Abstract
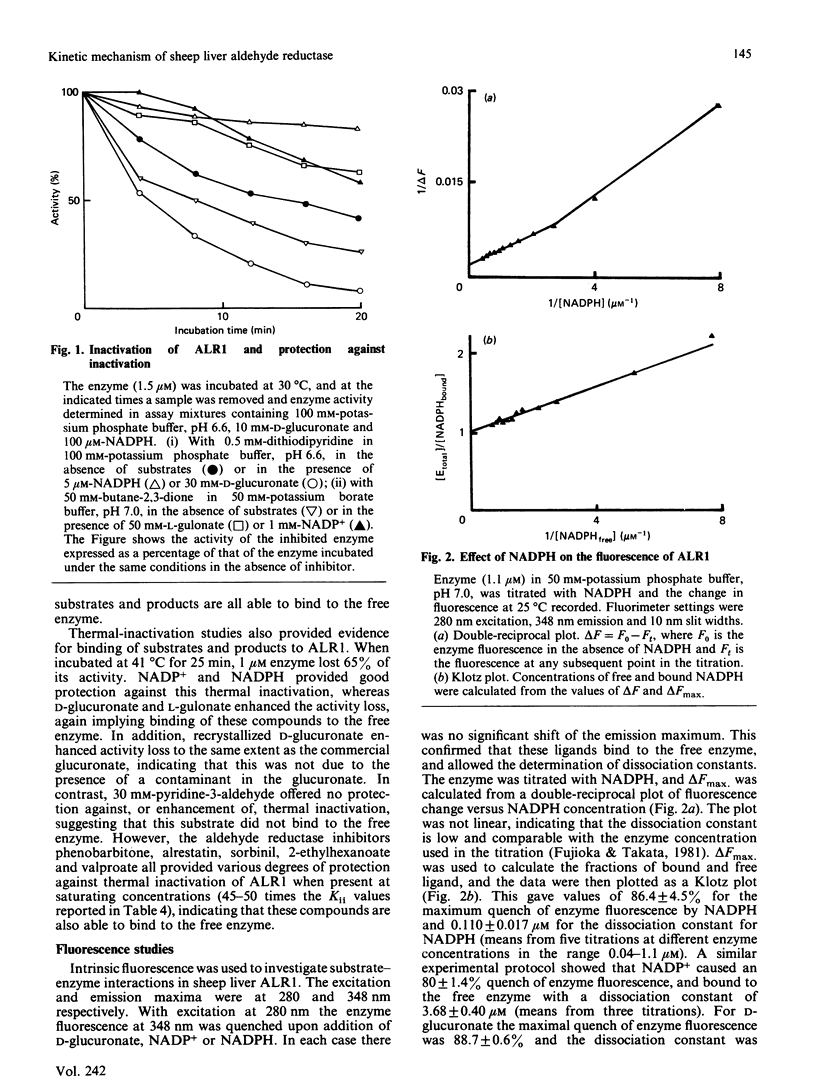
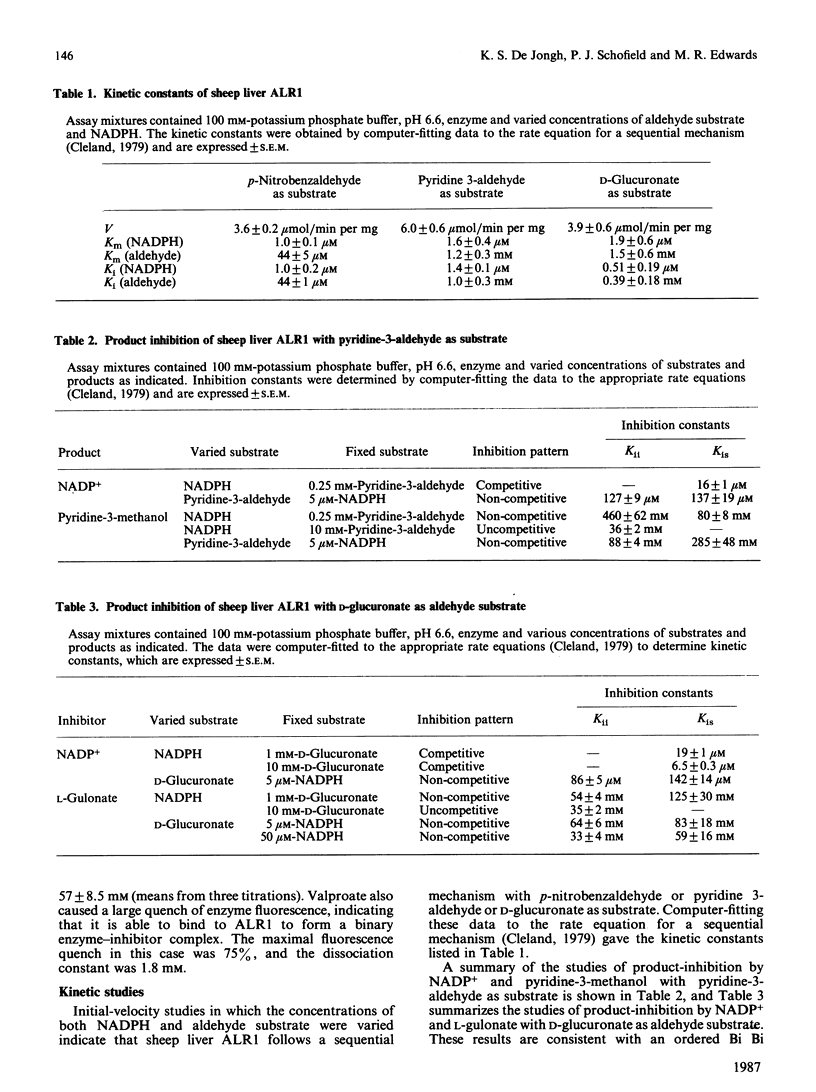
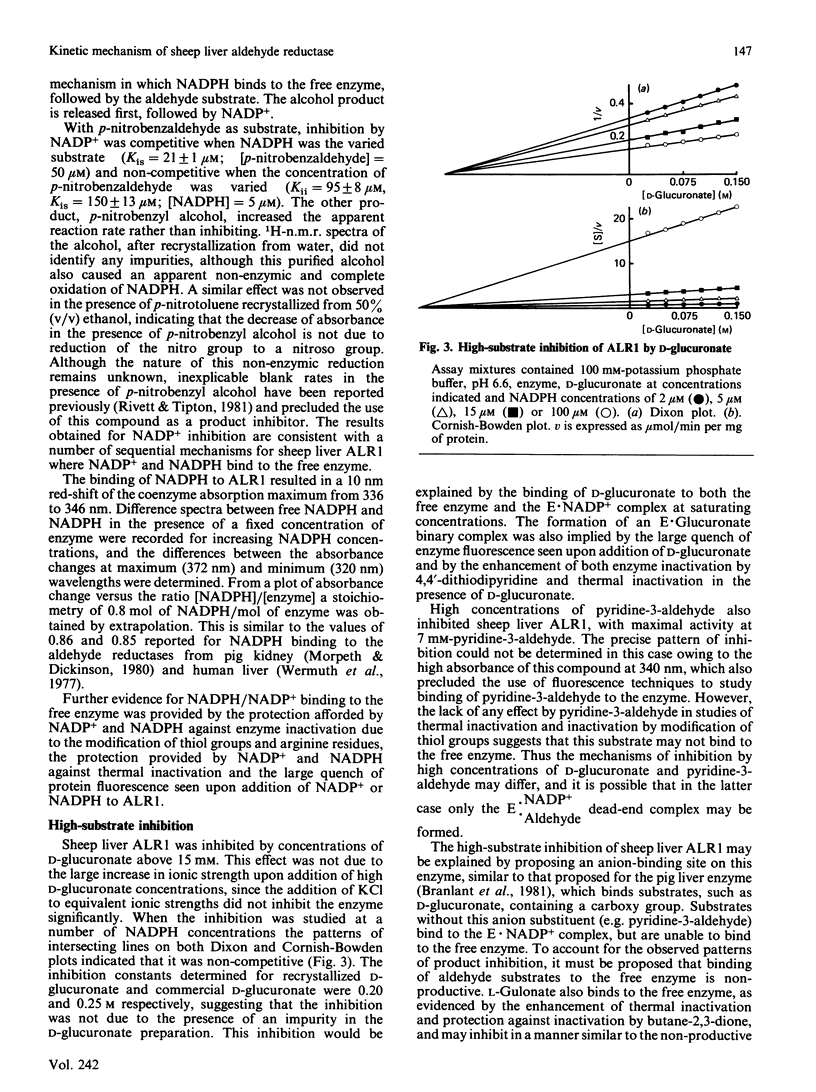
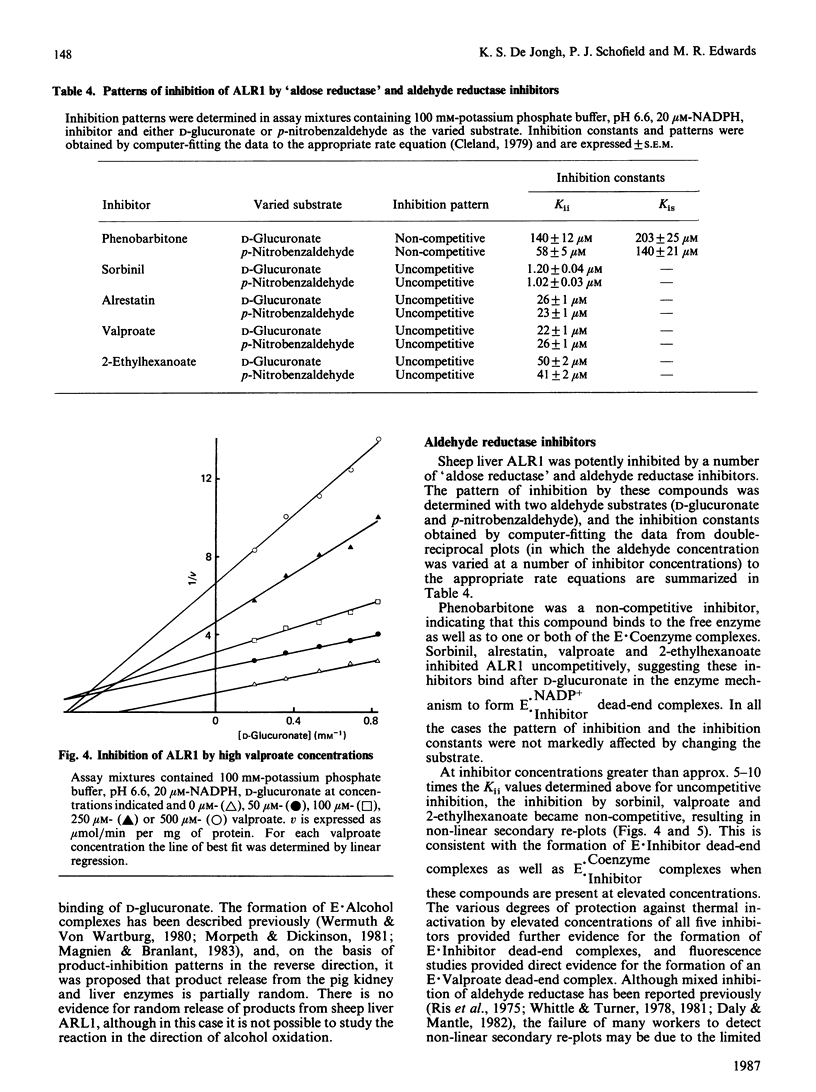
The kinetic mechanism of the major sheep liver aldehyde reductase (ALR1) was studied with three aldehyde substrates: p-nitrobenzaldehyde, pyridine-3-aldehyde and D-glucuronate. In each case the enzyme mechanism was sequential and product-inhibition studies were consistent with an ordered Bi Bi mechanism, with the coenzymes binding to the free enzyme. Binding studies were used to investigate the interactions of substrates, products and inhibitors with the free enzyme. These provided evidence for the binding of D-glucuronate, L-gulonate and valproate, as well as NADP+ and NADPH. The enzyme was inhibited by high concentrations of D-glucuronate in a non-competitive manner, indicating that this substrate was able to bind to the free enzyme and to the E X NADP+ complex at elevated concentrations. Although the enzyme was inhibited by high pyridine-3-aldehyde concentrations, there was no evidence for the binding of this substrate to the free enzyme. Sheep liver ALR1 was inhibited by the ionized forms of alrestatin, sorbinil, valproate, 2-ethylhexanoate and phenobarbitone, indicating the presence of an anion-binding site similar to that described for the pig liver enzyme, which interacts with inhibitors and substrates containing a carboxy group. Sorbinil, valproate and 2-ethylhexanoate inhibited the enzyme uncompetitively at low concentrations and non-competitively at high concentrations, whereas phenobarbitone and alrestatin were non-competitive and uncompetitive inhibitors respectively. The significance of these results with respect to inhibitor and substrate binding is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Branlant G., Tritsch D., Biellmann J. F. Evidence for the presence of anion-recognition sites in pig-liver aldehyde reductase. Modification by phenyl glyoxal and p-carboxyphenyl glyoxal of an arginyl residue located close to the substrate-binding site. Eur J Biochem. 1981 Jun 1;116(3):505–512. doi: 10.1111/j.1432-1033.1981.tb05365.x. [DOI] [PubMed] [Google Scholar]
- Bronaugh R. L., Erwin V. G. Further characterization of a reduced nicotinamide-adenine dinucleotide phosphate-dependent aldehyde reductase from bovine brain. Inhibition by phenothiazine derivatives. Biochem Pharmacol. 1972 May 15;21(10):1457–1464. doi: 10.1016/0006-2952(72)90370-x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Daly A. K., Mantle T. J. The kinetic mechanism of the major form of ox kidney aldehyde reductase with D-glucuronic acid. Biochem J. 1982 Aug 1;205(2):381–388. doi: 10.1042/bj2050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. S., Flynn T. G. A functional arginine residue in NADPH-dependent aldehyde reductase from pig kidney. J Biol Chem. 1979 May 25;254(10):3724–3729. [PubMed] [Google Scholar]
- Flynn T. G. Aldehyde reductases: monomeric NADPH-dependent oxidoreductases with multifunctional potential. Biochem Pharmacol. 1982 Sep 1;31(17):2705–2712. doi: 10.1016/0006-2952(82)90123-x. [DOI] [PubMed] [Google Scholar]
- Fujioka M., Takata Y. S-Adenosylhomocysteine hydrolase from rat liver. Purification and some properties. J Biol Chem. 1981 Feb 25;256(4):1631–1635. [PubMed] [Google Scholar]
- Luisi P. L., Olomucki A., Baici A., Karlovic D. Fluorescence properties of octopine dehydrogenase. Biochemistry. 1973 Oct 9;12(21):4100–4106. doi: 10.1021/bi00745a012. [DOI] [PubMed] [Google Scholar]
- Magnien A., Branlant G. The kinetics and mechanism of pig-liver aldehyde reductase. Comparative studies with pyridine-3-aldehyde and p-carboxybenzaldehyde. Eur J Biochem. 1983 Mar 15;131(2):375–381. doi: 10.1111/j.1432-1033.1983.tb07273.x. [DOI] [PubMed] [Google Scholar]
- Moonsammy G. I., Stewart M. A. Purification and properties of brain aldose reductase and L-hexonate dehydrogenase. J Neurochem. 1967 Dec;14(12):1187–1193. doi: 10.1111/j.1471-4159.1967.tb06166.x. [DOI] [PubMed] [Google Scholar]
- Morpeth F. F., Dickinson F. M. Kinetic studies of the mechanism of pig kidney aldehyde reductase. Biochem J. 1981 Feb 1;193(2):485–492. doi: 10.1042/bj1930485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth F. F., Dickinson F. M. Some properties of pig kidney-cortex aldehyde reductase. Biochem J. 1980 Nov 1;191(2):619–626. doi: 10.1042/bj1910619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris M. M., Deitrich R. A., Von Wartburg J. P. Inhibition of aldehyde reductase isoenzymes in human and rat brain. Biochem Pharmacol. 1975 Oct 15;24(20):1865–1869. doi: 10.1016/0006-2952(75)90405-0. [DOI] [PubMed] [Google Scholar]
- Rivett A. J., Tipton K. F. Kinetic studies of the reduction of succinic semialdehyde by rat-brain aldehyde reductase. Eur J Biochem. 1981 Sep 1;118(3):635–639. doi: 10.1111/j.1432-1033.1981.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Toews C. J. The kinetics and reaction mechanism of the nicotinamide-adenine dinucleotide phosphate-specific glycerol dehydrogenase of rat skeletal muscle. Biochem J. 1967 Dec;105(3):1067–1073. doi: 10.1042/bj1051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth B., Münch J. D., von Wartburg J. P. Purification and properties of NADPH-dependent aldehyde reductase from human liver. J Biol Chem. 1977 Jun 10;252(11):3821–3828. [PubMed] [Google Scholar]
- Wermuth B., von Wartburg J. P. Kinetic studies on NADPH-linked aldehyde reductase from human liver. Adv Exp Med Biol. 1980;132:189–195. doi: 10.1007/978-1-4757-1419-7_20. [DOI] [PubMed] [Google Scholar]
- Whittle S. R., Turner A. J. Anti-convulsants and brain aldehyde metabolism: inhibitory characteristics of ox brain aldehyde reductase. Biochem Pharmacol. 1981 Jun 1;30(11):1191–1196. doi: 10.1016/0006-2952(81)90296-3. [DOI] [PubMed] [Google Scholar]
- Whittle S. R., Turner A. J. Effects of the anticonvulsant sodium valproate on gamma-aminobutyrate and aldehyde metabolism in ox brain. J Neurochem. 1978 Dec;31(6):1453–1459. doi: 10.1111/j.1471-4159.1978.tb06572.x. [DOI] [PubMed] [Google Scholar]


