Abstract
The 2022 monkeypox virus (MPXV) outbreaks spurred global public health concern. In response, we undertook a living systematic review of its zoonotic characteristics, including potential reservoirs and susceptible species, transmissibility, and clinical presentation in nonhuman species. Electronic database searches yielded 148 eligible records published between 2000 and 2022. Primary reservoirs remain unidentified, with natural isolation identified in 2 species, the sooty mangabey monkey and rope squirrel. Transmission primarily occurs from animals to humans, but evidence of reverse zoonosis has emerged. Data on clinical infection and manifestations are sparse, with evidence of potentially susceptible species drawn primarily from experimental studies. Only 10% of articles were appropriate for quality assessment and most of these were rated as critically low. Overall, while evidence regarding MPXV exists, the quality of data are extremely poor, resulting in significant uncertainty regarding MPXV's zoonotic traits. High-quality empirical research to understand the impact of MPXV on animal and human populations is warranted.
Keywords: infectious diseases, living systematic review, monkeypox, public health, zoonosis
Recent mpox outbreaks prompted a systematic review of its zoonotic characteristics. Despite evidence suggesting transmission from animals to humans and reverse zoonosis, uncertainties persist, highlighting the need for high-quality empirical research to understand mpox's impact on animal and human populations.
The recent emergence and spread of zoonotic viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), demonstrate that animal-sourced viruses are a very real threat to global public health. There are >250 known zoonotic viruses that have previously spread from animals to humans, causing disease in people [1]. Virus discovery efforts have detected hundreds of new animal viruses with unknown zoonotic risk. Approximately 1.67 million undescribed viruses are thought to exist in mammals and birds, up to half of which are estimated to have the potential to infect humans [2, 3].
Monkeypox virus (MPXV), which belongs to the genus Orthopoxvirus in the family Poxviridae, is an enveloped double-stranded DNA virus with a large genome of approximately 200 000 nucleotide bases [4, 5]. Mpox (formerly known as monkeypox) is a disease caused by infection with MPXV. MPXV was discovered in 1958 and initially found to cause rash disease in nonhuman primates, with human MPXV infection first confirmed in 1970 by the World Health Organization (WHO) Commission to Certify Smallpox Eradication [4].
As a zoonotic virus, human MPXV outbreaks have occurred primarily in the tropical rainforests of West and Central Africa, including Congo, Sierra Leone, Ghana, Central African Republic, Nigeria, and Sudan [4]. The first outbreak outside Africa was reported in the United States (US) in 2003, which included 72 confirmed or suspected cases following the importation of MPXV-infected animals from Ghana [6]. In 2018 and 2021, human-to-human transmission outside Africa was also reported among healthcare workers and family members of travelers from Nigeria [7, 8]. The most recent outbreak of mpox in May 2022 has led to several cases in many countries. On 23 July 2022, the WHO declared a public health emergency of international concern over the global outbreak of mpox [9].
Considering the coronavirus disease 2019 pandemic, more attention is being placed on novel disease outbreaks with pandemic potential. Faced with an increasing number of reports of mpox outbreaks in Africa and globally (linked to transmission between humans, rodents, and nonhuman primates), there is an urgent need to understand the virus's natural host range, modes of transmission, clinical infection in animals, and management at the human–animal ecosystem interface. In this living systematic review, we aimed to evaluate accumulating evidence related to the zoonotic characteristics of MPXV and identify important evidence gaps.
METHODS
Overview
This living systematic review was requested by the Public Health Agency of Canada (PHAC) in response to the 2022 mpox outbreak. Its livingness is characterized by continual updates to incorporate new evidence as it became available, between July and October 2022 [10]. Following each search update, evidence was synthesized and presented to PHAC to inform their policy decision-making and were made publicly available on the Coronavirus Variants Rapid Response Network (CoVaRR-NET) website. No further search updates were requested from PHAC and updating was stopped in October 2022. We used a standardized protocol to ensure transparency and consistency of methods. Refinements to the search parameters were determined by the review team with input from PHAC and clearly described and justified between search updates. The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42022349554) [11]. Reporting of the review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement for reporting [12].
Search Strategy
Medline and Embase (both via the Ovid platform) were searched biweekly between 12 July 2022 and 28 October 2022, resulting in a total of 8 searches. Our search strategy was composed of a combination of Medical Subject Heading (MeSH) terms, subject headings, and keywords related to MPXV (Supplementary Appendix 1). A modification to the protocol was implemented midway through the project at the request of PHAC to include nonempirical evidence (editorials, letters, and invited commentaries) published prior to 2018 to capture information prior to, and including, the mpox outbreak in Nigeria in 2017.
Eligibility Criteria
English-language documents published between 1 January 2020 and 28 October 2022 were eligible for inclusion. The following document types were eligible for inclusion: guidelines (recommendation documents based on evidence synthesis activities), reviews (including systematic, narrative/nonsystematic, rapid, and scoping reviews), primary studies (including randomized trials, comparative nonrandomized studies, observational studies), published reports (eg, US Centers for Disease Control and Prevention [CDC] outbreak reports), and preprints. Preprints were reassessed in subsequent searches for updates in publication status. The most significant refinement to the eligibility criteria was the inclusion of nonempirical reports published between 1 January 2020 and 3 December 2017; the search for these papers began midway through the project (Supplementary Appendix 2).
The publications had to report on at least 1 of the following concepts: (1) reservoirs of MPXV, (2) transmissibility of MPXV, (3) clinical infection in animals (incubation period, shedding), (4) clinical presentation in animals, (5) susceptible animal species, and/or (6) handling of infected susceptible animals. For the first few search updates of the living review, data on the environmental and occupation aspects of MPXV infection in animals were sought. This ceased to be an eligibility criterion in searches conducted after 2 September 2022, because of the absence of data.
Data Selection and Extraction
Records identified in each search were imported into COVIDence systematic review software (Veritas Health Innovation, Melbourne, Australia), and duplicates were removed. Records were screened for eligibility using a 2-stage approach (title/abstract, then full text). Screening was done independently and in duplicate (K. A., J. H., E. C.), and conflicts and uncertainties were discussed and resolved by consensus or input from a third reviewer (M. B.) where appropriate.
We structured our data extraction for zoonotic information following the 6 concepts of the study selection criteria outlined above. The second concept of transmissibility of MPXV was further divided into 4 subdomains including (1) animal-to-animal transmission, (2) animal-to-human transmission, (3) human-to-animal transmission, or (4) transmission from animal waste or wastewater. The structure of our data extraction was designed in response to virology and infectious disease–related literature, expertise from study team members (including those with knowledge of veterinary infectious disease), and expertise and preferences of our public health partners.
Extractions of both bibliometric data (authors, title, abstract, date of publication, citations) and relevant organizing framework data were conducted by one team member and audited by a second team member (K. A., J. H., E. C.). For each domain, a narrative summary was prepared highlighting the total number of different types of highly relevant documents and their key findings.
Risk of Bias Assessment
Studies incorporating selected study designs were assessed for quality independently by one member (J. H.) and audited by a second member (E. C.). Randomized trials (using the Cochrane Risk of Bias tool [13]) and cohort and case-control studies (using the Newcastle-Ottawa Scale [14]) were appraised. Papers reporting nonsystematic reviews, noncomparative primary studies, and nonempirical documents (eg, commentaries, editorials), published reports, and animal-only experiments were not assessed because of their greater risk of bias or because international quality assessment tools have not been developed for these types of publications. Preprints were given provisionally “critically low” ratings when first included and revised if a peer-reviewed publication became available during the search period.
RESULTS
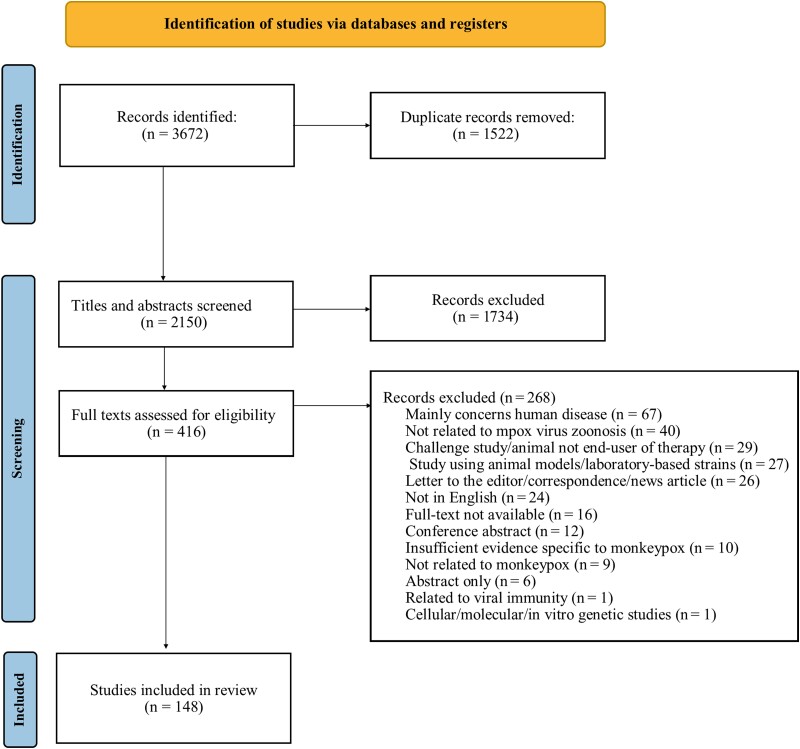
From our 8 electronic searches, we identified a total of 2150 articles after removing duplicates. The search resulted in the inclusion of 148 eligible articles (Figure 1). Table 1 reports the number and types of papers in each organizing domain. Supplementary Appendix 3 provides a description of the included studies.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram based on articles identified from 8 search updates.
Table 1.
Total Number and Types of Papers by Topic
| Type of Document | Total (N = 148) | Reservoirs of the Virus | Transmissibility of the Virus | Clinical Infection in Animals | Clinical Presentation in Animals | Susceptible Animals of the Virus | Handling of Infected Susceptible Animals |
|---|---|---|---|---|---|---|---|
| Guidelines | 2 | 1 | 2 | … | … | 1 | 1 |
| Systematic reviews | 7 | 4 | 6 | … | 2 | 1 | … |
| Rapid/scoping reviews | 5 | 1 | 5 | - | - | 1 | … |
| Nonsystematic reviews | 71 | 53 | 62 | 3 | 13 | 32 | 6 |
| Protocols for reviews or rapid reviews that are underway | 0 | … | … | … | … | … | … |
| Titles/questions for reviews that are being planned | 0 | … | … | … | … | … | … |
| Single study | 37 | 15 | 23 | 1 | 6 | 7 | 2 |
| Published reports | 11 | 9 | 8 | 1 | 1 | 1 | 1 |
| Dissertation/thesis | 0 | … | … | … | … | … | … |
| Preprints | 1 | 1 | 1 | … | … | … | … |
| Nonempirical: editorials, letter to editor, and commentary | 14 | 3 | 10 | … | 2 | 3 | 1 |
Characteristics of Included Studies
Among the included articles, most were nonsystematic narrative reviews (n = 71 [48%]). Other article types included single (primary) studies (n = 37 [25%]), nonempirical papers (n = 14 [10%]), published reports (n = 11 [8%]), systematic reviews (n = 7 [5%]), rapid/scoping reviews (n = 5 [3%]), and guidelines (n = 2 [1%]) (Table 1). The largest proportion of articles (n = 61 [41%]) were from the US, mostly following the 2003 and 2022 mpox outbreaks. Thirty-one studies were conducted in Central and Western African countries with few in Northern and Southern Africa.
Only 15 articles (10%) were eligible for risk of bias assessment. The quality of these publications ranged from critically low to moderate (Table 2), with 11 of the 15 (73%) publications assessed as critically low quality.
Table 2.
Quality Appraisal of Selected Studies
| Article Type | Quality Rating (Tool) | Citation |
|---|---|---|
| Scoping review | Critically low (AMSTAR-2) | Adnan et al, 2022 |
| Systematic review | Low (AMSTAR-2) | Beer and Rao, 2019 |
| Systematic review | Critically low (AMSTAR-2) | Brown and Leggat, 2016 [15] |
| Systematic review | Critically low (AMSTAR-2) | Bunge et al, 2022 |
| Scoping review | Critically low (AMSTAR-2) | Capobianchi et al, 2022 [16] |
| Systematic review | Critically low (AMSTAR-2) | Chauhan et al, 2020 |
| Scoping review | Critically low (AMSTAR-2) | Di Gennaro et al, 2022 |
| Rapid review | Critically low (AMSTAR-2) | Diaz et al, 2021 |
| Systematic review | Critically low (AMSTAR-2) | Kannan et al, 2022 |
| Scoping review | Critically low (AMSTAR-2) | Kipkorir et al, 2022 |
| Single study (prospective cohort study) | High (7/9, NOS) | Patrono et al, 2020 [17] |
| Systematic review | Critically low (AMSTAR-2) | Rahimi et al, 2022 |
| Systematic review | Critically low (AMSTAR-2) | Soheili et al, 2022 [18] |
| Single study (retrospective cohort study) | Moderate (5/9, NOS) | Tiee et al, 2018 |
| Single study (prospective cohort study) | High (8/9, NOS) | Whitehouse et al, 2021 |
Abbreviations: AMSTAR, A Meaurement Tool to Assess Systematic Reviews; NOS, Newcastle-Ottawa Scale.
Outcome: Virus Reservoirs
Eighty-seven papers provided data on MPXV reservoirs (Table 3 and Supplementary Appendix 4A). Based on these data, the primary reservoirs of MPXV have not been confirmed. In the Democratic Republic of Congo (DRC), archival animal samples indicated that Congo rope squirrels (Funisciurus congicus) have higher odds of being MPXV positive. In the DRC in 1985 and Cote d’Ivoire in 2012, MPXV was isolated from other species, such as Funisciurus squirrel and mangabey monkey [19–21]. A range of animals have been reported to be possible reservoirs of MPXV, but the primary natural reservoirs remain unidentified.
Table 3.
Reservoirs of the Monkeypox Virus
| Reservoir | Rodents | Nonhuman Primates | Others |
|---|---|---|---|
| Natural host | Rope squirrel (Funisciurus anerythrus) | Sooty mangabey monkey (Cercocebus atys) | None |
| Probable hosts |
|
|
|
aOne of the collaborators identified a contradiction between what was stated in the systematic review (Eur Rev Med Pharmacol Sci 2022; 26:5983–90) and what was published in the primary paper (Infect Dis Clin North Am 2019; 33:1027–43) in relation to mongoose being a possible reservoir of monkeypox virus. Hence, we recommend that mongoose not be considered as a possible reservoir unless further evidence is found.
Outcome: Virus Transmissibility
There were 117 papers that provided data on transmissibility of MPXV, from which 3 main types of transmission routes were identified: animal-to-human, human-to-animal (reverse zoonosis), and animal-to-animal (Supplementary Appendix 4B).
Animal-to-Human Transmission
A total of 107 papers discussed the potential for animal-to-human transmission of MPXV. Exposure to wild animals (either through trapping and hunting, or preparation and consumption of bushmeat) is a major risk factor for MPXV transmission. For example, one study demonstrated a greater risk of MPXV exposure among individuals living in rural communities near animal trapping sites in Africa compared to other living environments [21]. An investigation led by Reynolds in 2006 stated that MPXV could be transmitted to humans directly through traumatic injury to the skin via bites or scratches or indirectly through percutaneous, inhalation, or mucocutaneous exposures [22]. Direct contact with infected animals from a common distributor played a role in the 2003 US outbreak [23, 24], while direct contact combined with external climate and geopolitical factors has been implicated in the 2005 outbreak in Sudan [25] and the 2015 outbreak in DRC [26]. All cases during the US outbreak in 2003 resulted from close contact with infected prairie dogs, most commonly via bites, as noted in a case where a 3-year-old girl was hospitalized for mpox after being bitten by a prairie dog [6]. Guarner et al have found viral antigens and mature poxvirus particles in the tongues of prairie dogs, suggesting direct contact between saliva or lesions on infected prairie dogs and skin/mucous membranes of humans [27]. Another paper reported on a 1982 incident of a chimpanzee in the DRC seizing and biting a 6-month-old infant in the femur, resulting in MPXV infection later on [28]. Many of these papers did not provide concrete evidence supporting animal-to-human transmission in the cases they report; rather, they offered examples from which hypotheses could be drawn.
Human-to-Animal Transmission/Reverse Zoonosis
A total of 14 papers discussed human-to-animal transmission of MPXV. No evidence of such transmission has been reported prior to 2017. Recently, two possible instances of human-to-animal transmission of MPXV have been reported in France and Brazil [29]. In addition, one letter to the editor describes an instance of human-to-dog transmission. The authors suggest this is an example of human-to-animal transmission as samples from both the men and the dog corresponded to the same clade (B.1), which had been circulating in France at the time [30]. One editorial highlighted the possibility of reverse zoonosis during the 2022 outbreak, with domestic animals living near infected owners most likely to be infected [31].
However, there is a lack of evidence on the clinical and zoonotic consequences of these transmission patterns [32] or confirmation that detection of viral DNA on the animals represented true infection versus contamination of the animal’s haircoat from the infected person.
Animal-to-Animal Transmission
A total of 22 papers discussed animal-to-animal transmission of MPXV. These records have indicated direct transmission between animals can occur through respiratory droplets (aerosols), skin or eye abrasions, and ingestion of infected animal tissue, arthropods, and fomites. One prospective cohort study noted additional transmission opportunities through viral DNA shedding in fecal, urine, and fruit wedge bite samples from both symptomatic and clinically normal chimpanzees in the Ivory Coast [33]. Patrono et al represented the only investigated instance of MPXV transmission between suspected host animals (chimpanzees) [17]. The authors studied interactions with infected rodents as well as maggots and flesh flies (which primarily feed on urine and fecal matter and potentially carry infection) and through mutual grooming behaviors within social networks.
Papers have also indicated that certain animals, such as rodents, may play the role of intermediate hosts in the transmission of MPXV from one animal to another, before potential transmission to humans. An important example of animal-to-animal transmission with intermediate hosts is the outbreak in the US in 2003. Mpox had spread from rodents imported from Central Africa to prairie dogs when both species were kept in the same warehouse [34–36]. There are also reports of a rabbit becoming infected after exposure to a sick prairie dog at a veterinary clinic [36].
Other important reports of animal-to-animal transmission of MPXV include outbreaks among captive monkeys (Macaca fascicularis) in 1958, 1959, and 1962; in the latter outbreak, almost 90% of animals kept in the same room as 2 clinically affected monkeys were seropositive while only 11% of animals in other rooms were seropositive [37]. In a 1964 zoo outbreak, giant anteaters (Myrmecophaga tridactyla) infected Asian orangutans (Pongo pygmaeus) that were kept in a nearby enclosure, either through aerosol or fomite transmission [37].
Transmission Related to Animal Waste and Wastewater
A single primary study had reported on MPXV transmission via animal waste and wastewater. MPXV has been isolated from wastewater with possible DNA sources originating from human shedding or feces of animals living in sewers [38]. However, whether viable, infectious virus was present is not known.
Outcome: Clinical Infection in Animals
Five studies discussed clinical infection of MPXV in animals (Supplementary Appendix 4C). Nonhuman primates have been observed to have 2 phases of mpox disease similar to humans: a prodromal phase consisting of a nonspecific febrile syndrome, and an eruptive phase characterized by skin lesions [39]. The infection characteristics of prairie dogs infected with MPXV show an incubation period of 13–24 days with the duration of infection being 4 weeks long [40]. The infection period in Thomas's rope squirrels is reported to be 6–8 days, with viral shedding lasting up to 25 days afterward [40].
Outcome: Clinical Presentation in Animals
There were 24 included reports that discussed the clinical presentation of MPXV infection in animals. Common clinical presentation in rope squirrels (Funisciurus anerythrus) included skin lesions, lethargy, nasal discharge, and respiratory distress [18, 41], while prairie dogs presented commonly with ocular conjunctivitis, lymphadenopathy, skin lesions, and pulmonary consolidation [6, 27, 42–44]. In primates, common clinical presentation included skin lesions, respiratory distress, and lymphadenopathy [17, 32, 37, 40, 45, 46]. Clinical signs are summarized in Table 4 with more details provided in Supplementary Appendix 4D.
Table 4.
Clinical Presentations in Specific Susceptible Animals
| Susceptible Animals | Clinical Features |
|---|---|
| Prairie dogs | Reported symptoms:
|
| Rope squirrels | Reported symptoms:
|
| Monkeys | Generalized skin eruptions that develop into papules on the trunk, tail, limbs, face, palm, and soles within a week of onset. The papules then develop into vesicles and scabs that typically fall off approximately 10 days following the onset of the rash [40, 46, 50]. MPXV infection can also present as fever and facial edema in monkeys and apes [36]. Some nonhuman primates have developed severe respiratory signs with dyspnea and no skin lesions, or discrete and diffuse lesions and vesiculopustular skin eruptions in the trunk, tail, and limbs within a week of onset [39]. MPXV DNA and secondary bacterial infections were also noted in several organs in a fatal MPXV infection in an infant sooty mangabey monkey [40]. The severity of disease varies with the host species. For example, the disease is mild in cynomolgus monkeys (Macaca cynomolgus), but more severe in orangutans (Pongo sp) [46]. During the 1958 outbreak in Denmark among Asian monkeys (Macaca fascicularis), clinical signs included vesiculopustular skin eruptions across the body (face, trunk, limbs, tail, palms, and feet soles), which crusted over and healed on their own, resulting in scars [37]. In 1959, the first reported mpox cases involved cynomolgus macaques (M fascicularis) who presented with poxviral exanthema. Another outbreak occurred in 1959 in the US among cynomolgus and rhesus macaques (Macaca mulatta), where 2 manifestations of disease occurred. The first type of disease occurred in M fascicularis, presenting with facial and cervical edema, severe breathing difficulties that led to death by asphyxiation, papular eruptions across the body, ulcerative lesions in the oral mucosa, cutaneous lesions, and generalized lymphadenopathy. The second type of disease was more common and occurred in both M fascicularis and M mulatta, resulting in cutaneous lesions that became pustular and crusted, leaving scars behind all over the body (face, hands, feet, hind limbs, and buttocks) and hemorrhagic lesions linked with fatality. During the 1962 US outbreak among 2 M fascicularis monkeys, clinical presentation included pox-like eruptions, bloody diarrhea, dyspnea, hemorrhagic ulcerations, and facial and cervical edema [37, 43]. Cynomolgus monkeys imported from Singapore have also exhibited vesiculopustular skin eruptions over the entire trunk, tail, face, and limbs [16]. Cutaneous rash, varying levels of exanthema, coughing, and severe respiratory distress were also noted in chimpanzees in Tai National Park in the Ivory Coast [40, 51]. |
| Various animals | During the 1964 outbreak at Rotterdam Zoo, a South American squirrel monkey presented with pox lesions, while owl-faced monkeys presented with lesions on the lips. Other animals affected during this outbreak include giant anteaters who presented with skin lesions; Asian orangutans with erythema and nasal discharge with lesions on the body, legs, and face; African gorillas and chimpanzees with pox lesions; Asian gibbon with vesicles on the limbs, trunk, and face; and a South American common marmoset with red and swollen areas around the nose and eyes, as well as face and belly lesions [37]. |
| Dogs | A case of an Italian greyhound who tested positive for MPXV, most likely receiving infection from 2 men in the same household, presented with mucocutaneous lesions, abdominal pustules, and thin anal ulceration [32, 50]. |
Abbreviation: MPXV, monkeypox virus.
Outcomes: Susceptible Animals to the Virus
Forty-six reports identified animal species that were susceptible to infection from MPXV (Table 5 and Supplementary Appendix 4E). Many ecologic and serological studies have suggested nonhuman primates and rodents (particularly rope and tree squirrels, Gambian pouched rats, and dormice) as the most common species susceptible to infection from MPXV. Other animal species known to be susceptible include opossums, hedgehogs, anteaters, elephant shrew, wild pigs, rabbits, and coatimundis. The susceptibility of production animals (ie, cattle, sheep, goats, pigs, and poultry) is the least known; however, ruminants are susceptible to some other poxviruses (eg, cowpox). Last, the susceptibility of domestic pets is unknown. Table 5 provides a detailed list of genus and species summarized within this report.
Table 5.
Animal Species at Risk of Monkeypox Virus Infection
| Animal Groups | ||||
|---|---|---|---|---|
| Wild | Domestic | Pets | ||
| Nonhuman primates | Rodents | Other | ||
|
|
|
The susceptibility of production animals (cattle, goats, sheep) is the least known. However, as most ruminants are susceptible to other poxviruses (such as cowpox), this should not be overlooked |
The susceptibility of pets (such as cats) is unknown. May act as accidental hosts and cause viral spillover. Dogs and ferrets are also regarded in this instance. |
The following species have been experimentally found to be susceptible to monkeypox virus infection: CAST/EiJ, PERA/EiJ, and MOLF/EiJ mice; cynomolgus monkeys; Kellen's dormouse (Graphiurus kelleni); black-tailed prairie dogs (Cynomys ludovicianus); Thomas's rope squirrel (Funisciurus anerythrus); ground squirrels (Ictidomys tridecemlineatus); Gambian pouched rats (Cricetomys gambianus); and albino rabbits; as well as neonates of rats and exotic pet mice.
Outcome: Handling of Infected Susceptible Animals
Eleven reports discussed handling procedures for animals infected with MPXV (Supplementary Appendix 4F). A report from the WHO has stated that MPXV-infected animals need to be immediately quarantined. Specifically, animals potentially in contact with infected animals should also be quarantined and monitored for mpox signs for 30 days. Wearing personal protective equipment (PPE) when handling infected animals and tissues is recommended to prevent MPXV transmission from animals to humans [52].
The US Centers for Disease Control and Prevention has advised that sick animals not be released in the wild to prevent an enzootic MPXV cycle in local wildlife [53]. Infected animals or animals suspected to be infected must be isolated promptly, with reverse and positive contact tracing where possible. The authors also suggest strict importation limits on wildlife into countries to avoid contact with sick animals (especially African rodents, marsupials, nonhuman primates) which may carry MPXV. Any collected samples from suspected infected animals need to be handled with care and with appropriate protection by trained personnel [54].
In a veterinary or field setting, measures to prevent infection of MPXV from infected animals include isolation from other humans and the animal's environment, avoiding feeding them directly, isolation of direct contacts of the infected animal(s), and environmental disinfection of contacted surfaces [55]. The review by Haddad and Cordevant has advised that veterinary staff should take extra precautions, such as PPE and careful disinfection of the animal's isolation environment [39]. For presumed low-risk transmissors of MPXV (eg, domestic pet animals such as dogs and cats), pet owners should avoid contact with other animals in the event they suspect mpox-like disease in their pets. For high-risk transmissors (eg, rodents), closer monitoring is suggested; euthanasia is used as a last resort. Potentially contaminated environments from residues from rodents and other pets should be properly cleaned and disinfected [50].
DISCUSSION
Since its discovery in 1958, our understanding of the zoonotic features of MPXV has significantly envolved. MPXV infection was endemic in central and west Africa, with a large outbreak also occurring in the US in 2003. Several outbreaks in humans in nonendemic countries have occurred in the past 5 years including the recent 2022 outbreak that has raised concern regarding a global pandemic. To understand the full spectrum of mpox disease and its management at the animal–human interface, it is essential to know the inter-species and intra-species transmission risk of this virus. In this living systematic review, we systematically collected evidence on the reservoir, transmissibility, clinical infection, and presentation in animals, susceptible animals, and handling of infected animals.
The findings of this living review corroborated that the natural reservoir of MPXV is yet to be confirmed. Outside of Africa, the source of infection in the 2003 US outbreak was prairie dogs housed alongside Gambian rodents imported from Ghana into the US. Operational guidance in low-resource settings has suggested that species lists in local areas can be useful in informing risk reduction strategies for animal-to-human transmission. Our list of possible reservoirs can assist individual countries to build their own list and help to develop preventive strategies to be applied at the animal-to-human interface.
The transmissibility of MPXV is a topic that has the most contemporary relevance. Though evidence has indicated specific transmission methods, there remain unknown factors and transmission routes that need to be properly investigated and accurately documented. For example, reverse zoonosis and the zoonotic lifecycle in species in nonendemic countries are 2 areas where significant uncertainties exist according to the data collected in our review. Despite the importance of reservoir species in transmission, studies suggest that the survival of MPXV is also affected by environmental conditions and geopolitical factors [15, 23, 26]. Hence, future transmissibility research must address these external factors and elements.
Evidence related to infection phases and their duration was limited in our review. Very few studies reported on clinical features of mpox disease. These data are essential for epidemic management and development of appropriate surveillance strategies. Our review has presented the clinical manifestations of the MPXV infection in certain reservoir animals. However, it is important to identify the clinical features in other susceptible species. Awareness of the key clinical characteristic of mpox will aid clinical detection.
Our review identified a list of animals that may be susceptible to MPXV. Many of these species were included in experimental studies where animals were artificially injected with the virus. Natural infection may differ from that caused by experimentation; thus, caution should be taken in interpreting these results. Studies of natural infection of the virus are required to provide more definitive zoonotic features and can be applied to the development of infection prevention strategies.
Published reports on handling of MPXV-infected animals indicated specific guidance for both infected animals and those who handle them. However, the nonendemic outbreak in 2003 in the US and the recent global outbreak in 2022 strongly indicate the need for stronger surveillance and management of mpox disease. These outbreaks may reflect a broader range of zoonotic infections triggering and spreading during the interaction of pathogens with the natural environment and human behavior.
Surveillance of mpox is difficult due to limited resources and infrastructure, inappropriate diagnostic material, and lack of clinical recognition of MPXV infection [15]. The sylvatic component of the cycle means eradication is not possible and therefore, prevention becomes paramount [15]. In light of environmental impacts, further research to identify and specify the zoonotic characteristics of MPXV is necessary to protect those most vulnerable.
Strengths/Limitations
This living systematic review searched, systematically assessed, and synthesized a wide variety of evidence sources, including both published sources, as well as preprints and grey literature. This allowed for a comprehensive examination of the contemporary evidence on zoonotic characteristics of MPXV. Another strength was the adherence to high-quality methodological standards of knowledge synthesis. A protocol was created and posted publicly prior to review commencement to ensure transparency and consistency of methods between searches and minimize the opportunity for biases. Any protocol refinements were explicitly documented and justified. Study selection and data extraction were done independently and in duplicate or with data audit to enhance the reliability and validity of the review process. The most striking feature of this review, and its key limitation, is the paucity of high-quality evidence from which to make confident inferences regarding the zoonotic characteristics of MPXV. While the inclusion of noncomparative primary studies, nonempirical documents, and animal-based experimental studies expanded the evidence base, these sources were extremely inconsistent in their methodology and reporting. We were only able to formally assess the quality of 10% of included studies, and in most assessed studies, the quality was judged to be critically low. Further research and more transparent reporting are needed to resolve uncertainties related to the zoonotic characteristics of MPXV. Of particular note, innovative and rigorous investigations such as ecological studies or predictive modeling to explore factors associated with MPXV, especially on animal-to-animal transmission, spillover events, human-to-human transmission, and survivors, are warranted.
CONCLUSIONS
This living systematic review performed a comprehensive synthesis of the zoonotic characteristics of MPXV, including potential reservoirs and susceptible species, transmissibility, and clinical presentation in nonhuman species. While a large number of eligible records were included, the overall quality of evidence was found to be critically low. This review identified gaps in the current zoonotic understanding of MPXV, including the lack of certainty surrounding the primary reservoir species and the sparsity of data on clinical infection and manifestations. Additional high-quality empirical research is required to address these gaps and confirm the impact of MPXV on animal and human populations.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kawsari Abdullah, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Junayd Hussain, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Emilie Chan, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Kylie Tingley, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Valentina Ly, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
J Scott Weese, Centre for Public Health and Zoonoses, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada.
Nicole Shaver, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Alexandria Bennett, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Melissa Brouwers, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Notes
Author contributions. K. A., J. H., J. S. W., and M. C. B. made substantial contributions to the conception and design of the work and the acquisition, analysis, and interpretation of data. E. C., V. L., and M. B. contributed to the acquisition and analysis of data. K. A., K. T., and M. C. B. drafted the manuscript. N. S., A. B., and M. B. revised the manuscript, and all co-authors reviewed the manuscript for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Data availability. Previous reports are available on the CoVaRR-NET website. For additional data, please contact the corresponding author.
Financial support. This work was funded by the Canadian Institutes of Health Research (funding reference number 175622 to M. B.).
Supplement sponsorship. This article appears as part of the supplement “Mpox Outbreak 2022 and Beyond: Understanding Transmission to Inform Public Health Responses,” sponsored by the authors.
References
- 1. Mollentze N, Streicker DG. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc Natl Acad Sci U S A 2020; 117:9423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carroll D, Daszak P, Wolfe ND, et al. The Global Virome Project. Science 2018; 359:872–4. [DOI] [PubMed] [Google Scholar]
- 3. Grange ZL, Goldstein T, Johnson CK, et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc Natl Acad Sci U S A 2021; 118:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song TZ, Zheng YT. Monkeypox, wild animals, and potential big problem. Zool Res 2022; 43:612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shchelkunov SN, Totmenin A V, Safronov PF, et al. Analysis of the monkeypox virus genome. Virology 2002; 297:172–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ligon BL. Monkeypox: a review of the history and emergence in the western hemisphere. Semin Pediatr Infect Dis 2004; 15:280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hobson G, Adamson J, Adler H, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill 2021; 26:2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis 2020; 26:782–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng K, Guo Q, Shen Z, et al. Global research trends on four orthopoxviruses threatening human health: monkeypox is a neglected branch which deserves more attention. Int J Surg 2022; 105:106846. [DOI] [PubMed] [Google Scholar]
- 10. Abdullah K, Hussain J, Chan E, Ly V, Brouwers M, Weese S; CoVaRR-Net . Zoonotic characteristics of mpox: living evidence profile—CoVaRR-Net. 2022. Available at: https://covarrnet.ca/zoonotic-characteristics-of-mpox-living-evidence-profile/. Accessed 12 April 2023.
- 11. Abdullah K, Hussain J, Chan E, et al. PROSPERO . Monkey pox virus—zoonotic characteristics: living evidence synthesis. 2022. Available at: https://www.crd.york.ac.uk/prospero/display_record.php? ID=CRD42022349554. Accessed 12 April 2023.
- 12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 14. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2022. Available at: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp. Accessed 12 April 2023.
- 15. Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis 2016; 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capobianchi MR, Di CA, Piubelli C, Mori A, Bisoffi Z, Castilletti C. Monkeypox 2022 outbreak in non-endemic countries: open questions relevant for public health, nonpharmacological intervention and literature review. Front Cell Infect Microbiol 2022; 12:1005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patrono LV, Pléh K, Samuni L, et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat Microbiol 2020; 5:955–65. [DOI] [PubMed] [Google Scholar]
- 18. Soheili M, Nasseri S, Afraie M, et al. Monkeypox: virology, pathophysiology, clinical characteristics, epidemiology, vaccines, diagnosis, and treatments. J Pharm Pharm Sci 2022; 25:297–322. [DOI] [PubMed] [Google Scholar]
- 19. Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis 2001; 7:434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakazawa Y, Lash RR, Carroll DS, et al. Mapping monkeypox transmission risk through time and space in the Congo basin. PLoS One 2013; 8:e74816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds MG, Carroll DS, Olson VA, et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg 2010; 82:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis 2006; 194:773–80. [DOI] [PubMed] [Google Scholar]
- 23. Kile JC, Fleischauer AT, Beard B, et al. Transmission of monkeypox among persons exposed to infected prairie dogs in Indiana in 2003. Arch Pediatr Adolesc Med 2005; 159:1022–5. [DOI] [PubMed] [Google Scholar]
- 24. Reed KD, Melski JW, Beth Graham M, et al. The detection of monkeypox in humans in the western hemisphere from the departments of pathology. N Engl J Med 2004; 350:342–50. [DOI] [PubMed] [Google Scholar]
- 25. Nakazawa Y, Emerson GL, Carroll DS, et al. Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg Infect Dis 2013; 19:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nolen LD, Osadebe L, Katomba J, et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg 2015; 93:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guarner J, Johnson BJ, Paddock CD, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis 2004; 10:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wrangham R, Wilson M, Hare B, Wolfe ND. Chimpanzee predation and the ecology of microbial exchange. Microbial Ecol Health Dis 2000; 12:186–8. [Google Scholar]
- 29. Shepherd W, Beard PM, Brookes SM, et al. The risk of reverse zoonotic transmission to pet animals during the current global monkeypox outbreak, United Kingdom, June to mid-September 2022. Euro Surveill 2022; 27:2200758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022; 400:658–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afrooghe A, Damavandi AR, Ahmadi E. Reverse zoonosis and monkeypox: time for a more advanced global surveillance system for emerging pathogens. New Microbes New Infect 2022; 48:101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonilla-Aldana DK, Rodriguez-Morales AJ. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet Q 2022; 42:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suu-Ire RD, Obodai E, Bonney JHK, Bel-Nono SO, Ampofo W, Kelly TR. Viral zoonoses of national importance in Ghana: advancements and opportunities for enhancing capacities for early detection and response. J Trop Med 2021; 2021:8938530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med 2022; 387:1783–179. [DOI] [PubMed] [Google Scholar]
- 35. Stephenson J. Monkeypox outbreak a reminder of emerging infections vulnerabilities. JAMA 2003; 290:23–4. [DOI] [PubMed] [Google Scholar]
- 36. Sah R, Mohanty A, Hada V, et al. The emergence of monkeypox: a global health threat. Cureus 2022; 14:e29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol 2013; 8:129–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Jonge EF, Peterse CM, Koelewijn JM, et al. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci Total Environ 2022; 852:158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haddad N, Cordevant C. Les animaux hors d’Afrique peuvent-ils être concernés par la flambée de monkeypox en cours, voire en devenir des acteurs importants. Bull Acad Vet Fr 2022; 175:266–304. [Google Scholar]
- 40. MacNeill AL. Comparative pathology of zoonotic orthopoxviruses. Pathogens 2022; 11:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falendysz EA, Lopera JG, Doty JB, et al. Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.). PLoS Negl Trop Dis 2017; 11:e0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langohr IM, Stevenson GW, Thacker HL, Regnery RL. Extensive lesions of monkeypox in a prairie dog (Cynomys sp). Vet Pathol 2004; 41:702–7. [DOI] [PubMed] [Google Scholar]
- 43. Tack DM, Reynolds MG. Zoonotic poxviruses associated with companion animals. Animals (Basel) 2011; 1:377–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Croft DR, Sotir MJ, Williams CJ, et al. Occupational risks during a monkeypox outbreak, Wisconsin, 2003. Emerg Infect Dis 2007; 13:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radonić A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty Mangabey, Côte d’Ivoire. Emerg Infect Dis 2014; 20:1009–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol 2010; 140:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salzer JS, Carroll DS, Rwego IB, et al. Serologic evidence for circulating orthopoxviruses in peridomestic rodents from rural Uganda. J Wildl Dis 2013; 49:125–31. [DOI] [PubMed] [Google Scholar]
- 48. Gibbs EPJ. Emerging zoonotic epidemics in the interconnected global community. Vet Rec 2005; 157:673–9. [DOI] [PubMed] [Google Scholar]
- 49. Hutson CL, Lee KN, Abel J, et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg 2007; 76:757–68. [PubMed] [Google Scholar]
- 50. Gomez-Lucia E. Monkeypox: some keys to understand this emerging disease. Animals (Basel) 2022; 12:2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kulesh DA, Loveless BM, Norwood D, et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest 2004; 84:1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. World Health Organization . Weekly epidemiological record. Wkly Epidemiol Rec 2011; 86:445–56.21984985 [Google Scholar]
- 53. Centers for Disease Control and Prevention . Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep 2003; 52:537–40. [PubMed] [Google Scholar]
- 54. Zhu M, Ji J, Shi D, et al. Unusual global outbreak of monkeypox: what should we do? Front Med 2022; 16:507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haddad N. The presumed receptivity and susceptibility to monkeypox of European animal species. Infect Dis Now 2022; 52:294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.