Abstract
The ability of alpha interferon (IFN-α) and IFN-γ to inhibit transmission of herpes simplex virus type 1 (HSV-1) from neuronal axon to epidermal cells (ECs), and subsequent spread in these cells was investigated in an in vitro dual-chamber model consisting of human fetal dorsal root ganglia (DRG) innervating autologous skin explants and compared with direct HSV-1 infection of epidermal explants. After axonal transmission from HSV-1-infected DRG neurons, both the number and size of viral cytopathic plaques in ECs was significantly reduced by addition of recombinant IFN-γ and IFN-α to ECs in the outer chamber in a concentration-dependent fashion. Inhibition was maximal when IFNs were added at the same time as the DRG were infected with HSV-1. The mean numbers of plaques were reduced by 52% by IFN-α, 36% by IFN-γ, and by 62% when IFN-α and IFN-γ were combined, and the mean plaque size was reduced by 64, 43, and 72%, respectively. Similar but less-inhibitory effects of both IFNs were observed after direct infection of EC explants, being maximal when IFNs were added simultaneously or 6 h before HSV-1 infection. These results show that both IFN-α and IFN-γ can interfere with HSV-1 infection after axonal transmission and subsequent spread of HSV-1 in ECs by a direct antiviral effect. Therefore, both IFN-α and -γ could contribute to the control of HSV-1 spread and shedding in a similar fashion in recurrent herpetic lesions.
Primary infection with herpes simplex virus (HSV) of epidermal cells (ECs) in the skin or mucosa results in subsequent entry into intraepidermal sensory nerve twigs and then retrograde viral transport to the cell bodies of dorsal root ganglia (DRG) neurons where latent infection is established (20). HSV reactivates intermittently and, after anterograde transport back to the originally infected dermatome, causes clinical recurrences or asymptomatic shedding (6, 10, 35). Viral replication in genital mucosa or skin is restricted by a variety of immune modalities. These immune modalities consist of the early innate mechanisms, such as interferons (IFNs), macrophages, and probably NK cells, especially in primary infection (12), and the later specific effects of skin mucosal T lymphocytes via cytokines or cytotoxic T cells. Both CD4 and CD8 cytotoxic T cells are active in lesions, probably sequentially, and CD4 lymphocytes are the main initial source of cytokines (8, 9,17, 18, 28). Antibody plays only a modulatory role at most (19, 36). HSV infection of ECs induces secretion of alpha IFN (IFN-α) and IFN-β in vitro (30). HSV type 1 (HSV-1) antigen-stimulated CD4 T lymphocytes from patients with recurrent herpes simplex secrete IFN-α and -γ (8). IFNs have been detected at high concentrations in recurrent herpetic lesions (18, 32, 34). The frequency of recurrent herpetic lesions was shown to be proportional to blood and lesional IFN-γ (8, 34).
The exact role for cytokines, especially IFNs in controlling human HSV-1 infection needs further clarification. Cytokines may exert antiviral effects either directly or via immune effects. IFN-α and -β act directly by upregulating antiviral pathways within infected cells, especially 2′,5′-oligoadenylate synthetase and RNase L (3, 11), and also activate macrophages and NK cells (3, 25). IFN-γ and tumor necrosis factor alpha (TNF-α) both have direct antiviral and immunomodulatory effects. IFN-γ usually has weaker antiviral effects than IFN-α or β. However, it also enhances CD8 T-cell cytotoxicity, upregulates major histocompatibility complex (MHC) class II, and reverses downregulation of MHC class I by HSV-1 ICP47 (2, 13, 19, 25).
An in vitro model consisting of human DRG neurons and autologous ECs (DRG-EC model) in two separate chambers was originally developed in our laboratory to study anterograde axonal transport of HSV-1 (27). Our previous findings indicate that glycoproteins and capsids associated with tegument proteins are transported by different pathways and with different kinetics (14, 24, 27), and assembly into virions probably occurs before the glycoproteins and capsids cross the intercellular gap between axonal termini and ECs (19, 27). Glycoprotein and nucleocapsid antigens are detectable by immunohistochemistry and confocal microscopy at 20 h in ECs, and subsequent development of HSV-1 cytopathic plaques can be observed over the next 48 h (19, 27). Here we utilize the DRG-EC model to study the effects of IFNs on transmission of HSV-1 from human axon to epidermis in comparison with direct infection of ECs and to test the hypothesis that both IFN-α and -γ play a role in limiting the spread of infection in the skin after transmission from the axon termini.
MATERIALS AND METHODS
Human fetal tissue.
Human fetal tissue of age 16 to 18 weeks was obtained from therapeutic terminations with informed consent and with approval from the Western Sydney Area Health Service Ethics Committee as previously described (14, 19, 24)
Preparation of the fetal DRG-EC model.
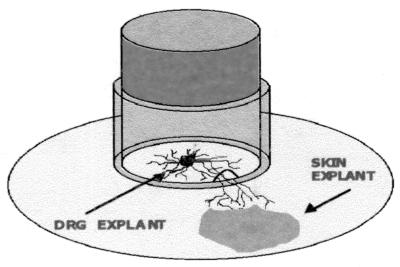
This in vitro model has been described extensively previously (14, 19, 27). It consists of two chambers. The inner chamber is a stainless steel cylinder attached to the plastic coverslip (Thermanox; Nalge Nunc International, Naperville, Ill.) placed in each well of a six-well tissue culture plate (outer chamber) (14, 19, 27). The ring has two grooves filled with 2% agarose on its opposite inferior surfaces. Two fetal skin explants were placed onto the coverslip in each well outside the ring, with each explant opposite two autologous DRGs in the inner chamber (14, 19, 27) (Fig. 1). Growth medium (Dulbecco modified minimal essential medium) supplemented with 9% fetal calf serum (FCS) (CSL, Sydney, Australia) and other cell growth ingredients were used. Axons grew out from the ganglia, penetrated the agarose without causing leaks, and interacted with ECs within 6 to 10 days. The integrity of the seal was tested by sampling for infectious HSV-1 in the outer chamber as previously described (14, 19). Axonal spread leads to the presence of immunoperoxidase-positive viral plaques in the vicinity of the termination of axonal fascicles in the cell sheet (which can be observed by light microscopy), whereas leaking virus infects the proximal edge of the epidermal cell sheet nearest to the cylinder separating inner and outer chambers. Less than 10% of outer chamber samples were scored positive, and they were excluded from further studies.
FIG. 1.
Diagram of the two-chamber DRG-EC model.
Determination of the optimal concentrations of IFN-α and IFN-γ used in direct infection of ECs and in DRG-EC model.
All cytokines were purchased from BD Pharmingen (San Diego, Calif.) and were tested at various concentrations to determine their optimal effect in the experiments involving direct infection of ECs and infection of ECs in the outer chamber of the DRG-EC model. The cytokines IFN-α and IFN-γ were tested separately and in combinations in concentrations ranging from 100 to 1,000 IU/ml for IFN-α and for IFN-γ (per 3 × 105 ECs) in directly infected ECs, as well as in the DRG-EC model. The cytokines (or growth medium) were incubated for 2 h with ECs in the outer chamber of the model at 24 and 12 h before and at 0, 12, 18, 26 and 32 h postinfection (hpi) of the DRG neurons in the inner chamber. These cytokines were tested on directly infected ECs at the same time points. IFNs were added approximately 1/2 to 1 h before HSV-1 at the 0 h time point.
HSV-1 infection and incubation of ECs with cytokines in the DRG-EC model.
The low-passage HSV-1 clinical isolate WM-1 was used to infect the DRG neurons of the model at approximately 0.1 50% tissue culture infective dose (TCID50) per ganglionic neuron (as previously described [19]). The HSV-1 inoculum was aspirated after incubation for 1 h, and the DRGs were washed once carefully with Hanks balanced salt solution (HBSS).
Cytokines or control medium was added to ECs in the outer chamber of the model at the different time points, and cells were examined for viral cytopathic effect 48 h later.
Direct HSV-1 infection of ECs and incubation with cytokines.
A single-cell suspension of ECs (prepared as described previously [12]) was seeded in 12-well plates using growth medium containing 9% fetal calf serum (FCS) (Nalge Nunc International). Twenty-four hours later, the autologous ECs were infected at a 0.1 TCID50/EC for 1 h, washed twice with HBSS, and incubated with cytokines at the same time points as in the DRG-EC model. Concentrations of cytokines were maintained throughout the experiment by replacing half of the growth medium supplemented with IFNs every 48 h. Cells were fixed with electron microscopy (EM)-grade methanol at 48 hpi prior to immunoperoxidase staining for HSV-1 antigen.
Development of HSV-1 cytopathic plaques in the DRG-EC model.
As previously reported, HSV-1 antigen was first detected in ECs at 20 hpi. At the time of fixation at 48 h, cytopathic plaques of marked size heterogeneity in direct relation to axon termini and surrounded by gC antigen-positive ECs were observed. The size heterogeneity partly represents the seeding of axons in the DRG at different times as HSV-1 infection proceeded through the DRG.
Detection of HSV-1 antigen in ECs (in the directly infected ECs and in the DRG-EC model).
After fixation with methanol, infected or mock-infected ECs were stained with anti-gC1 antibody (Goodwin Institute for Cancer Research, Plantation, Fla.) (diluted 1:100) for 45 min, washed with HBSS, and stained with secondary biotinylated sheep anti-mouse antibody (Biosource International, Camarillo, Calif.) (diluted 1:200) for 45 min at room temperature (RT). After the ECs were washed twice with HBSS, streptavidin/horseradish peroxidase conjugate (Biosource International) was used (12).
Detection of HSV-1 antigen in DRGs.
IFN-α or IFN-γ (or control medium) was added to the outer chamber for 12 h before HSV-1 infection of the DRG. HSV-1-infected and mock-infected DRG were snap-frozen for 30 s in liquid nitrogen at 26, 36, 48, and 72 hpi as previously described (19). They were mounted and sectioned on a freezing cryotome (Shandon E-600) at −20°C. Staining was performed with anti-gC1 monoclonal antibody (Goodwin Institute for Cancer Research) and then with biotinylated sheep anti-mouse antibody (Biosource International) (diluted 1:200). After the sections were washed twice, they were treated with streptavidin/horseradish peroxidase conjugate (Biosource International). The proportions of cross sections of DRGs which were gC antigen positive were quantified in frozen sections of the whole mounted DRG.
Statistical evaluation.
We compared the difference in the number and size of the HSV-1 plaques with and without different treatments in ECs. The size of plaques was measured as the greatest diameter of the approximately circular plaque (of bare substrate), using an ocular micrometer as previously described (19). When single infected cells with retracted cytoplasm were observed in the IFN-treated cultures (2 to 4% of plaques/foci), the maximum diameter of the area of exposed substrate was measured (image analysis was not suitable for quantification of both immunoperoxidase-positive cells and bare substrate because of the different color scales and variable intensity of staining). The results were calculated as the mean values of experimental data using 18 different sets of fetal tissue, with each experiment performed in triplicate. Differences between the size of plaques after various treatments were assessed for statistical significance by two-way analysis of variance with repeated measures and expressed as the percent mean reduction in the size or number of HSV-1 plaques.
In the experiments examining the effects of cytokines on HSV-1 spread through the DRG, the proportion of the DRG neurons which were HSV-1 infected (i.e., gC antigen positive) was determined as previously published (19). Briefly, 10 whole perpendicular sections (1 sampled every 30 serial sections of DRG) were examined by light microscopy after immunoperoxidase staining, and the proportion of infected neurons was estimated from each section (19). Two DRGs were examined for each experimental group (treated with control medium or IFN-α) at each time point in three different experiments. The proportions of HSV-1-infected DRG neurons were calculated as the means and standard error (SE) of readings from each section for each experimental group. Differences between the values for the control and IFN-treated DRG were evaluated for significance by the Student t test, adjusted for unequal variances.
RESULTS
Determination of the optimal concentrations of IFN-α and -γ for the inhibition of HSV-1 growth in ECs after direct infection and in the DRG-EC model.
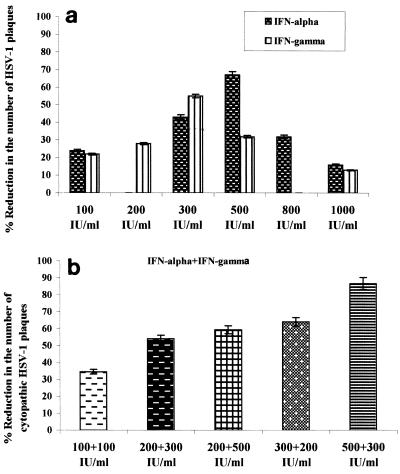
A range of concentrations of IFN-α (100, 300, 500, 800, and 1,000 IU/ml) and IFN-γ (100, 200, 300, 500, and 1,000 IU/ml) was preincubated with ECs for 1 h before the direct infection of ECs with HSV-1 (Fig. 2) or before infection of ECs (from six donors) in the central chamber of the DRG-EC model (data not shown) (each experiment was performed in triplicate). Combinations of IFN-α and IFN-γ at 100:100, 200:300, 200:500, 300:200, and 500:200 IU/ml, respectively, were tested similarly for ECs from six different donors (i.e., Fig. 2a shows a representative experiment from a single donor). Although there was interpatient variation in inhibition of 20 to 30%, the optimal concentrations were consistently determined to be 500 IU/ml for IFN-α, 300 IU/ml for IFN-γ, and 500:200 IU/ml for the combinations (Fig. 2). No differences were observed between direct infection of EC or via the DRG. Concentrations above 800 IU/ml per 3 × 105 cells resulted in cell toxicity.
FIG. 2.
(a) Effects of increasing concentrations of IFN-α or IFN-γ on inhibition of direct infection of ECs with HSV-1 (MOI = 0.1 TCID50/cell). (b) Effects of different combinations of concentrations of IFN-α and -γ on inhibition of HSV-1 infection of ECs (MOI = 0.1 TCID50/cell). IFNs were added 1 h before HSV-1 infection. IFN-α was used at 500 IU/ml and IFN-γ was used at 300 IU/ml whether alone or in combination. Panels a and b show the results of representative experiments with ECs from a single donor, with each experiment done in triplicate. Similar results were obtained for another five experiments with ECs from five different donors.
Effects of cytokines on HSV-1 cytopathic plaques in ECs after direct infection or axonal transmission.
Incubation of ECs with IFN-α or IFN-γ or a combination of both cytokines significantly reduced the size and number of HSV-1 cytopathic plaques in ECs of the DRG-EC model or in EC cultures alone (Fig. 3). In the IFN-treated EC cultures alone, single infected cells with retracted cytoplasm exposing bare substrate were occasionally observed (2 to 4% of plaques/foci). For the DRG-EC model, significant inhibition (P < 0.001) in both the number and size of plaques was observed when both IFNs were added at −6, 0, 12, and 18 hpi to the ECs from six different donors (each experiment performed in triplicate). Maximal inhibition was observed with simultaneous addition of HSV-1 and IFNs (0 hpi).
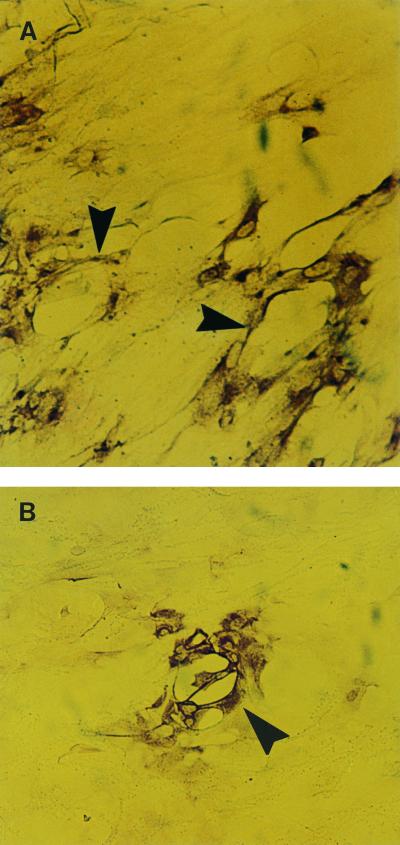
FIG. 3.
Effect of IFN-γ pretreatment on HSV-1 plaques following direct infection of ECs. (A) Two moderate-sized cytopathic plaques (arrowheads) produced by HSV-1 infection in ECs that were not treated with IFN-γ. Magnification, ×194. (B) Small cytopathic plaque (arrowhead) in ECs treated with IFN-α and -γ. Magnification, ×194.
At this time the addition of IFN to the ECs in the model reduced the size of EC plaques by a mean of 64% (range, 52 to 71%) for IFN-α, by 43% (range, 34 to 53%) for IFN-γ, and by 72% (range, 59 to 77%) for both IFNs combined (a representative experiment shown in Fig. 4a). The mean number of plaques after IFN addition was reduced by 58% (range, 52 to 63%) for IFN-α, 45% (range, 36 to 49%) for IFN-γ, and 64% (range, 55 to 71%) for both IFNs combined (a representative experiment shown in Fig. 4b).
FIG. 4.
(a) Effects of IFN-α, IFN-γ, and control cytokines on the size of cytopathic plaques induced by HSV-1 in ECs after axonal transmission in the DRG-EC model (MOI for HSV inoculum = 0.1 TCID50/neuron). Experimental data presented here are from one experiment with ECs from one donor. (b) Effects of IFN-α, IFN-γ, and control cytokines on the number of cytopathic plaques induced by HSV-1 in ECs after axonal transmission in the DRG-EC model (MOI for HSV-1 inoculum = 0.1 TCID50/neuron). Experimental data presented here are from one experiment with ECs from one donor. The results shown here are representative of those observed for five other experiments with ECs from this donor and two other donors. IFN-a+IFN-g, IFN-α plus IFN-γ.
In directly infected ECs, inhibition by IFN-α or IFN-γ alone or combined on both the number and size of plaques were less marked but still significant (P < 0.01) (Fig. 5) at −6, 0, and 12 hpi (and in some cases 18 hpi) for ECs from six different donors (each experiment performed in triplicate). The combination was significantly more inhibitory than either alone at −6, 0, and 12 h. Maximal inhibition was observed when IFNs were added at −6 and 0 hpi (Fig. 5). Maximal inhibition of the number and size of cytopathic plaques (of 30 to 37%) (and in one case 47%) was observed when both IFN-α and IFN-γ were added.
FIG. 5.
(a) Effects of IFN-α, IFN-γ, and control cytokines on the size of cytopathic plaques induced by HSV-1 in ECs after direct infection of the EC monolayers (MOI for HSV-1 inoculum = 0.1 TCID50/EC). Experimental data presented here are from one experiment with ECs from one donor. (b) Effects of IFN-α, IFN-γ, and control cytokines on the number of cytopathic plaques induced by HSV-1 in ECs after direct infection of the EC monolayers (MOI for HSV-1 inoculum = 0.1 TCID50/EC). Experimental data presented here are from one experiment with ECs from one donor. The results shown here are representative of those observed for five other experiments with ECs from this donor and two other donors. IFN-a+IFN-g, IFN-α plus IFN-γ.
Do the cytokines diffuse to the inner chamber and inhibit the viral transport within the ganglion in the DRG-EC cell model?
To test the possibility that cytokines could diffuse into the inner chamber and possibly inhibit the HSV-1 spread through the DRG as a confounding factor, the DRGs in the inner chamber were first infected (or mock infected) for 1 h, and then the supernatant fluid was carefully aspirated and replaced with growth medium. The ECs in the outer chamber were incubated with 500 IU of IFN-α per ml and 300 IU of IFN-γ per ml (or growth medium) for a longer period of 12 h. The DRGs were then fixed at 26, 36, and 48 h, sectioned, and stained for gC antigen by immunoperoxidase.
The proportions of DRGs positively stained for viral antigen were similar at 26, 36, and 48 h in viral controls and in the DRG-EC model treated with IFN-α or IFN-γ. There were no significant differences (P > 0.1 by Student t test) (Table 1).
TABLE 1.
Spread of HSV-1 through human fetal DRG in the presence or absence of IFN added to the outer chambera
| IFN treatment | Proportion (%) of HSV-1-infected DRG atb:
|
||||
|---|---|---|---|---|---|
| 0 hpi | 26 hpi | 36 hpi | 48 hpi | 72 hpi | |
| None (control) | 19 ± 3 | 24 ± 9 | 39 ± 8 | 53 ± 7 | 61 ± 9 |
| IFN-γ | 21 ± 7 | 32 ± 8 | 31 ± 5 | 49 ± 9 | 60 ± 9 |
| IFN-α | 38 ± 6 | 62 ± 8 | |||
Experiments with IFN-α were done only at 36 and 72 hpi.
The proportions of DRG sections containing gC antigen-positive neurons in this representative experiment were calculated as the mean ± SE of readings from each section for each experimental group with cells from three different donors.
DISCUSSION
Both IFN-α/β and IFN-γ have well-described inhibitory effects on HSV-1 replication in vitro, although their relative potencies may differ in different cell types (33). IFN-α acts early in the HSV-1 replication pathway, inhibiting immediate-early gene expression (15) IFN-α/β also appear to limit progress of infection from the periphery to trigeminal ganglion in mice (12). IFN-γ and/or its receptor play a role in the susceptibility of mice to HSV-1 infection and restrict viral replication in the DRG after reactivation (4, 5) and also assist in clearing viral infection from the skin or genital lesions of mice (23, 26, 31). However, the exact effects of IFN-α/β and IFN-γ in recurrent herpes simplex at the neurocutaneous interface after reactivation are unknown and are highly relevant to their roles in recurrent herpes simplex, perhaps in determining the size of lesions and clinical presentation.
We hypothesized that the transport and transmission of HSV from the axon and its terminus to ECs are bottlenecks, with relatively low transmission rates from the terminus per individual axon, which is an ideal site for the inhibitory effects of these IFNs. Furthermore, in vivo delivery from the axon termini is likely to be asynchronous, and the formation of viral lesions within the epidermis or mucosa must involve lateral cell-to-cell spread, which in the early stages may also involve low titers of virus. Therefore, activity of IFNs on individual cells in the epidermis may strongly inhibit viral replication during the initial transmission and subsequent spread. The results of this study appear to support such a hypothesis. IFN-α and -γ at optimal concentrations reduced both the number and size of viral plaques in human fetal EC explant monolayers, whether HSV-1 was delivered exogenously as low titers of cell-free virus or via axon termini in a previously well-characterized neuron-EC two-chamber model. The marked effect of IFN inhibition on HSV plaque size was shown by the occasional but characteristic finding of single HSV antigen-positive cells, probably demonstrating delayed cytopathicity. Inhibition appeared greater in the neuron-EC system, although standardization of multiplicity of infection (MOI) between the two systems is difficult and there is variation in the effect of IFN on cells from different donors. Both IFN-α and -γ had significant inhibitory effects, and the inhibitory effect of IFN-α was consistently greater than those for IFN-α and -γ. There was significant synergy between IFN-α and -γ, in that the inhibitory effect of the combination exceeded those of optimal concentrations of either IFN alone, suggesting activity on ECs through receptors for both IFN-α and -γ. In other cell types, the activity of these two IFNs is mediated through different pathways and is synergistic (1, 3, 15). The maximal inhibitory effect on the size or number of viral plaques was usually on the order of 60 to 70% at optimal IFN concentrations compared with consistently greater than 90% inhibition by high (pharmacological) doses of neutralizing monoclonal antibody to herpes simplex glycoprotein D in a previous study (19).
One potential confounding effect of the addition of IFN to the external chamber of the two-chamber model is the diffusion of the IFNs into the central chamber and an effect on spread of the virus through the DRG prior to virus being transported distally (anterogradely) via axons to the epidermal explants. This would require diffusion of the IFNs through the agarose or plug in the grooves on the inferior surface of the stainless steel chamber. Alternatively, IFNs may bind to receptors on the axons in the external chamber to induce a similar antiviral state in neurons. These potential confounding effects were examined as previously for the antibody inhibition studies (19). Spread through the DRG was examined by sampling individual replicate ganglia at times previously determined to be appropriate, namely, 24, 48, and 72 h (19). All parts of the ganglia were sampled by sectioning and counting every 30th section to achieve a random distribution throughout the DRG; this procedure was done twice. These studies showed no significant difference in the proportion of neurons staining positive for HSV antigen between controls and maximal IFN treatment. Thus, there was no evidence for penetration of concentrations of IFN sufficient to induce an antiviral state in DRG neurons, particularly as pretreatment for 12 h prior to infection had no effect on such spread. Future studies will examine interneuronal spread of HSV within the DRG after direct application of IFNs within the central chamber.
We have previously shown that IFN-γ plays an important immunologic role in recurrent herpes simplex lesions by reversing the downregulation of MHC class I molecules on the surfaces of infected ECs induced by the HSV-1 immediate-early protein ICP47. IFN-γ also upregulates MHC class II molecules, thus allowing infected epidermal keratinocytes to be targets for both CD8 and CD4 cytotoxic T lymphocytes (2, 22, 25, 29). This study shows that IFN-γ also has a significant direct antiviral effect, particularly in conjunction with IFN-α. The reduction in both the number and size of plaques suggests that the production of local IFN-α and -γ in skin, initially secreted by ECs and resident memory T cells, respectively, may restrict transmission of virus from the axon terminus and subsequent lateral spread. Later sources of IFN-α include both ECs and infiltrating macrophages and CD4 cells (9, 16, 18). The latter also secrete IFN-γ. High titers of IFN-α and -γ have been detected within the vesicles of recurrent herpetic lesions (32, 34), and HSV-1-infected ECs have been shown to secrete high levels of IFN-α (30). The restriction in the number and size of viral plaques may be translated into a restriction on the size of macroscopic lesions of the epidermis. Variability in such activity from patient to patient or from time to time within an individual patient might determine whether a lesion is macroscopically visible and causes symptoms, i.e., whether there is clinical recurrent herpes simplex or asymptomatic shedding. In our studies, the relatively low variability in inhibitory effects of both IFNs in EC donors suggests that variability in IFN induction is more likely to be important. Such a hypothesis is supported by earlier studies showing a direct correlation between blood and lesional IFN-γ and frequency of lesions of recurrent herpes labialis (7, 34). Furthermore, protection against mucosal and DRG infection by vaccines has been correlated with IFN-γ secretion in guinea pig models (23).
Other cytokines such as TNF-α also have direct antiviral effects and should be studied in this system in the future. However, in our recent studies TNF-α was found only at low concentrations in vesical fluid compared with other bullous immunopathologic conditions of skin such as pemphigoid (22). Therefore, the focus of this study was on IFN-α and -γ.
It is clear that both CD4 and CD8 lymphocytes may play a role in protection against recurrent herpes simplex infection, with CD4 lymphocytes predominating in the early stages of the lesions (9, 17, 18). Both types of lymphocytes have been shown to exert cytotoxic activity (20, 21, 28). However, this study demonstrates another potential effector activity by CD4 (or CD8) lymphocytes, a direct antiviral effect of IFN-γ which acts in synergy with IFN-α. As IFN-β is produced by infiltrating macrophages and HSV-1-infected ECs and also acts synergistically with IFN-γ in other settings, the same is likely to occur with this cytokine. In this study, synergy of IFN-γ with late induction of endogenous IFN-α or -β may have made a minor contribution to the reduction in HSV-1 plaque size but not to a reduction in the number of plaques. IFN-γ has been regarded as a relatively weak antiviral cytokine compared with IFN-α and -β, but recent studies with HSV-1 in mice (4, 5, 23, 31) and here in human tissue in vitro show it to be more potent. The synergy between IFN-α and -γ also enhances the antiviral effects of either IFN alone (1, 3, 25).
In summary, the concentrations of these effectors after neurocutaneous HSV-1 transmission may be important determinants of individual susceptibility or resistance to the occurrence or frequency of clinical recurrent herpes simplex. They may also play a role as indicators of response to HSV vaccines.
ACKNOWLEDGMENTS
This work was supported in part by the National Health and Medical Research Council of Australia (grant 970738 to A. L. Cunningham).
We thank Bill Sinai and Jane Milliken (Histopathology Unit at Westmead Hospital) for their help with sectioning and immunoperoxidase staining of DRGs and Karen Byth for statistical advice.
REFERENCES
- 1.Balish M J, Abrams M E, Pumfery A M, Brandt C R. Enhanced inhibition of herpes simplex virus type 1 growth in human corneal fibroblasts by combinations of interferon-alpha and gamma. J Infect Dis. 1992;166:1401–1403. doi: 10.1093/infdis/166.6.1401. [DOI] [PubMed] [Google Scholar]
- 2.Basham T Y, Nickoloff B J, Merigan T C, Morhenn V B. Recombinant gamma interferon differentially regulates class II antigen expression and biosynthesis on cultured normal human keratinocytes. J Interferon Res. 1985;5:23–32. doi: 10.1089/jir.1985.5.23. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;4:419–424. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 4.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantin E, Tanamachi B, Openshaw H, Mann J, Clarke K. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J Virol. 1999;73:5196–5200. doi: 10.1128/jvi.73.6.5196-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corey L, Adams H G, Brown Z A, Holmes K K. Genital herpes simplex virus infection: clinical manifestations, course and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham A L, Merigan T C. γ interferon production appears to predict time of recurrence of herpes labialis. J Immunol. 1983;130:2397–2400. [PubMed] [Google Scholar]
- 8.Cunningham A L, Merigan T C. Leu3+ T-cells produce γ interferon in patients with recurrent herpes labialis. J Immunol. 1984;132:197–202. [PubMed] [Google Scholar]
- 9.Cunningham A L, Turner R R, Miller A C, Para M F, Merigan T C. Evolution of recurrent herpes simplex virus lesions. J Clin Invest. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas R G, Couch R B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289–295. [PubMed] [Google Scholar]
- 11.Guidotti I G, Chisari F V. Cytokine-mediated control of viral infections. Virology. 2000;273:221–227. doi: 10.1006/viro.2000.0442. [DOI] [PubMed] [Google Scholar]
- 12.Halford W P, Veress L A, Gebhardt B M, Carr D J. Innate and acquired immunity to herpes simplex virus type 1. Virology. 1997;236:328–337. doi: 10.1006/viro.1997.8738. [DOI] [PubMed] [Google Scholar]
- 13.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 14.Holland D J, Miranda-Saksena M, Boadle R A, Armati P, Cunningham A L. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J Virol. 1999;73:8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotzbucher A, Mittnacht S, Kirchner H, Jacobsen H. Different effects of IFN gamma and IFN alpha/beta on “immediate early” gene expression of HSV-1. Virology. 1990;179:487–491. doi: 10.1016/0042-6822(90)90322-i. [DOI] [PubMed] [Google Scholar]
- 16.Kodukula P, Liu T, Rooijen N V, Jager M J, Hendricks R L. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 17.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koelle D M, Corey L, Burke R L, Eisenberg R J, Cohen G H, Pichyangkura R, Triezenberg S J. Antigenic specificities of human CD4 T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikloska Z, Sanna P P, Cunningham A L. Neutralizing antibodies inhibit the axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J Virol. 1999;73:5934–5944. doi: 10.1128/jvi.73.7.5934-5944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikloska Z, Kesson A M, Penfold M E T, Cunningham A L. Herpes simplex protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 21.Mikloska Z, Cunningham A L. Glycoproteins gB, gC, gD are targets for CD4 cytotoxic T lymphocytes in IFN-gamma pretreated human epidermal keratinocytes. J Gen Virol. 1998;79:353–361. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 22.Mikloska Z, Danis V A, Adams S, Lloyd A R, Adrian D L, Cunningham A L. In vivo production of cytokines and (C-C) chemokines in human recurrent herpes simplex lesions. Do virus infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 23.Milligan G N, Bernstein D I. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 24.Miranda-Saksena M, Armati P, Boadle R A, Holland D J, Cunningham A L. Anterograde transport of herpes simplex virus type 1 in cultured dissociated human and rat dorsal root ganglion neurons. J Virol. 2000;74:1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Shea J J, Visconti R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat Immunol. 2000;1:17–19. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]
- 26.Parr M B, Parr E L. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 27.Penfold M E T, Armati P, Cunningham A L. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posavad C M, Koelle D M, Corey L. High frequency of CD8 cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol. 1996;170:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid D S, Rouse B T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- 30.Schnipper L E, Leven M, Crumpacker C S, Gilchrest B A. Virus replication and induction of interferon in human epidermal keratinocytes following infection with herpes simplex virus. J Invest Dermatol. 1984;82:94–96. doi: 10.1111/1523-1747.ep12259193. [DOI] [PubMed] [Google Scholar]
- 31.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–78. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 32.Spruance S L, Green J A, Chiu G, Yeh T J, Wenerstrom G, Overall J C. Pathogenesis of herpes simplex labialis: correlation of vesicle fluid interferon with lesion age and virus titer. Infect Immun. 1982;36:907–910. doi: 10.1128/iai.36.3.907-910.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor J L, Little S D, O'Brien W J. The comparative anti-herpes simplex virus effects of human interferons. J Interferon Cytokine Res. 1998;18:159–165. doi: 10.1089/jir.1998.18.159. [DOI] [PubMed] [Google Scholar]
- 34.Torseth J W, Merigan T C. Significance of local γ interferon in recurrent herpes simplex infection. J Infect Dis. 1986;153:979–984. doi: 10.1093/infdis/153.5.979. [DOI] [PubMed] [Google Scholar]
- 35.Wald A, Zeh J, Selke S, Ashley R L, Corey L. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Investig. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zweerink H J, Stanton L W. Immune response to herpes simplex virus infections: virus-specific antibodies in sera from patients with recurrent facial infections. Infect Immun. 1981;31:624–630. doi: 10.1128/iai.31.2.624-630.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]