Abstract
Background/Objectives: Acute exacerbation (AE) of interstitial lung disease (ILD) is a major challenge. This study aimed to retrospectively investigate occurrences of AEs in patients with ILDs, including idiopathic pulmonary fibrosis (IPF), non-IPF (iNSIP: idiopathic nonspecific interstitial pneumonia), and connective tissue disease (CTD)-associated ILDs (CTD-ILDs), at a single tertiary center before and after the coronavirus disease 2019 (COVID-19) pandemic. The study aimed to clarify the seasonal and regional trends of AEs of ILDs, assess the roles of viral and bacterial infections, and identify key prognostic factors for patient outcomes. Methods: We conducted a retrospective review of hospitalized adult patients with AEs of ILDs from January 2019 to February 2024. Results: A total of 93 patients were enrolled: IPF (n = 42), iNSIP (n = 37), and CTD-ILDs (n = 14). The median age was 80 years (interquartile range: 74.0–86.0 years), with males comprising 64.5% (n = 60). AEs of ILDs predominantly occurred in winter and were particularly notable after summer 2023, coinciding with the lifting of COVID-19-related travel restrictions in Japan. Patient referrals from different areas (Northern Tama, East and/or Southern Tama, and other Tokyo metropolitan areas) were evenly distributed throughout the study period. Viral infections were detected in only two patients (SARS-CoV-2), and bacterial infections included methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. The Cox regression analysis identified serum lactate dehydrogenase levels ≥350 IU/L and tachypnea (respiratory rate ≥ 30 breaths per min) on admission as prognostic factors for mortality, with a hazard ratio [HR] of 2.783 (95% confidence interval [CI]: 1.480–5.235, p = 0.001) and an HR of 3.332 (95% CI: 1.710–6.492, p < 0.001), respectively. Conclusions: AEs of ILDs predominantly occur in winter, and viral and bacterial infections are infrequently detected. Elevated serum LDH levels and tachypnea are crucial prognostic markers for mortality. This study highlights the seasonal trend in the AE of ILD and emphasizes the importance of specific prognostic indicators in clinical practice.
Keywords: acute exacerbation of interstitial pneumonia, infection, prognostic factor, seasonal predilection
1. Introduction
Interstitial lung disease (ILD) refers to a group of pulmonary disorders characterized by inflammation and/or fibrosis of the lung parenchyma, leading to progressive dyspnea that often culminates in end-stage respiratory failure. ILDs are subcategorized based on etiology and include connective tissue disease-associated ILD (CTD-ILD), hypersensitivity pneumonitis, drug-induced ILD, postinfectious ILD, and idiopathic interstitial pneumonias. Regarding pathophysiology, idiopathic pulmonary fibrosis (IPF) progresses with an abnormal wound healing response in genetically susceptible individuals following repeated alveolar epithelial injury, and the biological pathways of CTD-ILD are poorly understood.
In United States, ILDs, including IPF, affect approximately 0.21% of the population. The prevalence of IPF specifically is estimated at around 14.0 to 27.9 per 100,000 people. The burden of ILDs, including progressive forms, has been increasing over the years, largely due to an aging population [1]. In European countries, the prevalence of ILDs varies, with IPF estimates ranging from 1.25 to 23.4 per 100,000 people. A study covering six European countries reported an ILD prevalence between 6.9 and 78 per 100,000 people [1]. The prevalence of IPF in Japan is estimated to be around 10.0 to 27.0 per 100,000 people, with a higher incidence among men and older adults, particularly those aged 75–79 years [2].
Acute exacerbation (AE) is a critical issue for patients with ILD, often leading to a rapid decline in respiratory status and death within a few months. The etiology of acute exacerbations of idiopathic pulmonary fibrosis (IPF) remains an area of active research, and the role of infections in these exacerbations has indeed been the subject of mixed findings [3,4,5,6]. Furthermore, the significance of viral infections in other types of ILDs for triggering AEs remains to be determined.
In the pre-coronavirus disease 2019 (COVID-19) era, our previous study reported that 19.2% of patients experiencing acute exacerbations of ILDs, including IPF, non-IPF, and CTD-associated pneumonia, had viral infections, primarily human herpes virus 7 and cytomegalovirus [7]. However, significant numbers of respiratory viruses were rarely identified in patients with an ILD during that period.
Conversely, during the same timeframe, patients with an exacerbation of asthma (75.3% of inpatients and 19.3% of outpatients) frequently harbored respiratory viruses, such as human rhinovirus (HRV), human metapneumovirus (hMPV), respiratory syncytial virus (RSV), and influenza virus (Inf-V), exhibiting seasonal variations [8]. This highlights disease-specific susceptibility to pathogens.
Following the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the clinical and radiological similarities between COVID-19-related pneumonia and acute exacerbation (AE) of ILDs prompted the development and widespread adoption of multiplex polymerase chain reaction (PCR) assays for pathogen detection in clinical settings. In this context, our study aimed to adopt a multidisciplinary approach to AEs of ILDs, encompassing the investigation of etiological agents, including SARS-CoV-2, using multiplex PCR, exploring seasonal and regional patterns, and identifying prognostic factors.
2. Materials and Methods
2.1. Patients and Study Design
We conducted a retrospective cohort study by enrolling hospitalized adult patients experiencing an AE of interstitial pneumonia at Kyorin University Hospital from January 2019 to February 2024. The definition of an AE of interstitial pneumonia (IP) followed the criteria established in a previous report [3]: (1) unexplained onset of dyspnea within 30 days; (2) new bilateral pulmonary ground-glass opacities or consolidations superimposed on a reticular and/or honeycomb pattern on chest computed tomography; (3) acute respiratory symptoms; (4) the absence of pathogenic bacteria in bronchoalveolar lavage fluid; and (5) the exclusion of alternative causes, such as left heart failure and pulmonary embolism. Patients with drug-induced pneumonia or hypersensitivity pneumonia were excluded.
2.2. Samples and Clinical Data Collection
Clinical data collected upon admission included age, sex, underlying diseases, vital signs, including delta heart rate (ΔHR)/body temperature (ΔBT) [9], symptoms, use of antifibrotic agents, previous exacerbation of IP, treatment regimens, serum laboratory data, and pulmonary function tests prior to exacerbation, if available. The Δ heart rate and ΔBT were defined as changes in HR and BT between admission and the non-illness state (prior to the AE or post-treatment, if previous data were unavailable). The post-pandemic period was defined as beginning in May 2023, following the lifting of COVID-19-related travel restrictions. Patient characteristics were compared between the pre- and post-COVID-19 pandemic periods.
2.3. Definition of Stages
The Ministry of Health, Labour and Welfare of Japan established the criteria for the stages of ILDs [10]. The classification of severity is determined by the following criteria: an arterial blood oxygen pressure (PaO2) at rest of 80 Torr or higher is classified as grade I, between 70 Torr and 80 Torr as grade II, between 60 Torr and 70 Torr as grade III, and below 60 Torr as grade IV. For patients with grade I or II PaO2 at rest, if the minimum SpO2 during a 6 min walk test is less than 90%, the severity is classified as grade III. Additionally, for patients with grade III PaO2 at rest, if the minimum SpO2 during a 6 min walk test is less than 90%, the severity is classified as grade IV. However, if PaO2 at rest is below 70 Torr, the minimum SpO2 during the 6 min walk test does not necessarily need to be measured.
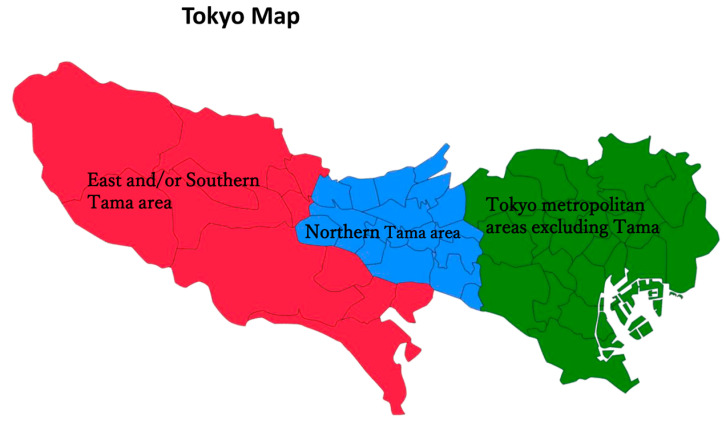
We analyzed the patients based on seasonal and regional distributions (Northern Tama area, East and/or Southern Tama area, and other Tokyo metropolitan areas, excluding Tama) during the study period (Figure 1). Kyorin University Hospital is in Northern Tama, which is home to approximately 4.23 million residents and covers a vast geographic area.
Figure 1.
The map of Tokyo consists of three areas: East and Southern Tama area (red color), Northern Tama area (blue color), and Tokyo metropolitan areas (green color).
Nasal swab samples collected upon admission were subjected to multiplex-nested PCR (FilmArray® Respiratory Panel 2.1, bioMérieux Co. Ltd., Tokyo, Japan), which is capable of detecting respiratory viruses such as SARS-CoV-2, adenovirus, human coronaviruses (229E, HKU1, NL63, and OC43), hMPV, HRV/enterovirus, Inf-V A (H1, H1-2009, and H3), Inf-V B, para-Inf-Vs 1-4, and RSV. Bacterial pathogens, including Bordetella parapertussis, Bordetella pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae, were also included in the panel. The FilmArray® Respiratory Panel 2.1 was introduced in our hospital in December 2020, nine months after the beginning of the COVID-19 pandemic on the Diamond Princess cruise ship in Japan.
This study was approved by the Ethics Committee of Kyorin University (approved number: 2421).
2.4. Statistical Analysis
Nonparametric data were analyzed using the Mann-Whitney U test or Wilcoxon signed-rank test. Categorical data were compared using Pearson’s chi-square test. The median overall survival (OS) was estimated using the Kaplan-Meier method with 95% confidence intervals (CIs), and the differences between survival curves were assessed using the log-rank test. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% CIs. All statistical tests were two-sided, with p < 0.05 considered statistically significant. Statistical analyses were performed using SPSS version 25.0 for Windows.
3. Results
3.1. Patient Characteristics
A total of 93 hospitalized patients with an AE of IP were examined during the study period. The median age was 80 years (interquartile range [IQR]: 74.0–86.0 years), with males comprising 64.5% (n = 60), and 66.7% (n = 62) were current or former smokers. The percentage of patients in stage I or II was 14.0% (n = 13), with a median duration of illness of 4.0 years (IQR: 2.0–5.0 years) (Table 1). Among the idiopathic interstitial pneumonia (IIP) patients, 42 had IPF, 37 had non-IPF (iNSIP), and 14 had connective tissue disease (CTD), including rheumatoid arthritis (n = 7), dermatomyositis (n = 4), systemic sclerosis (n = 2), and systemic lupus erythematosus (n = 1). The major comorbidities included cardiac disease (23.7%, n = 22), malignant disease (19.3%, n = 18), type 2 diabetes mellitus (14.0%, n = 13), and chronic obstructive pulmonary disease (14.0%, n = 13). Dyspnea was the most common symptom. Upper respiratory tract infections (e.g., nasal discharge or sore throat) were rare, and viral infections were only detected in two patients (SARS-CoV-2), with bacterial infections limited to methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. The value of ΔHR/ΔBT (median 27.8, IQR: 15.2–56.0) was noted, and most patients had experienced previous episodes of AEs. Before and after the COVID-19 pandemic, the number of patients was 73 and 20, respectively, with a frequency of 1.4 and 2.0 persons per month, suggesting a resurgence of AEs of ILDs.
Table 1.
Patient characteristics.
| Number of Patients (n = 93) | |
|---|---|
| Age | 80.0 (74.0–86.0) |
| Sex (Male) | 64.5% (n = 60) |
| Smoking | |
| Ex or Current | 66.7% (n = 62) |
| IIPs | |
| IPF | 44.1% (n = 42) |
| non-IPF (iNSIP) | 40.9% (n = 37) |
| CTD-ILD (N = 14) | |
| RA | 7.5% (n = 7) |
| DM | 4.3% (n = 4) |
| SLE | 1.1% (n = 1) |
| SSc | 2.2% (n = 2) |
| Stage I or II | 14.0% (n = 13) |
| Duration of illness (years) | 4.0 (2.0–5.0) |
| Comorbidities | |
| Asthma | 0% (n = 0) |
| COPD | 14.0% (n = 13) |
| Cardiac diseases | 23.7% (n = 22) |
| NIDDM type 2 | 14.0% (n = 13) |
| Maintenance hemodialysis | 1.1% (n = 1) |
| Malignant diseases | 19.3% (n = 18) |
| Respiratory viruses * | |
| SARS-CoV-2 | 2.5% (n = 2) |
| Bacteria | |
| MRSA | 1.1% (n = 1) |
| Pseudomonas aeruginosa | 1.1% (n = 1) |
| Vital signs | |
| BT (°C) | 37.0 ± 0.76 |
| RR (breaths/min) | 24.0 (18.0–28.0) |
| ΔHR/ΔBT | 27.8 (15.2–56.0) |
| SpO2 (%) | 82.1 (78.6–85.3) |
| BMI | 21.5 (18.7–24.1) |
| Symptoms | |
| nasal discharge | 5.4% (n = 5) |
| sore throat | 1.1% (n = 1) |
| dyspnea | 73.1% (n = 68) |
| Antifibrotic agents | 9.7% (n = 9) |
| Previous episodes of AEs | 14.0% (n = 13) |
* The FilmArray® Respiratory Panel 2.1 was applied for 79 admitted patients from Jan 2020 to 2023. Data are expressed as percentages (numbers) or medians (interquartile ranges). The stage classification was based on the Japanese Respiratory Society guideline 2022. AE, acute exacerbation; BMI, body mass index; BT, body temperature; COPD, chronic obstructive pulmonary disease; CTD-ILD, connective tissue disease–interstitial lung disease; IIPs, idiopathic interstitial pneumonias; IPF, idiopathic pulmonary fibrosis; non-IPF (iNSIP), idiopathic nonspecific interstitial pneumonia; MRSA, methicillin-resistant Staphylococcus aureus; NIDDM, non-insulin-dependent diabetes mellitus; RA, rheumatoid arthritis; RR, respiratory rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLE, systemic lupus erythematosus; SSc, systemic scleroderma.
The characteristics of these patients were comparable, except for the ratios of patients with COPD and NIDDM type 2 (Table 2).
Table 2.
Patient characteristics before and after the pandemic.
| Before the Pandemic (n = 73) |
After the Pandemic (n = 20) |
p Value | |
|---|---|---|---|
| Age | 80 (70–83) | 79 (70–87) | 0.918 |
| Sex (Male) | 68.5% (n = 50) | 50.0% (n = 10) | 0.186 |
| Smoking | |||
| Ex or Current | 72.6% (n = 53) | 50.0%(n = 10) | 0.065 |
| IIPs | 84.9% (n = 62) | 85.0% (n = 17) | 1 |
| IPF | 46.6% (n = 34) | 35.0% (n = 7) | 0.411 |
| non-IPF (iNSIP) | 38.4% (n = 28) | 50.0%(n = 10) | 0.414 |
| CTD-ILD | 15.1% (n = 11) | 15.0% (n = 3) | 1 |
| RA | 8.2% (n = 6) | 5.0% (n = 1) | 1 |
| DM PM | 6.8% (n = 5) | 0% (n = 0) | 0.581 |
| SLE | 1.4% (n = 1) | 0% (n = 0) | 1 |
| SSc | 0% (n = 0) | 10.0% (n = 2) | 0.044 |
| Stage I or II | 17.8% (n = 13) | 0% (n = 0) | 0.052 |
| Duration of illness (years) | 4.0 (3.0–5.0) | 4.0 (1.0–6.0) | 0.501 |
| Comorbidities | |||
| Asthma | 0% (n = 0) | 0% (n = 0) | N.D |
| COPD | 8.2%(n = 6) | 35.0%(n = 7) | 0.006 |
| Cardiac diseases | 19.2% (n = 14) | 40.0% (n = 8) | 0.074 |
| NIDDM type 2 | 8.2% (n = 6) | 35.0% (n = 7) | 0.006 |
| Maintenance hemodialysis | 1.4% (n = 1) | 0% (n = 0) | 1 |
| Malignant diseases | 17.8% (n = 13) | 25.0% (n = 5) | 0.526 |
| Respiratory viruses | |||
| SARS-CoV-2 | 0% (n = 0) | 10.0% (n = 2) | 0.044 |
| Bacteria | 1.4% (n = 1) | 5.0% (n = 1) | 0.386 |
| MRSA | 1.4% (n = 1) | 0% (n = 0) | 1 |
| Pseudomonas aeruginosa | 0% (n = 0) | 5.0% (n = 1) | 0.215 |
| Vital signs | |||
| BT (°C) | 36.6 (36.6–37.2) | 36.7 (36.6–37.1) | 0.191 |
| RR (breaths/min) | 22 (18–28) | 24 (20–32) | 0.209 |
| ΔHR/ΔBT | 36.3 (15.9–190.3) | 16.5 (7.5–32.0) | 0.109 |
| SpO2 (%) | 88.0 (82.0–95.0) | 86.0 (81.0–91.0) | 0.784 |
| BMI | 22.4 (19.4–25.3) | 21.5 (16.0–26.0) | 0.726 |
| Symptoms | |||
| nasal discharge | 5.5% (n = 4) | 5.0% (n = 1) | 1 |
| sore throat | 1.4% (n = 1) | 0% (n = 0) | 1 |
| dyspnea | 1.4% (n = 1) | 0% (n = 0) | 0.085 |
| Antifibrotic agents | 12.3% (n = 9) | 0% (n = 0) | 0.197 |
| Previous episodes of AEs | 16.4% (n = 12) | 5.0% (n = 1) | 0.285 |
The FilmArray® Respiratory Panel 2.1 was applied for 79 admitted patients from Jan 2020 to 2023. Data are expressed as percentages (numbers) or medians (interquartile ranges). The stage classification was based on the criteria of idiopathic interstitial pneumonia according to the Ministry of Health, Labour and Welfare of Japan [10]. AE, acute exacerbation; BMI, body mass index; BT, body temperature; COPD, chronic obstructive pulmonary disease; CTD-ILD, connective tissue disease–interstitial lung disease; IIPs, idiopathic interstitial pneumonias; IPF, idiopathic pulmonary fibrosis; non-IPF (iNSIP), idiopathic nonspecific interstitial pneumonia; MRSA, methicillin-resistant Staphylococcus aureus; NIDDM, non-insulin-dependent diabetes mellitus; RA, rheumatoid arthritis; RR, respiratory rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLE, systemic lupus erythematosus; SSc, systemic scleroderma.
3.2. Regional and Seasonal Distribution of Admitted Patients
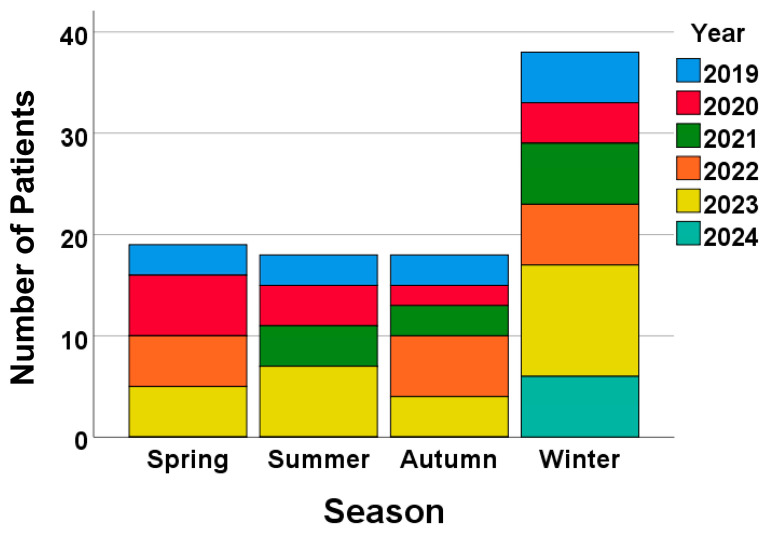
The annual and seasonal distribution of admitted patients indicated a resurgence of AEs of ILDs from the summer of 2023 following the relaxation of COVID-19-related travel restrictions in Japan (Figure 2), which allowed people to move across prefectures.
Figure 2.
The number of admitted patients increased from the summer of 2023 following the relaxation of COVID-19-related travel restrictions.
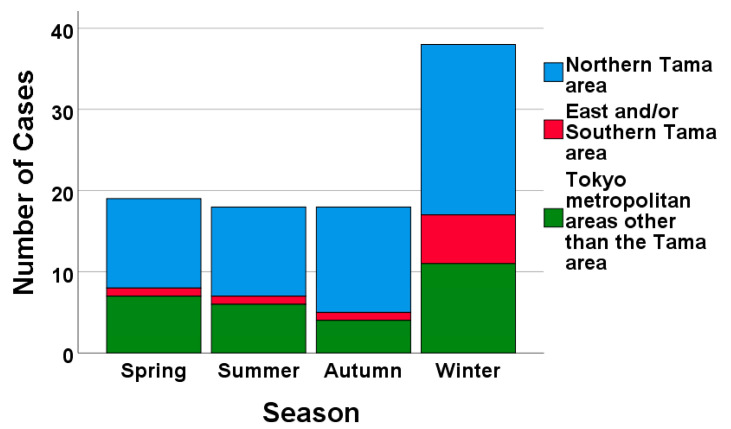
While the total number of patients was highest in winter, the proportions among the different regions (Northern Tama, East and/or Southern Tama, and other Tokyo metropolitan areas) remained consistent across regions throughout the study period (Figure 3).
Figure 3.
The number of admitted patients was highest in winter, but the proportions of patients in areas remained comparable during the study period.
3.3. Various Parameters on Admission between Survivor and Deceased Groups
The survivor group (N = 48) and the deceased group (N = 45) were similar in terms of age, sex, duration of illness, body mass index, smoking status, and the prevalence of underlying diseases or comorbidities (Table 3). However, the proportion of tachypnea (respiratory rate [RR] ≥ 30 breaths/min) was significantly higher in the deceased group than in the survivor group (33.3% vs. 12.5%, p = 0.025). On admission, the deceased group also exhibited significantly higher white blood cell counts (median 11,000/µL, IQR: 9100–13,650/µL, p = 0.004), lactate dehydrogenase (LDH) levels (median 384 IU/L, IQR: 295–517, p = 0.004), and surfactant protein D (SP-D) levels (median 401 IU/L, IQR: 270–719, p = 0.007) compared to the survivor group (WBC: median 9400/µL, IQR: 7275–11,925/µL; LDH: median 317 IU/L, IQR: 251–363; SP-D: median 252 IU/L, IQR: 127–434). Serum LDH levels ≥ 350 IU/L and SP-D levels ≥ 314 IU/L were more frequent in deceased patients than survivors (LDH: 51.1% vs. 39.6%, p = 0.005; SP-D ≥ 314 IU/L: 40.4% vs. 29.2%, p = 0.046). Although pulmonary function tests were not performed for all patients (survivor: n = 30, 62.5%; deceased: n = 15, 33.3%), the results were comparable between the groups.
Table 3.
Comparison between survivor and deceased groups.
| All Patients (n = 93) | Survivors (n = 48) | Deceased (n = 45) | p Value |
|---|---|---|---|
| Age | 79 (70–84) | 81 (72–85) | 0.142 |
| Sex | 62.5% (n = 30) | 66.7% (n = 30) | 0.829 |
| Duration of illness (years) | 4.0 (2.8–5.3) | 4.0 (2.5–5.0) | 0.634 |
| BMI | 21.6 (18.7–24.6) | 21.5 (19.0–24.0) | 0.860 |
| Smoker (ex or current) | 66.7% (n = 32) | 68.9% (n = 31) | 0.829 |
| SpO2 (%) | 90.0 (83.0–94.0) | 84.0 (73–91.0) | 0.008 ** |
| Hypoxemia (<SpO2 94%) | 79.2% (n = 48) | 88.9% (n = 45) | 0.264 |
| RR (breaths/min) | 20 (18–22) | 30 (24–36) | 0.013 * |
| HR (beats/min) | 91(80–106) | 85 (78–102) | 0.997 |
| RR ≥ 20 (breaths/min) | 58.3% (n = 28) | 73.3% (n = 33) | 0.190 |
| RR ≥ 30 (breaths/min) | 12.5%(n = 6) | 33.3% (n = 15) | 0.025 * |
| ΔHR/ΔBT | 27.8 (19.5–117.5) | 35.0(9.6–188) | 0.694 |
| Body temperature (°C) | 36.7 (36.6–37.1) | 36.6 (36.6–36.7) | 0.375 |
| IIPs | 85.4% (n = 41) | 84.4% (n = 38) | 1.0 |
| IPF | 43.8% (n = 21) | 44.4% (n = 20) | 0.824 |
| non-IPF (iNSIP) | 41.7% (n = 20) | 40.0% (n = 18) | 1.0 |
| CTD-ILD | |||
| RA | 6.3% (n = 3) | 11.1% (n = 5) | 0.205 |
| DM | 2.1% (n = 1) | 0% (n = 0) | 0.330 |
| SLE | 2.1% (n = 1) | 0% (n = 0) | 0.330 |
| Stage I or II * | 10.4% (n = 5) | 17.8% (n = 8) | 0.755 |
| Comorbidities | |||
| COPD | 12.5% (n = 6) | 15.6% (n = 7) | 0.769 |
| Cardiac diseases | 16.7% (n = 8) | 31.1% (n = 14) | 0.149 |
| NIDDM type 2 | 16.7% (n = 8) | 11.1% (n = 5) | 0.440 |
| Maintenance hemodialysis | 2.1% (n = 1) | 0% (n = 0) | 0.330 |
| Malignant diseases | 14.5% (n = 7) | 22.9% (n = 11) | 0.871 |
| Previous episodes of AE | 12.5% (n = 6) | 15.6% (n = 7) | 0.769 |
| HOT on admission | 22.9% (n = 11) | 17.8% (n = 8) | 0.612 |
| Hospital days | 28.0 (21.0–39.0) | 18.0 (13.0–33.0) | 0.003 ** |
| Duration of hypoxemia or needs more O2 supply than usual (days) |
9.5 (3.0–13.0) | 10.0(5.0–16.0) | 0.106 |
| Respiratory viruses | 4.2% (n = 2) | 0% (n = 0) | 0.495 |
| Bacteria | 0% (n = 0) | 4.4% (n = 2) | 0.231 |
| Symptoms | |||
| Nasal discharge | 6.3% (n = 3) | 4.4% (n = 2) | 1 |
| Sore throat | 2.1% (n = 1) | 0% (n = 0) | 1 |
| Dyspnea on efforts | 68.8% (n = 33) | 77.8% (n = 35) | 0.358 |
| Antifibrotic agents | 8.3% (n = 4) | 11.1% (n = 5) | 0.735 |
| Treatments | |||
| IVCY | 18.8% (n = 9) | 42.2% (n = 19) | 0.023 * |
| mPSL pulse | 81.3% (n = 39) | 91.1% (n = 41) | 0.235 |
| Laboratory data | |||
| WBC | 9400 (7275–10925) | 11,000 (9100–13,650) | 0.004 ** |
| Monocyte | 7.1 (6.1–10.9) | 6.2 (5.0–7.5) | 0.010 * |
| Albumin | 3.1 (2.7–3.4) | 2.9 (2.5–3.2) | 0.101 |
| Plt | 24.9 (18.1–32.2) | 20.4 (15.2–30.2) | 0.089 |
| CRP | 7.51 (3.60–12.8) | 10.2(5.5–14.4) | 0.114 |
| LDH | 317 (251–363) | 384 (295–517) | 0.004 ** |
| LDH ≥ 350 | 39.6% (n = 19) | 51.1% (n = 23) | 0.005 ** |
| KL-6 | 989 (691–1707) | 988 (547–2145) | 0.913 |
| SP-D | 252 (127–434) | 401 (270–719) | 0.007 ** |
| SP-D ≥ 314 | 29.2% (n = 14) | 40.4% (n = 18) | 0.046 * |
| Pulmonary function test | |||
| VC | 70.8 (55.2–86.6) | 82.6 (60.9–90.2) | 0.639 |
| FVC | 72.5 (56.4–92.6) | 80.9 (63.1–84.7) | 0.736 |
| FEV1.0% | 84.2 (80.1–86.9) | 79.9 (73.1–86.3) | 0.691 |
| %FEV1.0 | 74.8 (59.9–92.8) | 74.2 (70.0–87.0) | 0.791 |
| %DLCO | 41.6 (33.3–54.3) | 57.4 (30.0–70.6) | 0.336 |
| %DLCO/VA | 50.0(40.0–75.3) | 59.4 (49.8–74.3) | 0.428 |
AE, acute exacerbation; BMI, body mass index; BT, body temperature; CTD-ILD, connective tissue disease related to interstitial lung disease; COPD, chronic obstructive lung disease; DM, dermatomyositis; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO/VA, diffusing capacity of the lungs for carbon monoxide/alveolar volume; FEV1.0%, forced expiratory volume in one second/forced vital capacity; %FEV1.0, percent predicted forced expiratory volume in one second; FVC, forced vital capacity; IIPs, idiopathic interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; mPSL, methylprednisolone; NIDDM, non-insulin dependent diabetes mellitus; RA, rheumatoid arthritis; RR, respiratory rate; SLE, systemic lupus erythematosus; SpO2, oxygen saturation; IVCY, intravenous cyclophosphamide; VC, vital capacity, Pulmonary function tests were available for 62.5% of the survivor group (n = 30) and 33.3% of the deceased group (n = 15). * means p < 0.05, ** means p < 0.01.
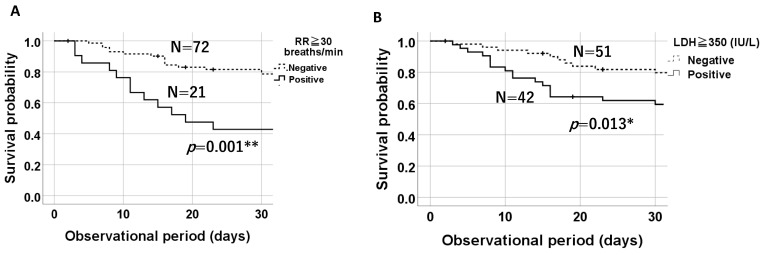
3.4. Comparison of 30-Day Survival Probabilities on a Kaplan–Meier Plot Based on the Presence of Tachypnea (RR ≥ 30 Breaths/Min) and Elevated LDH Levels (≥350 IU/L)
Kaplan–Meier survival curves over 30 days showed that patients with tachypnea (RR ≥ 30 breaths/min) had a 30-day survival rate of 42.9%, compared to 76.9% in those without tachypnea (p = 0.001) (Figure 4A). Similarly, patients with an LDH level ≥ 350 IU/L on admission exhibited significantly lower survival probabilities than those with an LDH level < 350 IU/L (positive: n = 42, 54.8%; negative: n = 51, 80.4%, p = 0.013) based on the log-rank test (Figure 4B).
Figure 4.
Kaplan–Meier plots demonstrating that tachypnea (RR ≥ 30 breaths/min) (A) and serum LDH levels ≥ 350 IU/L (B) were significant prognostic factors for 30-day survival probabilities. * means p < 0.05, ** means p < 0.01.
3.5. Prognostic Factors for an AE of IP
The multivariate analysis adjusted for age and sex demonstrated that serum LDH levels ≥ 350 IU/L and tachypnea (RR ≥ 30 breaths/min) were identified as risk factors for 30-day mortality, with hazard ratios of 4.0 (95% CI: 1.45–11.0, p = 0.007) and 4.85 (95% CI: 1.61–14.61, p = 0.005), respectively (Table 4). The Cox regression analysis further confirmed serum LDH levels ≥350 IU/L and tachypnea (RR ≥ 30 breaths/min) as prognostic factors for mortality, with hazard ratios of 2.78 (95% CI: 1.48–5.24, p = 0.001) and 3.33 (95% CI: 1.71–6.49, p < 0.001), respectively (Table 5), but not serum SP-D levels ≥ 314 IU/L (HR 2.009, 95% CI: 0.90–4.51, p = 0.09).
Table 4.
Multivariate analysis of 30-day mortality.
| Parameter | Hazard Ratio (95%CI) | p Value |
|---|---|---|
| Age, yr | 1.036 (0.974–1.103) | 0.262 |
| Male sex | 1.538 (0.493–4.801) | 0.459 |
| LDH ≥ 350 (IU/L) | 3.997(1.452–11.0) | 0.007 ** |
| RR ≥ 30 (breaths/min) | 4.854 (1.613–14.609) | 0.005 ** |
** means p < 0.01.
Table 5.
Cox regression analysis of mortality.
| Parameter | Hazard Ratio (95%CI) | p Value |
|---|---|---|
| Age, yr | 1.036 (0.997–1.076) | 0.069 |
| Male sex | 2.022 (0.961–4.254) | 0.064 |
| LDH ≥ 350 (IU/L) | 2.783 (1.480–5.235) | 0.001 ** |
| RR ≥ 30 (breaths/min) | 3.332 (1.710–6.492) | <0.001 *** |
** means p < 0.01, *** means p < 0.001.
4. Discussion
This study demonstrated that AEs of ILDs predominantly occurred in winter, particularly increasing from the summer of 2023, shortly after the lifting of COVID-19-related travel restrictions in Japan. Infections were infrequently detected during the study. Serum LDH levels ≥ 350 IU/L and tachypnea (RR ≥ 30) on admission emerged as potent prognostic factors for mortality, indicating the need for more intensive treatment (e.g., steroid pulse therapy and/or other immunosuppressive drugs).
Regarding infectious agents, Mostafaei et al. [11] reported a pooled prevalence of viral and bacterial infections in patients with IPF of 53.72% and 31.21%, respectively, based on a random-effects meta-analysis. However, these infections may not always act as direct risk factors for AEs of IPF but may be involved in the pathogenesis of interstitial pneumonia. Pathogen detection rates varied due to geographical differences, techniques for viral/bacterial detection, and the types of biological samples used. Our study did not perform PCR testing for some viruses, such as cytomegalovirus (CMV), Epstein–Barr virus (EBV), and human herpesvirus (HHV) 7 and 8, as in our previous study [8] or other studies [12], potentially affecting the detection rate. Nonetheless, the low frequency of upper respiratory tract infection symptoms and the absence of viruses other than SARS-CoV-2 raise the hypothesis that the higher detection rates reported in comprehensive studies may correspond to the reactivation of certain viruses (e.g., HHV7, HHV8, CMV, and EBV) in immunocompromised hosts or disease progression of ILDs, although their roles in AEs remain uncertain.
In terms of the annual and seasonal distributions of AEs of ILDs, the total number of cases was higher in winter than in other seasons and appeared to increase from summer 2023 following the easing of COVID-19-related travel restrictions. Previous studies focusing on AEs of IPF have shown higher exacerbation rates in winter to spring [13,14] or specifically in winter [15]. Although our study included patients with and without IPF (e.g., idiopathic NSIP and CTD-ILD), winter was the predominant season for AEs of ILDs. The reason for this seasonal predilection remain unclear; however, unidentified pathogens may contribute to the occurrence of AEs. Regarding regional distributions, we anticipated observing regional variations in patient referrals to our hospital, given that it serves as the referral center for Mitaka City. However, the regional variations appeared unchanged before and after the COVID-19 pandemic. Based on these data alone, we cannot assess the effects on healthcare delivery.
For clinicians, vital signs play a crucial role in hypothesizing etiologies. In healthy individuals, the normal physiological response involves an increase in heart rate by approximately 10 beats/min per 0.55 °C rise in body temperature, resulting in an expected ΔHR/ΔBT ≤ 20. However, the AE of ILDs in our study exhibited higher ΔHR/ΔBT values without bacterial infection, suggesting that this rule may not apply to AE of ILDs with bacterial infections. Conversely, RR ≥ 30 breaths/min on admission could serve as a pivotal prognostic sign for short-term (30-day) or long-term mortality, along with an elevated serum LDH level (≥350 IU/L). To the best of our knowledge, the severity of tachypnea in AEs of ILDs has rarely been reported; however, its correlation with mortality rates resembles that of community-acquired pneumonia [16].
The serum KL-6 level has been identified as a valuable biomarker for both diagnostic purposes and assessing the severity of ILDs in children with CTD [17], as well as in adults with CTD [18]. A systematic review of adults with CTD (rheumatoid arthritis-associated ILD) indicated that KL-6, SP-D, and interleukin-6 are associated with all-cause mortality [19]. In clinically amyopathic adults with dermatomyositis, Gono et al. reported that ferritin predicts the disease severity and prognosis of amyopathic dermatomyositis [20]. Furthermore, Zou J et al. reported that elevated serum ferritin levels and the extent of lung involvement, as calculated by high-resolution CT, were independent significant factors for 1-year mortality [21]. However, this was not the case for LDH levels, based on a multivariable analysis using Cox proportional hazards regression models.
On the other hand, Kishaba et al. found that high LDH (>280 IU/L) and KL-6 levels (>1000 IU/L) predict the 3-month mortality rate due to AEs of IPF [22]. Murohashi et al. reported the utility of the Charlson Comorbidity Index, sex, and serum LDH levels as mortality prediction tools for acute or subacute idiopathic interstitial pneumonia and AEs of CVD-IP [23].
The annual incidence of AEs of IPF is reported to be 5–19%, and AEs are of milder forms of IPF are less common. However, the post-exacerbation mortality of ILDs is reported to range from 33–83% with hospital mortality rates of 50–100% for patients with CTD-ILDs and 75–100% for patients with hypersensitivity pneumonitis [24]. Furthermore, many cases of ILDs rarely showed external triggers, including infection. Therefore, the early recognition of AE-ILDs, followed by urgent treatment using simple clinical parameters, is essential.
This study had limitations: (1) It was conducted as an observational study at a single center. (2) The FilmArray® Respiratory Panel 2.1 was applied to only 79 admitted patients from January 2020 to 2023, thus not covering all patients in the study. In addition, some viruses detected in previous studies were not examined. (3) The relatively small number of enrolled patients was due to the low incidence of AEs of ILDs. Nevertheless, this study provides clinical evidence that both serum LDH levels (≥350 IU/L) and tachypnea (RR ≥ 30 breaths/min) can serve as simple yet potent prognostic factors and that AEs of ILDs exhibit a paucity of infections with a seasonal predilection for winter.
5. Conclusions
Acute exacerbations of ILDs predominantly occur in winter, with limited evidence of bacterial and viral infections, even during the COVID-19 era. A serum LDH level ≥ 350 IU/L and tachypnea (RR ≥ 30 breaths/min) represent simple yet crucial prognostic factors for mortality.
Author Contributions
Conceptualization, T.S.; formal analysis, T.S.; investigation, R.T., S.Y., K.N. (Kei Nakajima), K.D., T.A., N.I., N.K., F.K., H.N., J.A., Y.N., M.I., M.S., K.H., K.N. (Keitaro Nakamoto), S.T. and H.I.; data curation, T.S.; writing—original draft, R.T. and T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Kyorin University School of Medicine (approved number-2421, at 6 August 2024).
Informed Consent Statement
Patient consent was waived due to the study character.
Data Availability Statement
The data used and/or analyzed in this study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this manuscript, and the database is available from the corresponding author (saraya@ks.kyorin-u.ac.jp) upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jeganathan N., Sathananthan M. The prevalence and burden of interstitial lung diseases in the USA. ERJ Open Res. 2022;8:00630-2021. doi: 10.1183/23120541.00630-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondoh Y., Suda T., Hongo Y., Yoshida M., Hiroi S., Iwasaki K., Takeshima T., Homma S. Prevalence of idiopathic pulmonary fibrosis in Japan based on a claims database analysis. Respir. Res. 2022;23:24. doi: 10.1186/s12931-022-01938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collard H.R., Moore B.B., Flaherty K.R., Brown K.K., Kaner R.J., King T.E., Jr., Lasky J.A., Loyd J.E., Noth I., Olman M.A., et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007;176:636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Y.W., Johnson J.E., Browning P.J., Cruz-Gervis R.A., Davis A., Graham B.S., Brigham K.L., Oates J.A., Jr., Loyd J.E., Stecenko A.A. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J. Clin. Microbiol. 2003;41:2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ushiki A., Yamazaki Y., Hama M., Yasuo M., Hanaoka M., Kubo K. Viral infections in patients with an acute exacerbation of idiopathic interstitial pneumonia. Respir. Investig. 2014;52:65–70. doi: 10.1016/j.resinv.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wootton S.C., Kim D.S., Kondoh Y., Chen E., Lee J.S., Song J.W., Huh J.W., Taniguchi H., Chiu C., Boushey H., et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care. Med. 2011;183:1698–1702. doi: 10.1164/rccm.201010-1752OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraya T., Kimura H., Kurai D., Tamura M., Ogawa Y., Mikura S., Sada M., Oda M., Watanabe T., Ohkuma K., et al. Clinical significance of respiratory virus detection in patients with acute exacerbation of interstitial lung diseases. Respir. Med. 2018;136:88–92. doi: 10.1016/j.rmed.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraya T., Kimura H., Kurai D., Ishii H., Takizawa H. The molecular epidemiology of respiratory viruses associated with asthma attacks. A single-center observational study in Japan. Medicine. 2017;96:e8204. doi: 10.1097/MD.0000000000008204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamano J., Tokuda Y. Changes in vital signs as predictors of bacterial infection in home care: A multi-center prospective cohort study. Postgrad. Med. 2017;129:283–287. doi: 10.1080/00325481.2017.1251819. [DOI] [PubMed] [Google Scholar]
- 10.The Ministry of Health LaWoJ Idiopathic Interstitial Pneumonia; 2024. [(accessed on 18 September 2024)]. Available online: https://www.nanbyou.or.jp/entry/302.
- 11.Mostafaei S., Sayad B., Azar M.E.F., Doroudian M., Hadifar S., Behrouzi A., Riahi P., Hussen B.M., Bayat B., Nahand J.S., et al. The role of viral and bacterial infections in the pathogenesis of IPF: A systematic review and meta-analysis. Respir. Res. 2021;22:53. doi: 10.1186/s12931-021-01650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng G., Chen P., Wei Y., Yue H., Chu J., Zhao J., Wang Y., Zhang W., Zhang H.L. Viral Infection Increases the Risk of Idiopathic Pulmonary Fibrosis: A Meta-Analysis. Chest. 2020;157:1175–1187. doi: 10.1016/j.chest.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collard H.R., Yow E., Richeldi L., Anstrom K.J., Glazer C. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir. Res. 2013;14:73. doi: 10.1186/1465-9921-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon-Blancal V., Freynet O., Nunes H., Bouvry D., Naggara N., Brillet P.Y., Denis D., Cohen Y., Vincent F., Valeyre D., et al. Acute exacerbation of idiopathic pulmonary fibrosis: Outcome and prognostic factors. Respiration. 2012;83:28–35. doi: 10.1159/000329891. [DOI] [PubMed] [Google Scholar]
- 15.Yamazoe M., Tomioka H. Acute exacerbation of idiopathic pulmonary fibrosis: A 10-year single-centre retrospective study. BMJ Open Respir. Res. 2018;5:e000342. doi: 10.1136/bmjresp-2018-000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindo Y., Ito R., Kobayashi D., Ando M., Ichikawa M., Goto Y., Fukui Y., Iwaki M., Okumura J., Yamaguchi I., et al. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: An observational cohort study. Lancet Infect. Dis. 2015;15:1055–1065. doi: 10.1016/S1473-3099(15)00151-6. [DOI] [PubMed] [Google Scholar]
- 17.El-Beheidy R., Domouky A.M., Zidan H., Amer Y.A. Serum KL-6 as predictive and prognostic marker of interstitial lung disease in childhood connective tissue diseases: A pilot study. Reumatismo. 2021;73:147–155. doi: 10.4081/reumatismo.2021.1399. [DOI] [PubMed] [Google Scholar]
- 18.Zheng P., Zheng X., Takehiro H., Cheng Z.J., Wang J., Xue M., Lin Q., Huang Z., Huang H., Liao C., et al. The Prognostic Value of Krebs von den Lungen-6 and Surfactant Protein-A Levels in the Patients with Interstitial Lung Disease. J. Transl. Int. Med. 2021;9:212–222. doi: 10.2478/jtim-2021-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groseanu L., Nita C. A Systematic Review of the Key Predictors of Progression and Mortality of Rheumatoid Arthritis-Associated Interstitial Lung Disease. Diagnostics. 2024;14:1890. doi: 10.3390/diagnostics14171890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gono T., Kawaguchi Y., Satoh T., Kuwana M., Katsumata Y., Takagi K., Masuda I., Tochimoto A., Baba S., Okamoto Y., et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. 2010;49:1713–1719. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 21.Zou J., Guo Q., Chi J., Wu H., Bao C. HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin. Rheumatol. 2015;34:707–714. doi: 10.1007/s10067-015-2866-5. [DOI] [PubMed] [Google Scholar]
- 22.Kishaba T., Tamaki H., Shimaoka Y., Fukuyama H., Yamashiro S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192:141–149. doi: 10.1007/s00408-013-9530-0. [DOI] [PubMed] [Google Scholar]
- 23.Murohashi K., Hara Y., Saigusa Y., Kobayashi N., Sato T., Yamamoto M., Kudo M., Kaneko T. Clinical significance of Charlson comorbidity index as a prognostic parameter for patients with acute or subacute idiopathic interstitial pneumonias and acute exacerbation of collagen vascular diseases-related interstitial pneumonia. J. Thorac. Dis. 2019;11:2448–2457. doi: 10.21037/jtd.2019.05.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb M., Bondue B., Pesci A., Miyazaki Y., Song J.W., Bhatt N.Y., Huggins J.T., Oldham J.M., Padilla M.L., Roman J., et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018;27:180071. doi: 10.1183/16000617.0071-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analyzed in this study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this manuscript, and the database is available from the corresponding author (saraya@ks.kyorin-u.ac.jp) upon reasonable request.