Abstract
Heterologous prime/boost regimens are AIDS vaccine candidates because of their potential for inducing cellular immune responses. Here, we have developed a prime/boost regimen leading to rapid control of highly pathogenic immunodeficiency virus infection in macaques. The strategy, priming by an env and nef deletion-containing simian-human immunodeficiency virus (SHIV) proviral DNA followed by a single booster with a Gag-expressing Sendai virus (SeV-Gag), efficiently induced virus-specific T cells, which were maintained for more than 3 months until challenge. While all naive control macaques showed acute CD4+ T-cell depletion at week 2 after an intravenous SHIV89.6PD challenge, all the macaques vaccinated with the prime/boost regimen were protected from depletion and showed greatly reduced peak viral loads compared with controls. Vaccination with the DNA alone or SeV-Gag alone was not enough to confer the consistent protection from the depletion, although it led to efficient secondary CD8+ T-cell responses at week 2 after challenge. At week 1, a difference in the secondary responses between the protected and the unprotected macaques was clear; rapid augmentation of virus-specific CD8+ T cells was detected in the former but not in the latter. Thus, our results indicate the importance of rapid secondary responses for reduction in the peak viral loads and protection from acute CD4+ T-cell depletion.
Cellular immune responses play a critical role in the control of immunodeficiency virus infections (8, 25). The importance of CD8+ T cells in this control has been indicated in human immunodeficiency virus type 1-infected individuals (7, 15, 22) and in macaque AIDS models (11, 20, 24). Also, virus-specific CD4+ T-cell responses have been shown to be essential for effective cytotoxic-T-lymphocyte responses and for controlling virus infections (3, 18, 23). Therefore, a strategy inducing virus-specific T-cell responses efficiently can be a promising AIDS vaccine candidate.
For efficient induction of the responses, we previously developed a DNA vaccine system (19) using FMSIV, which is a chimeric simian-human immunodeficiency virus (SHIV) with ecotropic Friend murine leukemia virus (FMLV) env in place of SHIV env, in combination with the FMLV receptor, mCAT1 (1), which is not normally expressed in primate cells. Vaccination of macaques with both the FMSIV proviral DNA and mCAT1 expression plasmid DNA allowed mCAT1-dependent FMSIV replication and induced resistance against intravenous challenge with a pathogenic strain of simian immunodeficiency virus, SIVmac239; the macaques vaccinated with FMSIV DNA plus mCAT1 DNA showed reduced viral loads at both the peak and the set point compared with controls (19). Further, we established an efficient antigen expression system using Sendai virus (SeV), which is a nonsegmented negative-strand RNA virus considered nonpathogenic for humans and nonhuman primates (13, 14, 21). Intranasal immunization of macaques with a recombinant SeV vector expressing SIV Gag (SeV-Gag) elicited resistance against intravenous SIVmac239 challenge, leading to marked reduction in the set-point plasma viral loads to below or just above the detectable level, although the primary acute viremia was not controlled (12). In this study, we combined these two systems to develop a prime/boost vaccine strategy and evaluated its protective efficacy in a macaque AIDS model using a highly pathogenic immunodeficiency virus. We used 14 rhesus macaques (Macaca mulatta) divided into four groups for the evaluation (Table 1).
TABLE 1.
Vaccination and challenge protocol in macaques
| Group (vaccination) and animala | DNA vaccinationb | SeV vaccination (wk)c | SHIV challenge (wk) |
|---|---|---|---|
| I (Naive) | |||
| R009 | SHIV89.6PD | ||
| R004 | pCMVmCAT1 | SeV control (12) | SHIV89.6PD (26) |
| R010 | pCMVmCAT1 | SeV control (12) | SHIV89.6PD (26) |
| R014 | SeV control (0) | SHIV89.6PD (14) | |
| II (DNA alone) | |||
| R002 | FMSIV + pCMVmCAT1 | SeV control (12) | SHIV89.6PD (26) |
| R021 | FMSIV + pCMVmCAT1 | SHIV89.6PD (14) | |
| R022 | FMSIV + pCMVmCAT1 | SHIV89.6PD (14) | |
| III (SeV-Gag alone) | |||
| R013 | SeV-Gag (0) | SHIV89.6PD (14) | |
| R015 | SeV-Gag (0) | SHIV89.6PD (14) | |
| R017 | SeV-Gag (0) | SHIV89.6PD (14) | |
| IV (DNA + SeV-Gag) | |||
| R007 | FMSIV + pCMVN | SeV-Gag (12) | SHIV89.6PD (26) |
| R011 | FMSIV + pCMVN | SeV-Gag (12) | SHIV89.6PD (26) |
| R005 | FMSIV + pCMVmCAT1 | SeV-Gag (12) | SHIV89.6PD (26) |
| R012 | FMSIV + pCMVmCAT1 | SeV-Gag (12) | SHIV89.6PD (26) |
All the macaques were male and negative for SIV and simian retrovirus type D before use. They were maintained in accordance with the institutional guidelines for laboratory animals.
The FMSIV proviral DNA, a plasmid expressing the FMLV receptor (pCMVmCAT1), and a control plasmid (pCMVN) were constructed as described previously (19). At each DNA vaccination, animals received 800 μg of individual DNA intramuscularly and 10 μg of individual DNA by gene gun as described previously (19). DNA vaccinations were performed at weeks 0, 0.5, 1, and 6 after the initial vaccination.
The frequency of specific T cells measured by flow-cytometric analysis of intracellular interferon-γ (IFN-γ) induction is regarded as an index of antigen-specific cellular immune responses, although it does not always correlate with antigen-specific cytolytic activity (5, 9, 10, 16). Then, we examined the frequencies of the T cells reactive to the SHIV antigens other than Env and Nef, which are expected to be induced by FMSIV DNA–SeV-Gag vaccinations. In brief, an SHIV proviral DNA with env and nef deleted, SIVGP1 DNA, was obtained by removing the whole FMLV env region from the FMSIV DNA. COS-1 cells were cotransfected with SIVGP1 DNA and a plasmid DNA expressing vesicular stomatitis virus G protein (pVSV-G; Clontech, Palo Alto, Calif.) to obtain a pseudotyped SHIV bearing VSV-G, SIVGP1(VSV-G). For the SHIV-specific stimulation, 106 peripheral blood mononuclear cells (PBMC) were cocultured with 105 herpesvirus papio-immortalized B-lymphoblastoid cells (26) infected with SIVGP1(VSV-G) for 6 h (in the presence of GolgiStop [monensin; Pharmingen, San Diego, Calif.] for the last 5 h). For nonspecific stimulation, a VSV-G-pseudotyped MLV, MLVGP(VSV-G), was used instead of SIVGP1(VSV-G). Then, intracellular IFN-γ staining was performed with a Cytofix-Cytoperm kit (Pharmingen) according to the manufacturer's protocol. Fluorescein isothiocyanate-conjugated anti-human CD4 (Pharmingen), peridinin chlorophyll protein-conjugated anti-human CD8 (Becton Dickinson, San Jose, Calif.), allophycocyanin-conjugated anti-human CD3 (Pharmingen), and phycoerythrin-conjugated anti-human IFN-γ (Pharmingen) antibodies were used. Stained samples were collected by FACScalibur and analyzed using CellQuest software (Becton Dickinson). Gating was performed on mononuclear cells and then on CD3+ CD4+ or CD3+CD8+ subpopulations. From the ratio of CD3+ CD4+ IFN-γ+ or CD3+ CD8+ IFN-γ+ cells to mononuclear cells, the frequency of CD4+ IFN-γ+ or CD8+ IFN-γ+ T cells per 106 cells was calculated. Then, the frequency of SHIV-specific IFN-γ+ cells was calculated by subtracting the frequency after the nonspecific stimulation from that after the SHIV-specific stimulation.
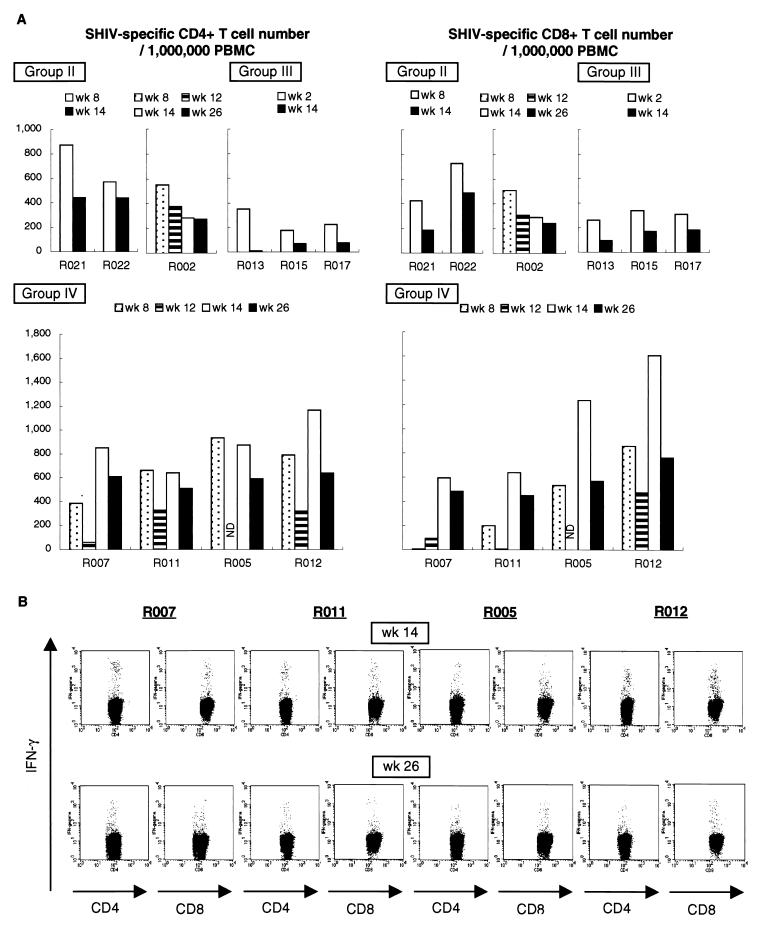
The frequencies of specific T cells in macaque PBMC after vaccination were examined by flow-cytometric analysis (Fig. 1A). None of the group I macaques showed SHIV-specific IFN-γ induction before SHIV challenge (data not shown). In all the group II macaques vaccinated with the DNA alone, SHIV-specific T cells were clearly induced and remained detectable until challenge. In all the group III macaques vaccinated with SeV-Gag alone, significant levels of specific T cells were seen at week 2 following vaccination, but their numbers declined to marginal levels before challenge. Four animals in group IV were given SeV-Gag boosters at week 12. Before the SeV-Gag booster, two (R007 and R011) received only FMSIV DNA (with control DNA), while the other two (R005 and R012) were vaccinated with FMSIV DNA plus mCAT1 DNA. In the former two, SHIV-specific T cells were detected faintly before the SeV-Gag booster, but only a single SeV-Gag vaccination resulted in efficient induction of SHIV-specific T cells. In the latter two, the DNA vaccination induced SHIV-specific T cells efficiently and the SeV-Gag booster led to more vigorous responses. Thus, SHIV-specific T cells expanded efficiently after a single SeV-Gag booster and were kept at high levels for more than 3 months until challenge in all the group IV macaques (Fig. 1B).
FIG. 1.
SHIV-specific T-cell frequencies after vaccination. (A) SHIV-specific CD4+ and CD8+ T-cell frequencies in PBMC obtained at the indicated time points after the initial vaccination in group II, III, and IV macaques. In all macaques, SHIV-specific IFN-γ induction was undetectable before the initial vaccination. ND, not determined. (B) Dot plots showing SHIV-specific IFN-γ induction in group IV macaques after a SeV-Gag booster (2 weeks after the booster [wk 14] and just before challenge [wk 26]). CD4, SHIV-specific CD4+ T cells, gated on CD3+ CD4+; CD8, SHIV-specific CD8+ T cells, gated on CD3+ CD8+.
For the challenge, we used a highly pathogenic SHIV89.6PD virus stock provided by Y. Lu (17). Before the present vaccine study, we had confirmed that its intravenous inoculation at the dose of 10 50% tissue culture infective doses caused almost complete depletion of peripheral CD4+ T cells within 2 weeks in rhesus macaques. Then, all 14 macaques (Table 1) were challenged intravenously with 10 50% tissue culture infective doses of SHIV89.6PD. We challenged macaques in group IV with SHIV89.6PD more than 3 months after the SeV-Gag booster to examine its long-term efficacy, although intravenous challenge was performed no more than 6 weeks after the last vaccination in many previous studies.
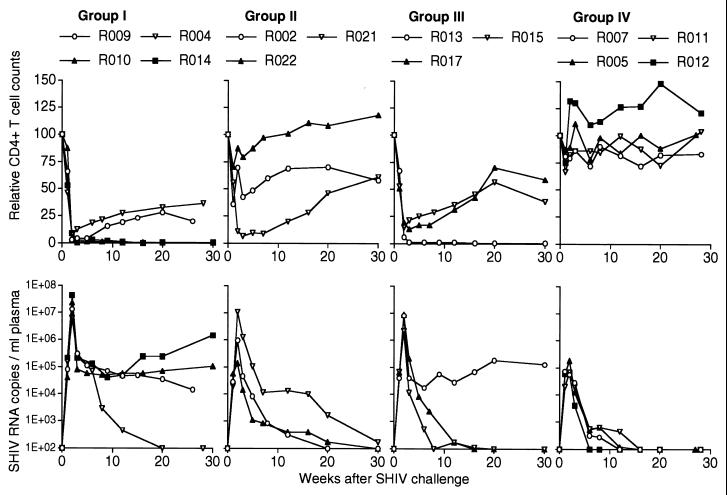
All the macaques in groups I and III showed almost complete depletion of peripheral CD4+ T cells at week 2 after challenge (Fig. 2). In group II, one animal (R022) was protected completely from depletion and a second (R002) was partially protected, but a third (R021) showed depletion. In marked contrast to these, all four macaques in group IV were completely protected from CD4+ T-cell depletion (Fig. 2 and Table 2).
FIG. 2.
Protection after SHIV89.6PD challenge. (Top) For each animal, the CD4 counts relative to that at challenge (set at 100) are shown. (Bottom) Changes in plasma SHIV RNA copy number were quantified as described previously (19).
TABLE 2.
Mean CD4 counts and viral loads at week 2 after challenge
| Group | Relative mean CD4 counta | Plasma viral loadb (geometric mean) |
|---|---|---|
| I | 5.1 | 1.67 × 107 |
| II | 56.1 | 1.10 × 106 |
| III | 13.0 | 5.33 × 106 |
| IV | 95.4 | 8.30 × 104 |
See Fig. 2, top. The CD4 counts in group IV were significantly higher than those in group I and those in group III (P = 0.0003 and 0.0026 by t test using StatView software, respectively).
See Fig. 2, bottom. The plasma SHIV RNA copy numbers in group IV were significantly lower than those in group I and those in group III (P < 0.0001 and P = 0.0004, respectively).
All the group IV macaques showed greatly reduced plasma SHIV loads compared with those in group I (Fig. 2). The reduction at the peak (week 2) was striking (Table 2), and viremia was undetectable at the set point in group IV. In group II, the peak viral load was greatly reduced in R022 protected from the CD4 depletion, while no reduction was observed in the unprotected R021. The peak level in the partially protected R002 was between the other two. In group III, no reduction in the peak viral loads was observed, but the set-point viral loads declined to undetectable levels in two animals.
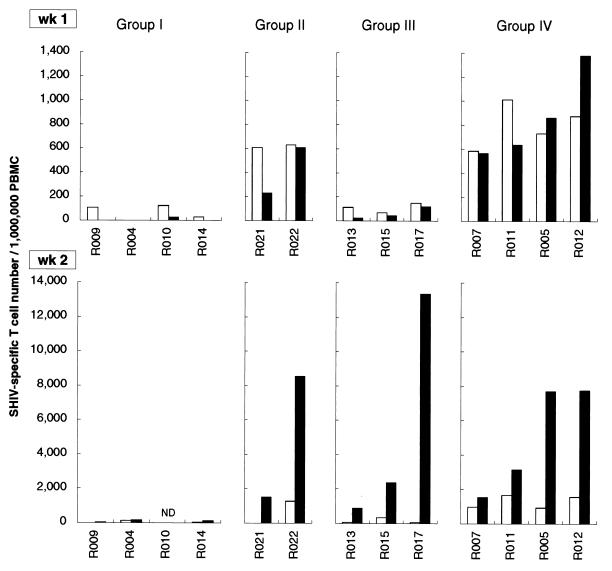
We examined frequencies of SHIV-specific T cells in PBMC at weeks 1 and 2 after challenge (Fig. 3). Augmented SHIV-specific CD4+ and CD8+ T cells appeared at week 1 in all the group IV macaques, while no significant secondary responses were observed at week 1 or 2 in the group I macaques. In group II, the protected macaque R022 showed augmented SHIV-specific CD4+ and CD8+ T cells at week 1. In contrast, the unprotected macaque R021 showed significant secondary responses of SHIV-specific CD4+ T cells at week 1, but SHIV-specific CD8+ T cell responses were delayed. Thus, poor protection in R021 can be explained by insufficient augmentation of SHIV-specific CD8+ T cells at week 1. The group III macaques showed no significant secondary responses at week 1, but efficient augmentation of SHIV-specific CD8+ T cells appeared at week 2.
FIG. 3.
SHIV-specific T-cell frequencies after SHIV89.6PD challenge. SHIV-specific CD4+ (open bars) and CD8+ (closed bars) T-cell frequencies in macaque PBMC at 1 and 2 weeks after challenge are shown. The frequencies in R002 were not determined because we failed to obtain enough samples from the animal. ND, not determined.
AIDS vaccine strategies have been evaluated in macaque models using pathogenic SIV or SHIV (2). While most macaques develop AIDS a few years after SIV challenge, the pathogenic SHIV model shows acute CD4 depletion a few weeks after challenge. In the latter model, this study showed excellent protection by our env-independent prime/boost vaccination against acute CD4 depletion. Interestingly, a single protein, Gag, was sufficient as the booster antigen for protection. The macaques vaccinated with the prime/boost regimen showed rapid secondary responses of virus-specific T cells after challenge.
At week 1 after challenge, augmented SHIV-specific CD8+ T cells were observed in all the protected macaques but not in any of the unprotected macaques. The group III macaques without SHIV-specific T-cell augmentation at week 1 were not protected from the CD4+ T-cell depletion at week 2, although they showed efficient secondary CD8+ T-cell responses at week 2. The secondary responses may lead to reduction in the set-point viral loads, as has been indicated (4, 6), but the appearance of SHIV-specific CD8+ T cells at week 2 would be too late for a reduction in peak viral loads and protection from acute CD4 depletion. Thus, the turning point determining whether macaques can be protected from acute CD4 depletion is within a week after challenge. This is consistent with a previous study showing that the effects of CD8 depletion on viral loads are greater in macaques treated with anti-CD8 antibody just before SHIV challenge than in those treated at week 1 after challenge (20). Taken together, our results indicate that rapid secondary responses of SHIV-specific T cells, particularly CD8+ T cells, were essential for the marked reduction in peak viral loads and the protection from acute CD4+ T-cell depletion after challenge.
In group IV, SHIV-specific T-cell induction was not efficient before SeV-Gag booster in two macaques (R007 and R011) primed with FMSIV DNA only, but they showed levels of protection similar to those in the other two (R005 and R012) primed with FMSIV plus mCAT1 DNA. In contrast, the group III macaques vaccinated with SeV-Gag alone were not protected from acute CD4+ T-cell depletion and showed delayed secondary responses. Thus, vaccination with FMSIV DNA (without mCAT1 DNA) may be required and sufficient as priming in our prime/boost regimen for the rapid secondary responses and the protection against acute CD4 depletion.
Recently, a DNA vaccine with cytokine augmentation succeeded in preventing SHIV89.6P-induced AIDS in macaques (6). Further, a DNA priming followed by a booster with a recombinant modified vaccinia virus Ankara expressing multiple proteins has been reported to control mucosal SHIV89.6P challenge (4). In vaccinated animals, however, the virus-specific T cells were undetectable in peripheral blood at week 1 after challenge, potentially reflecting the recruitment of specific T cells to the site of infection. The virus-specific secondary responses became detectable later, and the late responses correlated with set-point viral loads but not with peak viral loads. Although there were some differences between experimental conditions in those studies and in ours, the protected macaques in our study showed the fastest detectable secondary responses at week 1 after challenge. The rapid responses may explain the greater reduction in peak viral loads in our study. Thus, our prime/boost regimen showed rapid control of immunodeficiency virus infection, which could contribute to host immune function and help slow the virus epidemic.
Acknowledgments
We thank Y. Lu for providing SHIV89.6PD, Y. Ami, M. Honda, M. Nakasone, T. Sata, F. Ono, K. Komatsuzaki, K. Oto, K. Mori, R. Mukai, and A. Yamada for assistance in the animal experiments and A. Kato, M. Miyazawa, N. Watanabe, A. Iwamoto, and H. Yoshikura for helpful suggestions.
This work was supported by Health Sciences Research grants from the Ministry of Health, Labour and Welfare in Japan.
REFERENCES
- 1.Albritton L M, Tweng L, Scadden D, Cunningham J M. A putative murine retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Almond N M, Heeney J L. AIDS vaccine development in primate models. AIDS. 1998;12:S133–S140. [PubMed] [Google Scholar]
- 3.Altfeld M, Rosenberg E S. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr Opin Immunol. 2000;12:375–380. doi: 10.1016/s0952-7915(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 4.Amara R R, Villinger F, Altman J D, Lydy S L, O'Neil S P, Staprans S I, Montefiori D C, Xu Y, Herndon J G, Wyatt L S, Candido M A, Kozyr N L, Earl P L, Smith J M, Ma H-L, Grimm B D, Hulsey M L, Miller J, McClure H M, McNicholl J M, Moss B, Robinson H L. Control of a mucosal challenge and prevention of AIDS in rhesus macaques by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 5.Appay V, Nixon D F, Donahoe S M, Gillespie G M A, Dong T, King A, Ogg G S, Spiegel H M L, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T-M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M-E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn B H, Shaw G, M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brander C, Walker B D. T lymphocyte responses in HIV-1 infection: implication for vaccine development. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 9.Donahoe S M, Moretto W J, Samuel R V, Metzner K J, Marx P A, Hanke T, Connor R I, Nixon D F. Direct measurement of CD8+ T cell responses in macaques infected with simian immunodeficiency virus. Virology. 2000;272:347–356. doi: 10.1006/viro.2000.0404. [DOI] [PubMed] [Google Scholar]
- 10.Gea-Banacloche J C, Migueles S A, Martino L, Shupert W L, McNeil A C, Sabbaghian M S, Ehler L, Prussin C, Stevens R, Lambert L, Altman J, Hallahan C W, Lopez Bernaldo de Quiros J C, Connors M. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 11.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kano M, Matano T, Nakamura H, Takeda A, Kato A, Ariyoshi K, Mori K, Sata T, Nagai Y. Elicitation of protective immunity against simian immunodeficiency virus infection by a recombinant Sendai virus expressing the Gag protein. AIDS. 2000;14:1281–1282. doi: 10.1097/00002030-200006160-00030. [DOI] [PubMed] [Google Scholar]
- 13.Kato A, Kiyotani K, Sakai Y, Yoshida T, Nagai Y. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 1997;16:578–587. doi: 10.1093/emboj/16.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato A, Sakai Y, Shioda T, Kondo T, Nakanishi M, Nagai Y. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells. 1996;1:569–579. doi: 10.1046/j.1365-2443.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]
- 15.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavini A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Pauza C D, Lu X, Montefiori D C, Miller C J. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Maltoubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matano T, Kano M, Odawara T, Nakamura H, Takeda A, Mori K, Sata T, Nagai Y. Induction of protective immunity against pathogenic simian immunodeficiency virus by a foreign receptor-dependent replication of an engineered avirulent virus. Vaccine. 2000;18:3310–3318. doi: 10.1016/s0264-410x(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 20.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai Y. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev Med Virol. 1999;9:83–99. doi: 10.1002/(sici)1099-1654(199904/06)9:2<83::aid-rmv244>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 25.Seder R A, Hill A V S. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 26.Voss G, Nick S, Stahl-Hennig C, Ritter K, Hunsmann G. Generation of macaque B lymphoblastoid cell lines with simian Epstein-Barr-like viruses: transformation procedure, characterization of the cell lines and occurrence of simian foamy virus. J Virol Methods. 1992;39:185–195. doi: 10.1016/0166-0934(92)90137-3. [DOI] [PubMed] [Google Scholar]