Abstract
The distribution of N-sulphate groups within fibroblast heparan sulphate chains was investigated. The detergent-extractable heparan sulphate proteoglycan from adult human skin fibroblasts, radiolabelled with [3H]glucosamine and [35S]sulphate, was coupled to CNBr-activated Sepharose 4B. After partial depolymerization of the heparan sulphate with nitrous acid, the remaining Sepharose-bound fragments were removed by treatment with alkali. These fragments, of various sizes, but all containing an intact reducing xylose residue, were fractionated on Sephacryl S-300 and the distribution of the 3H and 35S radiolabels was analysed. A decreased degree of sulphation was observed towards the reducing termini of the chains. After complete nitrous acid hydrolysis of the Sepharose-bound proteoglycan, analysis of the proximity of N-sulphation to the reducing end revealed the existence of an extended N-acetylated sequence directly adjacent to the protein-linkage sequence. The size of this N-acetylated domain was estimated by gel filtration to be approximately eight disaccharide units. This domain appears to be highly conserved, being present in virtually all the chains derived from this proteoglycan, implying the existence of a mechanism capable of generating such a non-random sequence during the post-polymeric modification of heparan sulphate. Comparison with the corresponding situation in heparin suggests that different mechanisms regulate polymer N-sulphation in the vicinity of the protein-linkage region of these chemically related glycosaminoglycans.
Full text
PDF
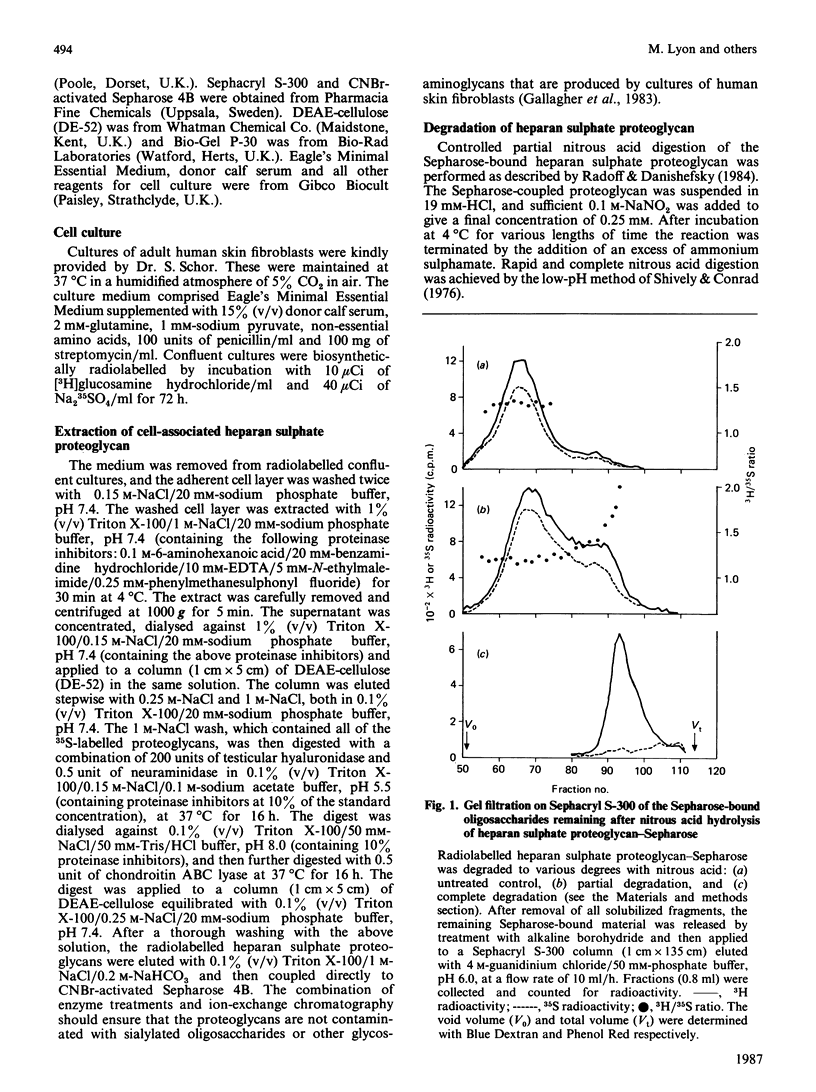
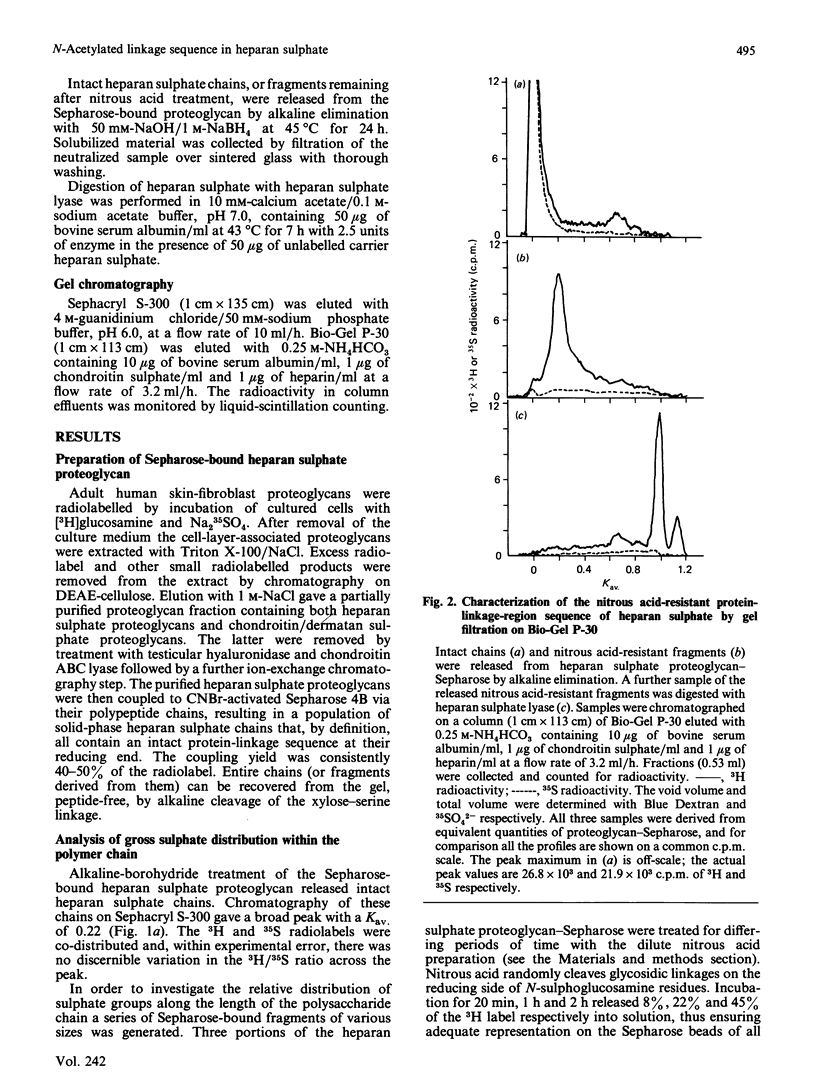
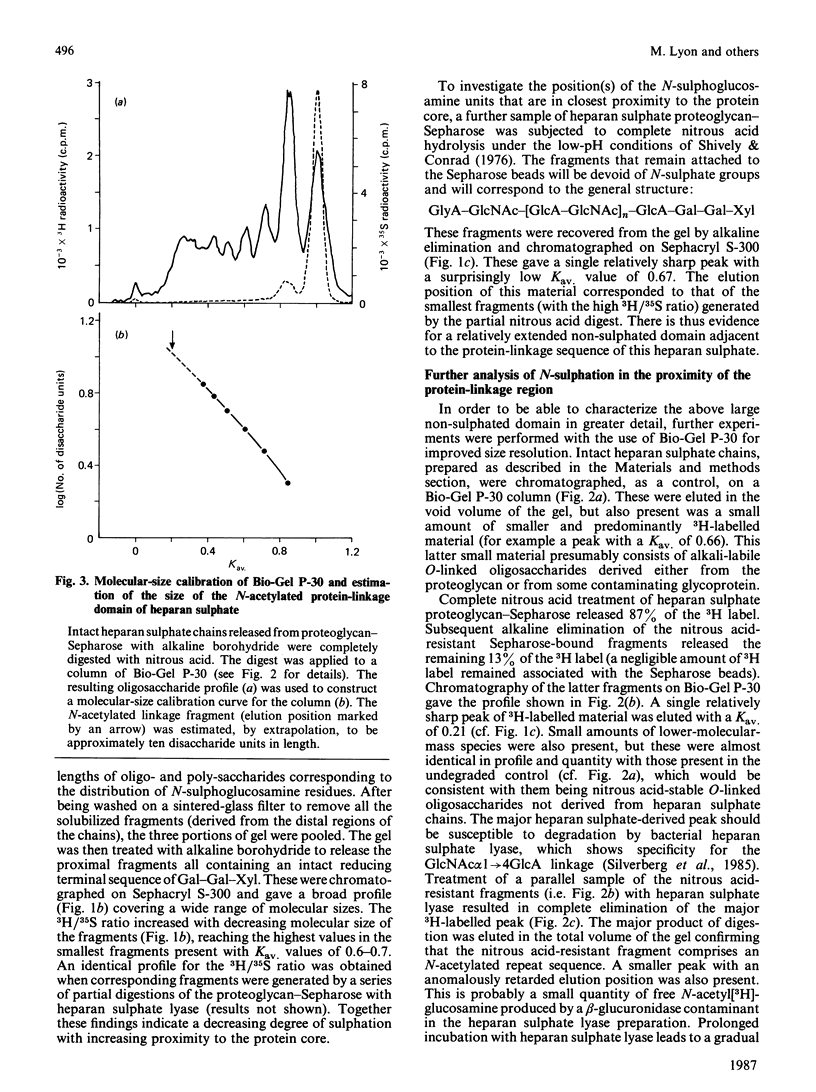


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlstedt I., Cöster L., Malmström A., Fransson L. A. Proteoheparan sulfate from human skin fibroblasts. Isolation and structural characterization. J Biol Chem. 1983 Oct 10;258(19):11629–11635. [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Silverberg I., Carlstedt I. Structure of the heparan sulfate-protein linkage region. Demonstration of the sequence galactosyl-galactosyl-xylose-2-phosphate. J Biol Chem. 1985 Nov 25;260(27):14722–14726. [PubMed] [Google Scholar]
- Gallagher J. T., Gasiunas N., Schor S. L. Specific association of iduronic acid-rich dermatan sulphate with the extracellular matrix of human skin fibroblasts cultured on collagen gels. Biochem J. 1983 Oct 1;215(1):107–116. doi: 10.1042/bj2150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A., Young E. Asymmetric distribution of sites with high affinity for antithrombin III in rat skin heparin proteoglycans. J Biol Chem. 1982 Aug 10;257(15):8749–8754. [PubMed] [Google Scholar]
- Knecht J., Cifonelli J. A., Dorfman A. Structural studies on heparitin sulfate of normal and Hurler tissues. J Biol Chem. 1967 Oct 25;242(20):4652–4661. [PubMed] [Google Scholar]
- Lindahl U. Further characterization of the heparin-protein linkage region. Biochim Biophys Acta. 1966 Dec 28;130(2):368–382. doi: 10.1016/0304-4165(66)90233-9. [DOI] [PubMed] [Google Scholar]
- Lindahl U. The structures of xylosylserine and galactosylxylosylserine from heparin. Biochim Biophys Acta. 1966 Dec 28;130(2):361–367. doi: 10.1016/0304-4165(66)90232-7. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Merchant Z. M., Rice K. G., Kim Y. S., Fitzgerald G. L., Grant A. C., Langer R. Evidence of random structural features in the heparin polymer. Biochemistry. 1985 Dec 17;24(26):7805–7810. doi: 10.1021/bi00347a045. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Smith J. A., Austen K. F., Silbert J. E. Polydispersity of rat mast cell heparin. Implications for proteoglycan assembly. J Biol Chem. 1980 Dec 25;255(24):11753–11758. [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Characterization of the glycosaminoglycan component of the renal glomerular basement membrane and its relationship to the peptide portion. J Biol Chem. 1981 Jan 10;256(1):507–513. [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Isolation and characterization of the heparan sulfate proteoglycan of the bovine glomerular basement membrane. J Biol Chem. 1984 Oct 25;259(20):12749–12755. [PubMed] [Google Scholar]
- Radoff S., Danishefsky I. Distribution of glucuronic and iduronic acid units in heparin chains. J Biol Chem. 1985 Dec 5;260(28):15106–15111. [PubMed] [Google Scholar]
- Radoff S., Danishefsky I. Location on heparin of the oligosaccharide section essential for anticoagulant activity. J Biol Chem. 1984 Jan 10;259(1):166–172. [PubMed] [Google Scholar]
- Radoff S., Danishefsky I. Solid-phase synthesis of fluorescent heparin. Anal Biochem. 1982 Mar 1;120(2):373–378. doi: 10.1016/0003-2697(82)90360-8. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]


