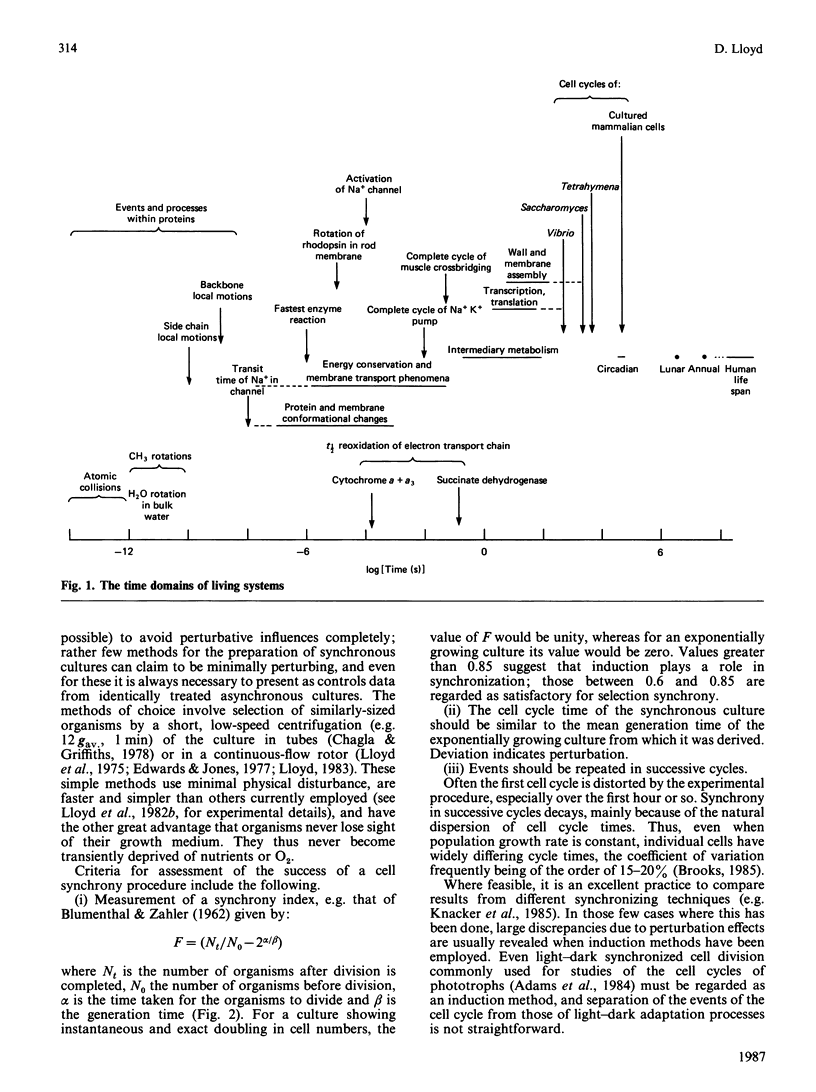
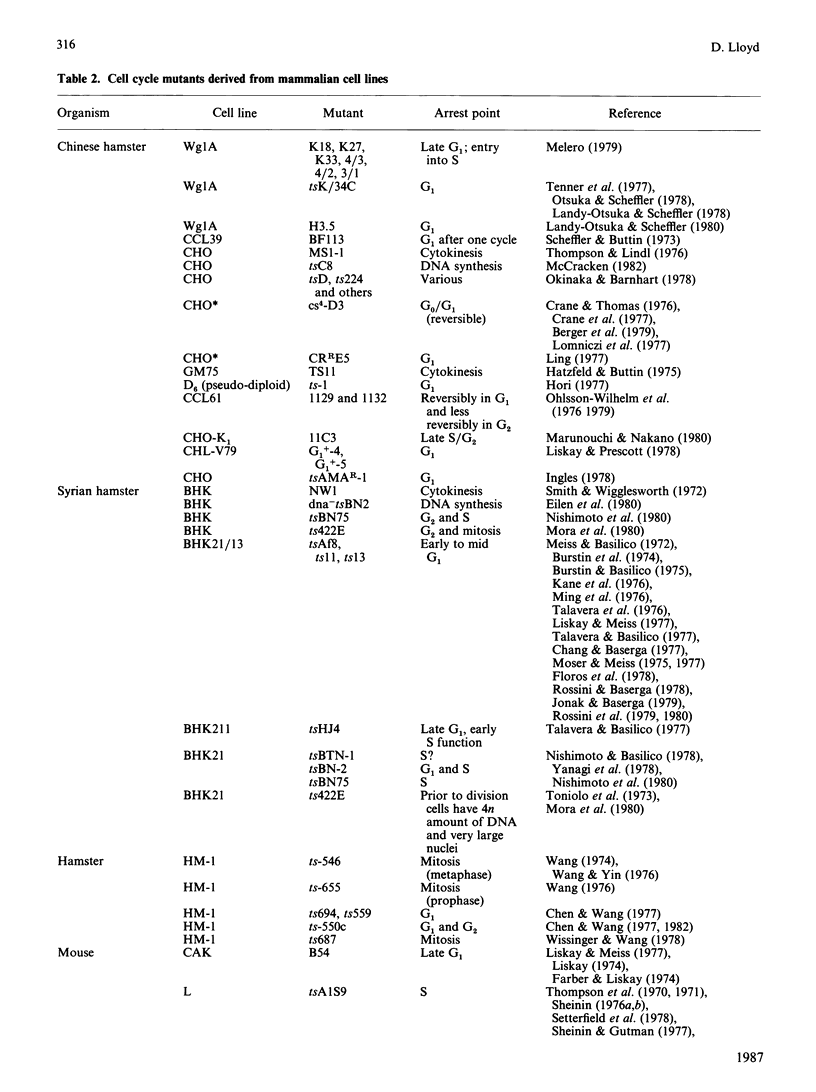
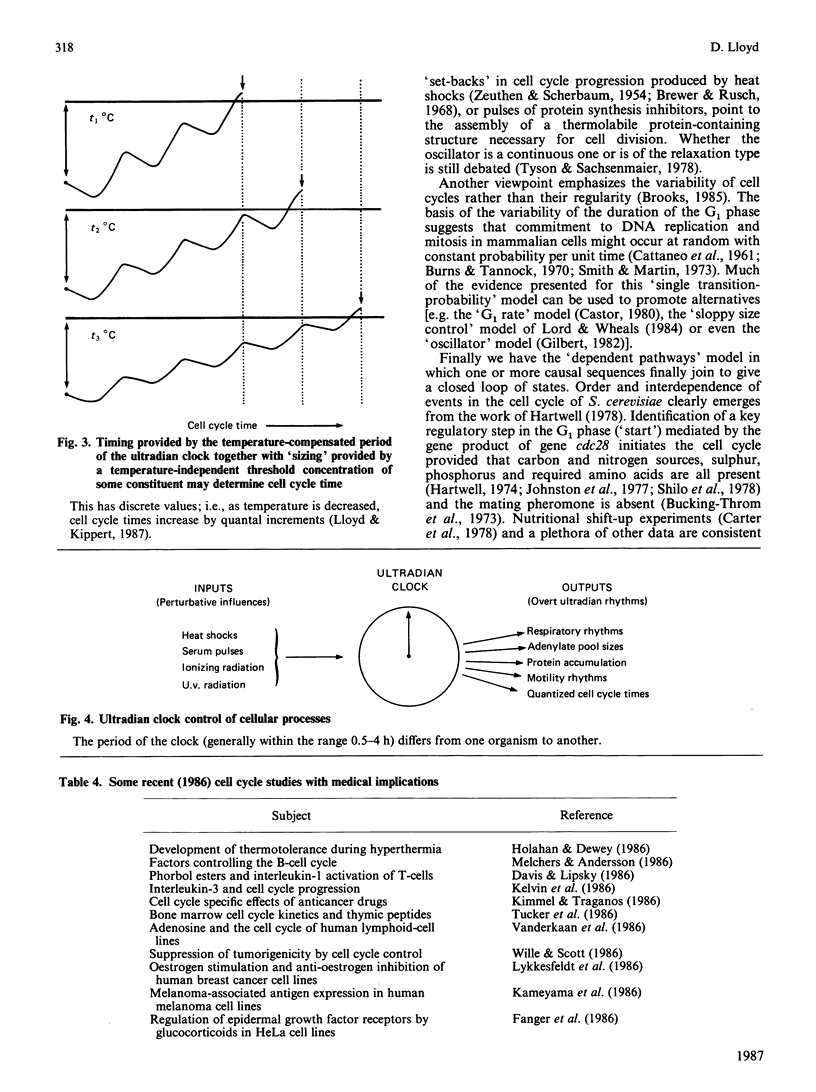
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Toda T., Niwa O., Yanagida M. Differential expressions of essential and nonessential alpha-tubulin genes in Schizosaccharomyces pombe. Mol Cell Biol. 1986 Jun;6(6):2168–2178. doi: 10.1128/mcb.6.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
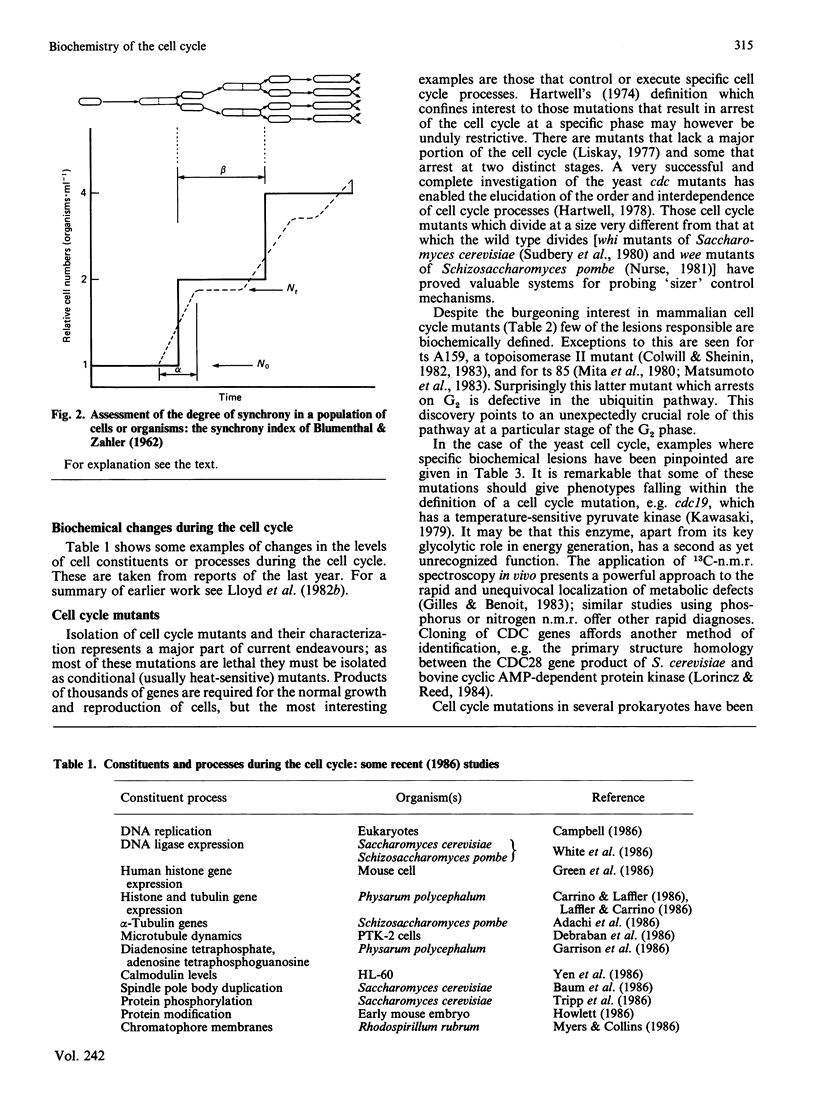
- BLUMENTHAL L. K., ZAHLER S. A. Index for measurement of synchronization of cell populations. Science. 1962 Mar 2;135(3505):724–724. doi: 10.1126/science.135.3505.724. [DOI] [PubMed] [Google Scholar]
- Baum P., Furlong C., Byers B. Yeast gene required for spindle pole body duplication: homology of its product with Ca2+-binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5512–5516. doi: 10.1073/pnas.83.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Petzold S. J., Berger S. J. Association of poly(ADP-rib) synthesis with cessation of DNA synthesis and DNA fragmentation. Biochim Biophys Acta. 1979 Aug 29;564(1):90–104. doi: 10.1016/0005-2787(79)90191-6. [DOI] [PubMed] [Google Scholar]
- Brewer E. N., Rusch H. P. Effect of elevated temperature shocks on mitosis and on the initiation of DNA replication in Physarum polycephalum. Exp Cell Res. 1968 Jan;49(1):79–86. doi: 10.1016/0014-4827(68)90521-1. [DOI] [PubMed] [Google Scholar]
- Burns F. J., Tannock I. F. On the existence of a G 0 -phase in the cell cycle. Cell Tissue Kinet. 1970 Oct;3(4):321–334. doi: 10.1111/j.1365-2184.1970.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Basilico C. Transformation by polyoma virus alters expression of a cell mutation affecting cycle traverse. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2540–2544. doi: 10.1073/pnas.72.7.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- Böhmer R. M. Flow cytometry and cell proliferation kinetics. Prog Histochem Cytochem. 1982;14(4):1–62. doi: 10.1016/s0079-6336(82)80001-6. [DOI] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- CATTANEO S. M., QUASTLER H., SHERMAN F. G. Proliferative cycle in the growing hair follicle of the mouse. Nature. 1961 Jun 3;190:923–924. doi: 10.1038/190923a0. [DOI] [PubMed] [Google Scholar]
- Campbell J. L. Eukaryotic DNA replication. Annu Rev Biochem. 1986;55:733–771. doi: 10.1146/annurev.bi.55.070186.003505. [DOI] [PubMed] [Google Scholar]
- Carrino J. J., Laffler T. G. Transcription of alpha-tubulin and histone H4 genes begins at the same point in the Physarum cell cycle. J Cell Biol. 1986 May;102(5):1666–1670. doi: 10.1083/jcb.102.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor L. N. A G1 rate model accounts for cell-cycle kinetics attributed to 'transition probability'. Nature. 1980 Oct 30;287(5785):857–859. doi: 10.1038/287857a0. [DOI] [PubMed] [Google Scholar]
- Chang H. L., Baserga R. Time of replication of genes responsible for a temperature-sensitive function in a cell cycle-specific ts mutant from a hamster cell line. J Cell Physiol. 1977 Sep;92(3):333–343. doi: 10.1002/jcp.1040920302. [DOI] [PubMed] [Google Scholar]
- Colwill R. W., Sheinin R. ts A1S9 locus in mouse L cells may encode a novobiocin binding protein that is required for DNA topoisomerase II activity. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4644–4648. doi: 10.1073/pnas.80.15.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane M. S., Thomas D. B. Cell-cycle, cell-shape mutant with features of the Go state. Nature. 1976 May 20;261(5557):205–208. doi: 10.1038/261205a0. [DOI] [PubMed] [Google Scholar]
- Davis L., Lipsky P. E. Signals involved in T cell activation. II. Distinct roles of intact accessory cells, phorbol esters, and interleukin 1 in activation and cell cycle progression of resting T lymphocytes. J Immunol. 1986 May 15;136(10):3588–3596. [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., Aerts F., De Mey J. Microtubule dynamics during the cell cycle: the effects of taxol and nocodazole on the microtubule system of Pt K2 cells at different stages of the mitotic cycle. Int Rev Cytol. 1986;101:215–274. doi: 10.1016/s0074-7696(08)60250-8. [DOI] [PubMed] [Google Scholar]
- Dickinson J. R. The cdc 22 mutation by Schizosaccharomyces pombe is a temperature-sensitive defect in nucleoside diphosphokinase. Eur J Biochem. 1981 Oct;119(2):341–345. doi: 10.1111/j.1432-1033.1981.tb05613.x. [DOI] [PubMed] [Google Scholar]
- Dickinson J. R., Williams A. S. The cdc30 mutation in Saccharomyces cerevisiae results in a temperature-sensitive isoenzyme of phosphoglucose isomerase. J Gen Microbiol. 1987 Jan;133(1):135–140. doi: 10.1099/00221287-133-1-135. [DOI] [PubMed] [Google Scholar]
- EAGON R. G. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J Bacteriol. 1962 Apr;83:736–737. doi: 10.1128/jb.83.4.736-737.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Evans J. B., Williams J. L., Lloyd D. The mitochondrial adenosine triphosphatase of Acanthamoeba castellanii. Oscillatory accumulation of enzyme activity, enzyme protein and F1-inhibitor during the cell cycle. Biochem J. 1982 Feb 15;202(2):453–458. doi: 10.1042/bj2020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Oscillations in protein and RNA content during synchronous growth of Acanthamoeba castellanii. Evidence for periodic turnover of macromolecules during the cell cycle. FEBS Lett. 1980 Jan 1;109(1):21–26. doi: 10.1016/0014-5793(80)81302-0. [DOI] [PubMed] [Google Scholar]
- Eilen E., Hand R., Basilico C. Decreased initiation of DNA synthesis in a temperature-sensitive mutant of hamster cells. J Cell Physiol. 1980 Nov;105(2):259–266. doi: 10.1002/jcp.1041050209. [DOI] [PubMed] [Google Scholar]
- Epifanova O. I., Polunovsky V. A. Cell cycle controls in higher eukaryotic cells: resting state or a prolonged G1 period? J Theor Biol. 1986 Jun 21;120(4):467–477. doi: 10.1016/s0022-5193(86)80040-6. [DOI] [PubMed] [Google Scholar]
- Fanger B. O., Currie R. A., Cidlowski J. A. Regulation of epidermal growth factor receptors by glucocorticoids during the cell cycle in HeLa S3 cells. Arch Biochem Biophys. 1986 Aug 15;249(1):116–125. doi: 10.1016/0003-9861(86)90566-7. [DOI] [PubMed] [Google Scholar]
- Farber R. A., Liskay R. M. Karyotypic analysis of a near-diploid established mouse cell line. Cytogenet Cell Genet. 1974;13(4):384–396. doi: 10.1159/000130288. [DOI] [PubMed] [Google Scholar]
- Floros J., Ashihara T., Baserga R. Characterization of ts13 cells a temperature-sensitive mutant of the G1 phase of the cell cycle. Cell Biol Int Rep. 1978 May;2(3):259–269. doi: 10.1016/0309-1651(78)90006-1. [DOI] [PubMed] [Google Scholar]
- Frankel J., Mohler J., Frankel A. K. The relationship between the excess-delay phenomenon and temperature-sensitive periods in Tetrahymena thermophila. J Cell Sci. 1980 Jun;43:75–91. doi: 10.1242/jcs.43.1.75. [DOI] [PubMed] [Google Scholar]
- Game J. C. Yeast cell-cycle mutant cdc21 is a temperature-sensitive thymidylate auxotroph. Mol Gen Genet. 1976 Aug 2;146(3):313–315. doi: 10.1007/BF00701257. [DOI] [PubMed] [Google Scholar]
- Garrison P. N., Mathis S. A., Barnes L. D. In vivo levels of diadenosine tetraphosphate and adenosine tetraphospho-guanosine in Physarum polycephalum during the cell cycle and oxidative stress. Mol Cell Biol. 1986 Apr;6(4):1179–1186. doi: 10.1128/mcb.6.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. A. An oscillator cell cycle model needs no first or second chance event. Biosystems. 1982;15(4):331–339. doi: 10.1016/0303-2647(82)90047-8. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Benoit A. G. NMR analysis of a cell division cycle mutant of Saccharomyces cerevisiae. Biochim Biophys Acta. 1983 Jun 2;762(3):466–470. doi: 10.1016/0167-4889(83)90013-7. [DOI] [PubMed] [Google Scholar]
- Green L., Schlaffer I., Wright K., Moreno M. L., Berand D., Hager G., Stein J., Stein G. Cell cycle-dependent expression of a stable episomal human histone gene in a mouse cell. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2315–2319. doi: 10.1073/pnas.83.8.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Cell division from a genetic perspective. J Cell Biol. 1978 Jun;77(3):627–637. doi: 10.1083/jcb.77.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld J., Buttin G. Temperature-sensitive cell cycle mutants: a chinese hamster cell line with a reversible block in cytokinesis. Cell. 1975 Jun;5(2):123–129. doi: 10.1016/0092-8674(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Holahan P. K., Dewey W. C. Effect of pH and cell cycle progression on development and decay of thermotolerance. Radiat Res. 1986 Apr;106(1):111–121. [PubMed] [Google Scholar]
- Howlett S. K. A set of proteins showing cell cycle dependent modification in the early mouse embryo. Cell. 1986 May 9;45(3):387–396. doi: 10.1016/0092-8674(86)90324-7. [DOI] [PubMed] [Google Scholar]
- Hyodo M., Suzuki K. A temperature-sensitive mutant isolated from mouse FM3A cells defective in DNA replication at a non-permissive temperature. Exp Cell Res. 1982 Jan;137(1):31–38. doi: 10.1016/0014-4827(82)90004-0. [DOI] [PubMed] [Google Scholar]
- Ide T., Ninomiya J., Ishibashi S. Isolation of a G0-specific ts mutant from a Fischer rat cell line, 3Y1. Exp Cell Res. 1984 Jan;150(1):60–67. doi: 10.1016/0014-4827(84)90701-8. [DOI] [PubMed] [Google Scholar]
- Ingles C. J. Temperature-sensitive RNA polymerase II mutations in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):405–409. doi: 10.1073/pnas.75.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. The role of ionic currents in establishing developmental pattern. Philos Trans R Soc Lond B Biol Sci. 1981 Oct 7;295(1078):553–566. doi: 10.1098/rstb.1981.0160. [DOI] [PubMed] [Google Scholar]
- James R., Bradshaw R. A. Polypeptide growth factors. Annu Rev Biochem. 1984;53:259–292. doi: 10.1146/annurev.bi.53.070184.001355. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Nasmyth K. A. Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase. Nature. 1978 Aug 31;274(5674):891–893. doi: 10.1038/274891a0. [DOI] [PubMed] [Google Scholar]
- Jonak G. J., Baserga R. Cytoplasmic regulation of two G1-specific temperature-sensitive functions. Cell. 1979 Sep;18(1):117–123. doi: 10.1016/0092-8674(79)90360-x. [DOI] [PubMed] [Google Scholar]
- Kameyama K., Takezaki S., Kanzaki T., Nishiyama S. HLA-DR and melanoma-associated antigen (p97) expression during the cell cycle in human melanoma cell lines, and the effects of recombinant gamma-interferon: two-color flow cytometric analysis. J Invest Dermatol. 1986 Sep;87(3):313–318. doi: 10.1111/1523-1747.ep12524381. [DOI] [PubMed] [Google Scholar]
- Kane G., Basilico C., Basterga R. Transcriptional activity and chromatin structural changes in a temperature-sensitive mutant of BHK cells blocked in early G1. Exp Cell Res. 1976 Apr;99(1):165–173. doi: 10.1016/0014-4827(76)90691-1. [DOI] [PubMed] [Google Scholar]
- Kauffman S., Wille J. J. The mitotic oscillator in Physarum polycephalum. J Theor Biol. 1975 Nov;55(1):47–93. doi: 10.1016/s0022-5193(75)80108-1. [DOI] [PubMed] [Google Scholar]
- Kelvin D. J., Chance S., Shreeve M., Axelrad A. A., Connolly J. A., McLeod D. Interleukin 3 and cell cycle progression. J Cell Physiol. 1986 Jun;127(3):403–409. doi: 10.1002/jcp.1041270308. [DOI] [PubMed] [Google Scholar]
- Knacker T., Harwood J. L., Hunter C. N., Russell N. J. Lipid biosynthesis in synchronized cultures of the photosynthetic bacterium Rhodopseudomonas sphaeroides. Biochem J. 1985 Aug 1;229(3):701–710. doi: 10.1042/bj2290701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLoyd D., John L., Edwards C., Chagla A. H. Synchronous cultures of micro-organisms: large-scale preparation by continuous-flow size selection. J Gen Microbiol. 1975 May;88(1):153–158. doi: 10.1099/00221287-88-1-153. [DOI] [PubMed] [Google Scholar]
- Laffler T. G., Carrino J. J. The tubulin and histone genes of Physarum polycephalum: models for cell cycle-regulated gene expression. Bioessays. 1986 Aug;5(2):62–65. doi: 10.1002/bies.950050205. [DOI] [PubMed] [Google Scholar]
- Landy-Otsuka F., Scheffler I. E. Enzyme induction in a temperature-sensitive cell cycle mutant of Chinese hamster fibroblasts. J Cell Physiol. 1980 Nov;105(2):209–220. doi: 10.1002/jcp.1041050204. [DOI] [PubMed] [Google Scholar]
- Landy-Otsuka F., Scheffler I. E. Induction of ornithine decarboxylase activity in a temperature-sensitive cell cycle mutant of Chinese hamster cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5001–5005. doi: 10.1073/pnas.75.10.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V. A membrane-altered mutant cold-sensitive for growth. J Cell Physiol. 1977 May;91(2):209–223. doi: 10.1002/jcp.1040910207. [DOI] [PubMed] [Google Scholar]
- Liskay R. M. A mammalian somatic "cell cycle" mutant defective in G1. J Cell Physiol. 1974 Aug;84(1):49–55. doi: 10.1002/jcp.1040840106. [DOI] [PubMed] [Google Scholar]
- Liskay R. M. Absence of a measurable G2 phase in two Chinese hamster cell lines. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1622–1625. doi: 10.1073/pnas.74.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Meiss H. K. Complementation between two temperature-sensitive mammalian cell mutants, each defective in the G1 phase of the cell cycle. Somatic Cell Genet. 1977 May;3(3):343–347. doi: 10.1007/BF01538752. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Prescott D. M. Genetic analysis of the G1 period: isolation of mutants (or variants) with a G1 perior from a Chinese hamster cell line lacking G1. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2873–2877. doi: 10.1073/pnas.75.6.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D., Edwards S. W., Fry J. C. Temperature-compensated oscillations in respiration and cellular protein content in synchronous cultures of Acanthamoeba castellanii. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3785–3788. doi: 10.1073/pnas.79.12.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Bosch F. X., Hay A. J., Skehel J. J. Influenza virus infection of a cell-cycle mutant of Chinese hamster ovary cells. J Gen Virol. 1977 Apr;35(1):187–190. doi: 10.1099/0022-1317-35-1-187. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp Cell Res. 1983 Jun;146(1):151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Yasuda H., Marunouchi T., Yamada M. Decrease in uH2A (protein A24) of a mouse temperature-sensitive mutant. FEBS Lett. 1983 Jan 10;151(1):139–142. doi: 10.1016/0014-5793(83)80359-7. [DOI] [PubMed] [Google Scholar]
- McCracken A. A. A temperature-sensitive DNA synthesis mutant isolated from the Chinese hamster ovary cell line. Somatic Cell Genet. 1982 Mar;8(2):179–195. doi: 10.1007/BF01538676. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Basilico C. Temperature sensitive mutants of BHK 21 cells. Nat New Biol. 1972 Sep 20;239(90):66–68. doi: 10.1038/newbio239066a0. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Factors controlling the B-cell cycle. Annu Rev Immunol. 1986;4:13–36. doi: 10.1146/annurev.iy.04.040186.000305. [DOI] [PubMed] [Google Scholar]
- Melero J. A. Isolation and cell cycle analysis of temperature-sensitive mutants from Chinese hamster cells. J Cell Physiol. 1979 Jan;98(1):17–30. doi: 10.1002/jcp.1040980104. [DOI] [PubMed] [Google Scholar]
- Ming P. M., Chang H. L., Baserga R. Release by human chromosome 3 of the block at G1 of the cell cycle, in hybrids between tsAF8 hamster and human cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2052–2055. doi: 10.1073/pnas.73.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Yasuda H., Marunouchi T., Ishiko S., Yamada M. A temperature-sensitive mutant of cultured mouse cells defective in chromosome condensation. Exp Cell Res. 1980 Apr;126(2):407–416. doi: 10.1016/0014-4827(80)90280-3. [DOI] [PubMed] [Google Scholar]
- Mora M., Darzynkiewicz Z., Baserga R. DNA synthesis and cell division in a mammalian cell mutant temperature sensitive for the processing of ribosomal RNA. Exp Cell Res. 1980 Jan;125(1):241–249. doi: 10.1016/0014-4827(80)90208-6. [DOI] [PubMed] [Google Scholar]
- Moser G. C., Meiss H. K. A cytological procedure to screen mammalian temperature-sensitive mutants for cell-cycle-related defects. Somatic Cell Genet. 1977 Jul;3(4):449–456. doi: 10.1007/BF01542973. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Collins M. L. Cell-cycle-specific oscillation in the composition of chromatophore membrane in Rhodospirillum rubrum. J Bacteriol. 1986 Jun;166(3):818–823. doi: 10.1128/jb.166.3.818-823.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naha P. M. Complementation of G1-phase variants of a mammalian cell cycle. J Cell Sci. 1979 Feb;35:53–58. doi: 10.1242/jcs.35.1.53. [DOI] [PubMed] [Google Scholar]
- Naha P. M. Early functional mutants of mammalian cells. Nat New Biol. 1973 Jan 3;241(105):13–14. doi: 10.1038/newbio241013a0. [DOI] [PubMed] [Google Scholar]
- Naha P. M. Induction of G1- cells at high frequency. J Cell Sci. 1979 Dec;40:33–42. doi: 10.1242/jcs.40.1.33. [DOI] [PubMed] [Google Scholar]
- Naha P. M., Meyer A. L., Hewitt K. Mapping of the G1 phase of a mammalian cell cycle. Nature. 1975 Nov 6;258(5530):49–53. doi: 10.1038/258049a0. [DOI] [PubMed] [Google Scholar]
- Naha P. M., Sorrentino R. Biochemical controls of the G1 phase of a mammalian cell cycle. I. Analysis of chromatin proteins in temperature-sensitive variants. Cell Biol Int Rep. 1980 Apr;4(4):365–378. doi: 10.1016/0309-1651(80)90218-0. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Sekiguchi T., Yamada M. A. A mammalian cell mutant with temperature-sensitive thymidine kinase. Somatic Cell Genet. 1978 Mar;4(2):169–178. doi: 10.1007/BF01538982. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A. Temperature-sensitive lethal mutants in the structural gene for DNA ligase in the yeast Schizosaccharomyces pombe. Cell. 1977 Dec;12(4):1109–1120. doi: 10.1016/0092-8674(77)90173-8. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Basilico C. Analysis of a method for selecting temperature-sensitive mutants of BHK cells. Somatic Cell Genet. 1978 May;4(3):323–340. doi: 10.1007/BF01542846. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Takahashi T., Basilico C. A temperature-sensitive mutation affecting S-phase progression can lead to accumulation of cells with a G2 DNA content. Somatic Cell Genet. 1980 Jul;6(4):465–476. doi: 10.1007/BF01539150. [DOI] [PubMed] [Google Scholar]
- Ohlsson-Wilhelm B. M., Freed J. J., Perry R. P. Selective isolation of reversible cold sensitive variants from Chinese hamster ovary cell cultures. J Cell Physiol. 1976 Sep;89(1):77–88. doi: 10.1002/jcp.1040890108. [DOI] [PubMed] [Google Scholar]
- Patterson M., Sclafani R. A., Fangman W. L., Rosamond J. Molecular characterization of cell cycle gene CDC7 from Saccharomyces cerevisiae. Mol Cell Biol. 1986 May;6(5):1590–1598. doi: 10.1128/mcb.6.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini M., Baserga R. RNA synthesis in a cell cycle-specific temperature sensitive mutant from a hamster cell line. Biochemistry. 1978 Mar 7;17(5):858–863. doi: 10.1021/bi00598a017. [DOI] [PubMed] [Google Scholar]
- Rossini M., Baserga S., Huang C. H., Ingles C. J., Baserga R. Changes in RNA polymerase II in a cell cycle-specific temperature-sensitive mutant of hamster cells. J Cell Physiol. 1980 Apr;103(1):97–103. doi: 10.1002/jcp.1041030114. [DOI] [PubMed] [Google Scholar]
- Rossini M., Weinmann R., Baserga R. DNA synthesis in temperature-sensitive mutants of the cell cycle infected by polyoma virus and adenovirus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4441–4445. doi: 10.1073/pnas.76.9.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Hama-Inaba H. A temperature-sensitive mammalian cell mutant exhibiting micronucleation. Exp Cell Res. 1978 Jul;114(2):484–486. doi: 10.1016/0014-4827(78)90515-3. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Buttin G. Conditionally lethal mutations in Chinese hamster cells. I. Isolation of a temperature-sensitive line and its investigation by cell cycle studies. J Cell Physiol. 1973 Apr;81(2):199–216. doi: 10.1002/jcp.1040810208. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Yeast gene CDC8 encodes thymidylate kinase and is complemented by herpes thymidine kinase gene TK. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5821–5825. doi: 10.1073/pnas.81.18.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sel'kov E. E. Dva al'ternativnykh avtokolebatel'nykh statsionarnykh sostoianiia v metabolizme tiolov--dva al'ternativnykh tipa razmnozheniia kletok: normal'nyi i zlokachestvennyi. Biofizika. 1970 Nov-Dec;15(6):1065–1073. [PubMed] [Google Scholar]
- Setterfield G., Sheinin R., Dardick I., Kiss G., Dubsky M. Structure of interphase nuclei in relation to the cell cycle. Chromatin organization in mouse L cells temperature-sensitive for DNA replication. J Cell Biol. 1978 Apr;77(1):246–263. doi: 10.1083/jcb.77.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]
- Sheinin Polyoma and cell DNA synthesis in mouse L cells temperature sensitive for the replication of cell DNA. J Virol. 1976 Mar;17(3):692–704. doi: 10.1128/jvi.17.3.692-704.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R., Guttman S. Semi-conservative and non-conservative replication of DNA in temperature-sensitive mouse L-cells. Biochim Biophys Acta. 1977 Nov 2;479(1):105–118. doi: 10.1016/0005-2787(77)90130-7. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Humbert J. Some aspects of eukaryotic DNA replication. Annu Rev Biochem. 1978;47:277–316. doi: 10.1146/annurev.bi.47.070178.001425. [DOI] [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Shilo B., Simchen G., Pardee A. B. Regulation of cell-cycle initiation in yeast by nutrients and protein synthesis. J Cell Physiol. 1978 Nov;97(2):177–187. doi: 10.1002/jcp.1040970207. [DOI] [PubMed] [Google Scholar]
- Shiomi T., Sato K. A temperature-sensitive mutant defective in mitosis and cytokinesis. Exp Cell Res. 1976 Jul;100(2):297–302. doi: 10.1016/0014-4827(76)90151-8. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Ozer H. L. Temperature-sensitive mutants of Balb/3T3 cells: description of a mutant affected in cellular and polyoma virus DNA synthesis. Cell. 1976 Feb;7(2):289–295. doi: 10.1016/0092-8674(76)90028-3. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Wigglesworth N. M. Cell line which is temperature-sensitive for cytokinesis. J Cell Physiol. 1972 Oct;80(2):253–259. doi: 10.1002/jcp.1040800212. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P. E., Goodey A. R., Carter B. L. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature. 1980 Nov 27;288(5789):401–404. doi: 10.1038/288401a0. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M. Interaction of the Circadian Cycle with the Cell Cycle in Pyrocystis fusiformis. Plant Physiol. 1982 Jul;70(1):272–276. doi: 10.1104/pp.70.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera A., Basilico C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J Cell Physiol. 1977 Sep;92(3):425–436. doi: 10.1002/jcp.1040920310. [DOI] [PubMed] [Google Scholar]
- Tenner A., Zieg J., Scheffler I. E. Glycoprotein synthesis in a temperature-sensitive Chinese hamster cell cycle mutant. J Cell Physiol. 1977 Feb;90(2):145–160. doi: 10.1002/jcp.1040900202. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Lindl P. A. A CHO-cell mutant with a defect in cytokinesis. Somatic Cell Genet. 1976 Sep;2(5):387–400. doi: 10.1007/BF01542720. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Till J. E., Siminovitch L., Whitmore G. F. Isolation of temperature-sensitive mutants of L-cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):377–384. doi: 10.1073/pnas.66.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Wright J. A., Till J. E., Siminovitch L., Whitmore G. F. Selective and nonselective isolation of temperature-sensitive mutants of mouse L-cells and their characterization. J Cell Physiol. 1971 Dec;78(3):431–440. doi: 10.1002/jcp.1040780312. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Meiss H. K., Basilico C. A temperature-sensitive mutation affecting 28S ribosomal RNA production in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1273–1277. doi: 10.1073/pnas.70.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp M. L., Piñon R., Meisenhelder J., Hunter T. Identification of phosphoproteins correlated with proliferation and cell cycle arrest in Saccharomyces cerevisiae: positive and negative regulation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5973–5977. doi: 10.1073/pnas.83.16.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. J., Hanaoka F., Nakano M. M., Yamada M. A mammalian DNA- mutant decreasing nuclear DNA polymerase alpha activity at nonpermissive temperature. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1190–1195. doi: 10.1016/0006-291x(79)92005-9. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., Hong L., Boorman G. A., Luster M. I. Alterations in bone marrow cell cycle kinetics by diphenylhydantoin and folate deficiency are restored by thymic peptides. Thymus. 1986;8(3):121–127. [PubMed] [Google Scholar]
- Tyson J., Sachsenmaier W. Is nuclear division in Physarum controlled by a continuous limit cycle oscillator? J Theor Biol. 1978 Aug 21;73(4):723–738. doi: 10.1016/0022-5193(78)90133-9. [DOI] [PubMed] [Google Scholar]
- Wang R. J. A novel temperature-sensitive mammalian cell line exhibiting defective prophase progression. Cell. 1976 Jun;8(2):257–261. doi: 10.1016/0092-8674(76)90009-x. [DOI] [PubMed] [Google Scholar]
- Wang R. J. Temperature-sensitive mammalian cell line blocked in mitosis. Nature. 1974 Mar 1;248(5443):76–78. doi: 10.1038/248076a0. [DOI] [PubMed] [Google Scholar]
- Wang R. J., Yin L. Further studies on a mutant mammalian cell line defective in mitosis. Exp Cell Res. 1976 Sep;101(2):331–336. doi: 10.1016/0014-4827(76)90385-2. [DOI] [PubMed] [Google Scholar]
- White J. H., Barker D. G., Nurse P., Johnston L. H. Periodic transcription as a means of regulating gene expression during the cell cycle: contrasting modes of expression of DNA ligase genes in budding and fission yeast. EMBO J. 1986 Jul;5(7):1705–1709. doi: 10.1002/j.1460-2075.1986.tb04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille J. J., Jr, Scott R. E. Suppression of tumorigenicity by the cell-cycle-dependent control of cellular differentiation and proliferation. Int J Cancer. 1986 Jun 15;37(6):875–881. doi: 10.1002/ijc.2910370613. [DOI] [PubMed] [Google Scholar]
- Williams R. J. Studies of larger motions within proteins by nuclear magnetic resonance spectroscopy. Biochem Soc Symp. 1981;(46):57–72. [PubMed] [Google Scholar]
- Wissinger W., Wang R. J. Studies on cell division in mammalian cells. IV. A temperature-sensitive cell line defective in post-metaphase chromosome movement. Exp Cell Res. 1978 Mar 1;112(1):89–94. doi: 10.1016/0014-4827(78)90528-1. [DOI] [PubMed] [Google Scholar]
- Yanagi K., Talavera A., Nishimoto T., Rush M. G. Inhibition of herpes simplex virus type 1 replication in temperature-sensitive cell cycle mutants. J Virol. 1978 Jan;25(1):42–50. doi: 10.1128/jvi.25.1.42-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Matsumoto Y., Mita S., Marunouchi T., Yamada M. A mouse temperature-sensitive mutant defective in H1 histone phosphorylation is defective in deoxyribonucleic acid synthesis and chromosome condensation. Biochemistry. 1981 Jul 21;20(15):4414–4419. doi: 10.1021/bi00518a028. [DOI] [PubMed] [Google Scholar]
- Yen A., Freeman L., Powers V., Van Sant R., Fishbaugh J. Cell cycle dependence of calmodulin levels during HL-60 proliferation and myeloid differentiation. No changes during pre-commitment. Exp Cell Res. 1986 Jul;165(1):139–151. doi: 10.1016/0014-4827(86)90539-2. [DOI] [PubMed] [Google Scholar]
- Zaitsu H., Kimura G. Arrest states in a set of mutants of rat 3Y1 cells temperature-sensitive for entering S phase. J Cell Physiol. 1984 Apr;119(1):82–88. doi: 10.1002/jcp.1041190114. [DOI] [PubMed] [Google Scholar]
- van der Kraan P. M., van Zandvoort P. M., De Abreu R. A., Bakkeren J. A., van Laarhoven J. P., de Bruyn C. H. Biphasic effect of adenosine on cell growth and cell cycle of human lymphoid cell lines. Adv Exp Med Biol. 1986;195(Pt A):553–559. doi: 10.1007/978-1-4684-5104-7_93. [DOI] [PubMed] [Google Scholar]


