Abstract
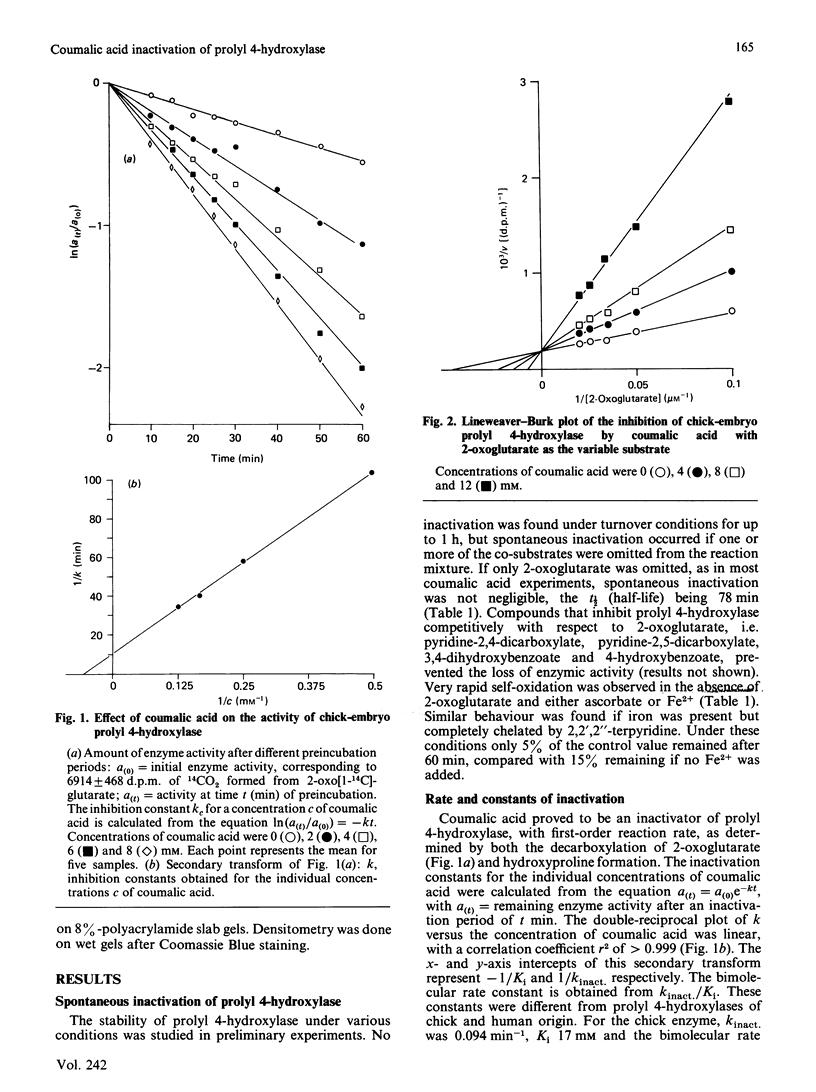
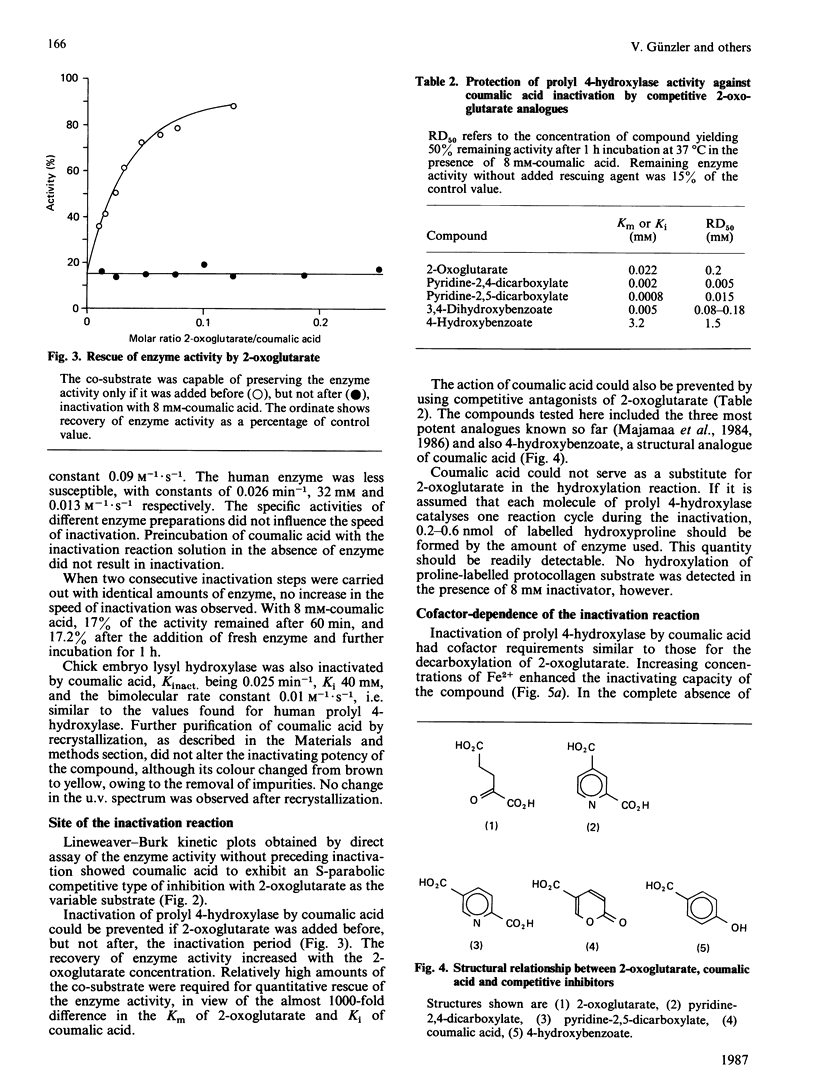
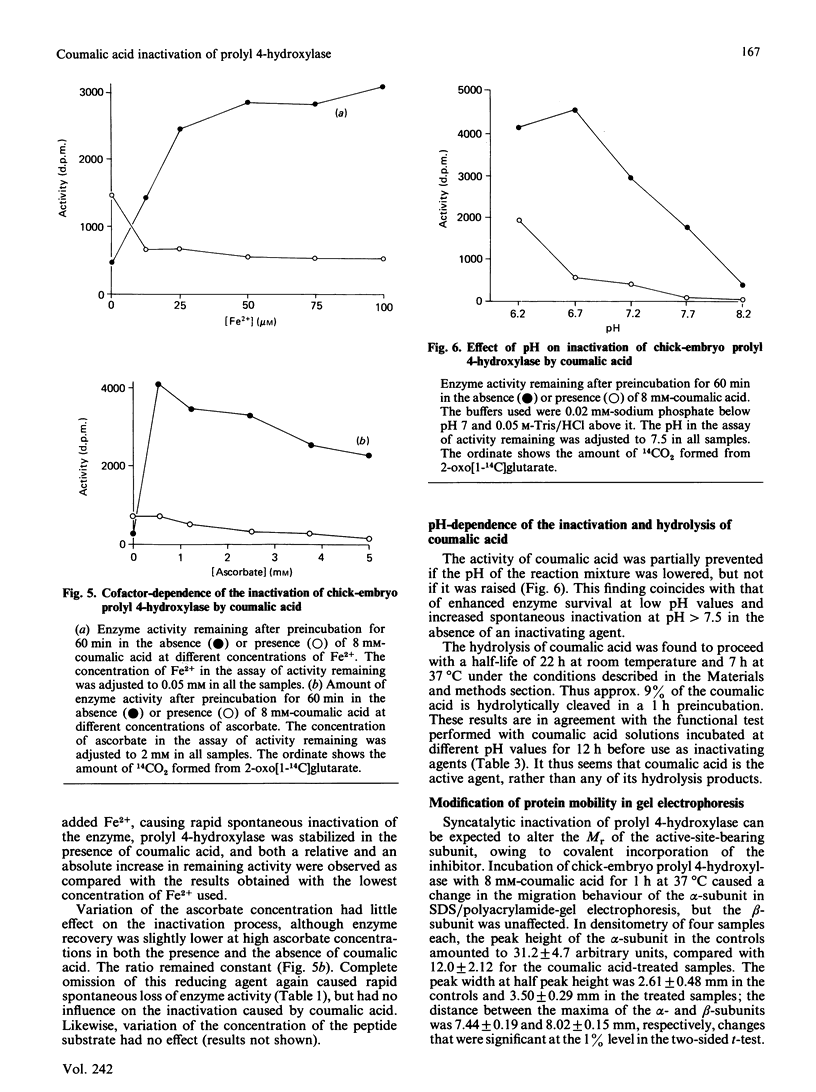
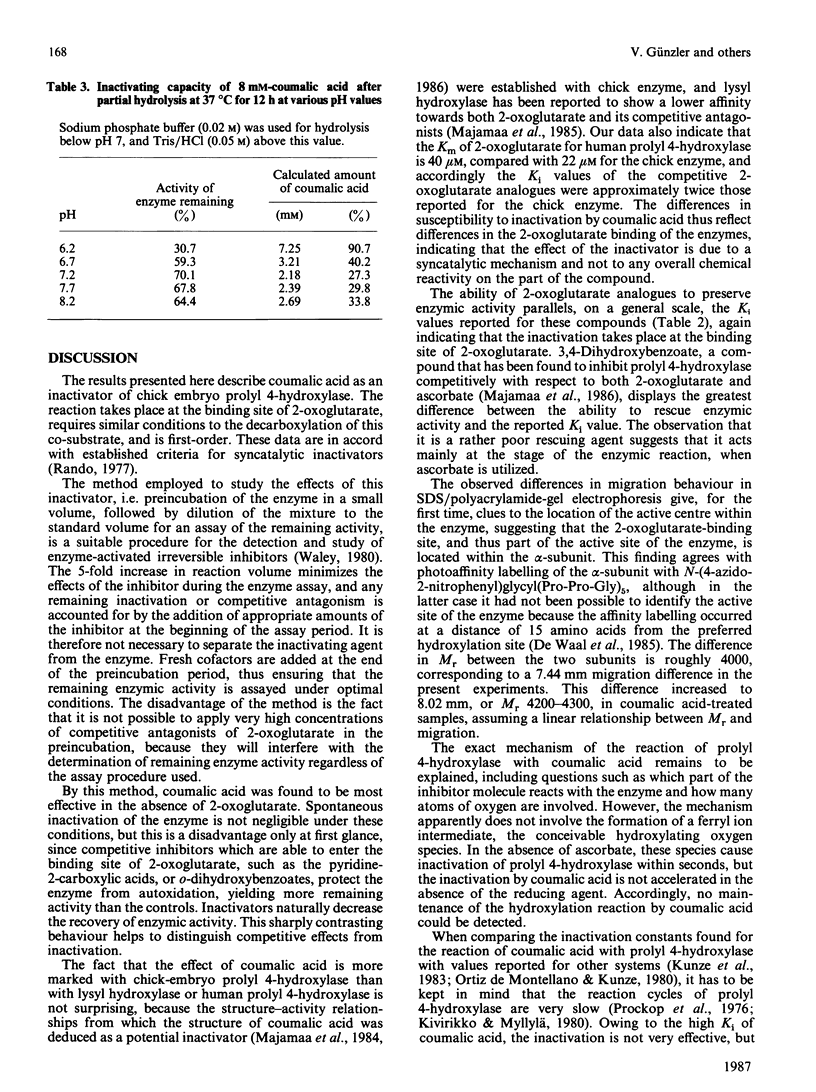
From the structure-activity relationships of known competitive inhibitors, coumalic acid (2-oxo-1,2H-pyran-5-carboxylic acid) was deduced to be a potential syncatalytic inhibitor for chick-embryo prolyl 4-hydroxylase. The compound caused time-dependent inactivation, the reaction rate being first-order. The inactivation constant was 0.094 min-1, the Ki 17 mM and the bimolecular rate constant 0.09 M-1 X S-1. Human prolyl 4-hydroxylase and chick embryo lysyl hydroxylase were also inactivated, though to a lesser extent. Inactivation could be prevented by adding high concentrations of 2-oxoglutarate or its competitive analogues to the reaction mixture. In Lineweaver-Burk kinetics, coumalic acid displayed S-parabolic competitive inhibition with respect to 2-oxoglutarate. The inactivation reaction had cofactor requirements similar to those for the decarboxylation of 2-oxoglutarate. Enzymic activity was partially preserved in the absence of iron, but the rescue was incomplete, owing to decreased stability of the enzyme under this condition. Coumalic acid also decreased the electrophoretic mobility of the alpha-subunit, but the beta-subunit was not affected. Prolonged incubation of coumalic acid above pH 6.8 led to loss of its inactivating potency, owing to hydrolysis. It is concluded that the inactivation of prolyl 4-hydroxylase by coumalic acid is due to a syncatalytic mechanism. The data also suggest that the 2-oxoglutarate-binding site of the enzyme is located within the alpha-subunit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Counts D. F., Cardinale G. J., Udenfriend S. Prolyl hydroxylase half reaction: peptidyl prolyl-independent decarboxylation of alpha-ketoglutarate. Proc Natl Acad Sci U S A. 1978 May;75(5):2145–2149. doi: 10.1073/pnas.75.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong L., Kemp A. Stoicheiometry and kinetics of the prolyl 4-hydroxylase partial reaction. Biochim Biophys Acta. 1984 May 31;787(1):105–111. doi: 10.1016/0167-4838(84)90113-4. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel H. M., Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982 Jan 21;94(2):421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Berg R. A. An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography. Coll Relat Res. 1981 Jul;1(4):345–353. doi: 10.1016/s0174-173x(81)80011-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Kunze K. L., Mangold B. L., Wheeler C., Beilan H. S., Ortiz de Montellano P. R. The cytochrome P-450 active site. Regiospecificity of prosthetic heme alkylation by olefins and acetylenes. J Biol Chem. 1983 Apr 10;258(7):4202–4207. [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Majamaa K., Hanauske-Abel H. M., Günzler V., Kivirikko K. I. The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem. 1984 Jan 16;138(2):239–245. doi: 10.1111/j.1432-1033.1984.tb07907.x. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Turpeenniemi-Hujanen T. M., Latipä P., Günzler V., Hanauske-Abel H. M., Hassinen I. E., Kivirikko K. I. Differences between collagen hydroxylases and 2-oxoglutarate dehydrogenase in their inhibition by structural analogues of 2-oxoglutarate. Biochem J. 1985 Jul 1;229(1):127–133. doi: 10.1042/bj2290127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Majamaa K., Günzler V., Hanauske-Abel H. M., Kivirikko K. I. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. J Biol Chem. 1984 May 10;259(9):5403–5405. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L. Self-catalyzed inactivation of hepatic cytochrome P-450 by ethynyl substrates. J Biol Chem. 1980 Jun 25;255(12):5578–5585. [PubMed] [Google Scholar]
- Rando R. R. Mechanism-based irreversible enzyme inhibitors. Methods Enzymol. 1977;46:28–41. doi: 10.1016/s0076-6879(77)46007-5. [DOI] [PubMed] [Google Scholar]
- Rao N. V., Adams E. Partial reaction of prolyl hydroxylase. (Gly-PRO-Ala)n stimulates alpha-ketoglutarate decarboxylation without prolyl hydroxylation. J Biol Chem. 1978 Sep 25;253(18):6327–6330. [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Myllylä R., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977 Nov 1;80(2):341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T. M., Puistola U., Kivirikko K. I. Isolation of lysyl hydroxylase, an enzyme of collagen synthesis, from chick embryos as a homogeneous protein. Biochem J. 1980 Aug 1;189(2):247–253. doi: 10.1042/bj1890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. Kinetics of suicide substrates. Biochem J. 1980 Mar 1;185(3):771–773. doi: 10.1042/bj1850771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong L., Albracht S. P., Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982 Jun 4;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L., Hartog A. F., Kemp A. Photoaffinity labeling of peptide binding sites of prolyl 4-hydroxylase with N-(4-azido-2-nitrophenyl)glycyl-(Pro-Pro-Gly)5. Biochemistry. 1985 Nov 5;24(23):6493–6499. doi: 10.1021/bi00344a028. [DOI] [PubMed] [Google Scholar]


