Abstract
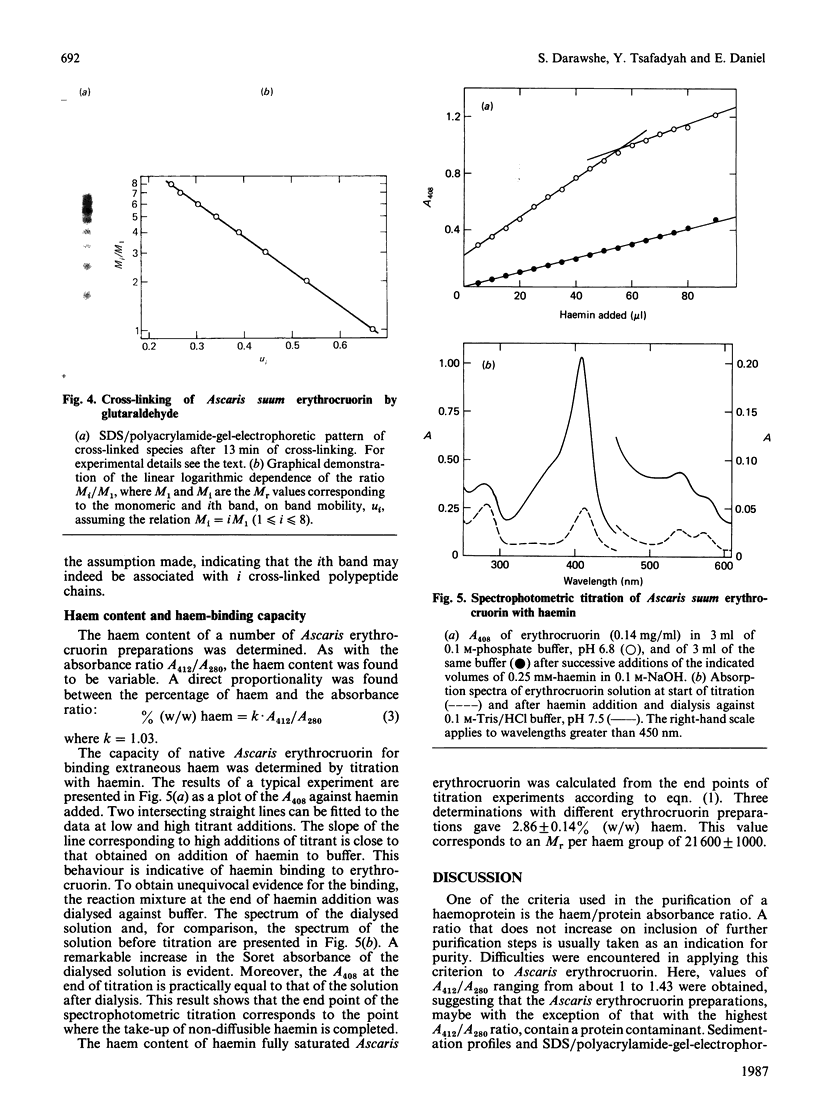
The quaternary structure of erythrocruorin from the nematode Ascaris suum was studied. The native protein had a sedimentation coefficient, at a protein concentration of 1 mg/ml, of 11.6 +/- 0.3 S and an Mr, as determined by sedimentation equilibrium, of 332,000 +/- 17,000. SDS/polyacrylamide-gel electrophoresis gave one band with a mobility corresponding to an Mr of 43,000 +/- 2000. The Mr of the polypeptide chain was determined to be 41,600 +/- 1,500 by sedimentation equilibrium in 6 M-guanidinium chloride and 0.1 M-2-mercaptoethanol. Cross-linking with glutaraldehyde followed by SDS/polyacrylamide-gel electrophoresis yielded a maximal number of eight bands. The haem content of Ascaris erythrocruorin was observed to vary from one preparation to another. This finding was shown to be due to non-realization of the full binding capacity for haem. By titration with haemin, the haem content was found to attain a maximal value of 2.86 +/- 0.14%, corresponding to a minimal Mr per haem group of 21,000 +/- 1,000. Our findings indicate that Ascaris suum erythrocruorin is composed of eight identical polypeptide chains, carrying two haem sites each.
Full text
PDF
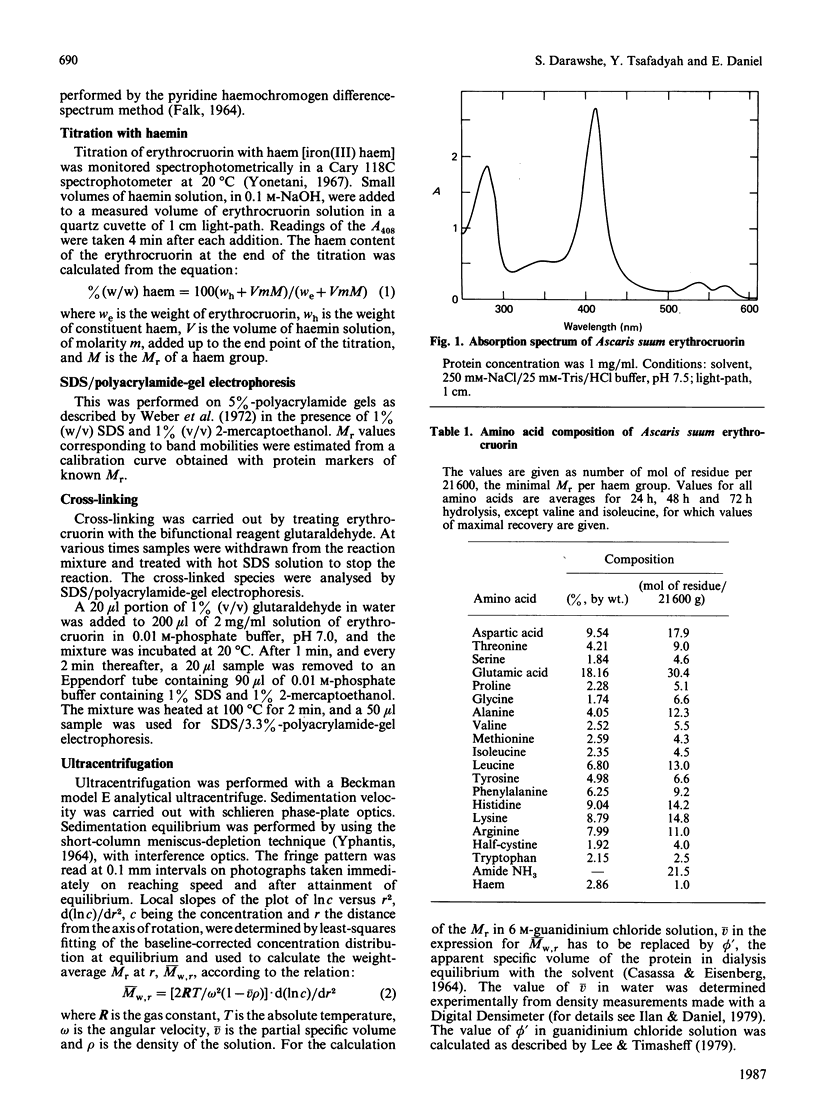
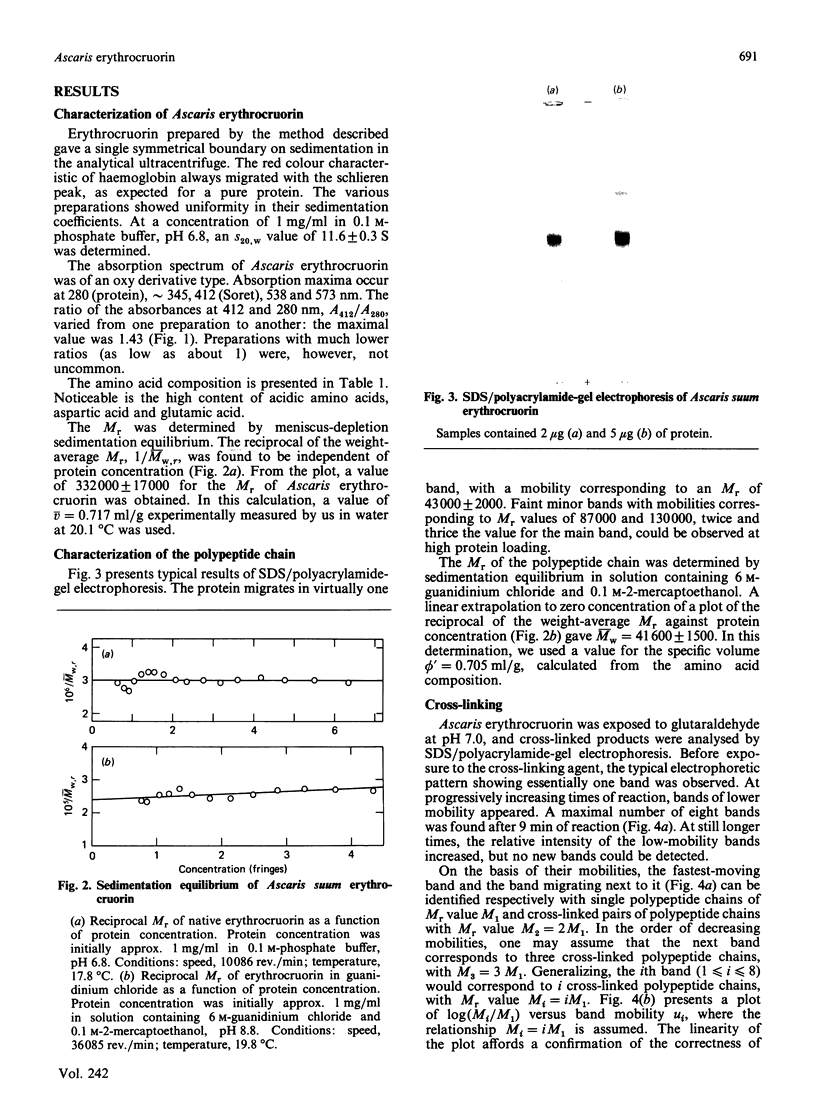


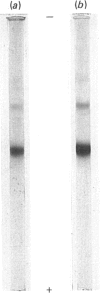
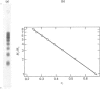
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASASSA E. F., EISENBERG H. THERMODYNAMIC ANALYSIS OF MULTICOMPONENT SOLUTIONS. Adv Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- Chiancone E., Rossi Fanelli M. R., Ascoli F., Vecchini P., Antonini E. Studies on erythrocruorin. VII. Reconstitution of earthworm erythrocruorin from the apoprotein. Biochim Biophys Acta. 1978 Jul 21;535(1):150–159. doi: 10.1016/0005-2795(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Chung M. C., Ellerton H. D. The physico-chemical and functional properties of extracellular respiratory haemoglobins and chlorocruorins. Prog Biophys Mol Biol. 1979;35(2):53–102. doi: 10.1016/0079-6107(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Dangott L. J., Terwilliger R. C. Structural studies of a branchiopod crustacean (Lepidurus bilobatus) extracellular hemoglobin. Evidence for oxygen-binding domains. Biochim Biophys Acta. 1979 Aug 28;579(2):452–461. doi: 10.1016/0005-2795(79)90072-2. [DOI] [PubMed] [Google Scholar]
- HAMADA K., OKAZAKI T., SHUKUYA R., KAZIRO K. Hemoglobins from Ascaris lumbricoides. III. A hemoglobin from perientric fluid. J Biochem. 1963 Jun;53:484–488. doi: 10.1093/oxfordjournals.jbchem.a127727. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Ilan E., Daniel E. Haemoglobin from the tadpole shrimp, Lepidurus apus lubbocki Characterization of the molecule and determination of the number of polypeptide chains. Biochem J. 1979 Nov 1;183(2):325–330. doi: 10.1042/bj1830325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan E., David M. M., Daniel E. Erythrocruorin from the crustacean Caenestheria inopinata. Quaternary structure and arrangement of subunits. Biochemistry. 1981 Oct 13;20(21):6190–6194. doi: 10.1021/bi00524a043. [DOI] [PubMed] [Google Scholar]
- Ilan E., Weisselberg E., Daniel E. Erythrocruorin from the water-flea Daphnia magna. Quaternary structure and arrangement of subunits. Biochem J. 1982 Nov 1;207(2):297–303. doi: 10.1042/bj2070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE D. L., SMITH M. H. HEMOGLOBINS OF PARASITIC ANIMALS. Exp Parasitol. 1965 Jun;16:392–424. doi: 10.1016/0014-4894(65)90062-7. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The calculation of partial specific volumes of proteins in 6 M guanidine hydrochloride. Methods Enzymol. 1979;61:49–57. doi: 10.1016/0076-6879(79)61006-6. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Briehl R. W., Wittenberg J. B., Wittenberg B. A. The hemoglobin of Ascaris perienteric fluid. II. Molecular weight and subunits. Biochim Biophys Acta. 1965 Dec 16;111(2):496–502. doi: 10.1016/0304-4165(65)90059-0. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Wittenberg J. B. The hemoglobin of Ascaris perienteric fluid. 3. Equilibria with oxygen and carbon monoxide. Biochim Biophys Acta. 1965 Dec 16;111(2):503–511. doi: 10.1016/0304-4165(65)90060-7. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Terwilliger N. B., Terwilliger R. C., Schabtach E. The quaternary structure of a molluscan (Helisoma trivolvis) extracellular hemoglobin. Biochim Biophys Acta. 1976 Nov 26;453(1):101–110. doi: 10.1016/0005-2795(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Verzili D., Santucci R., Ikeda-Saito M., Chiancone E., Ascoli F., Yonetani T., Antonini E. Studies on Scapharca hemoglobins. Properties of the dimeric protein reconstituted with Fe- or Co-porphyrin. Biochim Biophys Acta. 1982 Jun 4;704(2):215–220. doi: 10.1016/0167-4838(82)90148-0. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N. The structure of invertebrate extracellular hemoglobins (erythrocruorins and chlorocruorins). Comp Biochem Physiol B. 1985;82(1):1–15. doi: 10.1016/0305-0491(85)90120-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Okazaki T., Wittenberg J. B. The hemoglobin of Ascaris perienteric fluid. I. Purification and spectra. Biochim Biophys Acta. 1965 Dec 16;111(2):485–495. doi: 10.1016/0304-4165(65)90058-9. [DOI] [PubMed] [Google Scholar]
- Wood E. J. The oxygen transport and storage proteins of invertebrates. Essays Biochem. 1980;16:1–47. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. X. Crystalline apo-and reconstituted holoenzymes. J Biol Chem. 1967 Nov 10;242(21):5008–5013. [PubMed] [Google Scholar]