Abstract
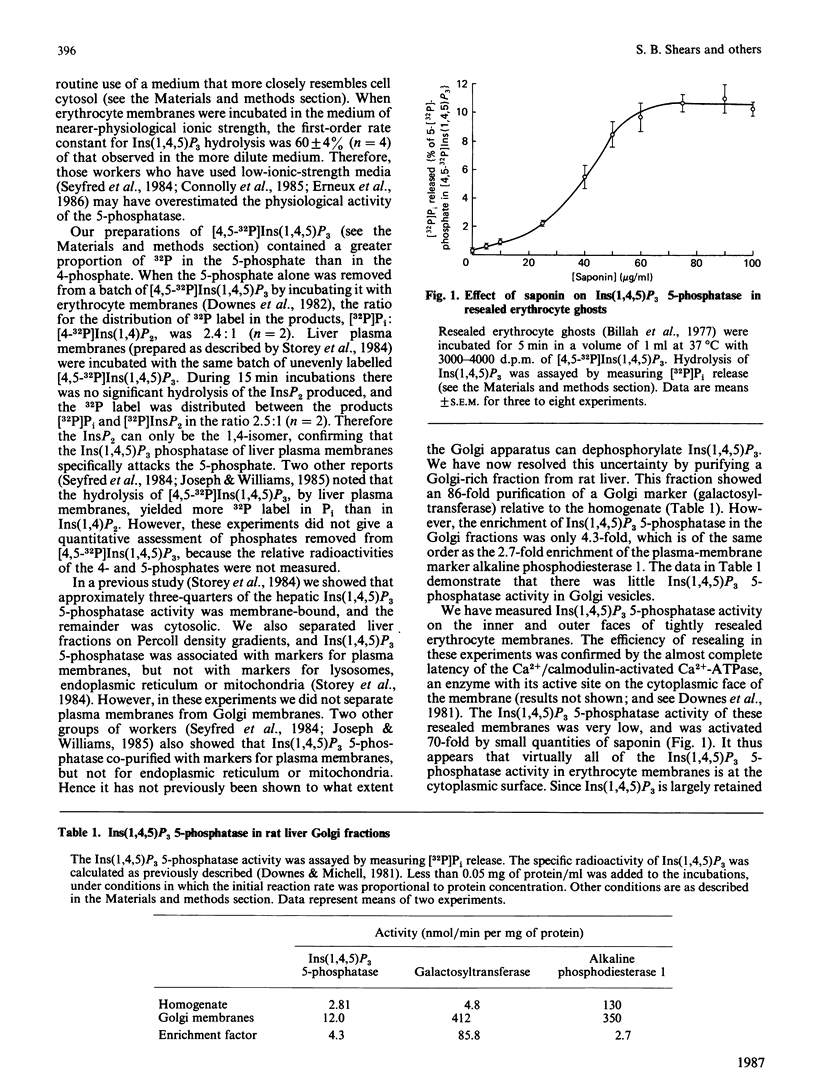
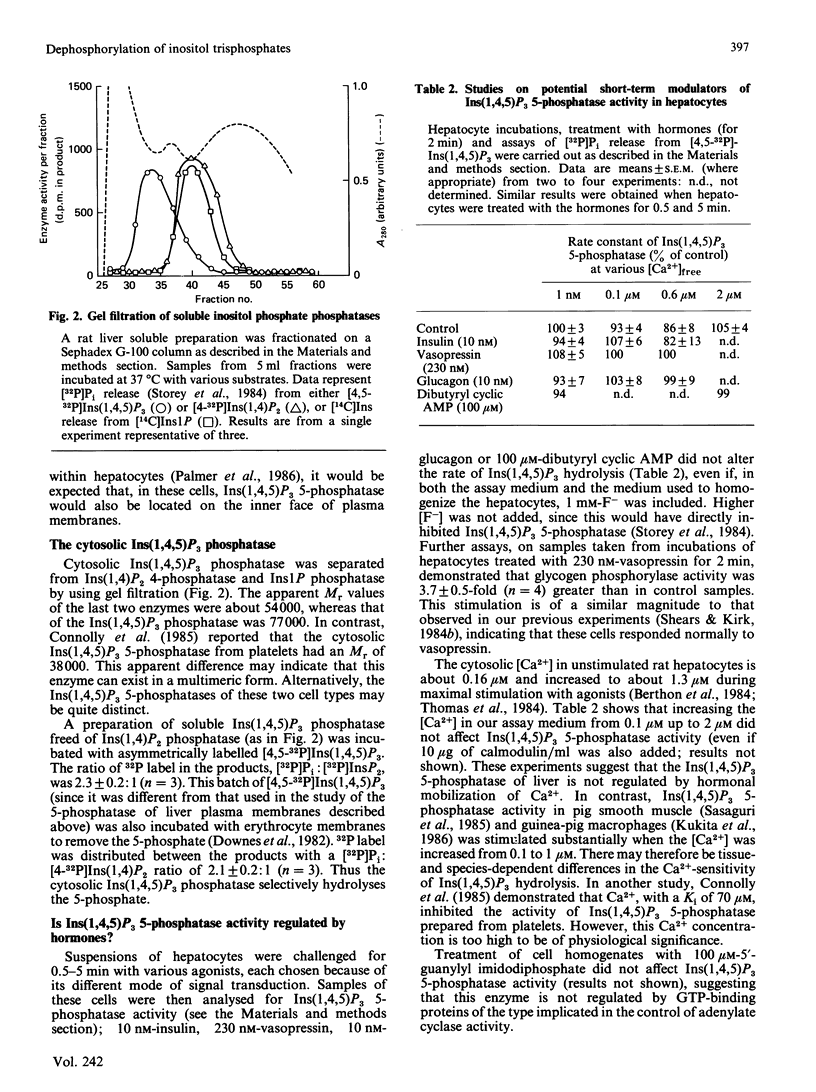
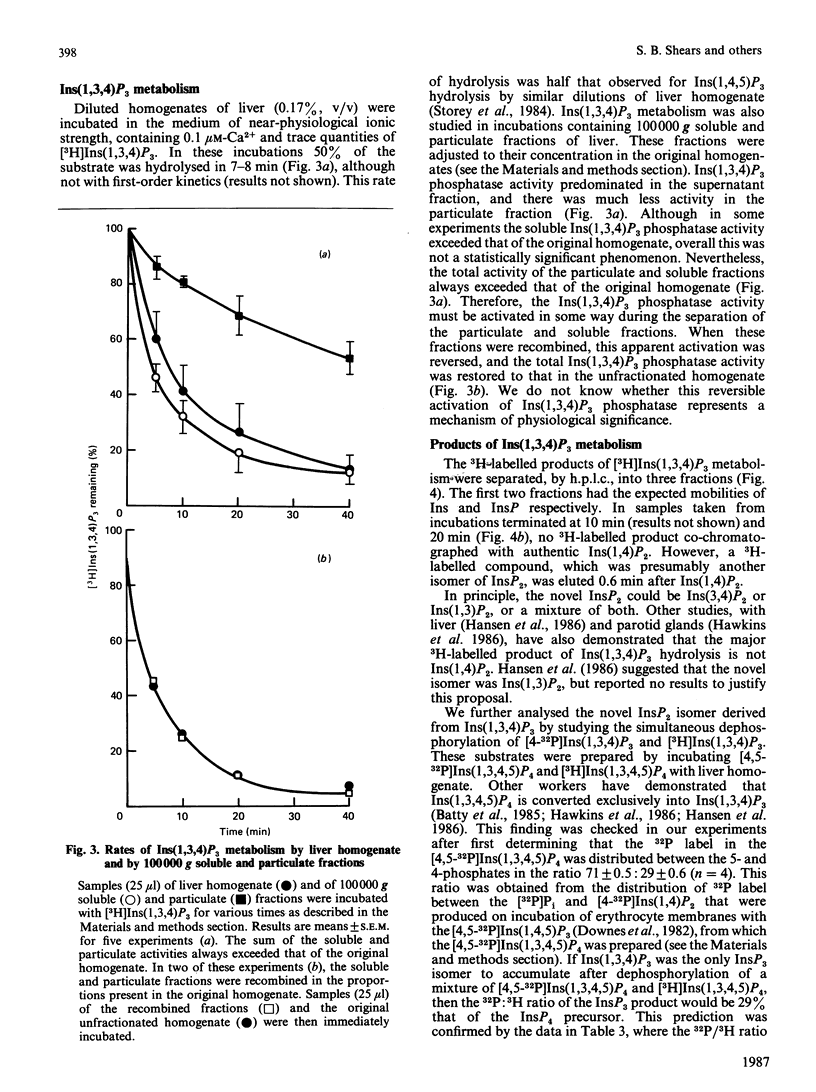
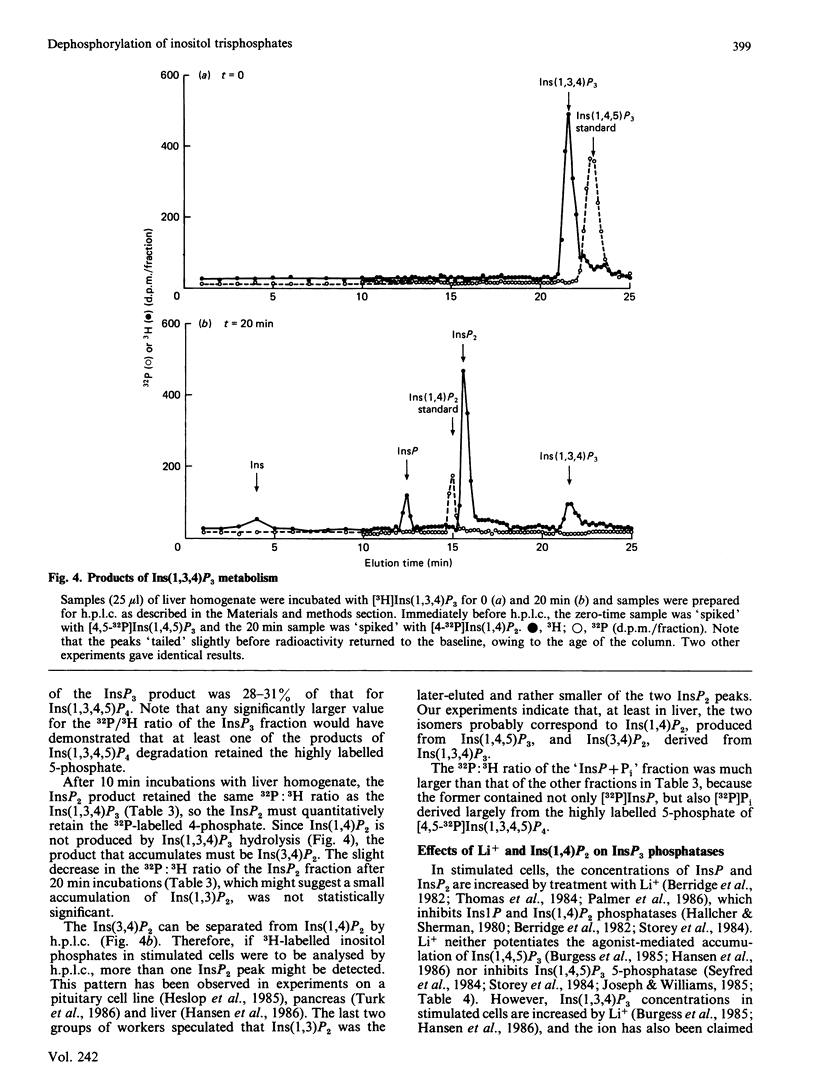
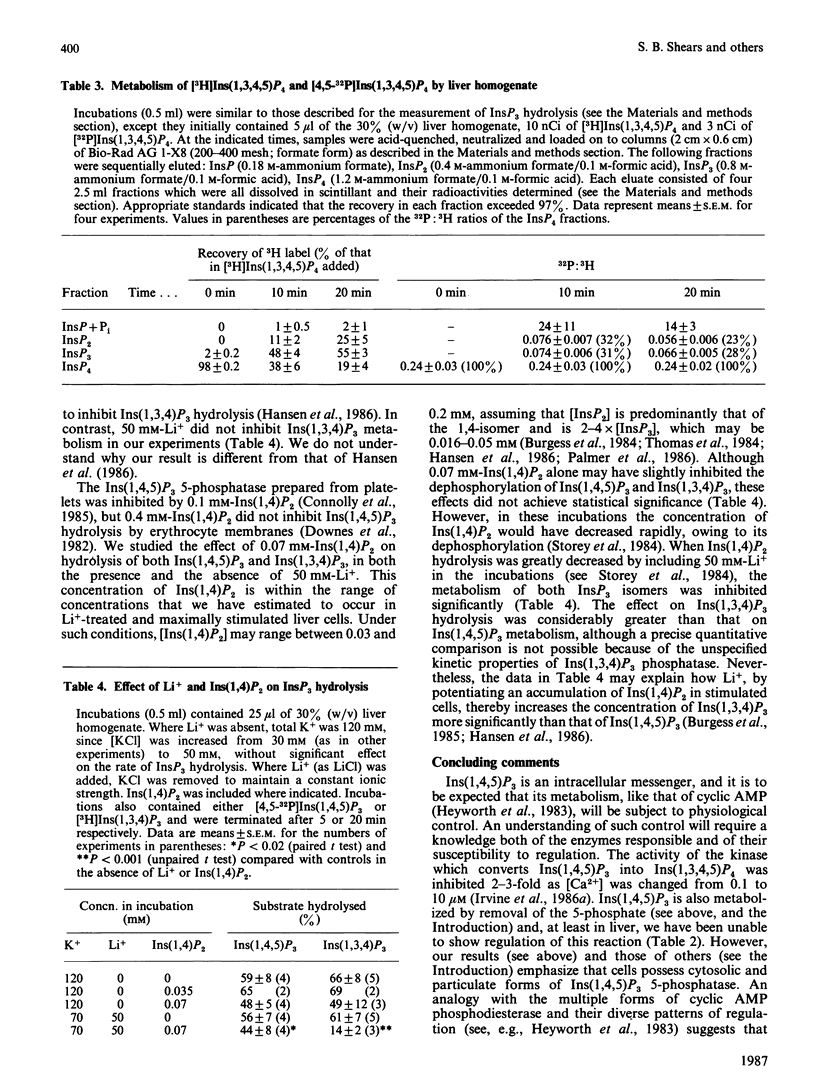
We have augmented our previous studies [Storey, Shears, Kirk & Michell (1984) Nature (London) 312, 374-376] on the subcellular location and properties of Ins(1,4,5)P3 (inositol 1,4,5-trisphosphate) phosphatases in rat liver and human erythrocytes. We also investigate Ins(1,3,4)P3 (inositol 1,3,4-trisphosphate) metabolism by rat liver. Membrane-bound and cytosolic Ins(1,4,5)P3 phosphatases both attack the 5-phosphate. The membrane-bound enzyme is located on the inner face of the plasma membrane, and there is little or no activity associated with Golgi apparatus. Cytosolic Ins(1,4,5)P3 5-phosphatase (Mr 77,000) was separated by gel filtration from Ins(1,4)P2 (inositol 1,4-bisphosphate) and inositol 1-phosphate phosphatases (Mr 54,000). Ins(1,4,5)P3 5-phosphatase activity in hepatocytes was unaffected by treatment of the cells with insulin, vasopressin, glucagon or dibutyryl cyclic AMP. Ins(1,4,5)P3 5-phosphatase activity in cell homogenates was unaffected by changes in [Ca2+] from 0.1 to 2 microM. After centrifugation of a liver homogenate at 100,000 g, Ins(1,3,4)P3 phosphatase activity was largely confined to the supernatant. The sum of the activities in the supernatant and the pellet exceeded that in the original homogenate. When these fractions were recombined, Ins(1,3,4)P3 phosphatase activity was restored to that observed in unfractionated homogenate. Ins(1,3,4)P3 was produced from Ins(1,3,4,5)P4 (inositol 1,3,4,5-tetrakisphosphate) and was metabolized to a novel InsP2 that was the 3,4-isomer. Ins(1,3,4)P3 phosphatase activity was not changed by 50 mM-Li+ or 0.07 mM-Ins(1,4)P2 alone, but when added together these agents inhibited Ins(1,3,4)P3 metabolism. In Li+-treated and vasopressin-stimulated hepatocytes, Ins(1,4)P2 may reach concentrations sufficient to inhibit Ins(1,3,4)P3 metabolism, with little effect on Ins(1,4,5)P3 hydrolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Finean J. B., Coleman R., Michell R. H. Permeability characteristics of erythrocyte ghosts prepared under isoionic conditions by a glycol-induced osmotic lysis. Biochim Biophys Acta. 1977 Mar 17;465(3):515–526. doi: 10.1016/0005-2736(77)90269-3. [DOI] [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat brain. Biochem Biophys Res Commun. 1986 Jan 14;134(1):351–358. doi: 10.1016/0006-291x(86)90570-x. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J. Subcellular localization and some properties of the enzymes hydrolysing inositol polyphosphates in rat liver. FEBS Lett. 1985 Jan 28;180(2):150–154. doi: 10.1016/0014-5793(85)81061-9. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Michell R. H., Parry J. B., Shears S. B. Inositol trisphosphate and tetrakisphosphate phosphomonoesterases of rat liver. Biochem Soc Trans. 1987 Feb;15(1):28–32. doi: 10.1042/bst0150028. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukita M., Hirata M., Koga T. Requirement of Ca2+ for the production and degradation of inositol 1,4,5-trisphosphate in macrophages. Biochim Biophys Acta. 1986 Jan 23;885(1):121–128. doi: 10.1016/0167-4889(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Michell B. Inositol phosphates. Profusion and confusion. Nature. 1986 Jan 16;319(6050):176–177. doi: 10.1038/319176a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hawkins P. T., Michell R. H., Kirk C. J. The labelling of polyphosphoinositides with [32P]Pi and the accumulation of inositol phosphates in vasopressin-stimulated hepatocytes. Biochem J. 1986 Sep 1;238(2):491–499. doi: 10.1042/bj2380491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri T., Hirata M., Kuriyama H. Dependence on Ca2+ of the activities of phosphatidylinositol 4,5-bisphosphate phosphodiesterase and inositol 1,4,5-trisphosphate phosphatase in smooth muscles of the porcine coronary artery. Biochem J. 1985 Nov 1;231(3):497–503. doi: 10.1042/bj2310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfred M. A., Farrell L. E., Wells W. W. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat liver plasma membranes. J Biol Chem. 1984 Nov 10;259(21):13204–13208. [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J. Characterization of a rapid cellular-fractionation technique for hepatocytes. Application in the measurement of mitochondrial membrane potential in situ. Biochem J. 1984 Apr 15;219(2):375–382. doi: 10.1042/bj2190375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J. Determination of mitochondrial calcium content in hepatocytes by a rapid cellular fractionation technique. Vasopressin stimulates mitochondrial Ca2+ uptake. Biochem J. 1984 Jun 1;220(2):417–421. doi: 10.1042/bj2200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Turk J., Wolf B. A., McDaniel M. L. Glucose-induced accumulation of inositol trisphosphates in isolated pancreatic islets. Predominance of the 1,3,4-isomer. Biochem J. 1986 Jul 1;237(1):259–263. doi: 10.1042/bj2370259. [DOI] [PMC free article] [PubMed] [Google Scholar]


