Abstract
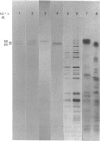
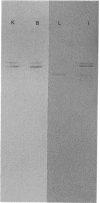
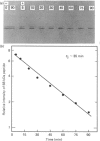
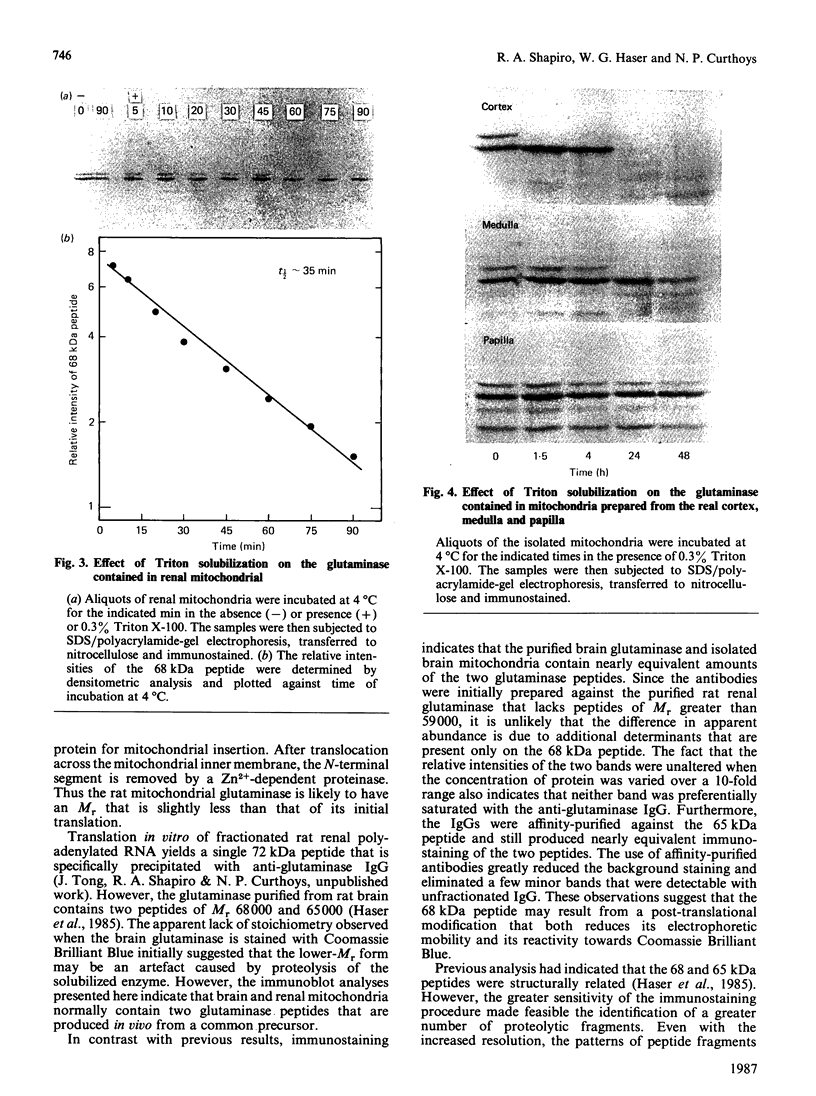
Antibodies were prepared against isolated rat renal glutaminase and affinity-purified against the 65 kDa peptide contained in the purified rat brain glutaminase. The affinity-purified IgGs were then used to compare the glutaminase immunoreactive peptides contained in samples that had been subjected to SDS/polyacrylamide-gel electrophoresis and transferred to nitrocellulose. The purified brain glutaminase and isolated brain mitochondria contain 68 and 65 kDa peptides that exhibit nearly equivalent immunostaining. Partial proteolysis of the isolated 68 and 65 kDa peptides with Staphylococcus aureus V8 proteinase produced an identical pattern of immunoreactive proteolytic fragments. However, digestion of the two peptides with chymotrypsin resulted in similar, but slightly different, patterns. The pattern of immunostaining was unaltered even when the brain mitochondria were solubilized with Triton X-100 and stored for 2 days at 4 degrees C. A very similar pattern was observed when intact renal mitochondria were subjected to immunoblot analysis. However, when renal mitochondria were solubilized, the 68 kDa peptide was rapidly degraded to the 65 kDa form. At 4 degrees C this reaction occurs with apparent first-order kinetics and a t1/2 of 35 min. Degradation of the 65 kDa form of the renal glutaminase occurs with much slower kinetics, but is nearly complete after 24 h. Solubilization of mitochondria isolated from various zones of the kidney indicated that the responsible endogenous proteinase was localized primarily in the cortex. Mitochondria isolated from intestinal or renal papillary tissue contain four glutaminase immunoreactive peptides (Mr 68,000, 65,000, 61,000 and 58,000). The smallest of these peptides is identical in size with the single immunoreactive peptide observed in liver tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Curthoys N. P., Kuhlenschmidt T., Godfrey S. S., Weiss R. F. Phosphate-dependent glutaminase from rat kidney. Cause of increased activity in response to acidosis and identity with glutaminase from other tissues. Arch Biochem Biophys. 1976 Jan;172(1):162–167. doi: 10.1016/0003-9861(76)90062-x. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Weiss R. F. Regulation of renal ammoniagenesis. Subcellular localization of rat kidney glutaminase isoenzymes. J Biol Chem. 1974 May 25;249(10):3261–3266. [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haser W. G., Shapiro R. A., Curthoys N. P. Comparison of the phosphate-dependent glutaminase obtained from rat brain and kidney. Biochem J. 1985 Jul 15;229(2):399–408. doi: 10.1042/bj2290399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular glutamine cycle during ureogenesis in perfused rat liver. Eur J Biochem. 1983 Jun 15;133(2):269–275. doi: 10.1111/j.1432-1033.1983.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z., McGivan J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983 Apr;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Pozefsky T., Most A. S., Cahill G. F., Jr Muscle and splanchnic glutmine and glutamate metabolism in postabsorptive andstarved man. J Clin Invest. 1971 Apr;50(4):814–817. doi: 10.1172/JCI106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., McGivan J. D. Partial purification and properties of rat liver glutaminase. Biochem J. 1984 Jun 1;220(2):583–590. doi: 10.1042/bj2200583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phromphetcharat V., Jackson A., Dass P. D., Welbourne T. C. Ammonia partitioning between glutamine and urea: interorgan participation in metabolic acidosis. Kidney Int. 1981 Nov;20(5):598–605. doi: 10.1038/ki.1981.182. [DOI] [PubMed] [Google Scholar]
- Regulation of renal ammoniagenesis. Purification and characterization of phosphate-dependent glutaminase from rat kidney. Arch Biochem Biophys. 1976 May;174(1):82–89. [PubMed] [Google Scholar]
- Schröck H., Goldstein L. Interorgan relationships for glutamine metabolism in normal and acidotic rats. Am J Physiol. 1981 May;240(5):E519–E525. doi: 10.1152/ajpendo.1981.240.5.E519. [DOI] [PubMed] [Google Scholar]
- Shapiro R. A., Haser W. G., Curthoys N. P. The orientation of phosphate-dependent glutaminase on the inner membrane of rat renal mitochondria. Arch Biochem Biophys. 1985 Nov 15;243(1):1–7. doi: 10.1016/0003-9861(85)90767-2. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Sastrasinh S. Response of ammonia metabolism to acute acidosis. Kidney Int. 1984 Jan;25(1):1–10. doi: 10.1038/ki.1984.1. [DOI] [PubMed] [Google Scholar]
- Tong J., Harrison G., Curthoys N. P. The effect of metabolic acidosis on the synthesis and turnover of rat renal phosphate-dependent glutaminase. Biochem J. 1986 Jan 1;233(1):139–144. doi: 10.1042/bj2330139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDMAN R. H., BURCH H. B. Rapid method for study of enzyme distribution in rat kidney. Am J Physiol. 1963 May;204:749–752. doi: 10.1152/ajplegacy.1963.204.5.749. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980 Jan 10;255(1):107–112. [PubMed] [Google Scholar]