Abstract
Individuals at increased risk for prostate cancer (PCa) are inconsistently defined in national and international guidelines. The National Comprehensive Cancer Network (NCCN) defines people at increased risk for PCa to include those with a concerning family history, West African/Caribbean/African-American individuals, and those who have germline mutations in known PCa-related genes. Recommendations for screening are also inconsistently defined in national and international guidelines. The NCCN and American Urological Association recommend that individuals at increased risk for PCa be screened with prostate-specific antigen and digital rectal exam starting at age 40. Defining increased risk groups and defining lifetime risk is an ongoing academic process that can be facilitated through patient registries of these cohorts at academic centers.
INTRODUCTION
In Canada, prostate cancer (PCa) is the leading cause of cancer in males and the fifth leading cause of cancer-related death.1 The current lifetime risk for a PCa diagnosis in Canada is 12–13%; however, this risk may be higher (Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society, Statistics Canada, and the Public Health Agency of Canada, Canadian Cancer Statistics 2021, Toronto, ON : Canadian Cancer Society; 2021).
In 2014, the Canadian Preventive Task Force recommended against PCa screening, and as a result, less people have been screened for, and diagnosed with, PCa.2 The frequency of prostate-specific antigen (PSA) screening in a population impacts the lifetime risk of being diagnosed with PCa, with less screening associated with lowered lifetime risk.3
Aside from aging, there are three risk factors for PCa: inheriting a germline pathogenic variant or likely pathogenic variant (PV/LPV) in a cancer-related gene, a strong family history of PCa, and West African/Caribbean (WA/C) ancestry. Screening and management approaches of people who have one or more of these risk factors remain inconsistent, and the cause of increased risk of PCa in people with a family history and/or of WA/C ancestry remains largely elusive.
WHO IS AT INCREASED RISK FOR PROSTATE CANCER?
Many cancer sites use the terms “increased risk” and “high-risk” synonymously; however, in PCa, high-risk disease has its own definition (Table 1) relating to PCa aggressiveness. Thus, using “high-risk” in the context of lifetime risk can be confusing and should be avoided.
Table 1.
Definition of prostate cancer risk groups
| Low-risk prostate cancer | Gleason score 6/grade group 1 and PSA <10 |
| Intermediate-risk prostate cancer | Gleason score 7/grade croup 2–3 and/or PSA 10–20 |
| High-risk prostate cancer | Gleason score 8–10/grade group 4–5 and/or PSA >20 |
PSA: prostate-specific antigen.
The Canadian Urological Association (CUA), the American Urological Association (AUA), and the National Comprehensive Cancer Network (NCCN) have each defined “increased risk” for PCa in their screening recommendations differently. The NCCN has the most comprehensive definition, describing individuals at “increased risk” for PCa as those with a family history, who are of WA/C ancestry, and/or who have a known inherited (germline) predisposition to PCa. Most cancers are sporadic in etiology and are caused by an accumulation of genetic mutations due to aging and environmental factors. Cancer can also be hereditary, where there is a known, typically Mendelian inherited genetic predisposition that can be identified through germline genetic testing. In contrast, familial cancers are caused by genetic factor(s) that are also inherited but cannot be identified through genetic testing as they are largely unknown.
Family history
Up to 57% of PCa is familial in etiology.4 Familial risk refers to shared genetic, environmental, and lifestyle factors that work together in ways that are too complex to understand at this time. With the mechanisms of risk unknown, association studies based on family history of PCa are used to help quantify familial risk in unaffected individuals.
For example, the CanRisk-Prostate model generates lifetime risk percentages for PCa in individuals based on family history of cancer, germline genetic status, and polygenic risk score analysis.5 This model is based on a multitude of studies that have generated empirical data from large family registries. An example of such a study is one based in Sweden that captured 98% of prostate cancers in the country and used family history to provide lifetime risks of low-risk PCa and high-risk PCa (Table 1) in unaffected individuals based on their family history alone (Table 2).6
Table 2.
Prostate cancer risk based on family history
| Family history | Average PCa risk by age 65 (%) | Average PCa risk by age 75 (%) | ||
|---|---|---|---|---|
| Any PCa | High-risk PCa | Any PCa | High-risk PCa | |
|
| ||||
| General population | 4.8 | 1.4 | 12.9 | 5.2 |
|
| ||||
| 1 brother, any PCa | 14.9 | 3.0 | 30.3 | 8.9 |
|
| ||||
| 1 brother, high-risk PCa | 16.1 | 3.4 | 31.7 | 9.3 |
|
| ||||
| Father, any PCa + 1 brother, any PCa | 29.8 | 5.6 | 47.8 | 13.8 |
|
| ||||
| Father died of PCa + brother, any PCa | 29.8 | 6.6 | 45.0 | 12.7 |
|
| ||||
| 2 brothers, any PCa | 34.4 | 12.0 | 55.1 | 13.4 |
|
| ||||
| Father, any PCa + 2 brothers, any PCa | 43.9 | 11.4 | 63.6 | 20.5 |
PCa: prostate cancer.
Although such assessment of the risk of developing PCa may be of benefit to an individual, it does not yet inform inclusion into an increased-risk screening program, as is the case with breast cancer screening. In Ontario, the definition of being at high risk (increased risk) for breast cancer is defined as 25% lifetime risk based on modelling.7 As such, whether using models as outlined above or more empirical data, it is reasonable to suggest that a threshold risk of 25% or higher, based on family history, should be factored into whether to include PCa screening in a patient’s care, especially in families whether there is an increased prevalence of high-risk.
Any individual with a prostate who has a sibling with PCa, or a sibling and a father with PCa is expected to have a higher than 25% lifetime risk for PCa themselves.6 It is important to note, however, that there is a dearth in data relating to lifetime risk of PCa based on family history of PCa in second-degree relatives (uncles and grandfathers) and third-degree relatives (cousins and great-grandparents). As a result, there is room for clinical opinion in defining whether a family history is strong enough to consider someone at “increased risk.”
Black/West African ancestry
Prostate cancer incidence and progression is highly variable across people of different geographical ancestral origins. In the U.S., non-Hispanic Black individuals have an increased risk of developing PCa, with an increased associated morbidity and mortality when compared to non-Hispanic White individuals.9 In comparison to non-Hispanic White individuals, the Centers for Disease Control (CDC) shows that PCa incidence is approximately 75% higher in non-Hispanic WA/C patients and 45% lower in non-Hispanic Asian and Pacific Islanders (U.S. Cancer Statistics Working Group, US Cancer Statistics Data Visualizations Tool, based on 2021 submission data [1999–2019]: US Department of Health and Human Services, CDC, and Prevention and National Cancer Institute; https://www.cdc.gov/cancer/dataviz, released in November 2022).
An English study from 2008–2010 showed the lifetime risk of a Black person to be diagnosed with PCa was 29%, and the lifetime risk of dying from their disease was 9%, whereas the lifetime risk for a White person to be diagnosed PCa was 13% and the lifetime risk of dying from it was 4%.10 These significant differences, although incredibly important for healthcare providers and policymakers, need to take into context the racial disparities in access to and utilization of healthcare, particularly for WA/C men in the U.S. As a result, it is unclear what component of the differences seen are genetic/inherited vs. due to the result of systemic bias and other confounders.11 Despite this uncertainty, it is important to note that Black individuals may have a greater than 25% lifetime risk for PCa regardless of family history.
Hereditary cancer
Hereditary cancer conditions are caused by inherited PV/LPV in cancer-protecting genes. The main genes associated with increased risk for PCa include ATM, BRCA1, BRCA2, CHEK2, HOXB13, MSH2, and PALB2 (Table 3), which are primarily involved in sensing and repairing DNA damage. Germline PV/LPVs have been documented in 11.8% of patients with metastatic prostate cancer (mPC) and 4.6% of patients with localized disease.12 There is increasing awareness that PV/LPVs in certain genes are not only of importance for estimating the risk of developing the disease but can also be used to help personalize treatment.
Table 3.
Prostate cancer risk, aggressiveness, and management based on germline mutation
| Gene | Prostate risk (meta-analysis) | Aggressiveness | Inform care | Other associated cancers |
|---|---|---|---|---|
| ATM | OR 4.437 | Uncertain | Yes (AS, PARPi) | Breast cancer, pancreatic cancer, ovarian cancer |
| BRCA1 | OR 1.3538 | Maybe | Yes (AS, PARPi) | Breast cancer, pancreatic cancer, ovarian cancer |
| BRCA2 | OR 2.6438 | Yes | Yes (AS, surgery, PARPi) | Breast cancer, pancreatic cancer, ovarian cancer |
| CHEK2 1100delC | OR 3.2939 | No | No | Breast cancer |
| I157T | OR 1.839 | No | No | Breast cancer |
| HOXB13 p.Gly84Glu | OR 3.2540 | Uncertain | No | No evidence |
| Lynch syndrome * | OR 2.1341 | Uncertain | Yes (PCDi) | Colon cancer, uterine cancer, ovarian cancer, pancreatic cancer, gastric cancer, genitourinary cancers |
| MSH2 | SIR 3.62 (retrospective study)42 | Uncertain | Yes (PCDi) | Colon cancer, uterine cancer, ovarian cancer, pancreatic cancer, gastric cancer, genitourinary cancers |
| PALB2 | OR 1.3843 | Uncertain | No | Breast cancer (male), ovarian, pancreatic cancer |
Lynch syndrome in this study included individuals with germline PV/LPVs in the genes MLH1, MSH2, and PMS2. AS: active surveillance; OR: odds ratio; PARPi: PARP-inhibitor; PCDi: programmed cell death inhibitor; SIR: standardized incidence ratio.
Patients with mPC who have inherited PV/LPVs (germline) or acquired PV/LPVs (somatic) in ATM, BRCA1, and BRCA2 are eligible for poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) treatment, which has been shown to increase progression-free and overall survival (OS) of their disease.13 In mPC patients with germline PV/LPVs in mismatch repair genes, such as MLH1, MSH2, and PMS2, programmed cell death inhibitors (PCDi) like pembrolizumab have been shown to decrease PSA levels by 50% in greater than half of treated men but do not affect OS.14
Additionally, knowledge of these genetic changes can be of value in the management of low-risk PCa with active surveillance (AS), where patients are closely monitored through regular PSA testing, magnetic resonance imaging (MRI), and prostate biopsy, with treatment being considered if there is progression in cancer grade.15 Germline ATM, BRCA1, and BRCA2 PV/LPVs have been shown to have increased risk of grade group reclassification in an AS cohort, with the strongest association being with BRCA2 carriers.16 Based on this evidence, BRCA2 carrier status should be factored into a patient’s candidacy for AS, and may warrant a modified AS protocol or upfront treatment at time of diagnosis.
Polygenic risk score or genetic risk score
Polygenic risk score (PRS) is a method of identifying genetic risk associated with a medical outcome. PRS uses genome-wide association studies to identify multiple genetic variants over-represented in the genomes of a cohort with a medical outcome compared to an unaffected cohort. The score generated by a PRS shows an individual’s risk for the medical outcome based on the number of variants associated with the outcome that provides a score. It is important to note that many conditions are multifactorial in nature, and genetics alone are not fully predictive. In the case of PCa, there are multi-ancestry-validated PRS designed to determine an individual’s PCa risk;8 however, the clinical utility of PRS, especially in the context of PSA and family history, remains unclear.
WHAT ARE CURRENT SCREENING GUIDELINES FOR PEOPLE AT INCREASED RISK FOR PCa?
The use of PSA screening for PCa, while currently the standard screening practice recommended by many urological organizations, is also not without controversy, stemming in part from the high rate of false-positive screening tests,17 as well as from the conflicting data regarding prostate mortality in cohorts receiving PSA screening.
Two examples of conflicting data include the ERS PC and Götenberg studies, which show decreased mortality,18,19 and the American PLCO trial showing no difference in mortality when comparing the screened and unscreened groups.20 The findings of the PLCO study had longstanding implications for policy development in North America despite the near complete contamination of PSA screening in the control arm of this study and no efficacy analysis.21,22
The major organizations providing guidelines for PCa screening may share some commonalities but are largely inconsistent (Table 4). The NCCN and two North American urological associations recommend that individuals with prostates who are at increased risk for PCa undergo decision-making for PSA screening between ages 40–45.
Table 4.
PSA screening recommendations from major North American guiding bodies
| Canadian Preventive Task Force (2014)2 | CUA (2017)44 | USPSTF (2018)45 | AUA (2023)46 | NCCN (2024)28 | |
|---|---|---|---|---|---|
| Screening for general population | Against PSA screening for all ages | Through shared decision-making with a healthcare provider: Undergo PSA screening ≥50 years |
Through shared decision-making with a healthcare provider: Undergo PSA screening between 55–69 years Against PSA screening >70 years |
Through shared decision-making with a healthcare provider: Undergo PSA screening beginning at age 45–50 years |
Through shared decision-making with a healthcare provider: Undergo PSA screening between age 45–75 years |
| Screening for increased risk population | N/A | Undergo PSA screening ≥45 years | N/A | Undergo PSA screening beginning at age 40–45 years | Undergo PSA screening between age 40–75 years |
AUA: American Urological Association; CUA: Canadian Urological Association; NCCN: National Comprehensive Cancer Network; PSA: prostate-specific antigen; USPSTF: U.S. Preventive Services Task Force.
As we become better at identifying people at increased risk for PCa, we have an opportunity to provide a standardized approach to screening in these individuals. Currently in Canada, prostate screening is largely based on patient-clinician conversations, with varying opinions based on inconsistent and sometimes conflicting data, which largely applies to the general population and not to increased-risk populations.
Increased risk for PCa can be defined as having a germline PV/LPV in a prostate cancer-related gene, having a family history of PCa in closely related family members, or being of WA/C ancestry. These criteria can be simplified as having a >25% lifetime risk for PCa. The NCCN and the AUA state that all individuals at increased risk of PCa should have annual PSA screening and a digital rectal exam (DRE) starting at age 40.
Enhanced screening beyond annual DRE and PSA testing is needed for those at increased risk of high-risk PCa. The use of MRI for PCa screening is of interest due to its high sensitivity for intermediate-risk and high-risk PCa (Table 5; NCCN 2020).23,34 The effectiveness of MRI as a screening tool has been found to be greater than for PSA in both studies of the general population25 and in an increased-risk population, such as people with BRCA1 and BRCA2 pathogenic variants;26 more studies are pending (NCT01990521).
Table 5.
Definition of increased risk for prostate cancer
| CUA (2022)44 | AUA (2023)46 | NCCN (2024)28 | |
|---|---|---|---|
| Definition of increased risk | Men with a first or second-degree relative with prostate cancer Men with germline mutations |
Men with Black ancestry, germline mutations, and strong family history. | Men who are of Black/African American ancestry Have a germline mutation that increases risk for prostate cancer Have a concerning family or personal history |
AUA: American Urological Association; CUA: Canadian Urological Association; NCCN: National Comprehensive Cancer Network.
A comprehensive review and meta-analysis of 12 randomized clinical trials and prospective cohort studies comparing MRI-based to PSA-only screening of 80 114 men showed the MRI-based pathway had increased odds of castrate-sensitive PCa, decreased odds of biopsies, and decreased odds of insignificant cancers identified without significant differences in identification of castrate-sensitive PCa.27 Due to the accumulating evidence of use of MRI in the prostate detection process, NCCN recommends the following MRI-based pathway:28
PSA >3 ng/mL or suspicious DRE in “high-risk” individuals;
PSA >4 ng/mL or suspicious DRE in general population.
Integration of MRI screening of patients at increased risk is equally a matter of debate and investigation, although it would appear clinically justifiable in those groups with a poorer prognosis than seen in the general population, such as those with a BRCA2 PV/LPV.29
HOW CAN SCREENING INCREASED-RISK POPULATIONS IMPACT OUTCOMES?
Among the individuals at increased risk for PCa are those at increased risk for high-risk PCa. Identifying PCa early through PSA decreases PCa mortality,18,19 and MRI-based pathways decrease odds of biopsies without significant differences in identified castrate-sensitive PCa.27 Clear PCa detection strategies for people at increased risk for PCa, such as provided by the NCCN, is expected to decrease mortality in this cohort.
Individuals with a significant family history of high-risk PCa and/or are of WA/C ancestry are at increased risk not only for PCa, but for high-risk PCa.6,9
Individuals with PV/LPVs in known genes associated with PCa are at increased risk for PCa and some are at increased risk for high-risk PCa. Of the known genes associated with PCa, the BRCA2 gene has the most consistent literature associating carriers with higher mortality rates. A retrospective analysis from 1998–2010 showed a 12-year PCa survival rate of 61.8% in BRCA2 carriers compared to 94.3% in participants without BRCA2 P/LPVs.29 The IMPACT study published their three-year prospective PSA-only screening protocol identifying BRCA2 carriers had higher rates of PCa, as well as more clinically significant PCa compared to BRCA1 and BRCA2 non-carriers.30 It remains to be determined whether people with PV/LPVs in ATM, BRCA1, HOXB13, and MSH2 are at increased risk for high-risk PCa.
WHO SHOULD BE OFFERED GENETIC TESTING?
The CUA developed genetic testing guidelines for germline and tumor genetic testing.31 The CUA’s two main criteria are 1) being diagnosed with mPC; and 2) being diagnosed with localized PCa and being of Ashkenazi Jewish ancestry and/or having a personal or family history of related cancers, such as breast, ovarian, colorectal, endometrial, or pancreatic cancer. The CUA highlights criteria for genetic testing of affected patients; however, once a germline PV/LPV is identified, potentially unaffected parents, siblings, children, and extended family are eligible for genetic testing for the identified variant. If the variant is identified in a family member, they can be screened more carefully for the cancer risk associated.
Each province may have more specific criteria for germline genetic testing (Figure 1) or for tissue genetic testing. To facilitate germline genetic testing, patients can be referred to a local genetics clinic. Some centers work directly with genetics clinics to facilitate genetic testing themselves through a model called physician-initiated genetic testing or mainstreaming.31 Universal germline genetic testing is a process in which all patients with a specific cancer, regardless of age, are offered germline genetic testing. Currently there are studies outlining how this process can be successfully implemented in rural and urban settings in the context of breast cancer.32,33
Figure 1.
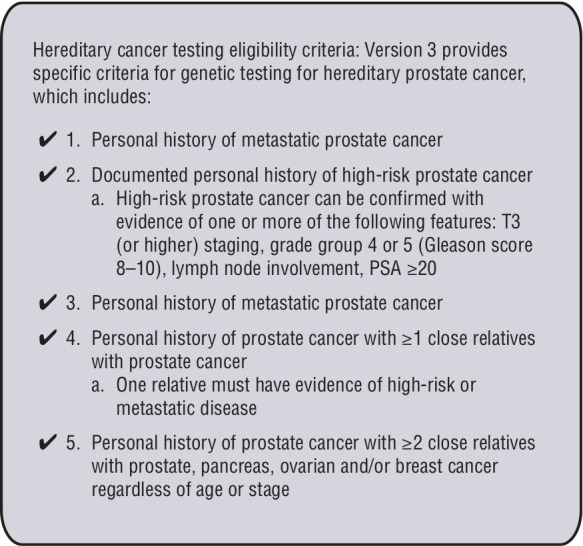
Cancer Care Ontario genetic testing criteria for prostate cancer.
HOW CAN WE CONTINUE TO RESEARCH PEOPLE AT INCREASED RISK?
Registries act as a foundation for facilitating research in a target population. Regarding high-risk PCa, there is the international IRONMAN registry (NCT03151629); in the U.S., there is the Prostate Cancer Genetic Risk, Experience, and Support Study (PROGRESS) registry and the PROMISE study (NCT05129605, NCT04995198); and in Canada, the MORE Registry.34 These registries catalogue and collect comprehensive clinical, pathological, and genomic information, which can be supplemented with biobanks to fill in gaps in our knowledge on target groups such as WA/C individuals with prostates and people with germline PV/LPVs that elevate risk for PCa.
Mistrust of medical research within the WA/C community due to healthcare discrimination and unethical research studies, such as the Tuskegee experiment, has made recruiting WA/C men to registries challenging, resulting in a lack of knowledge within the community.35 Building these registries cannot be emphasized enough to answer questions like why we see increased risk for high-risk PCa in this community, and the intersectionality between being of WA/C ancestry and having a germline PV/LPV. Although individually rare, registries can facilitate research on large groups of people with germline PV/LPVs to validate PCa prevention strategies such as ejaculation frequency,36 develop ways to predict PCa aggressiveness with use of polygenic risk scores, and develop enhanced PCa screening for people at increased risk for high-risk PCa.
CONCLUSIONS
Individuals at increased risk for developing prostate PCa include those with a family history, WA/C individuals, and those who have germline PV/LPVs in known PCa-related genes. A unifying factors among these three groups is a >25% lifetime risk for PCa. Despite knowing these populations are at increased risk, there is little consensus for screening of these individuals in Canada and each physician in Canada adopts their own model of care for screening in individuals at increased risk. The NCCN and AUA guidelines recommending all individuals at increased risk be screened at age 40 are the most comprehensive. We also encourage academic centers to establish opportunity to contribute to the body of research on causes of cancer risk in individuals at increased risk for PCa.
KEY MESSAGES.
■ Germline pathogenic variants/likely pathogenic variants can inform PCa screening and management.
■ Family history of PCa can be quantified into lifetime risk using models and family history data. Lifetime risks for prostate cancer in individuals with a brother with PCa can range from 30.3–63.6%.
■ Individuals of West African/Caribbean decsent with prostates have up to a 29% lifetime risk for PCa and an increased risk for developing high-risk PCa.
■ Who is at increased risk for PCa needs to be defined so it is clear who should access enhanced detection.
■ Individuals at increased risk should be offered annual PSA and DRE annually starting at age 40.
Footnotes
COMPETING INTERESTS: Dr. Lorentz has been an adversity board participant for AstraZeneca and has received honoraria for consulting work related to education materials, speaking engagements, and advisory boards from AstraZeneca, Merck, and UHN. The remaining authors do not report any competing personal or financial interests related to this work.
This paper has been peer reviewed.
REFERENCES
- 1.Brenner DR, Weir HK, Demers AA, et al. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192:E199–205. doi: 10.1503/cmaj.191292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell N, Connor Gorber S, Shane A, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ. 2014;186:1225–34. doi: 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith DP, Armstrong BK. Prostate-specific antigen testing in Australia and association with prostate cancer incidence in New South Wales. Med J Aust. 1998;169:17–20. doi: 10.5694/j.1326-5377.1998.tb141471.x. [DOI] [PubMed] [Google Scholar]
- 4.Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyberg T, Brook MN, Ficorella L, et al. CanRisk-Prostate: A comprehensive, externally validated risk model for the prediction of future prostate cancer. J Clin Oncol. 2023;41:1092–104. doi: 10.1200/JCO.22.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratt O, Drevin L, Akre O, et al. Family history and probability of prostate cancer, differentiated by risk category: A nationwide population-based study. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw110. [DOI] [PubMed] [Google Scholar]
- 7.Chiarelli AM, Prummel MV, Muradali D, et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: Results of the initial screen from the Ontario high risk breast screening program. J Clin Oncol. 2014;32:2224–30. doi: 10.1200/JCO.2013.52.8331. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Darst BF, Madduri RK, et al. Validation of a multi-ancestry polygenic risk score and age-specific risks of prostate cancer: A meta-analysis within diverse populations. Elife. 2022;11:e78304. doi: 10.7554/eLife.78304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeigler-Johnson CM, Spangler E, Jalloh M, et al. Genetic susceptibility to prostate cancer in men of African descent: Implications for global disparities in incidence and outcomes. Can J Urol. 2008;15:3872–82. [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd T, Hounsome L, Mehay A, et al. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Med. 2015;13:171. doi: 10.1186/s12916-015-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lillard JW, Jr, Moses KA, Mahal BA, et al. Racial disparities in Black men with prostate cancer: A literature review. Cancer. 2022;128:3787–95. doi: 10.1002/cncr.34433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 14.Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15:e0233260. doi: 10.1371/journal.pone.0233260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 16.Carter HB, Helfand B, Mamawala M, et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur Urol. 2019;75:743–9. doi: 10.1016/j.eururo.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry MJ. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N Engl J Med. 2001;344:1373–7. doi: 10.1056/NEJM200105033441806. [DOI] [PubMed] [Google Scholar]
- 18.Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur Urol. 2019;76:43–51. doi: 10.1016/j.eururo.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu CS, Huang WY, Pinsky PF, et al. The prostate, lung, colorectal and ovarian cancer (PLCO) screening trial pathology tissue resource. Cancer Epidemiol Biomarkers Prev. 2016;25:1635–42. doi: 10.1158/1055-9965.EPI-16-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooperberg MR. Prostate cancer: Why the prostate arm of the PLCO trial failed and what it has taught us. Nat Rev Urol. 2016;13:439–40. doi: 10.1038/nrurol.2016.116. [DOI] [PubMed] [Google Scholar]
- 22.Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795–6. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 23.Eldred-Evans D, Burak P, Connor MJ, et al. Population-based prostate cancer screening with magnetic resonance imaging or ultrasonography: The IP1-PROSTAGRAM study. JAMA Oncol. 2021;7:395–402. doi: 10.1001/jamaoncol.2020.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klotz L, Chin J, Black PC, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsynaive men at risk for prostate cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021;7:534–42. doi: 10.1001/jamaoncol.2020.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam RK, Wallis CJ, Stojcic-Bendavid J, et al. A pilot study to evaluate the role of magnetic resonance imaging for prostate cancer screening in the general population. J Urol. 2016;196:361–6. doi: 10.1016/j.juro.2016.01.114. [DOI] [PubMed] [Google Scholar]
- 26.Segal N, Ber Y, Benjaminov O, et al. Imaging-based prostate cancer screening among BRCA mutation carriers-results from the first round of screening. Ann Oncol. 2020;31:1545–52. doi: 10.1016/j.annonc.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Fazekas T, Shim SR, Basile G, et al. Magnetic resonance imaging in prostate cancer screening: A systematic review and meta-analysis. JAMA Oncol. 2024. p. e240734. [DOI] [PMC free article] [PubMed]
- 28.National Comprehensive Cancer Network. Prostate Cancer Early Detection (Version 2, 2024) https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf .
- 29.Akbari MR, Wallis CJD, Toi A, et al. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br J Cancer. 2014;111:1238–40. doi: 10.1038/bjc.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page EC, Bancroft EK, Brook MN, et al. Interim results from the impact study: Evidence for prostate-specific antigen screening in brca2 mutation carriers. Eur Urol. 2019;76:831–42. doi: 10.1016/j.eururo.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rendon RA, Selvarajah S, Wyatt AW, et al. 2023 Canadian Urological Association guideline: Genetic testing in prostate cancer. Can Urol Assoc J. 2023;17:314–25. doi: 10.5489/cuaj.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shelton C, Ruiz A, Shelton L, et al. Universal germline-genetic testing for breast cancer: Implementation in a rural practice and impact on shared decision-making. Ann Surg Oncol. 2024;31:325–34. doi: 10.1245/s10434-023-14394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Silva DL, Stafford L, Skandarajah AR, et al. Universal genetic testing for women with newly diagnosed breast cancer in the context of multidisciplinary team care. Med J Aust. 2023;218:368–73. doi: 10.5694/mja2.51906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorentz J, Liu SK, Vesprini D. Male Oncology Research and Education program for men at high risk for prostate cancer. Curr Oncol. 2018;25:170–5. doi: 10.3747/co.25.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharff DP, Mathews KJ, Jackson P, et al. More than Tuskegee: Understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–97. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rider JR, Wilson KM, Sinnott JA, et al. Ejaculation frequency and risk of prostate cancer: Updated results with an additional decade of follow-up. Eur Urol. 2016;70:974–82. doi: 10.1016/j.eururo.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsson Q, Brook MN, Dadaev T, et al. Rare germline variants in ATM predispose to prostate cancer: A PRACTICAL consortium study. Eur Urol Oncol. 2021;4:570–9. doi: 10.1016/j.euo.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh M, Alkhushaym N, Fallatah S, et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate. 2019;79:880–95. doi: 10.1002/pros.23795. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Dai B, Ye D. CHEK2 mutation and risk of prostate cancer: A systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:15708–15. [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Q, Wang X, Li X, et al. Germline HOXB13 p.Gly84Glu mutation and cancer susceptibility: A pooled analysis of 25 epidemiological studies with 145,257 participates. Oncotarget. 2015;6:42312–21. doi: 10.18632/oncotarget.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:437–49. doi: 10.1158/1055-9965.EPI-13-1165. [DOI] [PubMed] [Google Scholar]
- 42.Haraldsdottir S, Hampel H, Wei L, et al. Prostate cancer incidence in males with Lynch syndrome. Genet Med. 2014;16:553–7. doi: 10.1038/gim.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wokołorczyk D, Kluźniak W, Stempa K, et al. PALB2 mutations and prostate cancer risk and survival. Br J Cancer. 2021;125:569–75. doi: 10.1038/s41416-021-01410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason RJ, Marzoik K, Finelli A, et al. UPDATE – 2022 Canadian Urological Association recommendations on prostate cancer screening and early diagnosis: Endorsement of the 2021 Cancer Care Ontario guidelines on prostate multiparametric magnetic resonance imaging. Can Urol Assoc J. 2022;16:E184–96. doi: 10.5489/cuaj.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenton JJ, Weyrich MS, Durbin S, et al. Prostate-specific antigen-based screening for prostate cancer: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1914–31. doi: 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- 46.Wei JT, Barocas D, Carlsson S, et al. Early detection of prostate cancer: AUA/SUO guideline part I: Prostate cancer screening. J Urol. 2023;210:46–53. doi: 10.1097/JU.0000000000003491. [DOI] [PMC free article] [PubMed] [Google Scholar]


