Abstract
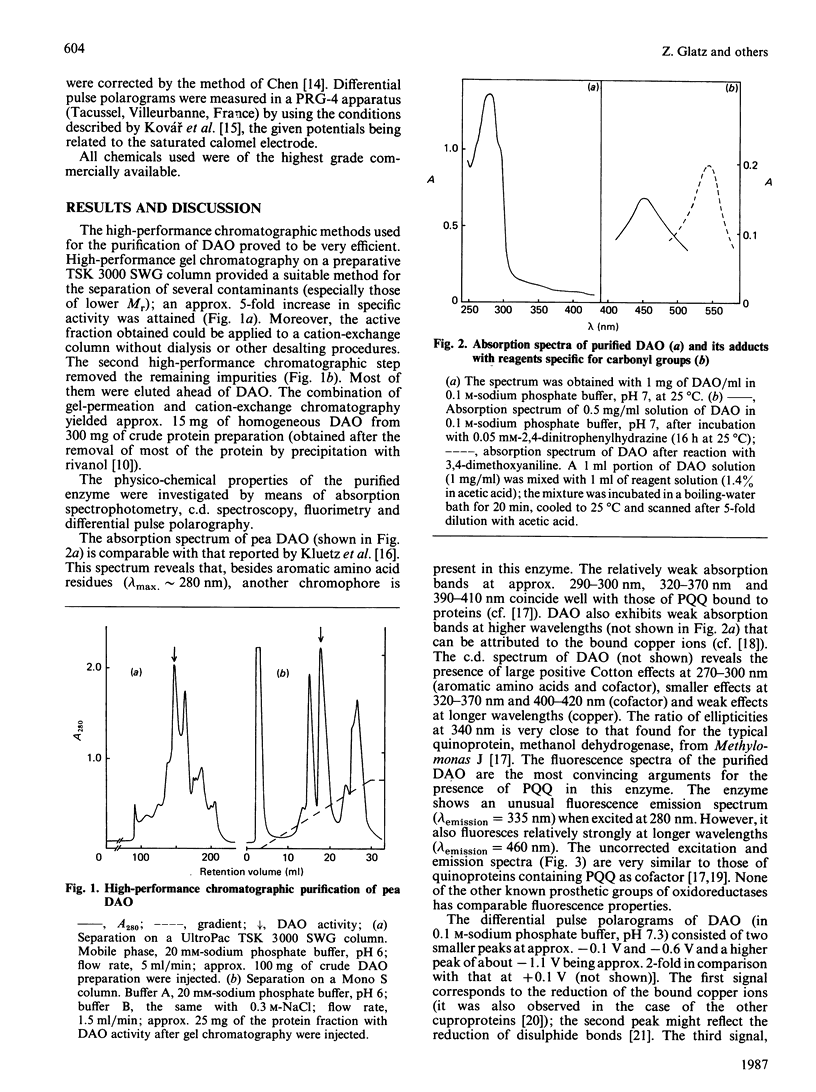
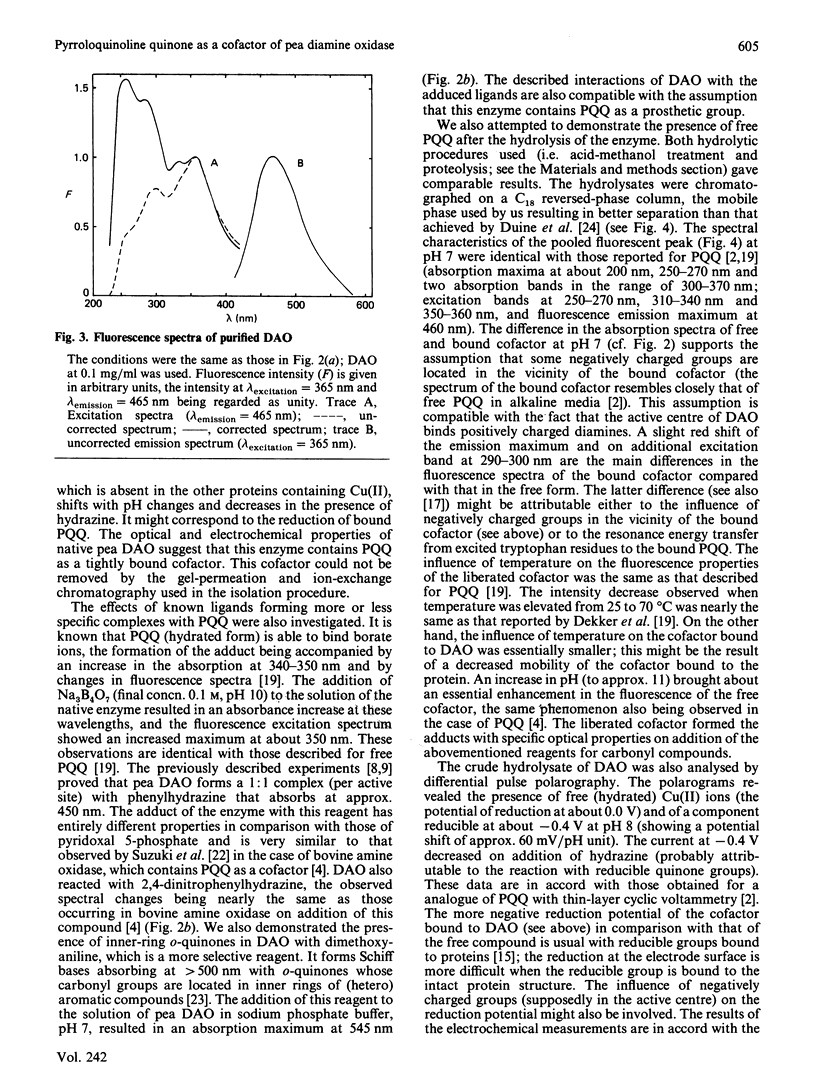
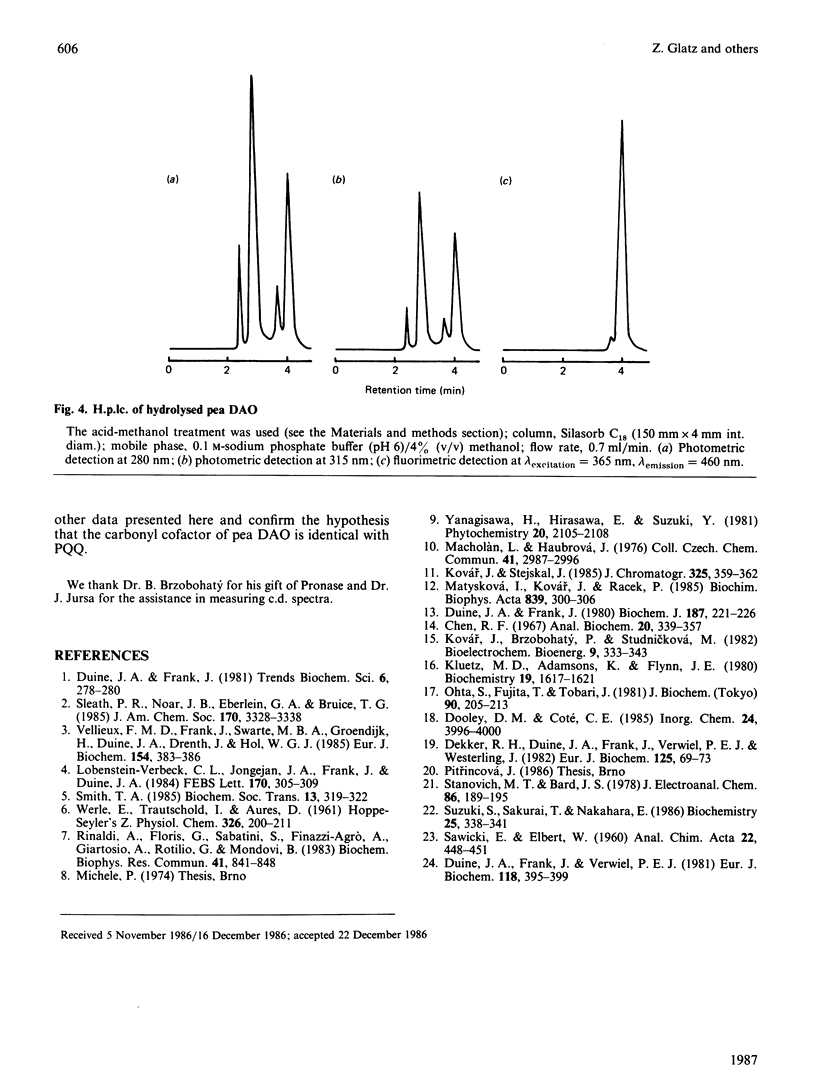
Diamine oxidase was prepared from pea (Pisum sativum) seedlings by a new purification procedure involving two h.p.l.c. steps. We studied the optical and electrochemical properties of the homogeneous enzyme and also analysed the hydrolysed protein by several methods. The data presented here suggest that the carbonyl cofactor of diamine oxidase is firmly bound pyrroloquinoline quinone.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. F. Practical aspects of the calibration and use of the Aminco-Bowman spectrophotofluorometer. Anal Biochem. 1967 Aug;20(2):339–357. doi: 10.1016/0003-2697(67)90040-1. [DOI] [PubMed] [Google Scholar]
- Dekker R. H., Duine J. A., Frank J., Verwiel P. E., Westerling J. Covalent addition of H2O, enzyme substrates and activators to pyrrolo-quinoline quinone, the coenzyme of quinoproteins. Eur J Biochem. 1982 Jun 15;125(1):69–73. doi: 10.1111/j.1432-1033.1982.tb06652.x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980 Apr 1;187(1):221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetz M. D., Adamsons K., Flynn J. E., Jr Cryoenzymology and spectrophotometry of pea seedling diamine oxidase. Biochemistry. 1980 Apr 15;19(8):1617–1621. doi: 10.1021/bi00549a014. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Ohta S., Fujita T., Tobari J. Methanol dehydrogenase of Methylomonas J: purification, crystallization, and some properties. J Biochem. 1981 Jul;90(1):205–213. doi: 10.1093/oxfordjournals.jbchem.a133451. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Floris G., Sabatini S., Finazzi-Agrò A., Giartosio A., Rotilio G., Mondovì B. Reaction of beef plasma and lentil seedlings Cu-amine oxidases with phenylhydrazine. Biochem Biophys Res Commun. 1983 Sep 30;115(3):841–848. doi: 10.1016/s0006-291x(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Smith T. A. The di- and poly-amine oxidases of higher plants. Biochem Soc Trans. 1985 Apr;13(2):319–322. doi: 10.1042/bst0130319. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sakurai T., Nakahara A. Roles of the two copper ions in bovine serum amine oxidase. Biochemistry. 1986 Jan 28;25(2):339–341. doi: 10.1021/bi00350a009. [DOI] [PubMed] [Google Scholar]
- Vellieux F. M., Frank J., Swarte M. B., Groendijk H., Duine J. A., Drenth J., Hol W. G. Purification, crystallization and preliminary X-ray investigation of quinoprotein methylamine dehydrogenase from Thiobacillus versutus. Eur J Biochem. 1986 Jan 15;154(2):383–386. doi: 10.1111/j.1432-1033.1986.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Verwiel P. E., Frank J., Verwiel E. J. Characterization of the second prosthetic group in methanol dehydrogenase from hyphomicrobium X. Eur J Biochem. 1981 Aug;118(2):395–399. doi: 10.1111/j.1432-1033.1981.tb06415.x. [DOI] [PubMed] [Google Scholar]
- WERLE E., TRAUTSCHOLD I., AURES D. [Purification and characterization of diamine oxidase from peas]. Hoppe Seylers Z Physiol Chem. 1961 Dec 21;326:200–211. doi: 10.1515/bchm2.1961.326.1.200. [DOI] [PubMed] [Google Scholar]


