Abstract
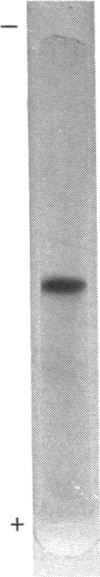
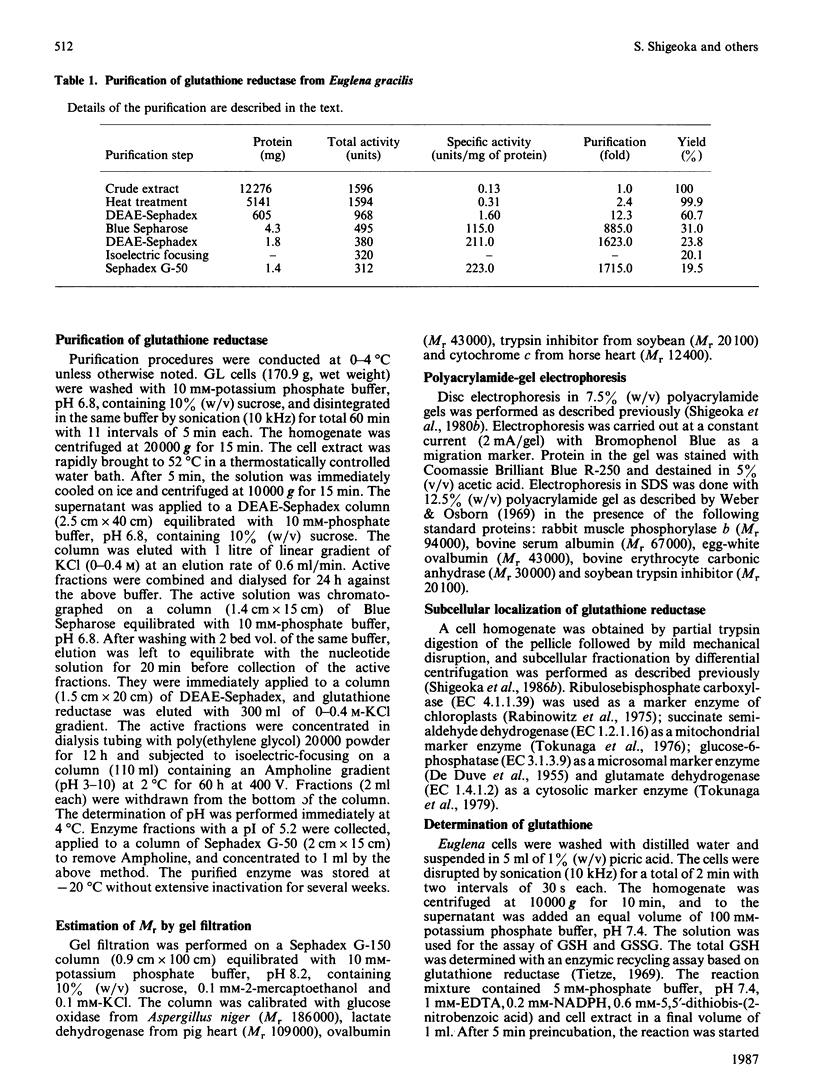
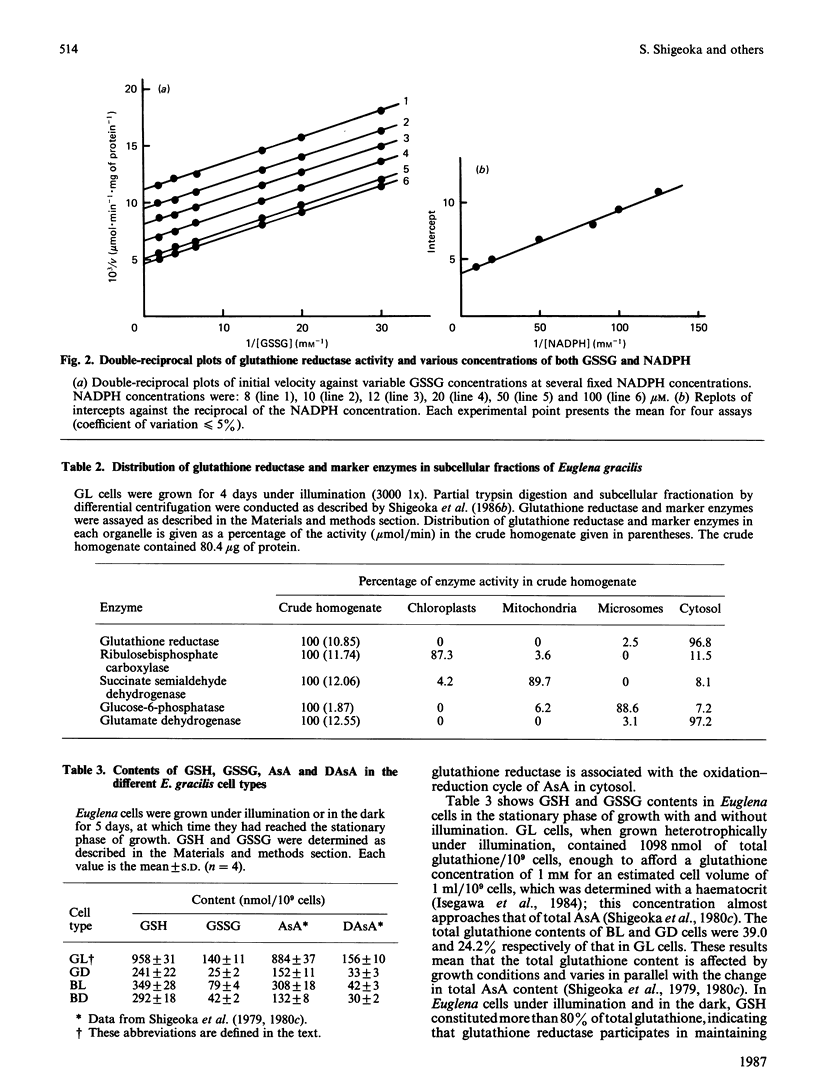
The purified glutathione reductase was homogeneous on polyacrylamide-gel electrophoresis. It had an Mr of 79,000 and consisted of two subunits with a Mr of 40,000. The activity was maximum at pH 8.2 and 52 degrees C. It was specific for NADPH but not for NADH as the electron donor; the reverse reaction was not observed. The Km values for NADPH and GSSG were 14 and 55 microM respectively. The enzyme activity was markedly inhibited by thiol inhibitors and metal ions such as Hg2+, Cu2+ and Zn2+. Euglena cells contained total glutathione at millimolar concentration. GSH constituted more than 80% of total glutathione in Euglena under various growth conditions. Glutathione reductase was located solely in cytosol, as were L-ascorbate peroxidase and dehydroascorbate reductase, which constitute the oxidation-reduction cycle of L-ascorbate [Shigeoka et al. (1980) Biochem. J. 186, 377-380]. These results indicate that glutathione reductase functions to maintain glutathione in the reduced form and to accelerate the oxidation-reduction of L-ascorbate, which scavenges peroxides generated in Euglena cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975 Jul 25;250(14):5475–5480. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Isegawa Y., Nakano Y., Kitaoka S. Conversion and Distribution of Cobalamin in Euglena gracilis z, with Special Reference to Its Location and Probable Function within Chloroplasts. Plant Physiol. 1984 Nov;76(3):814–818. doi: 10.1104/pp.76.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. A branching reaction mechanism of glutathione reductase. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1151–1158. doi: 10.1016/0006-291x(73)90585-8. [DOI] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Mavis R. D., Stellwagen E. Purification and subunit structure of glutathione reductase from bakers' yeast. J Biol Chem. 1968 Feb 25;243(4):809–814. [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Rabinowitz H., Reisfeld A., Sagher D., Edelman M. Ribulose Diphosphate Carboxylase from Autotrophic Euglena gracilis. Plant Physiol. 1975 Sep;56(3):345–350. doi: 10.1104/pp.56.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S., Nakano Y., Kitaoka S. Metabolism of hydrogen peroxide in Euglena gracilis Z by L-ascorbic acid peroxidase. Biochem J. 1980 Jan 15;186(1):377–380. doi: 10.1042/bj1860377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S., Nakano Y., Kitaoka S. Purification and some properties of L-ascorbic-acid-specific peroxidase in Euglena gracilis Z. Arch Biochem Biophys. 1980 Apr 15;201(1):121–127. doi: 10.1016/0003-9861(80)90495-6. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Nakano Y., Kitaoka S. Separation and properties of the NAD-linked and NADP-linked isozymes of succinic semialdehyde dehydrogenase in Euglena gracilis z. Biochim Biophys Acta. 1976 Mar 11;429(1):55–62. doi: 10.1016/0005-2744(76)90029-2. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Nakano Y., Kitaoka S. Subcellular localization of the GABA-shunt enzymes in Euglena gracilis strain Z. J Protozool. 1979 Aug;26(3):471–473. doi: 10.1111/j.1550-7408.1979.tb04655.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Worthington D. J., Rosemeyer M. A. Glutathione reductase from human erythrocytes. Catalytic properties and aggregation. Eur J Biochem. 1976 Aug 1;67(1):231–238. doi: 10.1111/j.1432-1033.1976.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Worthington D. J., Rosemeyer M. A. Human glutathione reductase: purification of the crystalline enzyme from erythrocytes. Eur J Biochem. 1974 Oct 1;48(1):167–177. doi: 10.1111/j.1432-1033.1974.tb03754.x. [DOI] [PubMed] [Google Scholar]
- Zanetti G. Rabbit liver glutathione reductase. Purification and properties. Arch Biochem Biophys. 1979 Nov;198(1):241–246. doi: 10.1016/0003-9861(79)90415-6. [DOI] [PubMed] [Google Scholar]