Abstract
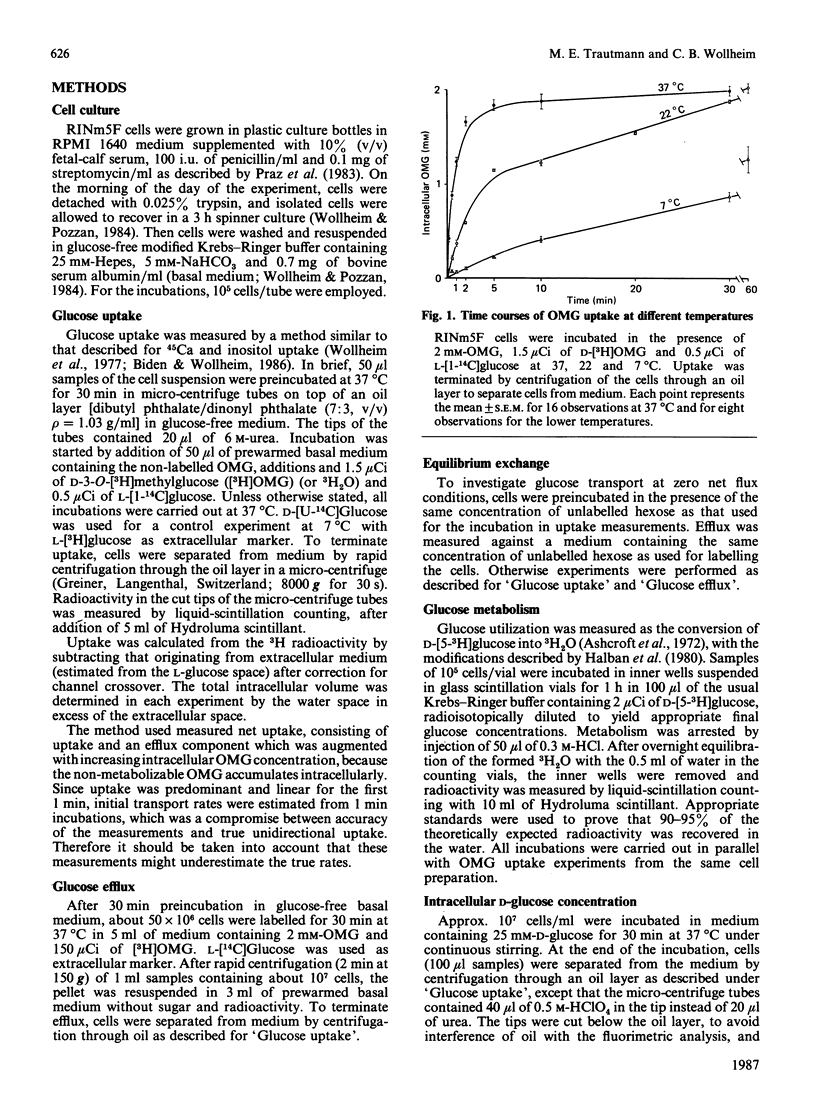
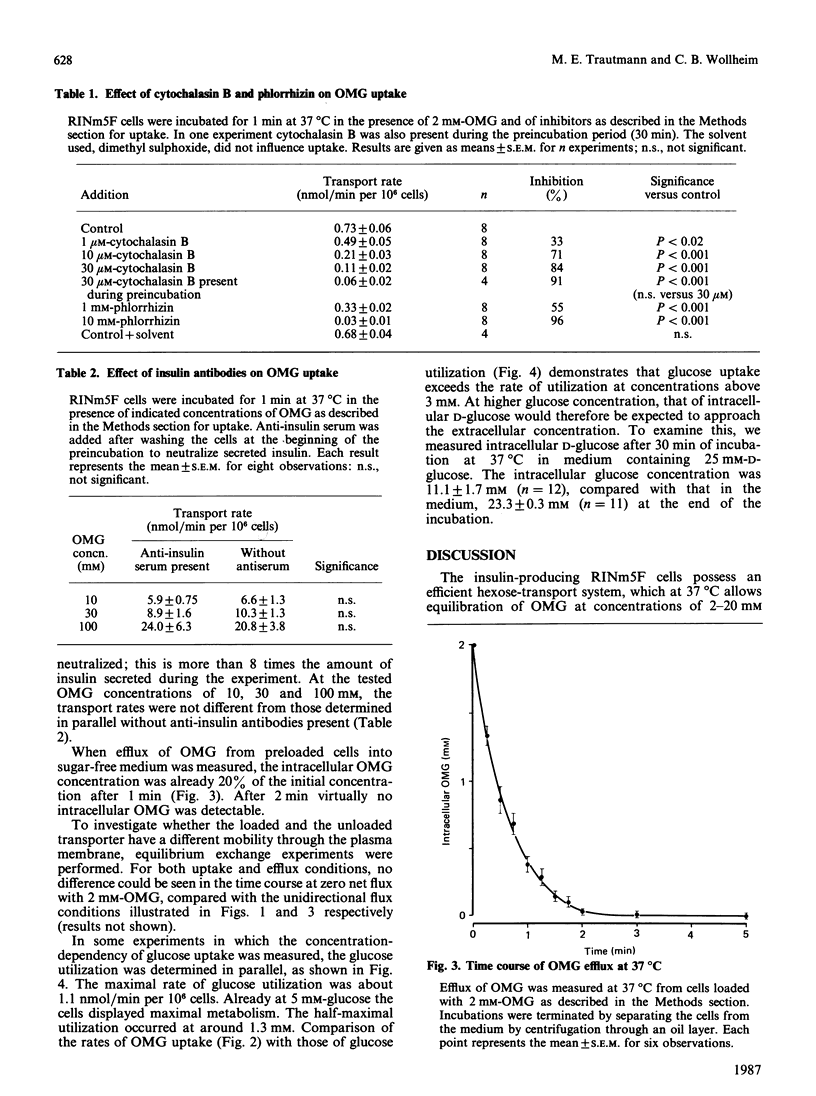
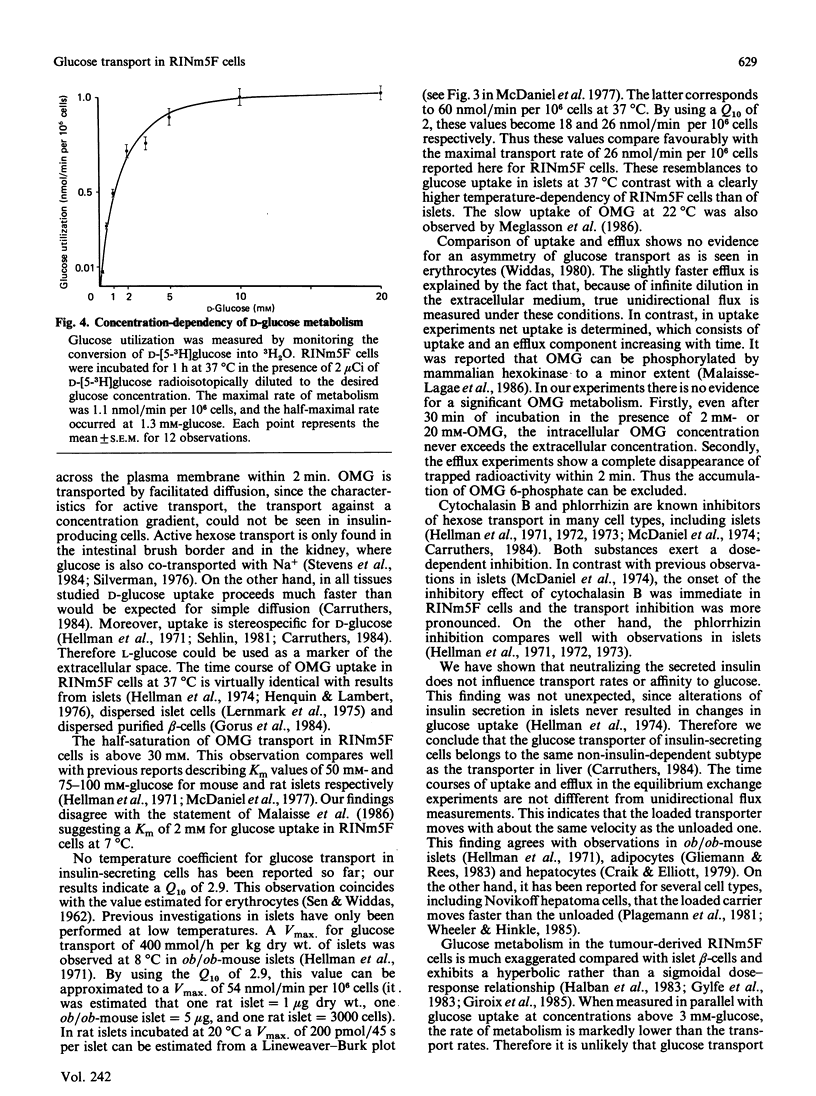
The rat insulinoma-derived RINm5F cell line retains many differentiated functions of islet beta-cells. However, it fails to recognize glucose as an insulin secretagogue in the physiological concentration range. With this cell line, glucose-transport kinetics were investigated, by using a double-label technique with the non-metabolizable glucose analogue 3-O-methylglucose (OMG). RINm5F cells possess a passive glucose-transport system with high capacity and low affinity. Equilibration across the plasma membrane of extracellular OMG concentrations up to at least 20 mM is achieved within 2 min at 37 degrees C. The half-saturation of OMG uptake occurs at 32 mM. At lower temperatures OMG uptake is markedly retarded, with a temperature coefficient (Q10) of 2.9. As indicated by efflux measurements, transport is symmetrical. Cytochalasin B at micromolar concentrations and phlorrhizin in millimolar concentrations are potent inhibitors of OMG uptake. Neutralization of the secreted insulin with antibodies does not alter OMG uptake kinetics. The glucose metabolism of RINm5F cells is much exaggerated compared with that of islet beta-cells. Nonetheless, when measured in parallel to uptake, transport exceeds by far the rate of metabolism at glucose concentrations above 3 mM. Measurements of intracellular D-glucose reveal a lower intracellular glucose concentration relative to the extracellular in RINm5F cells. This seems to be due to abnormalities in the subsequent steps of glucose metabolism, rather than to abnormalities in hexose uptake. The loss of glucose-induced insulin release in RINm5F cells cannot be explained by alterations in hexose transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Active transport of myo-inositol in rat pancreatic islets. Biochem J. 1986 Jun 15;236(3):889–893. doi: 10.1042/bj2360889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A. Sugar transport in animal cells: the passive hexose transfer system. Prog Biophys Mol Biol. 1984;43(1):33–69. doi: 10.1016/0079-6107(84)90003-8. [DOI] [PubMed] [Google Scholar]
- Craik J. D., Elliott K. R. Kinetics of 3-O-methyl-D-glucose transport in isolated rat hepatocytes. Biochem J. 1979 Aug 15;182(2):503–508. doi: 10.1042/bj1820503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H., Wollheim C. B. ATP-sensitive K+ channels in an insulin-secreting cell line are inhibited by D-glyceraldehyde and activated by membrane permeabilization. J Membr Biol. 1986;93(3):271–279. doi: 10.1007/BF01871181. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroix M. H., Sener A., Dufrane S. P., Malaisse-Lagae F., Malaisse W. J. Glucose metabolism in insulin-producing tumoral cells. Arch Biochem Biophys. 1985 Sep;241(2):561–570. doi: 10.1016/0003-9861(85)90581-8. [DOI] [PubMed] [Google Scholar]
- Gorus F. K., Malaisse W. J., Pipeleers D. G. Differences in glucose handling by pancreatic A- and B-cells. J Biol Chem. 1984 Jan 25;259(2):1196–1200. [PubMed] [Google Scholar]
- Gylfe E., Andersson T., Rorsman P., Abrahamsson H., Arkhammar P., Hellman P., Hellman B., Oie H. K., Gazdar A. F. Depolarization-independent net uptake of calcium into clonal insulin-releasing cells exposed to glucose. Biosci Rep. 1983 Oct;3(10):927–937. doi: 10.1007/BF01140662. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Praz G. A., Wollheim C. B. Abnormal glucose metabolism accompanies failure of glucose to stimulate insulin release from a rat pancreatic cell line (RINm5F). Biochem J. 1983 May 15;212(2):439–443. doi: 10.1042/bj2120439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B., Blondel B., Renold A. E. Long-term exposure of isolated pancreatic islets to mannoheptulose: evidence for insulin degradation in the beta cell. Biochem Pharmacol. 1980 Oct 1;29(19):2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974 Jan;138(1):33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Lernmark A., Sehlin J., Täljedal I. B. Effects of phlorizin on metabolism and function of pancreatic -cell. Metabolism. 1972 Jan;21(1):60–66. doi: 10.1016/0026-0495(72)90020-0. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Evidence for mediated transport of glucose in mammalian pancreatic -cells. Biochim Biophys Acta. 1971 Jul 6;241(1):147–154. doi: 10.1016/0005-2736(71)90312-9. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Transport of 3-O-methyl-D-glucose into mammalian pancreatic -cells. Pflugers Arch. 1973;340(1):51–58. doi: 10.1007/BF00592196. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Bicarbonate modulation of glucose-9nduced biphasic insulin release by rat islets. Am J Physiol. 1976 Sep;231(3):713–721. doi: 10.1152/ajplegacy.1976.231.3.713. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Giroix M. H., Sener A., Malaisse W. J. Phosphorylation of 3-O-methyl-D-glucose by yeast and beef hexokinase. FEBS Lett. 1986 Mar 31;198(2):292–294. doi: 10.1016/0014-5793(86)80423-9. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- McDaniel M. L., King S., Anderson S., Fink J., Lacy P. E. Effect of cytochalasin B on hexose transport and glucose metabolism in pancreatic islets. Diabetologia. 1974 Aug;10(4):303–308. doi: 10.1007/BF02627731. [DOI] [PubMed] [Google Scholar]
- McDaniel M. L., Weaver D. C., Roth C. E., Fink C. J., Swanson J. A., Lacy P. E. Characterization of the uptake of the methylxanthines theophylline and caffeine in isolated pancreatic islets and their effect on D-glucose transport. Endocrinology. 1977 Dec;101(6):1701–1708. doi: 10.1210/endo-101-6-1701. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M., Graff J., Erbe J., Wilkie P. Broad specificity hexose transport system with differential mobility of loaded and empty carrier, but directional symmetry, is common property of mammalian cell lines. J Biol Chem. 1981 Mar 25;256(6):2835–2842. [PubMed] [Google Scholar]
- Praz G. A., Halban P. A., Wollheim C. B., Blondel B., Strauss A. J., Renold A. E. Regulation of immunoreactive-insulin release from a rat cell line (RINm5F). Biochem J. 1983 Feb 15;210(2):345–352. doi: 10.1042/bj2100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Determination of the temperature and pH dependence of glucose transfer across the human erythrocyte membrane measured by glucose exit. J Physiol. 1962 Mar;160:392–403. doi: 10.1113/jphysiol.1962.sp006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Giroix M. H., Malaisse W. J. Impaired uptake of D-glucose by tumoral insulin-producing cells. Biochem Int. 1986 Jun;12(6):913–919. [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. Nutrient metabolism in islet cells. Experientia. 1984 Oct 15;40(10):1026–1035. doi: 10.1007/BF01971448. [DOI] [PubMed] [Google Scholar]
- Silverman M. Glucose transport in the kidney. Biochim Biophys Acta. 1976 Dec 14;457(3-4):303–351. doi: 10.1016/0304-4157(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Kaunitz J. D., Wright E. M. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- Vischer U., Blondel B., Wollheim C. B., Höppner W., Seitz H. J., Iynedjian P. B. Hexokinase isoenzymes of RIN-m5F insulinoma cells. Expression of glucokinase gene in insulin-producing cells. Biochem J. 1987 Jan 1;241(1):249–255. doi: 10.1042/bj2410249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Somatostatin- and epinephrine-induced modifications of 45Ca++ fluxes and insulin release in rat pancreatic islets maintained in tissue culture. J Clin Invest. 1977 Nov;60(5):1165–1173. doi: 10.1172/JCI108869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984 Feb 25;259(4):2262–2267. [PubMed] [Google Scholar]


