Abstract
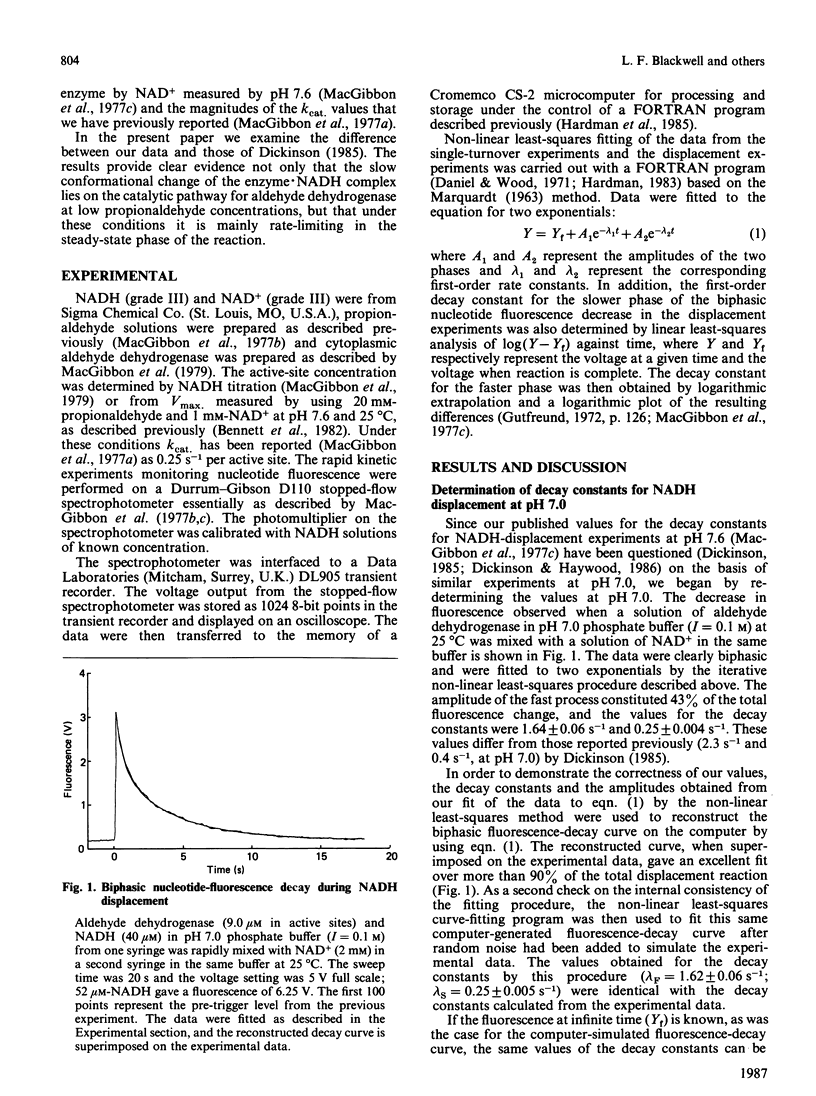
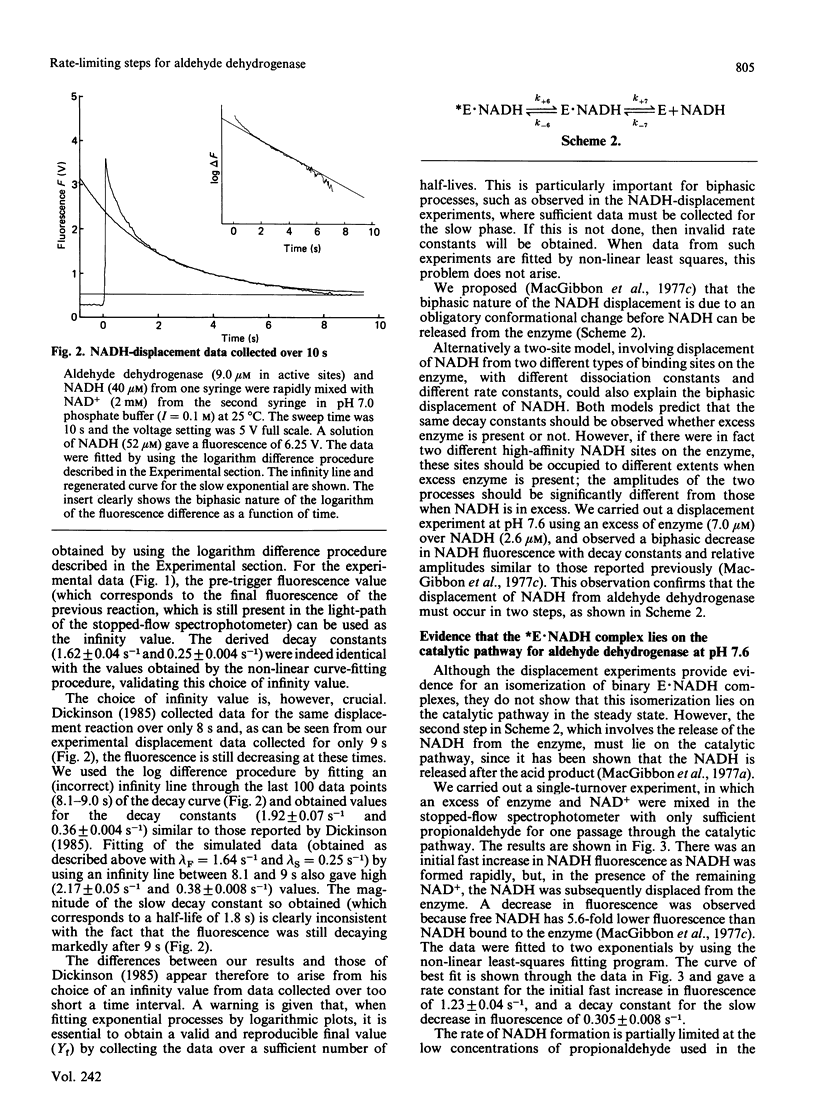
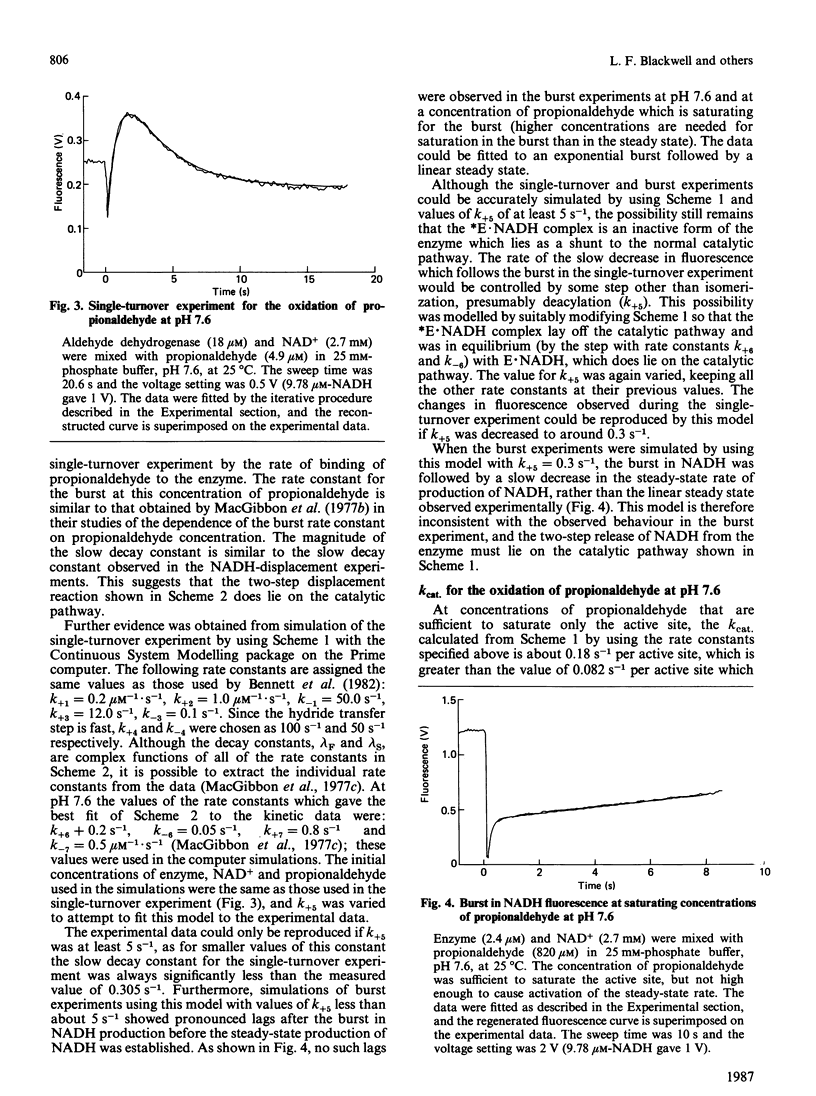
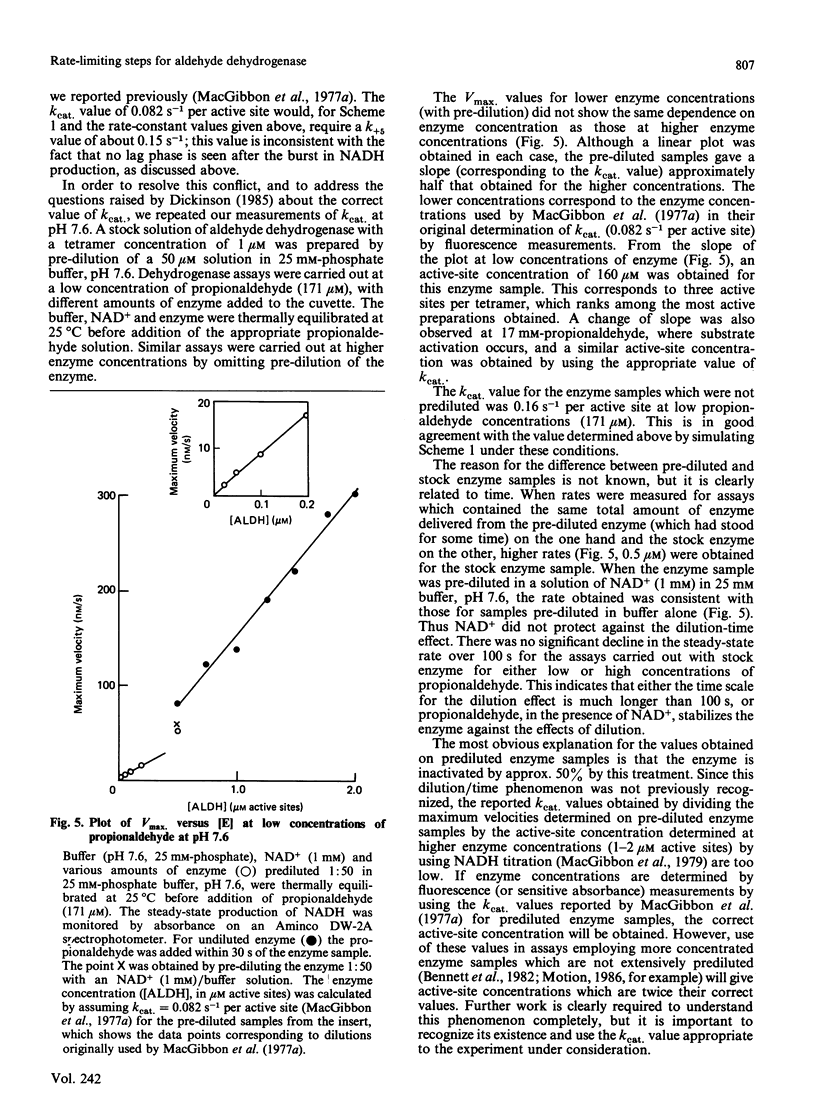
The displacement of NADH from the aldehyde dehydrogenase X NADH complex by NAD+ was followed at pH 7.0, and the data were fitted by a non-linear least-squares iterative procedure. At pH 7.0 the decay constants for the dissociation of NADH from aldehyde dehydrogenase X NADH complexes (1.62 +/- 0.09 s-1 and 0.25 +/- 0.004 s-1) were similar to the values previously determined by MacGibbon, Buckley & Blackwell [(1977) Biochem. J. 165, 455-462] at pH 7.6, and apparent differences between these values and those reported by Dickinson [(1985) Biochem. J. 225, 159-165] are resolved. Experiments at low concentrations of propionaldehyde show that isomerization of a binary E X NADH complex is part of the normal catalytic mechanism of the enzyme. Evidence is presented that the active-site concentration of aldehyde dehydrogenase is halved when enzyme is pre-diluted to low concentrations before addition of NAD+ and substrate. The consequences of this for the reported values of kcat. are discussed. A general mechanism for the aldehyde dehydrogenase-catalysed oxidation of propionaldehyde which accounts for the published kinetic data, at concentrations of aldehyde which bind only at the active site, is presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. F., Buckley P. D., Blackwell L. F. Proton release during the pre-steady-state oxidation of aldehydes by aldehyde dehydrogenase. Evidence for a rate-limiting conformational change. Biochemistry. 1982 Aug 31;21(18):4407–4413. doi: 10.1021/bi00261a033. [DOI] [PubMed] [Google Scholar]
- Buckley P. D., Dunn M. F. Observation of acyl-enzyme intermediates in the sheep liver aldehyde dehydrogenase catalytic mechanism via rapid-scanning UV-visible spectroscopy. Prog Clin Biol Res. 1982;114:23–35. [PubMed] [Google Scholar]
- Dickinson F. M., Haywood G. W. The effects of Mg2+ on certain steps in the mechanisms of the dehydrogenase and esterase reactions catalysed by sheep liver aldehyde dehydrogenase. Support for the view that dehydrogenase and esterase activities occur at the same site on the enzyme. Biochem J. 1986 Feb 1;233(3):877–883. doi: 10.1042/bj2330877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Studies on the mechanism of sheep liver cytosolic aldehyde dehydrogenase. Biochem J. 1985 Jan 1;225(1):159–165. doi: 10.1042/bj2250159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckfeldt J. H., Yonetani T. Kinetics and mechanism of the F1 isozyme of horse liver aldehyde dehydrogenase. Arch Biochem Biophys. 1976 Mar;173(1):273–281. doi: 10.1016/0003-9861(76)90260-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Iniguez L., Powers L., Chance B., Sellin S., Mannervik B., Mildvan A. S. X-ray absorption studies of the Zn2+ site of glyoxalase I. Biochemistry. 1984 Feb 14;23(4):685–689. doi: 10.1021/bi00299a016. [DOI] [PubMed] [Google Scholar]
- Hardman M. J., Crow V. L., Cruickshank D. S., Pritchard G. G. Kinetics of activation of L-lactate dehydrogenase from Streptococcus lactis by fructose 1,6-bisphosphate. Eur J Biochem. 1985 Jan 2;146(1):179–183. doi: 10.1111/j.1432-1033.1985.tb08636.x. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Kinetic properties of highly purified preparations of sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1982 Jun 1;203(3):617–627. doi: 10.1042/bj2030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Partial reversal of the acetaldehyde and butyraldehyde oxidation reactions catalysed by aldehyde dehydrogenases from sheep liver. Biochem J. 1978 Nov 1;175(2):753–756. doi: 10.1042/bj1750753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Blackwell L. F., Buckley P. D. Kinetics of sheep-liver cytoplasmic aldehyde dehydrogenase. Eur J Biochem. 1977 Jul 1;77(1):93–100. doi: 10.1111/j.1432-1033.1977.tb11645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Blackwell L. F., Buckley P. D. Pre-steady-state kinetic studies on cytoplasmic sheep liver aldehyde dehydrogenase. Biochem J. 1977 Nov 1;167(2):469–477. doi: 10.1042/bj1670469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Buckley P. D., Blackwell L. F. Evidence for two-step binding of reduced nicotinamide-adenine dinucleotide to aldehyde dehydrogenase. Biochem J. 1977 Sep 1;165(3):455–462. doi: 10.1042/bj1650455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Motion R. L., Crow K. E., Buckley P. D., Blackwell L. F. Purification and properties of sheep-liver aldehyde dehydrogenases. Eur J Biochem. 1979 Jun 1;96(3):585–595. doi: 10.1111/j.1432-1033.1979.tb13073.x. [DOI] [PubMed] [Google Scholar]


