Abstract
Epilepsy remains a disease that affects many people around the world. With the development of new drugs to treat this condition, the importance of therapeutic drug monitoring continues to rise and remains a challenge for the medical community. This review article explores recent advances in the detection of antiepileptic drugs across various sample types commonly used for drug monitoring, with a focus on their applications and impact. Some of these new methods have proven to be simpler, greener, and faster, making them easier to apply in the context of therapeutic drug monitoring. Additionally, besides the classic use of blood and its derivatives, there has been significant research into the application of alternative matrices due to their ease of sample collection and capacity to reflect drug behavior in blood. These advances have contributed to increasing the efficacy of therapeutic drug monitoring while enhancing its accessibility to the population.
Keywords: antiepileptic drugs, monitoring, blood and derivates, urine, saliva, hair, exhaled air
1. Introduction
Epilepsy is one of the most prevalent neurological diseases worldwide, affecting approximately 50 million individuals across all age groups. Besides neurological and cognitive complications, epilepsy also imposes significant psychological and social burdens due to stigma and discrimination [1]. Epilepsy is defined by the spontaneous recurrence of unprovoked seizures, meaning seizures not induced by transient systemic, metabolic, or toxic disorders. It can be classified as generalized, focal, unknown, and combined generalized and focal epilepsy. Generalized epilepsy is further divided into motor onset seizures, such as tonic and clonic, and non-motor onset seizures, such as myoclonic absence [1,2].
Factors such as the global increase in life expectancy and the rising proportion of individuals surviving events that often lead to epilepsy, such as birth trauma, traumatic brain injury, brain infections, and stroke, are expected to contribute to a higher prevalence of this condition worldwide. Thus, it is crucial to be attentive to the first symptoms [1]. A clinical diagnosis of epilepsy is made if there have been at least two unprovoked seizures occurring more than 24 h apart, or one unprovoked seizure with a recurrence probability of more than 60% over the subsequent 10 years [1,3].
The mainstay treatment strategy for seizures is medication management. However, much like the prescription of any other pharmaceutical agent, a clinician must balance efficacy with adverse events, and consider cost, drug interactions, patient preference, and availability [4]. Up to 70% of individuals with epilepsy could achieve seizure freedom with appropriate diagnosis and the use of cost-effective, commonly available antiseizure medicines, which can ultimately enable people with epilepsy to continue or return to a full and productive life [1,4].
While there is a multitude of different antiepileptic agents used in clinical practice today, they primarily act by interfering with one or more cellular mechanisms believed to cause seizures [5,6].
Antiepileptic drugs (AEDs) are categorized into two types: broad spectrum and narrow spectrum. Broad-spectrum AEDs treat a wide variety of seizure types and are a good initial choice, especially when the classification of seizure type is uncertain. These AEDs include, but are not limited to, levetiracetam, lamotrigine, zonisamide, topiramate, valproic acid, clonazepam, perampanel, clobazam, and rufinamide. Narrow-spectrum AEDs are primarily used for the treatment of focal or partial seizures. These include, but are not limited to, lacosamide, pregabalin, gabapentin, carbamazepine, oxcarbazepine, ezogabine, phenytoin, and vigabatrin [4].
Monotherapy is the ideal pathway for the treatment of seizures, but newer AEDs have had difficulty obtaining Food and Drug Administration approval as monotherapy agents due to the stringent requirements for approval. However, both the anecdotal evidence and current research suggest that second-generation AEDs appear to be an appropriate choice, as they have demonstrated similar efficacy compared to older AEDs and may be better tolerated [4].
There are several ways to classify AEDs, for example, Table 1 describes some AEDs according to their mechanisms of action. Some AEDs act on sodium channels by either blocking their repetitive activation (e.g., phenytoin and carbamazepine) or by enhancing their slow inactivation (e.g., lacosamide). Others target calcium channels by blocking T-type calcium channels (e.g., ethosuximide and valproic acid) or the N- and L-type calcium channels (e.g., zonisamide). Lamotrigine functions by blocking sodium channels, blocking N- and L-type calcium channels, and modulating the H-current. Topiramate acts by blocking sodium channels, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, and inhibiting carbonic anhydrase. Other mechanisms of AED action include enhancing gamma-aminobutyric acid (GABA)-A receptors (e.g., phenobarbital and benzodiazepines), blocking N-methyl-D-aspartic acid (NMDA) receptors (e.g., felbamate), and opening neuronal potassium channels (e.g., ezogabine) [4].
Table 1.
Classification of some AEDs according to their mechanism of action.
| Mechanism of Action | Drugs | Reference |
|---|---|---|
| Modulation of voltage-gated sodium channels |
|
[4,7] |
| GABA receptors modulation |
|
[4,7,8,9,10] |
| Modulation of calcium channels |
|
[7,11,12,13] |
| Carbonic anhydrase modulation |
|
[7,14] |
| Modulation of glutamate receptors and others |
|
[7,15] |
| Unknown mechanism of action |
|
[4,7] |
| Several mechanisms of action |
|
[4,7,16,17,18,19,20,21,22,23,24,25] |
AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ATPase: adenosin triphosphatase; CRMP2: collapsin response mediator protein 2; GABA: gamma-aminobutyric acid; GABA-A: gamma-aminobutyric acid type A; GABAtransaminase: gamma-aminobutyric acid transaminase; GAT1: gamma-aminobutyric acid (GABA) transporter 1; NMDA: N-methyl-D-aspartate; SV2A: synaptic vesicle protein 2.
AEDs can also be classified by their therapeutic usage. For instance:
Simple partial seizures (carbamazepine, phenytoin, phenobarbital, primidone, valproate, gabapentin, and lamotrigine).
Complex partial seizures (carbamazepine, phenobarbital, phenytoin, primidone, valproate, gabapentin, and lamotrigine).
Partial with secondary generalized tonic–clonic seizures (carbamazepine, phenobarbital, phenytoin, primidone, valproate, gabapentin, and lamotrigine).
Generalized absence seizures (clonazepam, ethosuximide, and valproate).
Generalized myoclonic seizures (valproate).
Tonic–clonic seizures (carbamazepine, phenobarbital, phenytoin, primidone, and valproate) [26].
Additionally, AEDs can be classified based on their chemical properties, such as barbiturates (e.g., phenobarbitone, mephobarbitone, and primidone), hydantoins (e.g., phenytoin and mephenytoin), iminostilbenes (e.g., carbamazepine), oxazolidinediones (e.g., trimethadione (troxidone)), succinimides (e.g., ethosuximide), aliphatic carboxylic acids (e.g., valproic acid), benzodiazepines (e.g., clonazepam and diazepam), acetylureas (e.g., phenacemide), newer drugs (e.g., progabide, vigabatrin, gabapentin, lamotrigine, felbamate, topiramate, and tiagabine), and miscellaneous (e.g., acetazolamide and dexamphetamine) [26].
Currently, approximately 30% of patients do not achieve satisfactory seizure control [5]. Additionally, many patients suffer from significant treatment-related adverse reactions, making therapeutic drug monitoring essential in epilepsy treatment to maximize clinical efficacy and minimize adverse drug reactions [2]. Drug monitoring is defined as the measurement and clinical use of drug concentrations in serum/plasma (or saliva) to adjust individual patient dosages, thereby tailoring it to each patient’s individual therapeutic requirements. It is most commonly applied to medications with a narrow therapeutic range; in this situation, we are referring to therapeutic drug monitoring (TDM). This monitoring has been used over the last 50 years to manage pharmacological variability within and between patients, during which time many drugs have been developed, enabling continuous advancements in this field and its impact on clinical practice [2,5,27].
TDM is crucial for all drugs where the serum concentration is expected to reflect the concentration and pharmacological action at the brain’s target site. The only exception is vigabatrin, an irreversible inhibitor of the enzyme responsible for GABA degradation and GABA transaminase. Vigabatrin can produce a prolonged effect on the brain, even when its serum concentration is declining or zero. Pharmacokinetic interactions may also be controlled through the use of TDM as changes in serum concentrations reflect alterations in metabolism [27].
In recent years, several new AEDs have been introduced to the market, with 27 AEDs now available internationally. The selection of the appropriate AED for different seizure types is of paramount importance, as some AEDs are specifically effective in certain seizure types. However, the efficacy and safety of these treatments rely heavily not only on the selection of the appropriate drug, but also on careful clinical monitoring throughout the course of therapy. TDM is a valuable tool for optimizing and individualizing AED treatment, allowing clinicians to adjust doses to achieve optimal therapeutic levels while minimizing the risk of toxicity or subtherapeutic dosing. Given the narrow therapeutic index of many AEDs, small changes in blood concentration can result in significant clinical consequences, including increased seizure frequency or adverse effects. Monitoring AED levels in the clinic provides real-time information on drug absorption, metabolism, and elimination, which can be influenced by factors, such as patient age, organ function, drug–drug interactions, and genetic variations [27]. Regular clinical monitoring is essential for ensuring that patients maintain therapeutic levels of AEDs, especially in cases where there are changes in the patient’s condition, the introduction of other medications, or alterations in adherence to treatment. Furthermore, TDM plays a critical role in long-term epilepsy management, helping to reduce the risk of treatment failure and minimizing potential side effects. The ability to individualize AED therapy through TDM not only improves seizure control, but also enhances patient safety and quality of life, making it a cornerstone of epilepsy treatment in clinical practice [28,29].
2. Strategies to Determine AEDs in Biological Specimens
As research progresses, the field of analytical techniques is constantly evolving, enabling the detection of drugs and their metabolites at extremely low concentrations. To achieve high performance metrics for any test, effective sample preparation before the detection step is crucial [30].
The three primary purification methods used for the extraction and concentration of analytes from biological samples are solid-phase extraction (SPE), liquid–liquid extraction (LLE), and protein precipitation (PP). Although these techniques are still in use today. They have several drawbacks. They require large sample volumes, emulsion formations in some cases, and the use of organic solvents, which generate significant waste. Additionally, they involve considerable manual work, making them less attractive [30,31].
Contemporary preconcentration techniques are generally divided into two major categories: liquid phase and solid-phase microextractions [32]. Solid-phase microextraction (SPME) is notably fast, widely applicable across various research domains, and demonstrates excellent sample purification outcomes. It is regarded as an extremely efficient technique for sample pretreatment that can be seamlessly integrated with separation or detection equipment. In-tube SPME (IT-SPME) is another advanced microextraction technique. This simple SPME format retains the benefits of traditional methods (solvent-free, fast, and cost-effective) and introduces a new “online extraction” mode that can be used with various mass spectrometry (MS) systems [32,33].
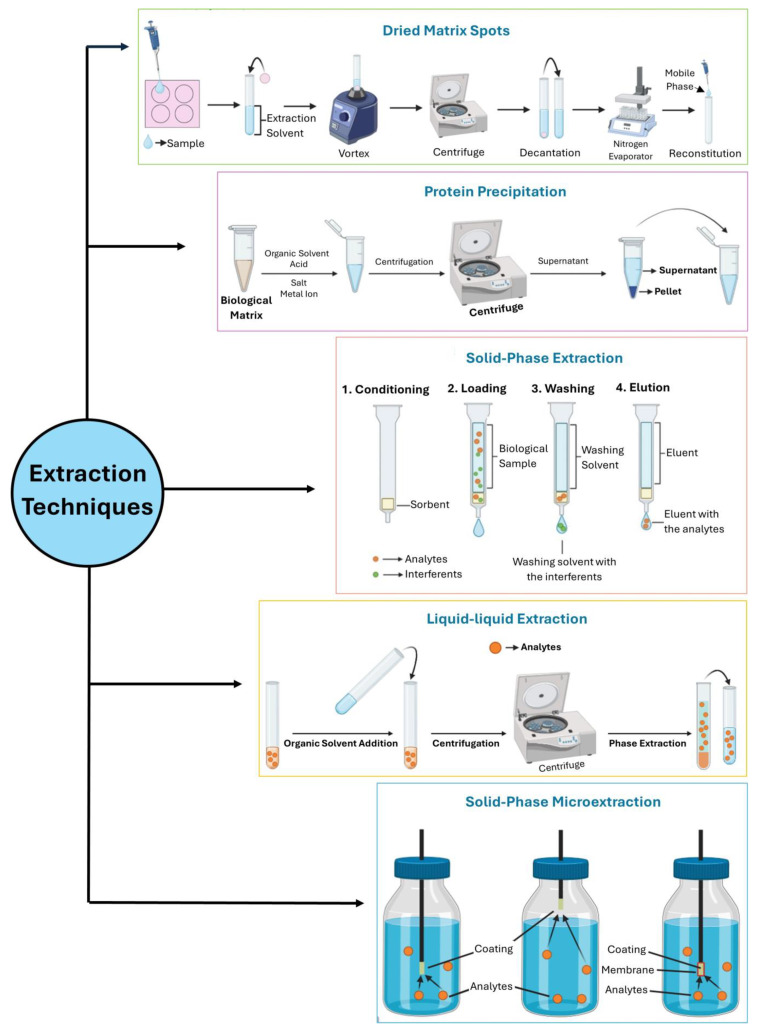
An alternative method gaining popularity is dried blood spot (DBS) analysis, a technique developed based on the recent advancements in analytical sensitivity and technology [27]. Initially used for screening newborn metabolic abnormalities over the past 50 years, DBS analysis has recently become increasingly relevant for determining both small and large compounds, particularly in clinical practice, toxicological and pharmacokinetic investigations, and sports drug testing. This technique offers several advantages for therapeutic drug monitoring in future clinical practice due to its reduced invasiveness, potential for automation, lower risk of infection, cost-effectiveness, streamlined sampling, storage, and transportation, and the ability to enhance the stability of many analytes [34]. Building on the advantages of DBS sampling, an adaptation to oral fluids has been developed and termed dried saliva spots (DSSs). This technique has proven to be an excellent alternative to neat oral fluids for pharmacokinetic evaluations of drugs [35]. By facilitating sample collection, DSS sampling reduces the risk of sample substitution or adulteration, owing to the possibility of supervision during collection. This method is also particularly valuable for monitoring AEDs, offering new solutions for handling, sampling, storage, and transportation due to its non-invasive collection method [27,34,35]. In Figure 1, the previously described extraction procedures are shown.
Figure 1.
The five main extraction procedures that have been recently used.
The absence of derivatization steps and the high specificity and sensitivity of liquid chromatography tandem mass spectrometry (LC-MS/MS) and liquid chromatography with mass spectrometry (LC-MS) make these methods highly relevant for detection. Additionally, these methods can handle complex matrices with ease. Their high sensitivity compensates for the low volume of alternative samples typically available. However, LC-MS still faces challenges, such as ion suppression or enhancement, when dealing with complex matrices [36].
As mentioned earlier, the number of available AEDs on the market has increased, highlighting the importance of selecting the appropriate medication for specific seizure types. Concurrently, the development and validation of new methods have expanded to address the need for effective control in maximizing clinical efficacy and minimizing adverse drug reactions. Given that therapeutic drug monitoring is crucial for drugs where plasma concentrations reflect the drug’s concentration and pharmacological action at the target site, researchers have been developing more methods for determining AEDs in serum, plasma, and whole blood. These new methods aim to address the limitations of previous approaches while adapting alternative matrices that offer less invasive collection methods and can reflect drug concentrations in the blood [27].
Based on this information, the present work provides a comprehensive review of the various applications and methods for determining AEDs in biological samples. For clarity, this section is organized by the type of biological sample in which AEDs can be detected. For each type of biological sample, the main developments in the determination of these compounds are discussed.
A search was conducted in both PubMed and ISI Web of Science databases using the following combinations of keywords: “determination of antiepileptic drugs” with the biological samples “blood”, “urine”, “oral fluid”, “hair”, “exhaled air”, “breast milk”, “alternative specimens”, and "other samples”, restricting the search to the last three years (from 2019 onward).
2.1. Blood and Derivates
Blood is considered a traditional matrix due to its historical use for drug testing in clinical and forensic toxicology, being one of the matrices in which higher drug levels can be detected [37]. The quantitative analysis of whole blood and blood-derived matrices is preferred due to their ability to correlate drug concentration with potential pharmacological effects. These matrices can also indicate recent drug use (hours) and are less prone to adulteration. Despite these advantages, blood and its derivatives still present several drawbacks, such as the invasive nature of collection, which requires qualified personnel, a short detection window, and the need for matrix extraction [37,38]. Table 2 summarizes the analytical procedures developed and published for the determination of AEDs in blood and its derivatives from 2020 to the present year.
Table 2.
Sample pretreatment and determination of AEDs in blood samples and derivatives.
| Compounds | Matrix | Volume | Extraction Method | Detection Method | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Lacosamide | Blood | 500 µL | LLE (methanol, NaOH 0.01 M, and ethyl acetate) | GC-MS | 0.1 µg/mL | 0.5 µg/mL | 78.6–121.9 | [39] |
| Valproic Acid | Plasma | 300 μL | PP (85:15 (v/v) solution of perchloric acid–ethylene glycol and 50% ammonium acetate solution) | 2D-LC-UV | 1.00 μg/mL | n.s. | 95.2–98.0 | [40] |
| (a) Valproic Acid (b) CBZ (c) CBZ-E (d) Phenobarbital |
Blood | n.s. | DBS | LC-MS/MS | n.s. | (a) 25 µg/mL (b) 2 µg/mL (c) n.s. (d) 1 µg/mL |
(a) 58.7 ± 8.33 (b) 62.6 ± 9.36 (c) 61.0 ± 9.99 (d) 61.2 ± 9.79 |
[41] |
| (a) Valproic Acid (b) CBZ (c) CBZ-E (d) Phenobarbital |
Blood | n.s. | VAMS (acetonitrile/water (80/20 v/v)) | LC-MS/MS | n.s. | (a) 25 µg/mL (b) 2 µg/mL (c) n.s. (d) 1 µg/mL |
(a) 85.2 ± 6.1 (b) 86.4 ± 5.9 (c) 91.4 ± 4.6 (d) 93.7 ± 4.6 |
[41] |
| Valproic Acid | Plasma | 200 μL | CF-UF/PP (dichloromethane) | GC-FID | n.s. | 0.56 µg/mL | 101.45 ± 2.08 to 102.58 ± 3.38 | [42] |
| Oxcarbazepine | Plasma | 1.0 g | VA–SHS–LPME (N,N-dimethylbenzylamine, distilled water (1:1, v/v), dry ice, and sodium hydroxide) | GC-MS | 6.2 μg/kg | 21 μg/kg | 97–100 | [32] |
| Lacosamide | Plasma | n.s. | PP (methanol:water (50:50, v/v), acetonitrile:methanol, 50:50 (v/v), and 0.1% formic acid in water (80:20, v/v)) | UHPLC-MS/MS | n.s. | n.s. | 97.2–99.7 | [43] |
| Levetiracetam | Plasma | 300 μL | PP (methanol) | HCLC-UV-PDA | n.s. | 6 µg/mL | n.s. | [44] |
| Phenytoin | Plasma | n.s. | EME | CE-DAD | 0.005 μg/mL | 0.03 μg/mL | n.s. | [45] |
| Sulthiame | Serum/Plasma | 50 μL | PP (acetonitrile) | RP-HPLC–UV | 0.19 μL/mL | 0.58 μL/mL | ≈100 | [46] |
| (a) Lacosamide (b) Oxcarbazepine (c) Lamotrigine |
Serum | 90 μL | PP (protein precipitator) | UHPLC-DAD | (a) 0.25 μg/mL (b) 0.50 μg/mL (c) 0.25 μg/mL |
(a) 0.5 µg/mL (b) 2.5 µg/mL (c) 0.5 µg/mL |
96.6–106.2 | [47] |
| (a) Perampanel (b) Lamotrigine |
Plasma | 200 μL | LLE (ethyl acetate) | HPLC-DAD | n.s. | (a) 0.03 µg/mL (b) 0.25 µg/mL |
(a) 90.0–114.6 (b) 93.3–112.9 |
[48] |
| Pregabalin | Serum | (a) n.s. (b) 100 μL |
(a) n.s. (b) n.s. |
(a) IT (b) LC-MS/MS |
n.s. | n.s. | n.s. | [49] |
| Valproic Acid | Plasma | (a) 40 μL (b) 30 μL |
(a) DPS/PP (water–ACN, 50:50, v/v)) (b) PP (acetonitrile) |
(a) LC–MS/MS (b) LC–MS/MS |
n.s. | 10 µg/mL | (a) 82.6–86.0 (b) 98.4–99.8 |
[50] |
| Perampanel | Plasma | (a) 50 µL (b) 1000 µL |
n.s. | LC-MS/MS | n.s. | 0.5 ng/mL | n.s. | [51] |
| Rufinamide | Plasma | 250 µL | LLE (methanol, ammonium hydroxide solution pH 9.25, and dichloromethane) | HPLC-UV | 0.05 μg/mL | 0.25 μg/mL | 94.1 ± 4.7 | [52] |
|
(a) Brivaracetam
(b) Carbamazepine (c) Carbamazepine-epoxide (g) Gabapentin (h) Lacosamide (i) Lamotrigine (j) Lamotrigine-13C,15N4 (k) Levetiracetam (m) 10-OH-Carbazepine (n) Perampanel (o) Phenytoin (p) Pregabalin (q) Primidone (u) Rufinamide (w) Stiripentol (x) Sultiame (y) Topiramate (b’) Zonisamide |
Blood | 20 μL | DBS | LC-MS/MS | n.s. | n.s. | n.s. | [34] |
| Rufinamide | Plasma | 50 μL | PP (methanol) | RP-HPLC-UV | n.s. | 0.5 µg/mL | n.s. | [53] |
| Valproic Acid | Serum | 50 µL | BioSPME (HCl 0.1 M, methanol) | GC-MS | n.s. | 10 µg/mL | n.s. | [54] |
| Gabapentin | Serum | 20 μL | PP (methanol, 95:5(v/v) 10 mM ammonium formate:methanol, and 0.1% formic acid) | LC-MS/MS | n.s. | 0.1 µg/mL | n.s. | [55] |
| Clonazepam | Plasma | n.s. | UA-EME | CE-DAD | 3.0 ng/mL | 0.01 µg/mL | 58 | [56] |
| Clonazepam | Plasma | 50 μL | n.s. | LC-MS | n.s. | 2 µg/mL | n.s. | [57] |
| (a) Fenfluramine (b) Norfenfluramine |
Plasma | n.s. | PP | LC-MS/MS | n.s. | n.s. | n.s. | [58] |
| Carbamazepine | Plasma | 5 µL | PP (methanol) | LC-MS3 | 0.5 µg/mL | 0.5 µg/mL | 110.5 ± 7.0 | [59] |
|
(a) Eslicarbazepine acetate
(b) Eslicarbazepine (c) Oxcarbazepine (d) (R)-licarbazepine |
Plasma | (a) and (c) 50 μL (b) and (d) 200 μL |
PP (50% acetonitrile for eslicarbazepine acetate and oxcarbazepine; 100% acetonitrile for eslicarbazepine and (R)-licarbazepine) | LC-MS/MS | n.s. | n.s. | n.s. | [60] |
| Zonisamide | (a) Blood (b) Plasma |
(a) 50 μL (b) 30 μL |
(a) DBS (b) DPS |
UHPLC–MS/MS | n.s. | (a) 0.125 µg/mL (b) 0.250 µg/mL |
n.s. | [61] |
| Valproic Acid | Blood | 200 µL | PP (acetonitrile) | LC-MS/MS | 2 μg/mL | 5 μg/mL | n.s. | [62] |
| Lacosamide | Plasma | 100 µL | ODS | UHPLC-DAD | n.s. | 0.25 µg/mL | 96.6–106.2 | [63] |
| Valproic Acid | Blood | 100 µL | LLE (MTBE, TMSDM, and methanol) | GC-MS | 1 µg/mL | n.s. | 86.7–91.6 | [64] |
| Gabapentin | (a) Serum (b) Plasma |
n.s. | PP (75% methanol in Milli-Q water (v/v)) | ID-LC-MS/MS | n.s. | n.s. | 99–108 | [65] |
| Topiramate | (a) Serum (b) Plasma |
n.s. | PP (75% methanol in Milli-Q water (v/v)) | ID-LC-MS/MS | 0.0239 μg/mL | n.s. | 95–102 | [65] |
| (a) Carbamazepine (b) Oxcarbazepine |
Plasma | n.s. | IT-SPME | MS | (a) 0.0002 µg/mL (b) 0.00025 µg/mL |
(a) 0.00008 µg/mL (b) 0.0001 µg/mL |
(a) 102.4–117.7 (b) 90.7–104.8 |
[33] |
| Levetiracetam | Plasma | 40 μL | PP (protein precipitation solution) | UPLC-MS/MS | n.s. | 0.1 µg/mL | 97.4–101.1 | [66] |
| Carbamazepine | Blood | 15 μL | MI-IPN (DBS) | CE | n.s. | 4 µg/mL | 89.7–94.7 | [67] |
| Levetiracetam | Serum | n.s. | n.s. | (a) HPLC-UV (b) IT |
n.s. | n.s. | n.s. | [68] |
| Brivaracetam | Serum | n.s. | n.s. | LC-MS/MS | 0.02 µg/mL | 0.1 µg/mL | n.s. | [69] |
| (a) Carbamazepine (b) Carbamazepine-epoxide |
Plasma | 2 mL | PP (dichloromethane) | ICA | (a) 0.25 ng/mL (b) 1 ng/mL |
n.s. | (a) 89.0–95.2 (b) 89.1–94.6 |
[70] |
| Padsenovil | (a) Blood (b) Plasma |
n.s. | (a) VAMS (b) n.s. |
n.s. | n.s. | n.s. | n.s. | [71] |
| Levetiracetam | Plasma | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [72] |
| (a) Lamotrigine (b) Levetiracetam (c) Carbamazepine (d) Carbamazepine-epoxide (e) Topiramate (f) Valproic acid (g) Zonisamide (h) Oxcarbazepine |
Plasma | n.s. | (a) n.s. (b) DBS |
LC-MS | n.s. | (a) 0.1 µg/mL (b) 1.8 µg/mL (c) 0.7 µg/mL (d) 0.1 µg/mL (e) 1.6 µg/mL (f) 13.1 µg/mL (g) 1.0 µg/mL (h) 0.1 µg/mL |
n.s. | [73] |
| Lamotrigine | Plasma | 50 μL | SPE | LC-MS/MS | n.s. | 0.2 µg/mL | 93.8–98.6 | [74] |
| Valproic Acid | Plasma | n.s. | SPE | UHPLC-MS/MS | n.s. | 0.05 µg/mL | 81.4–110 | [75] |
| Valproic Acid | Serum | 50 μL | PP (sulfuric acid, ether, and tetramethylammonium hydroxide) | HPLC-UV | 0.4 µg/mL | 1.0 µg/mL | 91.6–97.4 | [76] |
| (a) Carbamazepine (b) Carbamazepine-epoxide |
Serum | 100 μL | PP (acetonitrile) | UHPLC-MS/MS | n.s. | (a) 0.05 µg/mL (b) 0.01 µg/mL |
74.7– 93.48 | [77] |
| Lamotrigine | Serum | 200 μL | PP (methanol/acetonitrile 1:1, v/v) and SPME | HPLC-DAD | n.s. | 0.625 µg/mL | 75.4–82.5 | [78] |
| (a) Carbamazepine (b) Valproic acid (c) Phenobarbital (d) Phenytoin (e) Carbamazepine-epoxide |
Blood | 25 μL | DBS | LC-MS/MS | n.s. | (a) 2 µg/mL (b) 25 µg/mL (c) 1 µg/mL (d) 4 µg/mL (e) 0.25 µg/mL |
(a) 53.24–71.96 (b) 50.37–67.03 (c) 51.41–70.99 (d) 50.75–68.25 (e) 51.21–70.79 |
[79] |
| Topiramate | Blood | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [80] |
|
(a) Levetiracetam
(b) Lamotrigine (c) Zonisamide (d) Topiramate (e) Carbamazepine (f) Phenytoin (g) Valproic Acid (h) Oxcarbazepine (i) 10,11-dihydro- 10-hydroxycarbamazepine |
Plasma | 50 μL | PP (acetonitrile) | LC-MS/MS | n.s. | (a) 0.005 µg/mL (b) 0.005 µg/mL (c) 0.01 µg/mL (d) 0.01 µg/mL (e) 0.005 µg/mL (f) 0.010 µg/mL (g) 0.05 µg/mL (h) 0.005 µg/mL (i) 0.005 µg/mL |
(a) 93.7–102.9 (b) 95.6–103.8 (c) 93.7–105.7 (d) 100.1–109.3 (e) 98–104.4 (f) 98.6–104 (g) 68.9–73.9 (h) 98–104.6 (i) 93.7–103.6 |
[81] |
| Valproic Acid | Blood | n.s. | DBS | GC-MS | n.s. | n.s. | n.s. | [82] |
| Oxcarbazepine | Plasma | 200 μL | PP (methanol) | HPLC–n.s. | n.s. | 2 µg/mL | n.s. | [83] |
| Phenobarbital | Blood | 0.1 g | PP (acetonitrile) | LC-HRMS | 0.25 mg/kg | 0.5 mg/kg | n.s. | [84] |
| Lamotrigine | Serum | n.s. | PP (methanol) | HPLC–n.s. | n.s. | n.s. | n.s. | [85] |
| Carbamazepine | Serum | n.s. | (a) MIP-SBSE (b) MIP-MSPE |
HPLC-UV | n.s. | n.s. | n.s. | [86] |
| Carbamazepine | Serum | n.s. | n.s. | µ-BIS-LOV ELISA | n.s. | 1.0 µg/L | 93–110 | [87] |
| Brivaracetam | Plasma | n.s. | n.s. | UHPLC-MS/MS | n.s. | n.s. | n.s. | [88] |
| Sultiame | (a) Blood (b) Plasma |
100 μL | PP (methanol) | HPLC-MS/MS | n.s. | n.s. | (a) n.s. (b) 1.61–16.73 |
[89] |
| Phenobarbital | Plasma | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [90] |
| (a) Oxcarbazepine (b) Licarbazepine |
Plasma | n.s. | SPE | LC-HRMS | 0.008 µg/mL | 92.34–104.27 | [91] | |
| Zonisamide | Serum | n.s. | n.s. | (a) LTIA (b) HPLC-UV |
n.s. | (a) 3 µg/mL (b) 0.5 µg/mL |
n.s. | [92] |
| (a) Lamotrigine (b) Carbamazepine (c) Oxcarbazepine (d) Cabamazepine-Epoxide |
Plasma | 1 mL | PP (acetonitrile) and MSPE | HPLC-UV | (a) 0.01 µg/mL (b) 0.009 µg/mL (c) 0.007 µg/mL (d) 0.009 µg/mL |
(a) 0.031 µg/mL (b) 0.027 µg/mL (c) 0.02 µg/mL (d) 0.028 µg/mL |
(a) 86.8–101.8 (b) 82.5–99.2 (c) 80.6–98.9 (d) 78.7–98.5 |
[93] |
| Levetiracetam | Serum/Plasma | n.s. | n.s. | EI | n.s. | n.s. | n.s. | [94] |
| Perampanel | Plasma | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [95] |
| Lamotrigine | Serum | n.s. | n.s. | TI | n.s. | n.s. | n.s. | [96] |
| (a) Fenfluramine (b) Norfenfluramine |
Plasma | 100 μL | PP (acetonitrile) | LC-MS/MS | n.s. | (a) 1.6 ng/mL (b) 0.82 ng/mL |
n.s. | [97] |
| Perampanel | Plasma | n.s. | n.s. | HPLC-UV | n.s. | n.s. | n.s. | [98] |
| Primidone | Serum/Plasma | 50 μL | PP (75% methanol v/v) | ID-LC-MS/MS | 0.0620 µg/mL | n.s. | 97–103 | [99] |
| Carbamazepine | Serum/Plasma | 50 μL | PP (75% methanol v/v) | ID-LC-MS/MS | 0.115 µg/mL | n.s. | 101–105 | [99] |
| Phenobarbital | Serum/Plasma | n.s. | PP (75% methanol v/v) | ID-LC-MS/MS | 0.697 µg/mL | n.s. | 100–107 | [99] |
| Zonisamide | Serum/Plasma | 50 μL | PP(75% methanol v/v) | ID-LC-MS/MS | 0.317 µg/mL | 1.50 µg/mL | 98–101 | [99] |
| Levetiracetam | Plasma | 40 μL | PP (acetonitrile) | UHPLC-MS/MS | n.s. | 0.1 µg/mL | n.s. | [100] |
| Carbamazepine-epoxide | Serum/Plasma | 50 μL | PP (75% methanol v/v) | ID-LC-MS/MS | 0.0111 µg/mL | n.s. | 94–105 | [99] |
| Pregabalin | Serum | 2 mL | PP (methanol) | SM | 2.81 × 10−8 mol/L | 8.5 × 10−8 mol/L | 99.02–104.78 | [101] |
| (a) Vigabatrin (b) Levetiracetam (c) Pregabalin (d) Gabapentin (e) Lamotrigine (f) Lacosamide (g) Zonisamide (h) Rufinamide (i) Topiramate (j) Oxcarbazepine (k) Carbazepine |
Serum | 150 μL | PP (acetonitrile) | LC-MS/MS | n.s. | n.s. | >93 | [102] |
µ-BIS-LOV ELISA: micro-bead injection spectroscopy lab on valve enzyme-linked immunosorbent assay; 2D-LC-UV: 2-dimension liquid chromatography with ultraviolet spectroscopy; BioSPME: bioanalytical solid-phase microextraction; CE: capillary electrophoresis; CE-DAD: capillary electrophoresis coupled with diode array detection; CF-UF: centrifugal ultrafiltration; DAD: diode array detector; DBSs: dried blood spots; DPSs: dried plasma spots; EI: enzyme immunoassay; EME: electromembrane extraction; GC-FID: gas chromatography coupled with a flame ionization detector; GC-MS: gas chromatography coupled with mass spectrometry; HCLC-UV-PDA: heart-cutting liquid chromatography with ultraviolet spectroscopy and photodiode array detection; HPLC-DAD: high-performance liquid chromatography with diode array detection; HPLC-UV: high-performance liquid chromatography with ultraviolet spectroscopy; ICA: immunochromatographic assay; ID-LC-MS/MS: isotope dilution liquid chromatography-tandem mass spectrometry; IT: immunoassay test; IT-SPME: in-tube solid-phase microextraction; LC-HRMS: liquid chromatography coupled with high-resolution mass spectrometry; LC-MS: liquid chromatography mass spectrometry; LC-MS3: liquid chromatography tandem mass spectrometry cubed; LC-MS/MS: liquid chromatography tandem mass spectrometry; LC-TOF-MS: liquid chromatography time-of-flight mass spectrometry; LLE: liquid–liquid extraction; LTIA: latex particle-enhanced turbidimetric immunoassay; MSPE: magnetic solid-phase extraction; MI-IPN: paper-based molecularly imprinted interpenetrating polymer network; MIP-MSPE: molecularly imprinted polymers–magnetic solid-phase extraction; MIP-SBSE: molecularly imprinted polymers–stir bar sorptive extraction; MS: mass spectrometry; NaOH: sodium hydroxide; ODS: organic deproteinization solution; PP: protein precipitation; RP-HPLC-UV: reverse-phase high-performance liquid chromatography with ultraviolet spectroscopy; SM: spectrofluorimetric method; SPR: surface plasmon resonance; TI: turbidimetric immunoassay; UA-EME: ultrasound-assisted electromembrane extraction; UHPLC-DAD: ultra-high-performance liquid chromatography coupled with diode array detection; UHPLC-MS/MS: ultra-high-performance liquid chromatography tandem mass spectrometry; VAMS: volumetric absorptive microsampling; VA-SHS-LPME: vortex-assisted switchable hydrophilicity solvent-based liquid phase microextraction.
Overall, plasma is the most commonly used sample for these methods, representing over 60% of the published analytical developments for the determination of AEDs since 2020. This preference stems from plasma’s ability to effectively correlate drug concentrations with their potential pharmacological effects, making it a reliable choice for therapeutic drug monitoring, where therapeutic and toxic levels are well-documented. Traditional sample preparation techniques, particularly PP, remain the most widely applied for blood and its derivatives, accounting for more than 50% of the methods developed. LLE follows with approximately 15% of published methods. Despite their advantages, these classic procedures are typically applied to smaller sample volumes (<100 µL), aligning with the trend toward miniaturization in new methodological approaches. This trend is facilitated by the increasing sensitivity of modern analytical equipment, as reflected by the substantial use of LC-MS/MS methods, which account for around 56% of developments for AEDs in blood and its derivatives. Mass spectrometry (MS) is the chosen detector in 78% of publications since 2020. The enhanced sensitivity and specificity of these advanced instruments often allow for lower sample volumes and simpler sample preparation techniques, explaining the continued popularity of PP, especially when paired with LC-MS or LC-MS/MS. However, for gas chromatography-mass spectrometry (GC-MS) analyses, LLE is preferred.
Valproic acid is the most frequently detected AED in blood and its derivatives (see Table 2). Gu et al. [42] developed a method for detecting the free fraction of valproic acid in human plasma that is applicable across various medical facilities and requires only 200 µL of plasma. By combining centrifugal ultrafiltration and PP with dichloromethane, they achieved a limit of quantification (LOQ) of 0.56 µg/mL, demonstrating good specificity, stability, and cost-effectiveness. An interesting article was published by Schaefer et al. [54]. In this work, an LOQ of 10 µg/mL was achieved using 50 µL of serum samples and bioanalytical solid-phase microextraction (BioSPME). BioSPME, a new microextraction strategy, involves serum acidification, the direct immersion of BioSPME tips, agitation, and methanol desorption. This method provided comparable results to an LLE-based GC-MS method, highlighting its potential as an alternative sample preparation technique. In recent years, volumetric absorptive microsampling (VAMS) and dried blood spot (DBS) sampling have emerged as valuable techniques for sample collection and analysis, thanks to their numerous advantages. Both methods offer a minimally invasive approach to blood sampling, which greatly reduces patient discomfort and the risks associated with traditional venipuncture. VAMS employs a specialized device to absorb a precise volume of blood [103], while DBS involves depositing blood onto filter paper [104]. These techniques simplify the sample collection process, making them suitable for use in various settings, including remote locations. One of the significant benefits of VAMS and DBS methods is the stability of the samples. Both types can be stored at room temperature, which streamlines transportation and long-term storage, eliminating the need for cold chain logistics [105]. This stability is crucial for accurate analysis over time. Additionally, the cost-effectiveness of these methods is evident in the reduced need for extensive laboratory equipment and complex sample preparation procedures, leading to lower costs for both sample collection and analysis. The reduced risk of infection, owing to the less invasive nature of these techniques, further adds to their appeal. The applications of VAMS and DBS methods have expanded significantly [106]. In clinical settings, these methods are increasingly employed for monitoring drug levels, including AEDs, due to their convenience and effectiveness, and they facilitate routine therapeutic drug monitoring with minimal patient discomfort. An example of this application is the work of Velghe et al. [41]. The authors obtained an LOQ of 25 µg/mL using both DBS and VAMS techniques with LC-MS/MS. They also detected carbamazepine with an LOQ of 2 µg/mL, and carbamazepine-10,11-epoxide and phenobarbital with an LOQ of 1 µg/mL. Although both methods showed similar LOQs, the VAMS technique demonstrated better recovery rates. Li et al. [50] presented a robust method for quantifying valproic acid using LC-MS/MS and dried plasma spots (DPSs), achieving an LOQ of 10 µg/mL with just 40 µL of plasma. The simplicity, flexibility, and affordability of the PP method with acetonitrile provided high recovery rates [41,42,50,54,103,104,105,106].
Other applications using DBS and DPS methods are the studies of Möller et al. [34] and Rmandić et al. [61]. Möller et al. [34] developed an LC-MS/MS method to quantify five drug metabolites and 22 medications used by epilepsy patients with the DBS method. Their findings confirmed that DBS analysis is feasible, with all analytes detected in 20 μL of blood. However, conversion factors are needed to compare DBSs and serum concentrations accurately, and further investigation is required to clinically validate the results [34,61].
Rmandić et al. [61] developed a ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) method for zonisamide determination in DPSs and DBSs. With a runtime under 2.5 min and volumes of 50 μL for DBSs and 30 μL for DPSs, they achieved LOQs of 0.125 µg/mL for DBSs and 0.250 µg/mL for DPSs. This method proved economical and environmentally friendly, facilitating sample preparation by directly dissolving zonisamide from DBS/DPS cards in the mobile phase, and was effective for both blood and plasma quantifications [61].
Lacosamide is another AED frequently detected in blood and its derivatives. Mouskeftara et al. [39] achieved an LOD of 0.1 µg/mL and an LOQ of 0.5 µg/mL using LLE and GC-MS. The application of alkaline LLE with NaOH of 0.01 M and ethyl acetate effectively extracted lacosamide from blood samples, allowing for the detection of concentrations ten-times lower than the therapeutic range. Zhao et al. [47] developed and validated an ultra-high-performance liquid chromatography coupled diode array detector (UHPLC-DAD) method for quantifying lamotrigine, oxcarbazepine, and lacosamide in serum, obtaining the same LOQ of 0.5 µg/mL as Mouskeftara et al. [39] using a PP technique. Both methods exhibited high recovery rates, with Mouskeftara et al. [39] achieving recoveries between 78.56% and 121.90%, and Zhao et al. [47] between 96.58% and 106.22%. Notably, Zhao et al.’s method required an approximately five-times-less sample volume than Mouskeftara et al.’s [39,47].
Regarding the use of microextraction techniques, there are several interesting applications. To determine oxcarbazepine concentrations in human plasma and urine, Erarpat et al. [32] developed a sensitive, rapid, and environmentally friendly method involving vortex-assisted switchable hydrophilicity solvent-based liquid phase microextraction (VA–SHS–LPME) before GC-MS analysis. The switchable hydrophilicity solvents’ miscibility, altered by carbon dioxide, enabled efficient analyte extraction without the need for disperser solvents. The VA-SHS-LPME method achieved an LOD of 6.2 μg/kg and an LOQ of 21 μg/kg with 1 g of plasma. Hu et al. [33] coupled in-tube solid-phase microextraction (IT-SPME) directly with mass spectrometry (MS) using an open tubular column coated with a polymer for quantifying carbamazepine and oxcarbazepine in plasma and urine samples. This new “online extraction” mode allows IT-SPME to integrate seamlessly with various MS equipment, offering a high extraction efficiency and low LODs and LOQs [32,33].
As previously indicated, the most commonly used instrumental techniques are GC and LC coupled with MS and/or MS/MS. However, there are simpler methods that utilize more economical detection systems. One such study is described by Seyfinejad et al. [45]. The authors developed a method for determining the free fraction of phenytoin in plasma using electromembrane extraction (EME) and capillary electrophoresis coupled with diode array detection (CE-DAD). EME, based on extracting charged compounds using an electric field, achieved an LOD of 0.005 μg/mL and an LOQ of 0.03 μg/mL [45].
2.2. Urine
Like blood, urine is also considered a traditional matrix. It is the preferred matrix for systematic toxicological analysis because metabolites are usually present and concentrations are comparatively high. Metabolite detection can reduce the risk of a false negative and aid in the substance identification process. Additionally, urine has relatively extended detection windows, making it particularly suitable for workplace drug testing and forensic investigations, such as drug-facilitated crimes [38]. Although urine collection is less invasive and potentially more attractive for medication monitoring, it lacks established clinical ranges, which results in it not being applied often in the TDM process. This makes it exceedingly challenging to create new techniques that reveal appropriate concentration ranges, resulting in a decreased number of publications on this matter [107]. Moreover, urine samples can be easily adulterated [38,107].
While researching the literature for articles on the determination of antiepileptic drugs in urine from 2020 to the present year, only five different articles were found (Table 3). GC-MS instrumentation was mainly used when a single AED was to be quantified. In contrast, the LC-MS/MS system, due to its high sensitivity, was applied to a multi-method development for nine target compounds.
Table 3.
Sample pretreatment and determination of AEDs in urine samples.
| Compounds | Volume | Extraction Method | Detection Method | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Oxcarbazepine | 0.9 g | VA–SHS–LPME (N,N-dimethylbenzylamine, distilled water (1:1, v/v), and sodium hydroxide 1 M) | GC-MS | 6.2 μg/kg | 21 μg/kg | 97–100 | [32] |
|
(a) Carbamazepine (b) Carbamazepine-10,11-epoxide (c) Eslicarbazepine (d) Lamotrigine (e) Levetiracetam (f) Oxcarbazepine (g) Phenytoin (h) 4-hydroxyphenytoin (i) Topiramate |
25 µL | SPE (80:18:2 DCM/IPA/NH4OH) | LC-MS/MS | (a) 0.05 µg/mL (b) 0.05 µg/mL (c) 0.5 µg/mL (d) 500 ng/mL (e) 0.5 µg/mL (f) 0.5 µg/mL (g) 0.5 µg/mL (h) 0.5 µg/mL (i) 0.5 µg/mL |
(a) 0.05 µg/mL (b) 0.05 µg/mL (c) 0.5 µg/mL (d) 500 ng/mL (e) 0.5 µg/mL (f) 0.5 µg/mL (g) 0.5 µg/mL (h) 0.5 µg/mL (i) 0.5 µg/mL |
(a) 106.2 (b) 24.7 (c) 102.0 (d) 102.9 (e) 14.9 (f) 92.6 (g) 105.6 (h) 100.8 (i) 92.8 |
[107] |
| (a) Carbamazepine (b) Oxcarbazepine |
n.s. | IT-SPME | MS | (a) 0.00008 µg/mL (b) 0.0001 µg/mL |
(a) 0.0003 µg/mL (b) 0.0003 µg/mL |
(a) 98.7–108.6 (b) 90.2–107.2 |
[33] |
| Valproic Acid | 100 µL | LLE (MTBE, TMSDM, and methanol) | GC-MS | 1 µg/mL | n.s. | 86.2–98.0 | [64] |
| Carbamazepine | n.s. | n.s. | Electrochemical Sensor | 0.0018 μM | 0.006 μM | 99.0–100.7 | [108] |
| (a) Levetiracetam (b) Lacosamide |
1 mL | Filtration | UHPLC-DAD | (a) 0.026 µg/mL (b) 0.023 µg/mL |
(a) 0.096 µg/mL (b) 0.093 µg/mL |
98.69–101.87 | [109] |
| Carbamazepine | n.s. | CEC | MHPLC-MS/MS | n.s. | n.s. | n.s. | [110] |
| Valproic Acid | n.s. | SPE | UHPLC-MS/MS | n.s. | 0.2 µg/mL | 74.1–112.3 | [75] |
| Sultiame | n.s. | PP (methanol) | LC-MS/MS | n.s. | n.s. | 1.61–16.73 | [89] |
| Pregabalin | 1 mL | n.s. | SM | 2.81 × 10−8 mol/L | 8.5 × 10−8 mol/L | 99.08–104.96 | [101] |
CEC: capillary extraction column; GC-MS: gas chromatography coupled with mass spectrometry; IT-SPME: in-tube solid-phase microextraction; LC-MS/MS: liquid chromatography-tandem mass spectrometry; LLE: liquid–liquid extraction; MHPLC-MS/MS: multidimensional high-performance liquid chromatography tandem mass spectrometry; MS: mass spectrometry; SM: spectrofluorimetric method; SPE: solid-phase extraction; UHPLC-DAD: ultra-high-performance liquid chromatography coupled with diode array detection; VA-SHS-LPME: vortex-assisted switchable hydrophilicity solvent-based liquid phase microextraction.
As mentioned before, Erarpat et al. [32] developed a new, sensitive, rapid, and environmentally friendly analytical method that included VA–SHS–LPME prior to GC-MS analysis. With urine, the authors achieved an LOD of 6.2 µg/kg, an LOQ of 21 µg/kg, and a recovery rate ranging from 97% to 100%. These results were particularly impressive as this was the first application of VA-SHS-LPME for determining oxcarbazepine in the literature [32].
Feng et al. [107] developed a new, fast, LC-MS/MS method capable of determining eslicarbazepine, carbamazepine-10,11-epoxide, topiramate, phenytoin, oxcarbazepine, carbamazepine, levetiracetam, lamotrigine, and 4-hydroxyphenytoin simultaneously in under four minutes of runtime. By combining SPE for sample preparation and LC-MS/MS, the authors achieved low LODs and LOQs for all nine AEDs and good recoveries for most of them, using only 25 µL of urine. In comparison to Erarpat et al.’s green method, Feng et al. [107] managed to achieve lower LODs and LOQs for oxcarbazepine determination in urine with a shorter runtime [107].
The method developed by Hu et al. [33] for detecting carbamazepine and oxcarbazepine in plasma is also an excellent tool for analyzing urine samples. The LODs of 0.08 ng/mL and 0.10 ng/mL, and LOQs of 0.30 ng/mL for both carbamazepine and oxcarbazepine in urine, are significantly lower than those obtained using previous methods [33].
Regarding valproic acid, Namera et al. [64] developed a simple and cost-effective method using LLE and GC-MS, achieving an LOD of 1 µg/mL and recoveries ranging from 86.2% to 98% with 100 µL of urine [64].
Lastly, Mariyappan et al. [108] developed an electrochemical sensor for the determination of carbamazepine in urine. By functionalizing a glassy carbon electrode with produced carbon nanofiber, they created an electrochemical sensor based on a functionalized gadolinium vanate nanostructure. Using this electrochemical sensor, they achieved an LOD of 0.0018 µM and an LOQ of 0.006 µM [108].
2.3. Oral Fluid
Oral fluid’s safe (or more usually named saliva), straightforward, and non-invasive collection process has contributed to the recent increase in interest in its toxicological analysis applications. This specimen can be considered simpler than traditional matrices and might present fewer interferents, subsequently resulting in more accurate drug analysis. Oral fluid can be a useful tool for drug monitoring, as its drug levels are assumed to represent the concentration of free drug in plasma. It also reflects recent drug use (hours) and does not require privacy during sample collection, reducing the chances of adulteration. Nonetheless, oral fluid can be easily contaminated during collection, and only a small volume of sample can be obtained at a time. Additionally, the collection method can influence oral fluid’s pH and drug concentrations [38,111,112].
Interest in alternative specimens, such as oral fluid, for drug determination has been growing. Studies have shown a strong correlation between saliva and the plasma levels of certain AEDs, making saliva a reliable alternative for drug monitoring [111,113]. While the interest is growing, the number of comprehensive studies and standardized protocols for saliva monitoring of AEDs remains limited. In fact, only five articles related to the determination of AEDs in oral fluid were found from 2020 to the present year (Table 4).
Table 4.
Sample pretreatment and determination of AEDs in oral fluid samples.
| Compounds | Volume | Extraction Method | Detection Method | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Clonazepam | 20 μL | n.s. | LC-MS | n.s. | n.s. | n.s. | [57] |
| Perampanel | 10 μL | PSP (chloroform) | SERS | n.s. | n.s. | n.s. | [114] |
| (a) Carbamazepine (b) Carbamazepine-10,11-epoxide (c) S-licarbazepine (d) Lacosamide (e) Levetiracetam |
100 μL | PP (dichloromethane) | HPLC-DAD | n.s. | n.s. | (a) 80.1–95.4 (b) 82.5–95.1 (c) 80.0–94.2 (d) 79.8 –93.9 (e) 78.0–94.4 |
[115] |
| Perampanel | (a) 50 µL (b) 1 mL |
PP (acetonitrile) | LC-MS/MS | n.s. | n.s. | n.s. | [51] |
| Levetiracetam | 40 μL | PP (protein precipitation solution) | UHPLC-MS/MS | n.s. | 0.001 μg/mL | 108.8–113.5 | [66] |
| Rufinamide | 250 μL | LLE (methanol, ammonium hydroxide solution pH 9.25, and dichloromethan(e)) | HPLC-UV | 0.05 μg/mL | 0.25 μg/mL | 87.2 ± 3.9 | [52] |
| (a) Phenobarbital (b) Phenytoin (c) Carbamazepine (d) Carbamazepine-epoxide |
50 μL | LLE (acidified methanol pH 5.5) | HPLC-DAD | 0.05 μg/mL | 0.1 μg/mL | (a) 43–57.1 (b) 48.1–64.4 (c) 38.7–49.1 (d) 38.8–55 |
[35] |
| (a) Carbamazepine (b) Carbamazepine-epoxide |
n.s. | SPE | UHPLC-DAD | n.s. | n.s. | (a) 46.82–49.18 (b) 41.4–41.72 |
[116] |
| Levetiracetam | 40 μL | PP (acetonitrile) | UHPLC-MS/MS | n.s. | 0.1 µg/mL | n.s. | [100] |
HPLC-DAD: high-performance liquid chromatography with diode array detection; HPLC-UV: high-performance liquid chromatography with ultraviolet spectroscopy; LC-MS: liquid chromatography coupled with mass spectrometry; LC-MS/MS: liquid chromatography tandem mass spectrometry; LLE: liquid–liquid extraction; PP: protein precipitation; PSP: phase separation process; SERS: surface-enhanced Raman scattering; UHPLC-DAD: ultra-high-performance liquid chromatography coupled with diode array detection; UHPLC-MS/MS: ultra-high-performance liquid chromatography tandem mass spectrometry.
Kruizinga et al. [57] developed an LC-MS method to determine clonazepam in 20 µL of oral fluid to investigate the correlation between clonazepam levels in this sample and plasma. To forecast plasma concentrations from oral fluid samples, the authors evaluated a population pharmacokinetics model to explain this correlation. This novel approach proved to be effective [57].
In a different approach, Tommasini et al. [114] utilized surface-enhanced Raman scattering (SERS) to detect perampanel in saliva samples. SERS can identify low drug concentrations with analytical capabilities comparable to HPLC. Thus, the study explored the use of SERS for the therapeutic drug monitoring of perampanel for the first time. This new method required only 10 µL of oral fluid [114].
Similar to the previous studies, the next four articles focus on developing or optimizing methods for the drug monitoring of various antiepileptic drugs. Carona et al. [115] developed an HPLC-DAD method for monitoring levetiracetam, S-licarbazepine, carbamazepine, lacosamide, and carbamazepine-10,11-epoxide, achieving satisfactory recovery ranges for all five AEDs. This method achieved statistically significant correlations between the analyzed AEDs [115].
Kim et al. [51] utilized an LC-MS/MS system to detect perampanel in human saliva samples, determining both the total and free concentrations of perampanel with only 50 µL and 1 mL of oral fluid, respectively. This study demonstrated that oral fluid could be used for the drug monitoring of perampanel, as the total concentration of perampanel in oral fluid showed a linear correlation with the free concentration in plasma [32].
Zhang et al. [66] developed a straightforward, sensitive, and reliable UHPLC-MS/MS method to determine levetiracetam concentrations in oral fluid samples from pregnant Chinese women with epilepsy. This method achieved an LOQ of 1 ng/mL with a simple sample treatment (PP) and recoveries ranging from 108.8% to 113.5% [48].
Finally, Franco et al. [52] used a validated HPLC-UV method to compare rufinamide concentrations in saliva and plasma samples to assess the viability of saliva as an alternative for the TDM of rufinamide. The authors achieved excellent LOQ and LOD values, 0.25 µg/mL and 0.05 µg/mL, respectively, confirming the applicability of saliva for the TDM of rufinamide, despite the lower concentrations in saliva compared to plasma samples [33].
2.4. Hair
Hair samples can play a significant role in drug monitoring due to their unique properties. Unlike blood and urine, hair provides a long-term record of drug exposure, as substances incorporated into hair are retained for months or even years, depending on hair growth and length. This makes hair analysis particularly useful for assessing chronic drug use or long-term compliance with a prescribed regimen. One of the key advantages of using hair samples for drug monitoring is their stability. Hair is less likely to degrade or be contaminated compared to other biological matrices, making it a reliable source for historical drug exposure data. Additionally, hair samples can be collected non-invasively and without the need for specialist medical staff, which can simplify the monitoring process. However, this specimen is prone to environmental contamination, particularly from cosmetic products, which can affect the results. Furthermore, the interpretation of hair drug concentrations can be complex, as they may not directly correlate with current drug levels or effects. This can make it challenging to assess recent drug use accurately [117,118].
The study by Kim et al. [119] is the only article found from 2020 to the present that presents a method for determining AEDs in hair samples. These authors developed a novel, rapid, and efficient analytical method based on LC-MS/MS that allows for the simultaneous detection of topiramate, phenytoin, and six barbiturates in hair samples. For sample preparation, they explored three different conditions: methanolic extraction, LLE after basic digestion, and methanolic extraction with 1% hydrochloric acid. In the methanolic extraction process, hair samples are evaporated to dryness at 45 °C in glass tubes under a mild nitrogen stream after being incubated with 2 mL of methanol (for simple methanolic extraction) or acidified methanol (for acidified methanolic extraction) at 38 °C for 16 h. The residues are then treated in a glass insert micro-vial with a 0.22 µm hydrophilic syringe filter after reconstitution in 100 µL of mobile phase. For the LLE procedure, the hair samples undergo hydrolysis with 1 mL of 1 M NaOH at 90 °C for 30 min. Following this, 200 µL of acetic acid and 300 µL of 0.1 M sodium acetate buffer (pH 4.5) are added to acidify and digest the hair samples before extraction with 2 mL of n-hexane/ethyl acetate (1/1, v/v) for 5 min. The residues are then reconstituted in 100 µL of mobile phase prior to injection. The LOD and LOQ were 0.01 and 0.02 ng/mg for topiramate and in the ranges of 0.25–0.5 and 0.5–1 ng/mg for the other drugs, respectively. This method was applied to authentic hair samples from two drug users. In a segmental analysis of one female subject, phenobarbital concentrations ranged from 0.2 to 17.1 ng/mg. In another female subject, topiramate concentrations ranged from 0.19 to 0.93 ng/mg [119].
Certainly, the use of hair for the determination of AEDs is more prevalent in forensic toxicology than in clinical toxicology, due to the characteristics of this sample mentioned earlier.
2.5. Exhaled Air
Breath analysis has recently emerged as a valuable diagnostic tool, on par with blood and urine, for a broad range of analytical applications. These include monitoring biological responses, assessing health conditions, evaluating metabolic kinetics, studying toxicological effects, and chemical exposures, as well as conducting multiple time-point assessments [120]. The ease of sample collection, the ability for continuous sampling, and the lack of need for sample preparation are key reasons why exhaled air has long been used to monitor blood alcohol levels and enhance road safety. Given these advantages, breath analysis is now being explored as a tool for drug monitoring, particularly in pediatric cases [120,121,122].
The study by Awchi et al. [55] exemplifies this application and is notably the only article found on the detection of AEDs in alternative matrices from 2020 to the present. This study demonstrated the potential of real-time breath analysis for predicting valproic acid concentrations in clinical settings. It introduced a hybrid method combining offline breath specimen collection with secondary electrospray ionization coupled with high-resolution mass spectrometry (SESI-HRMS) for real-time analysis [55].
While real-time analysis eliminates sample manipulation, thus preserving the biochemical integrity of the sample, it is not feasible for screening large populations due to the difficulty some patients may have in performing controlled exhalations. To address this issue, the authors employed a custom-made nalophan bag, with a capacity of approximately 2 liters, to collect samples away from the mass spectrometer. This approach facilitated the transport and subsequent analysis of the stability of exhaled compounds associated with valproic acid by SESI-HRMS. The results showed that this hybrid method could detect around 55% of approximately 1200 mass spectral traits commonly found in breath, and its performance was comparable to real-time analysis. Additionally, the method demonstrated a stable signal intensity over four years of data collection, highlighting its potential for long-term patient monitoring [55].
It is worth noting that the authors compared three different analytical methods with varying quantitative capabilities in terms of sensitivity, accuracy, and precision. These included the enzyme-multiplied immunoassay technique (EMIT) for total valproic acid quantification in serum, versus offline and GC-MS for free valproic acid in serum, versus offline methods. This comparison underscores the importance of carefully evaluating the prediction capabilities of these methods, especially when interpreting results outside the therapeutic range [55].
Overall, this new technique shows significant promise for clinical applications, particularly when personalizing treatment for patients with epilepsy. The authors believe that this approach could enable widespread screening using a CE-marked in vitro diagnostic breath test, beneficial for a diverse patient population, including children and individuals with intellectual disabilities [55].
Another interesting study is the one published by Seyfinejad et al. [123]. The authors analyzed phenytoin in exhaled breath condensate using electromembrane extraction, a selective technique for isolating ionized molecules from samples. This method utilized a supported liquid membrane impregnated with 1-octanol, through which phenytoin was extracted from the exhaled breath condensate into an alkaline aqueous solution under a controlled electric field. The extraction process achieved optimal results with a 102-fold enrichment factor at 15 V over a 15 min period, with stirring at 750 rpm and a donor pH of 11.
Following extraction, the samples were analyzed using CE. The results demonstrate that this method is highly selective and precise, with no interfering peaks detected. The limit of detection for phenytoin was 0.001 µg/mL, indicating excellent sensitivity. The intra- and inter-day precision values were reported to be below 14%.
This method was successfully applied to real samples from patients receiving phenytoin therapy, proving it to be an effective, non-invasive alternative to blood sampling for TDM. Furthermore, the study concluded that electromembrane extraction combined with CE is a robust, low-cost, and highly sensitive approach, with potential for broader applications in clinical settings.
The researchers suggest that this method may offer a more convenient way to monitor AED levels in patients, supporting more frequent and less invasive monitoring.
2.6. Breast Milk
Breast milk serves as a vital biological sample in toxicology, especially when studying drug transfer and exposure in neonates. Its unique composition, including nutrients, hormones, and immune factors, makes it the optimal nutrition source for infants. However, it is also a route through which infants can be exposed to medications and environmental toxins ingested or absorbed by the mother. Analyzing breast milk is critical in evaluating the safety of maternal drug use during breastfeeding and assessing the potential risks of toxic substance exposure to the infant. Given its non-invasive collection process and the direct link between maternal and infant exposure, breast milk is an important medium for monitoring drug excretion and informing clinical decisions on safe breastfeeding practices for mothers on medication [124]. Dinavitser et al. [125] published a study concerning the use of this sample in the monitoring of AEDs. The study investigates the excretion of levetiracetam, an antiepileptic drug, into human breast milk and its impact on breastfed infants. Twenty women with epilepsy, treated with levetiracetam, participated in the study, and breast milk and serum samples were collected to measure the drug’s concentrations. The results indicate that the breast milk/serum ratio of levetiracetam is close to 1, suggesting that the drug passes efficiently into breast milk. The relative infant dose was above the safety threshold of 10% in fully breastfed infants, with three cases of somnolence reported in infants, which resolved after switching to partial breastfeeding. These findings suggest a need for the careful monitoring of breastfeeding infants exposed to levetiracetam, considering potential short- and long-term safety risks.
No studies have been found, following the previously established search criteria, on the determination of antiepileptic drugs in other types of samples.
3. Conclusions
To conclude, this review highlights several emerging areas and methods that have shown promising results in detecting AEDs in biological samples. Over the past four years, researchers have increasingly focused on utilizing alternative samples to blood/plasma, primarily due to the advantages of less invasive collection procedures. Additionally, efforts have been made to optimize existing methods and develop new ones to reduce analysis time and simplify procedures, with a focus on making them more environmentally friendly.
As anticipated, blood and derivatives remain the most extensively studied matrices, while oral fluid and urine show positive results as viable alternatives. Both matrices have shown promise due to their ease of collection, and innovative approaches have even led to the development of an electrochemical sensor for detecting carbamazepine in urine with high precision and efficiency. Unfortunately, despite its potential for monitoring long-term drug exposure, hair has not seen significant advancements in the detection of AEDs. It is surprising that only one relevant study has been identified from 2020 to the present year, given the advantages hair offers. Similarly, exhaled air remains an underexplored matrix, though it has shown potential in a method capable of predicting the free fraction of valproic acid.
Overall, the evolution of methodologies for detecting AEDs in biological samples has unveiled numerous new avenues for improving therapeutic drug monitoring. Continued advancements in traditional sample analysis are expected, and the exploration of alternative matrices promises to yield a variety of new methods, given the potential demonstrated in the limited number of studies to date.
Author Contributions
Conceptualization, A.Y.S., T.R. and E.G.; methodology, J.M.; validation, J.M., A.Y.S., M.B. and E.G.; formal analysis, J.M.; investigation, J.M.; writing—original draft preparation, J.M., A.Y.S., T.R., M.B. and E.G.; writing—review and editing, J.M., A.Y.S., T.R., M.B. and E.G.; supervision, A.Y.S., T.R. and E.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This systematic search was performed via the databases Web of Science and SCOPUS.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was developed within the scope of the CICS-UBI projects (https://doi.org/10.54499/UIDB/00709/2020 and https://doi.org/10.54499/UIDP/00709/2020, accessed on 2 August 2024), financed by national funds through the Portuguese Foundation for Science and Technology (FCT). Ana Y. Simão acknowledges the PhD fellowship received from FCT (Reference: https://doi.org/10.54499/2020.09070.BD, accessed on 2 August 2024).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization . WHO | Epilepsy: A Public Health Imperative. WHO; Geneva, Switzerland: 2019. [Google Scholar]
- 2.Hassan S.A., Helmy A.H., Youssef N.F., Weshahy S.A., El-zeany B.A. Fluorescence imaging approaches for eco-friendly determination of perampanel in human plasma and application for therapeutic drug monitoring. Luminescence. 2023;38:729–735. doi: 10.1002/bio.4501. [DOI] [PubMed] [Google Scholar]
- 3.Nasir M. Clinical Characteristics, Treatment Outcome and Associated Factors of Epilepsy Among Children at Hospitals of North-West Ethiopia. Pediatr. Health Med. Ther. 2023;14:385–404. doi: 10.2147/PHMT.S436022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbarao B.S., Silverman A., Eapen B. Seizure Medications. In: Springer C., Nappe T.M., editors. Anticonvulsants Toxicity. StatPearls Publishing; St. Petersburg, FL, USA: 2023. pp. 1–7. [Google Scholar]
- 5.Landazuri P., Lecumberri M.L., Bertran L.V., Farrengurg M., Lhatoo S. Seizure and Epilepsy Care The Pocket Epileptologist. Part of Cambridge Manuals in Neurology. Cambridge University Press; Cambidge, UK: 2023. [Google Scholar]
- 6.Aaryashree, Choudhary A.K., Yoshimi Y. Disposable Sensor Chips with Molecularly Imprinted Carbon Paste Electrodes for Monitoring Anti-Epileptic Drugs. Sensors. 2023;23:3271. doi: 10.3390/s23063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sills G.J., Rogawski M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. doi: 10.1016/j.neuropharm.2020.107966. [DOI] [PubMed] [Google Scholar]
- 8.Sigel E., Ernst M. The Benzodiazepine Binding Sites of GABA(A) Receptors. Trends Pharmacol. Sci. 2018;39:659–671. doi: 10.1016/j.tips.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Twyman R.E., Rogers C.J., Macdonald R.L. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann. Neurol. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- 10.Borden L.A., Murali Dhar T.G., Smith K.E., Weinshank R.L., Branchek T.A., Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur. J. Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 11.Coulter D.A., Huguenard J.R., Prince D.A. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann. Neurol. 1989;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- 12.Hendrich J., Van Minh A.T., Heblich F., Nieto-Rostro M., Watschinger K., Striessnig J., Wratten J., Davies A., Dolphin A.C. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trimethadione: Uses, Interactions, Mechanism of Action | DrugBank Online. [(accessed on 19 July 2024)]. Available online: https://go.drugbank.com/drugs/DB00347.
- 14.Reiss W.G., Oles K.S. Acetazolamide in the treatment of seizures. Ann. Pharmacother. 1996;30:514–519. doi: 10.1177/106002809603000515. [DOI] [PubMed] [Google Scholar]
- 15.Rogawski M.A., Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol. Scand. 2013;127:19–24. doi: 10.1111/ane.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefani A., Pisani A., De Murtas M., Mercuri N.B., Marciani M.G., Calabresi P. Action of GP 47779, the active metabolite of oxcarbazepine, on the corticostriatal system. II. Modulation of high-voltage-activated calcium currents. Epilepsia. 1995;36:997–1002. doi: 10.1111/j.1528-1157.1995.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 17.Sofia R.D. Mechanism of action of the anticonvulsant felbamate: Opposing effects on N-methyl-D-aspartate and gamma-aminobutyric acidA receptors. Ann. Neurol. 1994;36:677–678. doi: 10.1002/ana.410360423. [DOI] [PubMed] [Google Scholar]
- 18.Temperini C., Innocenti A., Scozzafava A., Parkkila S., Supuran C.T. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: The antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J. Med. Chem. 2010;53:850–854. doi: 10.1021/jm901524f. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M., Cho J.-H., Shin H., Jang I.-S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019;855:175–182. doi: 10.1016/j.ejphar.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Sharma R., Song W.S., Nakamura M., Neupane C., Shin H., Melnick S.M., Glenn K.J., Jang I.-S., Kim M.-H., Park J.B. Effects of Cenobamate on GABA-A Receptor Modulation (P1.5-033) Neurology. 2019;92:15. doi: 10.1212/WNL.92.15_supplement.P1.5-033. [DOI] [Google Scholar]
- 21.Löscher W. Valproate: A reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 1999;58:31–59. doi: 10.1016/S0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 22.Ticku M.K., Davis W.C. Effect of valproic acid on [3H]diazepam and [3H]dihydropicrotoxinin binding sites at the benzodiazepine-GABA receptor-ionophore complex. Brain Res. 1981;223:218–222. doi: 10.1016/0006-8993(81)90828-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X., Velumian A.A., Jones O.T., Carlen P.L. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia. 2000;41:52–60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 24.Simeone T.A., Wilcox K.S., White H.S. Subunit selectivity of topiramate modulation of heteromeric GABAA receptors. Neuropharmacology. 2006;50:845–857. doi: 10.1016/j.neuropharm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Gibbs J.W., 3rd, Sombati S., DeLorenzo R.J., Coulter D.A. Cellular actions of topiramate: Blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41:10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 26.Potnis V.V., Albhar K.G., Nanaware P.A., Pote V.S. A Review on Epilepsy and its Management. J. Drug Deliv. Ther. 2020;10:273–279. doi: 10.22270/jddt.v10i3.4090. [DOI] [Google Scholar]
- 27.Johannessen Landmark C., Johannessen S.I., Patsalos P.N. Therapeutic drug monitoring of antiepileptic drugs: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2020;16:227–238. doi: 10.1080/17425255.2020.1724956. [DOI] [PubMed] [Google Scholar]
- 28.Patsalos P.N., Berry D.J., Bourgeois B.F.D., Cloyd J.C., Glauser T.A., Johannessen S.I., Leppik I.E., Tomson T., Perucca E. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49:1239–1276. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- 29.Patsalos P.N., Perucca E. Clinically important drug interactions in epilepsy: Interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2:473–481. doi: 10.1016/S1474-4422(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 30.Opuni K.F.M., Boadu J.A., Amponsah S.K., Okai C.A. High performance liquid chromatography: A versatile tool for assaying antiepileptic drugs in biological matrices. J. Chromatogr. B. 2021;1179:122750. doi: 10.1016/j.jchromb.2021.122750. [DOI] [PubMed] [Google Scholar]
- 31.Feriduni B., Farajzadeh M.A., Jouyban A. Determination of two antiepileptic drugs in urine by homogenous liquid-liquid extraction performed in a narrow tube combined with dispersive liquid-liquid microextraction followed by gas chromatography-flame ionization detection. Iran. J. Pharm. Res. 2019;18:620–630. doi: 10.22037/ijpr.2019.1100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erarpat S., Bodur S., Ayyıldız M.F., Günkara Ö.T., Erulaş F., Chormey D.S., Turak F., Budak T.B., Bakırdere S. Accurate simple determination of oxcarbazepine in human plasma urine samples using switchable-hydrophilicity solvent in, G.C.–.M.S. Biomed. Chromatogr. 2020;34:e4915. doi: 10.1002/bmc.4915. [DOI] [PubMed] [Google Scholar]
- 33.Hu W., Zhou W., Wang C., Liu Z., Chen Z. Direct coupling in-tube solid-phase microextraction with mass spectrometry using polymer coated open-tubular column for rapid analysis of antiepileptic drugs in biofluids. Anal. Chim. Acta. 2023;1240((Suppl. S2022)):340775. doi: 10.1016/j.aca.2022.340775. [DOI] [PubMed] [Google Scholar]
- 34.Möller I., Held K., Klimpel D., Nadulski T., Dufaux B. Development and validation of an LC–MS/MS method for relevant drugs in epilepsy patients using dried blood spots. Biomed. Chromatogr. 2021;35:e5130. doi: 10.1002/bmc.5130. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho J., Rosado T., Barroso M., Gallardo E. Determination of antiepileptic drugs using dried saliva spots. J. Anal. Toxicol. 2019;43:61–71. doi: 10.1093/jat/bky064. [DOI] [PubMed] [Google Scholar]
- 36.Gallardo E., Barroso M., Queiroz J.A. LC-MS: A powerful tool in workplace drug testing. Drug Test. Anal. 2009;1:109–115. doi: 10.1002/dta.26. [DOI] [PubMed] [Google Scholar]
- 37.de Campos E.G., da Costa B.R.B., dos Santos F.S., Monedeiro F., Alves M.N.R., Santos Junior W.J.R., De Martinis B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2022;40:1–18. doi: 10.1007/s11419-021-00596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters F.T., Wissenbach D.K., Busardo F.P., Marchei E., Pichini S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2018;23:5455–5467. doi: 10.2174/1381612823666170622113331. [DOI] [PubMed] [Google Scholar]
- 39.Mouskeftara T., Alexandridou A., Krokos A., Gika H., Mastrogianni O., Orfanidis A., Raikos N. A Simple Method for the Determination of Lacosamide in Blood by, G.C.-M.S. J. Forensic Sci. 2020;65:288–294. doi: 10.1111/1556-4029.14151. [DOI] [PubMed] [Google Scholar]
- 40.Liu W., Shang X., Yao S., Wang F. A novel and nonderivatization method for the determination of valproic acid in human serum by two-dimensional liquid chromatography. Biomed. Chromatogr. 2020;34:e4695. doi: 10.1002/bmc.4695. [DOI] [PubMed] [Google Scholar]
- 41.Velghe S., Delahaye L., Ogwang R., Hotterbeekx A., Colebunders R., Mandro M., Idro R., Stove C.P. Dried Blood Microsampling-Based Therapeutic Drug Monitoring of Antiepileptic Drugs in Children With Nodding Syndrome and Epilepsy in Uganda and the Democratic Republic of the Congo. Ther. Drug Monit. 2020;42:481–490. doi: 10.1097/FTD.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 42.Gu X., Yu S., Peng Q., Ma M., Hu Y., Zhou B. Determination of unbound valproic acid in plasma using centrifugal ultrafiltration and gas chromatography:Application in TDM. Anal. Biochem. 2020;588:113475. doi: 10.1016/j.ab.2019.113475. [DOI] [PubMed] [Google Scholar]
- 43.Bharwad K.D., Shah P.A., Shrivastav P.S., Sharma V.S. Selective quantification of lacosamide in human plasma using UPLC–MS/MS: Application to pharmacokinetic study in healthy subjects with different doses. Biomed. Chromatogr. 2020;34:e4928. doi: 10.1002/bmc.4928. [DOI] [PubMed] [Google Scholar]
- 44.Önal C., Kul A., Ozdemir M., Sagirli O. Determination of levetiracetam in human plasma by online heart-cutting liquid chromatography: Application to therapeutic drug monitoring. J. Sep. Sci. 2020;43:3590–3596. doi: 10.1002/jssc.202000504. [DOI] [PubMed] [Google Scholar]
- 45.Seyfinejad B., Khoubnasabjafari M., Ziaei S.E., Ozkan S.A., Jouyban A. Electromembrane extraction as a new approach for determination of free concentration of phenytoin in plasma using capillary electrophoresis. DARU J. Pharm. Sci. 2020;28:615–624. doi: 10.1007/s40199-020-00366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madej K., Paprotny Ł., Wianowska D., Kasprzyk J., Herman M., Piekoszewski W. A fully validated HPLC–UV method for determination of sulthiame in human serum/plasma samples. Biomed. Chromatogr. 2021;35:e5002. doi: 10.1002/bmc.5002. [DOI] [PubMed] [Google Scholar]
- 47.Zhao T., Yu L.H., Wang T.T., Feng J., Ma L., Yu J., Sun L., Li H., Sun Y. Development and validation of an innovative UPLC method to quantify lacosamide, oxcarbazepine, and lamotrigine in the serum of children with epilepsy in China. Biomed. Chromatogr. 2021;35:e5022. doi: 10.1002/bmc.5022. [DOI] [PubMed] [Google Scholar]
- 48.Sabença R., Bicker J., Silva R., Carona A., Silva A., Santana I., Sales F., Falcão A., Fortuna A. Development and application of an HPLC-DAD technique for human plasma concentration monitoring of perampanel and lamotrigine in drug-resistant epileptic patients. J. Chromatogr. B. 2021;1162:122491. doi: 10.1016/j.jchromb.2020.122491. [DOI] [PubMed] [Google Scholar]
- 49.Hess C., Zeidler S., Roehrich J. Forensic application and evaluation of a commercially available pregabalin immunoassay test in serum on an Olympus AU480. Drug Test. Anal. 2021;13:1216–1218. doi: 10.1002/dta.2994. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., Jiang Y., Cao H., Lin H., Ren W., Huang J., Zhang J. Therapeutic drug monitoring of valproic acid using a dried plasma spot sampling device. J. Mass Spectrom. 2021;56:e4603. doi: 10.1002/jms.4603. [DOI] [PubMed] [Google Scholar]
- 51.Kim D.Y., Moon J., Shin Y.W., Lee S.T., Jung K.H., Park K.I.L., Jung K.Y., Kim M., Lee S.H., Yu K.S., et al. Usefulness of saliva for perampanel therapeutic drug monitoring. Epilepsia. 2020;61:1120–1128. doi: 10.1111/epi.16513. [DOI] [PubMed] [Google Scholar]
- 52.Franco V., Gatti G., Mazzucchelli I., Marchiselli R., Fattore C., Rota P., Galimberti C.A., Capovilla G., Beccaria F., De Giorgis V., et al. Relationship between saliva and plasma rufinamide concentrations in patients with epilepsy. Epilepsia. 2020;61:e79–e84. doi: 10.1111/epi.16584. [DOI] [PubMed] [Google Scholar]
- 53.Hassib S.T., Hashem H.M.A., Mahrouse M.A., Mostafa E.A. Development and Bio-Analytical Validation of Chromatographic Determination Method of Rufinamide in Presence of its Metabolite in Human Plasma. J. Chromatogr. Sci. 2021;59:458–464. doi: 10.1093/chromsci/bmaa142. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer V.D., de Lima Feltraco Lizot L., Hahn R.Z., Schneider A., Antunes M.V., Linden R. Simple determination of valproic acid serum concentrations using BioSPME followed by gas chromatography-mass spectrometric analysis. J. Chromatogr. B. 2021;1167:122574. doi: 10.1016/j.jchromb.2021.122574. [DOI] [PubMed] [Google Scholar]
- 55.Phillips S.J., Oliveto A., Mancino M.J., Hendrickson H.P. Development and validation of a rapid liquid chromatography/tandem mass spectrometry method to quantitate gabapentin and buprenorphine in human serum. Rapid Commun. Mass Spectrom. 2021;35:e9104. doi: 10.1002/rcm.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seyfinejad B., Ozkan S.A., Jouyban A. Ultrasound-assisted electromembrane extraction of clonazepam from plasma and determination using capillary electrophoresis. J. Chromatogr. B. 2021;1181:122928. doi: 10.1016/j.jchromb.2021.122928. [DOI] [PubMed] [Google Scholar]
- 57.Kruizinga M.D., Zuiker R.G.J.A., Bergmann K.R., Egas A.C., Cohen A.F., Santen G.W.E., van Esdonk M.J. Population pharmacokinetics of clonazepam in saliva and plasma: Steps towards noninvasive pharmacokinetic studies in vulnerable populations. Br. J. Clin. Pharmacol. 2022;88:2236–2245. doi: 10.1111/bcp.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoonjans A.S., Roosens L., Dewals W., Paelinck B.P., Ceulemans B. Therapeutic drug monitoring of fenfluramine in clinical practice: Pharmacokinetic variability and impact of concomitant antiseizure medications. Epilepsia. 2022;63:686–696. doi: 10.1111/epi.17162. [DOI] [PubMed] [Google Scholar]
- 59.Ma D., Ji Z., Cao H., Huang J., Zeng L., Yin L. LC–MS 3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules. 2022;27:1224. doi: 10.3390/molecules27041224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang S., Lee S., Kim E., Hwang I., Cho J.Y., Chung J.Y., Jang I., Oh J. The pharmacokinetic, safety, and tolerability profiles of eslicarbazepine acetate are comparable between Korean and White subjects. Clin. Transl. Sci. 2022;15:2116–2126. doi: 10.1111/cts.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rmandić M., Stajić A., Jančić J., Samardžić J., Jović N., Malenović A. Quantification of Zonisamide in Dried Blood Spots and Dried Plasma Spots by UPLC–MS/MS: Application to Clinical Practice. Molecules. 2022;27:4899. doi: 10.3390/molecules27154899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascali J.P., Giorgetti A., Barone R., Pelletti G., Fais P. Valproic acid determination by liquid chromatography coupled to mass spectrometry (LC–MS/MS) in whole blood for forensic purposes. Drug Test. Anal. 2023;15:128–133. doi: 10.1002/dta.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao T., Li H.J., Zhang H.L., Feng J., Yu J., Wang T.T., Sun Y., Yu L.H. Impact of ABCC2 1249G>A and-24C>T Polymorphisms on Lacosamide Efficacy and Plasma Concentrations in Uygur Pediatric Patients with Epilepsy in China. Ther. Drug Monit. 2023;45:117–125. doi: 10.1097/FTD.0000000000001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Namera A., Uekusa K., Saito T., Yoshimoto K., Ishiuchi N., Murata K., Nagao M. A method for determining valproic acid in human whole blood and urine via gas chromatography-mass spectrometry and small-scale inter-laboratory trial. Leg. Med. 2022;59:102133. doi: 10.1016/j.legalmed.2022.102133. [DOI] [PubMed] [Google Scholar]
- 65.Salzmann L., Wild J., Singh N., Schierscher T., Liesch F., Bauland F., Geistanger A., Risch L., Geletneky C., Seger C., et al. An isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS)-based candidate reference measurement procedure (RMP) for the quantification of gabapentin in human serum and plasma. Clin. Chem. Lab. Med. 2023;61:1955–1966. doi: 10.1515/cclm-2022-0998. [DOI] [PubMed] [Google Scholar]
- 66.Zhang M., Jin Y., Li W., He C., Di X., Duan Y., Chen L., Wang Z. Quantitation of levetiracetam concentrations in plasma and saliva samples by ultra-performance liquid chromatography–tandem mass spectrometry: Application to therapeutic drug monitoring for pregnant women with epilepsy. Biomed. Chromatogr. 2023;38:e5777. doi: 10.1002/bmc.5777. [DOI] [PubMed] [Google Scholar]
- 67.Nuchtavorn N., Dvořák M., Kubáň P. Paper-based molecularly imprinted-interpenetrating polymer network for on-spot collection and microextraction of dried blood spots for capillary electrophoresis determination of carbamazepine. Anal. Bioanal. Chem. 2020;412:2721–2730. doi: 10.1007/s00216-020-02523-w. [DOI] [PubMed] [Google Scholar]
- 68.Mendoza Aguilera M., Bellés Medall M.D., Álvarez Martín T., Pascual Marmaneu Ó., Liñana Granell C., Ferrando Piqueres R. Therapeutic drug monitoring of levetiracetam in daily clinical practice: High-performance liquid chromatography versus immunoassay. Eur. J. Hosp. Pharm. Sci. Pract. 2020;27:e2–e6. doi: 10.1136/ejhpharm-2018-001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagemann A., Klimpel D., Bien C.G., Brandt C., May T.W. Influence of dose and antiepileptic comedication on brivaracetam serum concentrations in patients with epilepsy. Epilepsia. 2020;61:e43–e48. doi: 10.1111/epi.16500. [DOI] [PubMed] [Google Scholar]
- 70.Zhou S., Xu L., Liu L., Kuang H., Xu C. Development of a monoclonal antibody-based immunochromatographic assay for the detection of carbamazepine and carbamazepine-10, 11-epoxide. J. Chromatogr. B. 2020;1141:122036. doi: 10.1016/j.jchromb.2020.122036. [DOI] [PubMed] [Google Scholar]
- 71.Li X.-Y., Hu C., Zhu X.-H., Wang Y., Shu S.-Q., Luo Z. Pharmacokinetics and safety of Padsevonil in healthy Chinese subjects and comparison of two sampling methods for Padsevonil quantification. Eur. Rev. Med. Pharmacol. Sci. 2023;27:4698–4707. doi: 10.26355/eurrev_202305_32482. [DOI] [PubMed] [Google Scholar]
- 72.Mavri A., Ilc S. The efficacy of direct oral anticoagulants in patients on concomitant treatment with levetiracetam. Sci. Rep. 2023;13:9257. doi: 10.1038/s41598-023-33876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birnbaum A.K., Meador K.J., Karanam A., Brown C., May R.C., Gerard E.E., Gedzelman E.R., Penovich P.E., Kalayjian L.A., Cavitt J., et al. Antiepileptic Drug Exposure in Infants of Breastfeeding Mothers With Epilepsy. JAMA Neurol. 2020;77:441–450. doi: 10.1001/jamaneurol.2019.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Itabashi S., Bito R., Nishina M., Fukumoto M., Soda M., Doi M., Usui S., Kitaichi K. Determination of lamotrigine in human plasma using liquid chromatography-tandem mass spectrometry. Neuropsychopharmacol. Rep. 2019;39:48–55. doi: 10.1002/npr2.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y., Zhan H., Fan Y., Zhang J., Cao G., Yu J., Chen Y., Guo B. Determination of DP-VPA and its active metabolite, VPA, in human plasma, urine, and feces by UPLC-MS/MS: A clinical pharmacokinetics and excretion study. Drug Test. Anal. 2019;11:1035–1047. doi: 10.1002/dta.2579. [DOI] [PubMed] [Google Scholar]
- 76.Mao G., Zhao K., Sun S., Lu Y., Li X. A new derivatization method for the determination of valproic acid in serum using tetramethylammonium hydroxide as a catalyst. Biomed. Chromatogr. 2019;33:e4440. doi: 10.1002/bmc.4440. [DOI] [PubMed] [Google Scholar]
- 77.Jiang W., Xia T., Yun Y., Li M., Zhang F., Gao S., Chen W. UHPLC-MS/MS method for simultaneous determination of carbamazepine and its seven major metabolites in serum of epileptic patients. J. Chromatogr. B. 2019;1108:17–24. doi: 10.1016/j.jchromb.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 78.Guo M., Shao L., Du Y., Qian Z., Huang T., Tang D. Microporous polymer based on the new compound “bi-(4-vinyl phenylquinoline) amide” for enrichment and quantitative determination of lamotrigine in rat and human serum. Anal. Bioanal. Chem. 2019;411:3353–3360. doi: 10.1007/s00216-019-01812-3. [DOI] [PubMed] [Google Scholar]
- 79.Velghe S., Deprez S., Stove C.P. Fully automated therapeutic drug monitoring of anti-epileptic drugs making use of dried blood spots. J. Chromatogr. A. 2019;1601:95–103. doi: 10.1016/j.chroma.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 80.Toki T., Iwasaki T., Ishii M. Topiramate Blood Levels During Polytherapy for Epilepsy in Children. Am. J. Ther. 2019;26:e18–e24. doi: 10.1097/MJT.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 81.Liu T., Kotha R.R., Jones J.W., Polli J.E., Kane M.A. Fast liquid chromatography-tandem mass spectrometry method for simultaneous determination of eight antiepileptic drugs and an active metabolite in human plasma using polarity switching and timed selected reaction monitoring. J. Pharm. Biomed. Anal. 2019;176:112816. doi: 10.1016/j.jpba.2019.112816. [DOI] [PubMed] [Google Scholar]
- 82.Guo M.-Z., Shao L., Chen X., Li H.-J., Wang L., Pan Y.-J., Tang D.-Q. Assay of dried blood spot from finger prick for sodium valproate via ink auxiliary headspace gas chromatography mass spectrometry. J. Chromatogr. A. 2019;1601:335–339. doi: 10.1016/j.chroma.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 83.Chen C.-Y., Zhou Y., Cui Y.-M., Yang T., Zhao X., Wu Y. Population pharmacokinetics and dose simulation of oxcarbazepine in Chinese paediatric patients with epilepsy. J. Clin. Pharm. Ther. 2019;44:300–311. doi: 10.1111/jcpt.12792. [DOI] [PubMed] [Google Scholar]
- 84.Høj L.J., Mollerup C.B., Rasmussen B.S., Johansen S.S., Linnet K., Dalsgaard P.W. Identification of phenobarbital and other barbiturates in forensic drug screening using positive electrospray ionization liquid chromatography-high resolution mass spectrometry. Drug Test. Anal. 2019;11:1258–1263. doi: 10.1002/dta.2603. [DOI] [PubMed] [Google Scholar]
- 85.Han X., Huang J., Lv J., Ma L., Peng L., Wang J., Nie X., Xia L., Zan X. The influence of concomitant antiepileptic drugs on lamotrigine serum concentrations in Northwest Chinese Han population with epilepsy. PLoS ONE. 2019;14:e0210600. doi: 10.1371/journal.pone.0210600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alvani-Alamdari S., Jouyban A., Khoubnasabjafari M., Nokhodchi A., Rahimpour E. Efficiency comparison of nylon-6-based solid-phase and stir bar sorptive extractors for carbamazepine extraction. Bioanalysis. 2019;11:899–911. doi: 10.4155/bio-2018-0321. [DOI] [PubMed] [Google Scholar]
- 87.Ramos I.I., Carl P., Schneider R.J., Segundo M.A. Automated lab-on-valve sequential injection ELISA for determination of carbamazepine. Anal. Chim. Acta. 2019;1076:91–99. doi: 10.1016/j.aca.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 88.Aicua-Rapun I., André P., Rossetti A.O., Decosterd L.A., Buclin T., Novy J. Intravenous brivaracetam in status epilepticus: Correlation between loading dose, plasma levels and clinical response. Epilepsy Res. 2019;149:88–91. doi: 10.1016/j.eplepsyres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Dao K., Thoueille P., Decosterd L.A., Mercier T., Guidi M., Bardinet C., Lebon S., Choong E., Castang A., Guittet C., et al. Sultiame pharmacokinetic profile in plasma and erythrocytes after single oral doses: A pilot study in healthy volunteers. Pharmacol. Res. Perspect. 2020;8:e00558. doi: 10.1002/prp2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Favié L.M.A., Groenendaal F., van den Broek M.P.H., Rademaker C.M.A., de Haan T.R., van Straaten H.L.M., Dijk P.H., van Heijst A., Simons S.H.P., Dijkman K.P., et al. Phenobarbital, Midazolam Pharmacokinetics, Effectiveness, and Drug-Drug Interaction in Asphyxiated Neonates Undergoing Therapeutic Hypothermia. Neonatology. 2019;116:154–162. doi: 10.1159/000499330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qu L., Pan L., Wang L., Liu C., Tian Y., Hao Z. Development of an online solid-phase extraction-liquid chromatography-mass spectrometric analysis of oxcarbazepine and its active metabolite licarbazepine from plasma with a direct injection step. J. Chromatogr. B. 2019;1125:121710. doi: 10.1016/j.jchromb.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 92.Eto D., Tanaka R., Suzuki Y., Sato Y., Itoh H. Comparison of performance characteristics between high-performance liquid chromatography and latex agglutination turbidimetric immunoassay for therapeutic drug monitoring of zonisamide. J. Clin. Lab. Anal. 2019;33:e22940. doi: 10.1002/jcla.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Q., Shi X., Tang S.-F., Wang H., Chen Y., Zhang N. Preparation of a β-cyclodextrin grafted magnetic biochar for efficient extraction of four antiepileptic drugs in plasma samples. J. Chromatogr. A. 2024;1724:464893. doi: 10.1016/j.chroma.2024.464893. [DOI] [PubMed] [Google Scholar]
- 94.Fallik N., Trakhtenbroit I., Fahoum F., Goldstein L. Therapeutic drug monitoring in pregnancy: Levetiracetam. Epilepsia. 2024;65:1285–1293. doi: 10.1111/epi.17925. [DOI] [PubMed] [Google Scholar]
- 95.Zhao T., Li H.-J., Zhang H.-L., Feng J.-R., Yu J., Sun K.-F., Feng J., Sun Y., Yu L.-H. Effects of CYP3A4 genetic polymorphisms on the pharmacokinetics and efficacy of perampanel in Chinese pediatric patients with epilepsy. Seizure. 2024;120:142–149. doi: 10.1016/j.seizure.2024.07.006. [DOI] [PubMed] [Google Scholar]
- 96.Hagemann A., Herting A., Klimpel D., Bien C.G., Polster T. Ethosuximide lowers lamotrigine serum concentrations: Evidence for a clinically relevant interaction. Epilepsia. 2024;65:e73–e78. doi: 10.1111/epi.17952. [DOI] [PubMed] [Google Scholar]
- 97.Pigliasco F., Cafaro A., Barco S., Stella M., Mattioli F., Riva A., Mancardi M.M., Lattanzi S., Bandettini R., Striano P., et al. Innovative LC-MS/MS method for therapeutic drug monitoring of fenfluramine and cannabidiol in the plasma of pediatric patients with epilepsy. J. Pharm. Biomed. Anal. 2024;245:116174. doi: 10.1016/j.jpba.2024.116174. [DOI] [PubMed] [Google Scholar]
- 98.Wang H., Wang J., Lin B., Zhang H., Sun Y., Wu Y., Ye W., Miao J. Effect of Age, Comedications, and CYP3A4/5 Polymorphisms on Perampanel Exposure in Chinese Pediatric Patients with Epilepsy. J. Clin. Pharmacol. 2024;64:737–743. doi: 10.1002/jcph.2415. [DOI] [PubMed] [Google Scholar]
- 99.Schierscher T., Salzmann L., Singh N., Fischer V., Kobel A., Bauland F., Geistanger A., Risch L., Geletneky C., Seger C., et al. An isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS)-based candidate reference measurement procedure (RMP) for the quantification of primidone in human serum and plasma. Clin. Chem. Lab. Med. 2024;62:1327–1338. doi: 10.1515/cclm-2023-1032. [DOI] [PubMed] [Google Scholar]
- 100.Li W., Yang X., Chen Q., Wang Z., Duan Y., Chen L. Monitoring levetiracetam concentration in saliva during pregnancy is stable and feasible. CNS Neurosci. Ther. 2024;30:e14827. doi: 10.1111/cns.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohamed A.A., Younis H.M. A novel spectrofluorimetric method using optical sensor Eu(3+)-ACAC as a highly selective photo probe to determine Pregabalin in biological samples and pharmaceutical form. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024;322:124811. doi: 10.1016/j.saa.2024.124811. [DOI] [PubMed] [Google Scholar]
- 102.Abady M.M., Jeong J.-S., Kwon H.-J. Simultaneous quantification of 11 antiepileptic drugs using limited isotope-labeled internal standards in LC-MS/MS: An accuracy assessment. J. Chromatogr. B. 2024;1240:124143. doi: 10.1016/j.jchromb.2024.124143. [DOI] [PubMed] [Google Scholar]
- 103.Protti M., Mandrioli R., Mercolini L. Tutorial: Volumetric absorptive microsampling (VAMS) Anal. Chim. Acta. 2019;1046:32–47. doi: 10.1016/j.aca.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Zailani N.N.B., Ho P.C.L. Dried Blood Spots—A Platform for Therapeutic Drug Monitoring (TDM) and Drug/Disease Response Monitoring (DRM) Eur. J. Drug Metab. Pharmacokinet. 2023;48:467–494. doi: 10.1007/s13318-023-00846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dodeja P., Giannoutsos S., Caritis S., Venkataramanan R. Applications of Volumetric Absorptive Microsampling Technique: A Systematic Critical Review. Ther. Drug Monit. 2023;45:431–462. doi: 10.1097/FTD.0000000000001083. [DOI] [PubMed] [Google Scholar]
- 106.Cafaro A., Conti M., Pigliasco F., Barco S., Bandettini R., Cangemi G. Biological Fluid Microsampling for Therapeutic Drug Monitoring: A Narrative Review. Biomedicines. 2023;11:1962. doi: 10.3390/biomedicines11071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feng S., Bridgewater B., Strickland E.C., McIntire G. A rapid LC–MS-MS method for the quantitation of antiepileptic drugs in urine. J. Anal. Toxicol. 2020;44:688–696. doi: 10.1093/jat/bkaa095. [DOI] [PubMed] [Google Scholar]
- 108.Mariyappan V., Sundaresan R., Chen S.M., Ramachandran R. Ultrasensitive electrochemical sensor for the detection of carbamazepine based on gadolinium vanadate nanostructure decorated functionalized carbon nanofiber nanocomposite. Chemosphere. 2022;307:135803. doi: 10.1016/j.chemosphere.2022.135803. [DOI] [PubMed] [Google Scholar]
- 109.Mohamed F.A., Ali M.F.B., Rageh A.H., Mostafa A.M. Highly sensitive UHPLC-DAD method for simultaneous determination of two synergistically acting antiepileptic drugs; levetiracetam and lacosamide: Application to pharmaceutical tablets and human urine. Biomed. Chromatogr. 2019;33:e4554. doi: 10.1002/bmc.4554. [DOI] [PubMed] [Google Scholar]
- 110.Maciel E.V.S., Toffoli A.L.D., Alves J.d.S., Lanças F.M. Multidimensional Liquid Chromatography Employing a Graphene Oxide Capillary Column as the First Dimension: Determination of Antidepressant and Antiepileptic Drugs in Urine. Molecules. 2020;25:1092. doi: 10.3390/molecules25051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eugenia Gallardo T.R., Barroso M. The Potential of Oral Fluid in 1Drug Monitoring: An Update. Bioanalysis. 2023;15:657–660. doi: 10.4155/bio-2023-0122. [DOI] [PubMed] [Google Scholar]
- 112.Gallardo E., Queiroz J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008;22:795–821. doi: 10.1002/bmc.1009. [DOI] [PubMed] [Google Scholar]
- 113.Chiș I.-A., Andrei V., Muntean A., Moldovan M., Mesaroș A., Dudescu M.C., Ilea A. Salivary Biomarkers of Anti-Epileptic Drugs: A Narrative Review. Diagnostics. 2023;13:1962. doi: 10.3390/diagnostics13111962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tommasini M., Lucotti A., Stefani L., Trusso S., Ossi P.M. SERS Detection of the Anti-Epileptic Drug Perampanel in Human Saliva. Molecules. 2023;28:4309. doi: 10.3390/molecules28114309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carona A., Bicker J., Silva R., Silva A., Santana I., Sales F., Falcão A., Fortuna A. HPLC method for the determination of antiepileptic drugs in human saliva and its application in therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2021;197:113961. doi: 10.1016/j.jpba.2021.113961. [DOI] [PubMed] [Google Scholar]
- 116.Dziurkowska E., Wesolowski M. Simultaneous Quantification of Antipsychotic and Antiepileptic Drugs and Their Metabolites in Human Saliva Using UHPLC-DAD. Molecules. 2019;24:2953. doi: 10.3390/molecules24162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosado T., Barroso M., Vieira D.N., Gallardo E. Trends in microextraction approaches for handling human hair extracts—A review. Anal. Chim. Acta. 2021;1185:338792. doi: 10.1016/j.aca.2021.338792. [DOI] [PubMed] [Google Scholar]
- 118.Barroso M., Gallardo E., Vieira D.N., López-Rivadulla M., Queiroz J.A. Hair: A complementary source of bioanalytical information in forensic toxicology. Bioanalysis. 2011;3:67–79. doi: 10.4155/bio.10.171. [DOI] [PubMed] [Google Scholar]
- 119.Kim J., Kim J., Yum H., Jang M., Rhee J., Lee S., Han S.B. Simultaneous determination of barbiturates, phenytoin and topiramate in hair by LC-MS/MS and application to real samples. J. Pharmacol. Toxicol. Methods. 2020;106:106931. doi: 10.1016/j.vascn.2020.106931. [DOI] [PubMed] [Google Scholar]
- 120.Pleil J.D. Breath biomarkers in toxicology. Arch. Toxicol. 2016;90:2669–2682. doi: 10.1007/s00204-016-1817-5. [DOI] [PubMed] [Google Scholar]
- 121.Awchi M., Singh K.D., Dill P.E., Frey U., Datta A.N., Sinues P. Prediction of systemic free and total valproic acid by off-line analysis of exhaled breath in epileptic children and adolescents. J. Breath Res. 2023;17:046013. doi: 10.1088/1752-7163/acf782. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J., Zhang Y., Xu C., Huang Z., Hu B. Detection of abused drugs in human exhaled breath using mass spectrometry: A review. Rapid Commun. Mass Spectrom. 2023;37:e9503. doi: 10.1002/rcm.9503. [DOI] [PubMed] [Google Scholar]
- 123.Seyfinejad B., Meshkini A., Habibolahi P., Ozkan S.A., Jouyban A. Determination of phenytoin in exhaled breath condensate using electromembrane extraction followed by capillary electrophoresis. Electrophoresis. 2020;41:666–677. doi: 10.1002/elps.201900440. [DOI] [PubMed] [Google Scholar]
- 124.Hale T.W., Krutsch K. Hale’s Medications & Mothers’ Milk 2021: A Manual of Lactational Pharmacology. 19th ed. Springer Publishing Company; New York, NY, USA: 2021. [Google Scholar]
- 125.Dinavitser N., Kohn E., Berlin M., Brandriss N., Bar-Chaim A., Keidar R., Livne A., Stepensky D., Berkovitch M., Sheinberg R. Levetiracetam in lactation: How much is excreted into human breast milk? Br. J. Clin. Pharmacol. 2022;88:199–205. doi: 10.1111/bcp.14940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This systematic search was performed via the databases Web of Science and SCOPUS.