Abstract
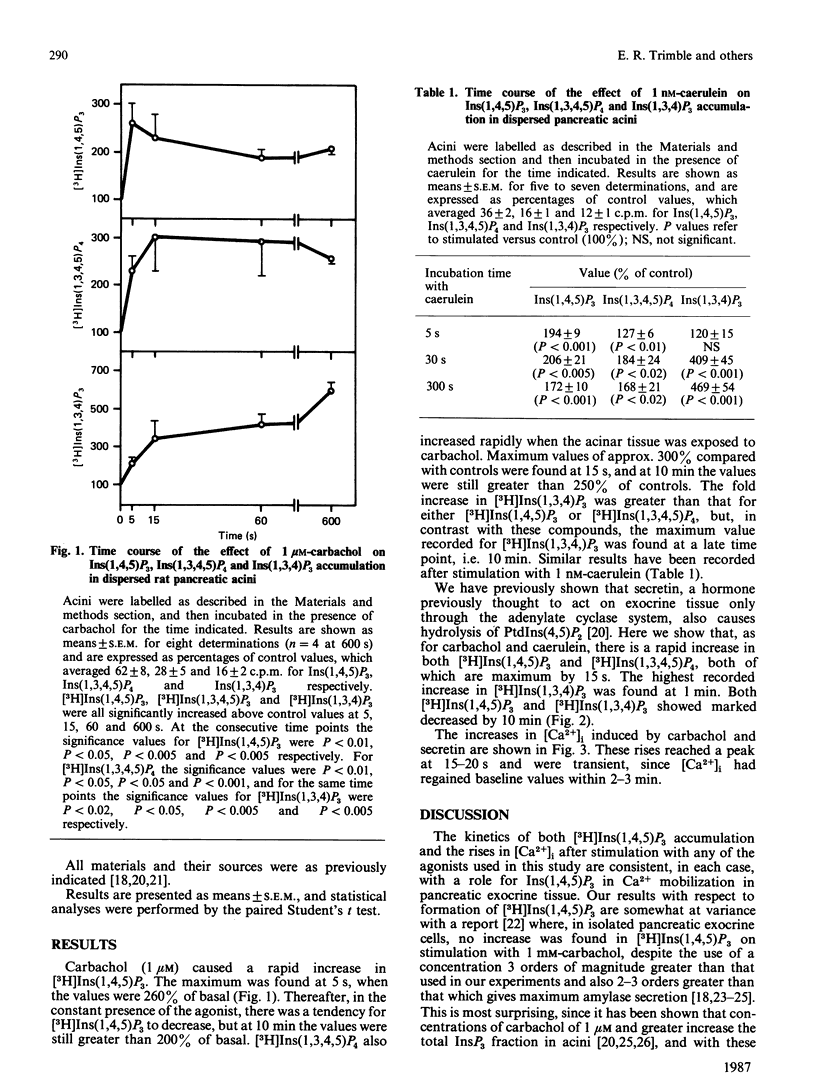
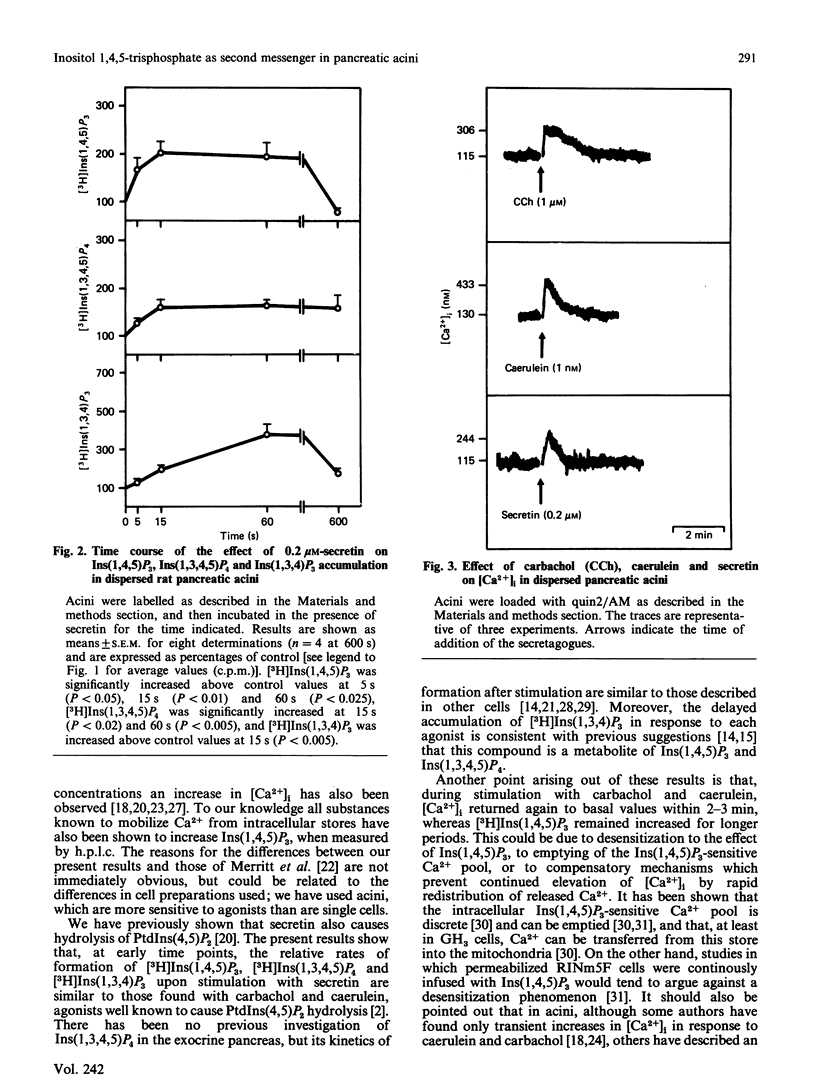
We compared the time course of increases in isomers of inositol trisphosphate [Ins(1,4,5)P3] and Ins(1,3,4)P3] and the tetrakisphosphate [Ins(1,3,4,5)P4] with changes in cytosolic free Ca2+ [( Ca2+]i) in dispersed pancreatic acini of the rat. There were rapid (5s) increases in Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in response to carbachol, caerulein and secretin, whereas Ins(1,3,4)P3 increased more slowly. All three secretagogues induced increases in [Ca2+]i, which reached a peak at 15-20 s. Our results indicate that the very rapid formation of Ins(1,4,5)P3 is compatible with its second-messenger role in the initial elevation of [Ca2+]i.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B., Schlegel W. Inositol 1,4,5-trisphosphate and intracellular Ca2+ homeostasis in clonal pituitary cells (GH3). Translocation of Ca2+ into mitochondria from a functionally discrete portion of the nonmitochondrial store. J Biol Chem. 1986 Jun 5;261(16):7223–7229. [PubMed] [Google Scholar]
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Pozzan T., Wollheim C. B. Caerulein and carbamoylcholine stimulate pancreatic amylase release at resting cytosolic free Ca2+. Biochem J. 1986 Apr 1;235(1):139–143. doi: 10.1042/bj2350139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Rubin R. P. Pancreatic amylase secretion and cytoplasmic free calcium. Effects of ionomycin, phorbol dibutyrate and diacylglycerols alone and in combination. Biochem J. 1985 Aug 15;230(1):151–159. doi: 10.1042/bj2300151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Isomers of inositol trisphosphate in exocrine pancreas. Biochem J. 1986 Sep 15;238(3):825–829. doi: 10.1042/bj2380825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Relation between free cytosolic calcium and amylase release by pancreatic acini. Am J Physiol. 1985 Sep;249(3 Pt 1):G389–G398. doi: 10.1152/ajpgi.1985.249.3.G389. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985 Aug 25;260(18):10081–10086. [PubMed] [Google Scholar]
- Powers R. E., Saluja A. K., Houlihan M. J., Steer M. L. Inositol trisphosphate production and amylase secretion in mouse pancreatic acini. Biochem Biophys Res Commun. 1985 Aug 30;131(1):284–288. doi: 10.1016/0006-291x(85)91800-5. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Di Virgilio F., Vicentini L. M., Meldolesi J. Activation of muscarinic receptors in PC12 cells. Stimulation of Ca2+ influx and redistribution. Biochem J. 1986 Mar 15;234(3):547–553. doi: 10.1042/bj2340547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Corkey B. E., Matschinsky F. M. Inositol 1,4,5-trisphosphate and the endoplasmic reticulum Ca2+ cycle of a rat insulinoma cell line. J Biol Chem. 1985 Aug 5;260(16):9185–9190. [PubMed] [Google Scholar]
- Rubin R. P., Godfrey P. P., Chapman D. A., Putney J. W., Jr Secretagogue-induced formation of inositol phosphates in rat exocrine pancreas. Implications for a messenger role for inositol trisphosphate. Biochem J. 1984 Apr 15;219(2):655–659. doi: 10.1042/bj2190655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Wollheim C. B. Thyrotropin-releasing hormone increases cytosolic free Ca2+ in clonal pituitary cells (GH3 cells): direct evidence for the mobilization of cellular calcium. J Cell Biol. 1984 Jul;99(1 Pt 1):83–87. doi: 10.1083/jcb.99.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Heslop J. P., Irvine R. F., Schulz I., Berridge M. J. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985 Jun 25;260(12):7309–7315. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Biden T. J., Farese R. V. Secretin induces rapid increases in inositol trisphosphate, cytosolic Ca2+ and diacylglycerol as well as cyclic AMP in rat pancreatic acini. Biochem J. 1986 Oct 15;239(2):257–261. doi: 10.1042/bj2390257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Ambrosini A., Di Virgilio F., Pozzan T., Meldolesi J. Muscarinic receptor-induced phosphoinositide hydrolysis at resting cytosolic Ca2+ concentration in PC12 cells. J Cell Biol. 1985 Apr;100(4):1330–1333. doi: 10.1083/jcb.100.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]


