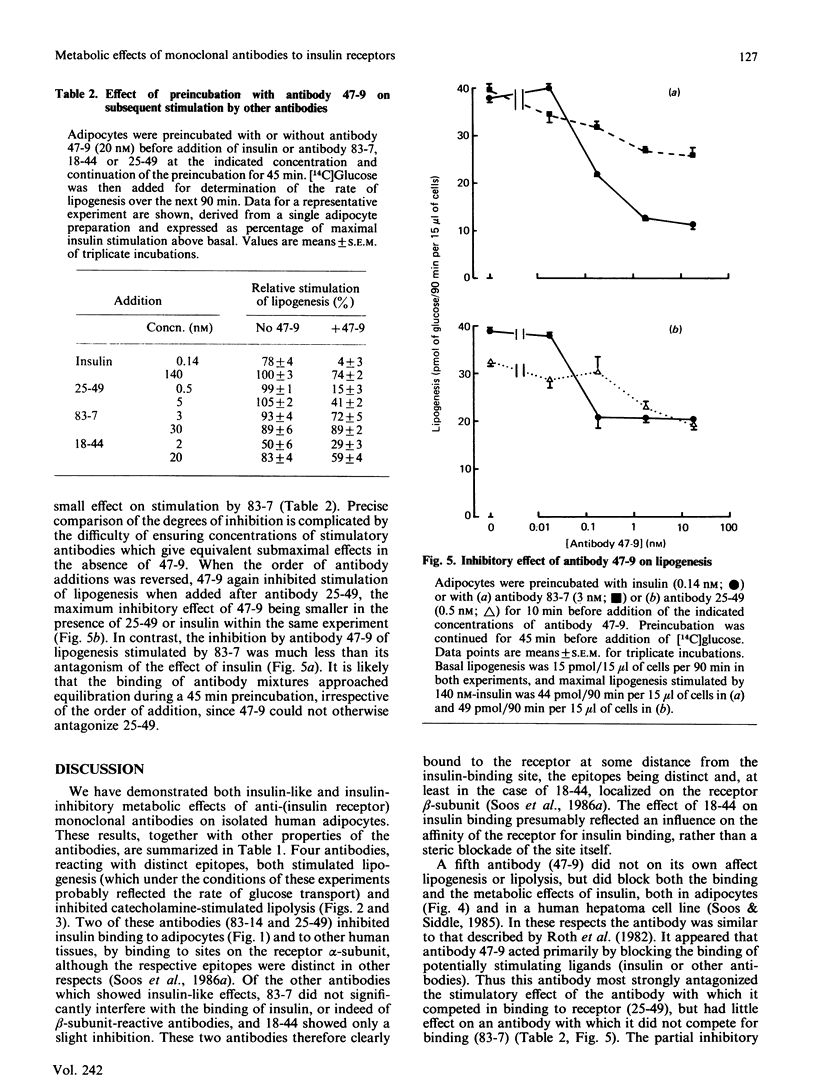
Monoclonal antibodies previously shown to react with five distinct epitopes on the human insulin receptor were tested for their metabolic effects on isolated human adipocytes. Two antibodies which reacted with receptor alpha-subunit and completely inhibited 125I-insulin binding mimicked the actions of insulin to stimulate lipogenesis from [14C]glucose and to inhibit catecholamine-induced lipolysis. On a molar basis, these antibodies were comparable in potency with insulin itself. Two other antibodies which decreased insulin binding only slightly or not at all also mimicked these metabolic effects of insulin. One of these antibodies reacted with receptor beta-subunit. In contrast, a further antibody which reacted with alpha-subunit and inhibited insulin binding did not affect basal lipogenesis or catecholamine-induced lipolysis, but was able to antagonize the effects of insulin on these processes. The same antibody antagonized the insulin-like effect of another antibody with which it competed in binding to insulin receptor, but not the effect of an antibody which bound independently to the receptor. It is concluded that binding of ligand at or close to the insulin-binding site is neither necessary nor sufficient to trigger insulin-like metabolic effects, which may rather depend on some general property of antibodies, such as their ability to cross-link and aggregate receptor molecules.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler L. P., Chandler C. E., Hosang M., Shooter E. M. A monoclonal antibody which inhibits epidermal growth factor binding has opposite effects on the biological action of epidermal growth factor in different cells. J Biol Chem. 1985 Mar 25;260(6):3360–3367. [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Djiane J., Dusanter-Fourt I., Katoh M., Kelly P. A. Biological activities of binding site specific monoclonal antibodies to prolactin receptors of rabbit mammary gland. J Biol Chem. 1985 Sep 25;260(21):11430–11435. [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Jarrett D. B., Roth J. Characterization of antibodies to the insulin receptor: a cause of insulin-resistant diabetes in man. J Clin Invest. 1976 Dec;58(6):1442–1449. doi: 10.1172/JCI108600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammeltoft S. Insulin receptors: binding kinetics and structure-function relationship of insulin. Physiol Rev. 1984 Oct;64(4):1321–1378. doi: 10.1152/physrev.1984.64.4.1321. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Kawamoto T., Cochet C., Le A., Sato J. D., Masui H., McLeod C., Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Jun 25;259(12):7755–7760. [PubMed] [Google Scholar]
- Gliemann J., Sonne O. Binding and receptor-mediated degradation of insulin in adipocytes. J Biol Chem. 1978 Nov 10;253(21):7857–7863. [PubMed] [Google Scholar]
- Gregorou M., Rees A. R. Properties of a monoclonal antibody to epidermal growth factor receptor with implications for the mechanism of action of EGF. EMBO J. 1984 May;3(5):929–937. doi: 10.1002/j.1460-2075.1984.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffetz D., Zick Y. Receptor aggregation is necessary for activation of the soluble insulin receptor kinase. J Biol Chem. 1986 Jan 15;261(2):889–894. [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Flier J. S., Grunfeld C., Harmon J. T., Harrison L. C., Karlsson F. A., Kasuga M., King G. L., Lang U. C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Prog Horm Res. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Beck-Nielsen H., Lindskov H. O., Sonne O., Gliemann J. Insulin receptor binding and receptor-mediated insulin degradation in human adipocytes. Diabetologia. 1981 Jun;20(6):636–641. [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Lindskov H. O. Insulin binding and action on fat cells from young healthy females and males. Am J Physiol. 1982 Aug;243(2):E158–E167. doi: 10.1152/ajpendo.1982.243.2.E158. [DOI] [PubMed] [Google Scholar]
- Podestá E. J., Solano A. R., Attar R., Sánchez M. L., Molina y Vedia L. Receptor aggregation induced by antilutropin receptor antibody and biological response in rat testis Leydig cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3986–3990. doi: 10.1073/pnas.80.13.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert N. D., Willingham M. C., Pastan I. Epidermal growth factor receptor. Characterization of a monoclonal antibody specific for the receptor of A431 cells. J Biol Chem. 1983 Jul 25;258(14):8902–8907. [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Maddux B. A., Goldfine I. D. Regulation of insulin receptor kinase activity by insulin mimickers and an insulin antagonist. Biochem Biophys Res Commun. 1983 Aug 30;115(1):245–252. doi: 10.1016/0006-291x(83)90996-8. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Wong K. Y., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor block insulin binding and inhibit insulin action. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7312–7316. doi: 10.1073/pnas.79.23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Maddux B. A., Cassell D. J., Goldfine I. D. Regulation of the insulin receptor by a monoclonal anti-receptor antibody. Evidence that receptor down regulation can be independent of insulin action. J Biol Chem. 1983 Oct 25;258(20):12094–12097. [PubMed] [Google Scholar]
- Schreiber A. B., Lax I., Yarden Y., Eshhar Z., Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Siddle K., Hales C. N. The effect of bivalent cation chelating agents on some actions of adrenalin and insulin in rat isolated fat cells. Horm Metab Res. 1980 Oct;12(10):509–515. doi: 10.1055/s-2007-999188. [DOI] [PubMed] [Google Scholar]
- Siddle K., Soos M. The production of monoclonal antibodies to rat liver plasma membranes. Biochem Soc Trans. 1981 Feb;9(1):142–143. doi: 10.1042/bst0090142. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos M., Taylor S. J., Gard T., Siddle K. A rapid, sensitive two-site immunometric assay for TSH using monoclonal antibodies: investigation of factors affecting optimisation. J Immunol Methods. 1984 Oct 26;73(2):237–249. doi: 10.1016/0022-1759(84)90398-3. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Valente W. A., Vitti P., Yavin Z., Yavin E., Rotella C. M., Grollman E. F., Toccafondi R. S., Kohn L. D. Monoclonal antibodies to the thyrotropin receptor: stimulating and blocking antibodies derived from the lymphocytes of patients with Graves disease. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6680–6684. doi: 10.1073/pnas.79.21.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente W. A., Yavin Z., Yavin E., Grollman E. F., Schneider M., Rotella C. M., Zonefrati R., Toccafondi R. S., Kohn L. D. Monoclonal antibodies to the thyrotropin receptor: the identification of blocking and stimulating antibodies. J Endocrinol Invest. 1982 Sep-Oct;5(5):293–301. doi: 10.1007/BF03350517. [DOI] [PubMed] [Google Scholar]
- Zick Y., Rees-Jones R. W., Taylor S. I., Gorden P., Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984 Apr 10;259(7):4396–4400. [PubMed] [Google Scholar]
- Zidovetzki R., Yarden Y., Schlessinger J., Jovin T. M. Microaggregation of hormone-occupied epidermal growth factor receptors on plasma membrane preparations. EMBO J. 1986 Feb;5(2):247–250. doi: 10.1002/j.1460-2075.1986.tb04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



