Abstract
Glioblastoma (GBM) is the most malignant brain tumor frequently characterized by a hypoxic microenvironment. In this investigation, we unveiled unprecedented role of Ribonuclease 4 (RNASE4) in GBM pathogenesis through integrative methodologies. Leveraging The Cancer Genome Atlas (TCGA) dataset and clinical specimens from normal brain tissues, low- and high-grade gliomas, alongside rigorous in vitro and in vivo functional analyses, we identified a consistent upregulation of RNASE4 correlating with advanced GBM pathological stages and poor clinical survival outcomes. Functional assays corroborated the pivotal influences of RNASE4 on key tumorigenic processes such as cell proliferation, migration, invasion, stemness properties and temozolomide (TMZ) resistance. Further, Gene Set Enrichment Analysis (GSEA) illuminated the involvement of RNASE4 in modulating epithelial-mesenchymal transition (EMT) via activation of AXL, AKT and NF-κB signaling pathways. Furthermore, recombinant human RNASE4 (hRNASE4)-mediated NF-κB activation through IκBα phosphorylation and degradation could result in the upregulation of inhibitors of apoptosis proteins (IAPs), such as cIAP1, cIAP2, and SURVIVIN. Notably, treating RNASE4-induced TMZ-resistant cells with the SURVIVIN inhibitor YM-155 significantly restored cellular sensitivity to TMZ therapy. Herein, this study positions RNASE4 as a potent prognostic biomarker and therapeutic target, offering new insights into molecular pathogenesis of GBM and new avenues for future therapeutic interventions.
Keywords: RNASE4, hypoxia, GBM, AXL, NF-κB, cIAP1, cIAP2, SURVIVIN
Introduction
Glioblastoma (GBM) represents the epitome of malignancy among primary adult cerebral neoplasm, typified by a median survival of 15 months post-diagnosis, notwithstanding aggressive therapeutic regimens [1]. Therapeutic options for GBM remain woefully inadequate, further compounded by the paucity of prognostic biomarkers as well as druggable molecular targets [2,3]. Elucidating pivotal molecular determinants underlying the pathogenesis and progression of GBM is thus of great significance, with the potential to substantially enhance clinical outcomes for patients [4,5]. Ribonuclease 4 (RNASE4) is one of the 8 members of RNase A family implicated in the extracellular degradation of RNA [6]. Despite an early research documents correlation of RNASE4 upregulation to plasticity and astrocytic differentiation in Sox2-depleted GBM cells [7], association of RNASE4 with cancer progression has currently been limited to prostate cancer [8]. RNase A family members other than RNASE4, nonetheless, have been implicated in promoting tumorigenesis in several neoplasms.
RNASE1, for instance, is overexpressed and contributes to cellular proliferation and stemness of breast and pancreatic cancers [9,10]. In addition, up-regulation of RNASE2 enhances PI3K/AKT signaling to drive tumor proliferation and metastasis in GBM [11], while RNASE3 overexpression is correlated with augmented tumor burden and advanced pathological stages in head and neck squamous cell carcinoma [12]. Further, RNASE5, also recognized as angiogenin (ANG), is implicated in epithelial-mesenchymal-transition (EMT) induction via epidermal growth factor receptor (EGFR) interaction to facilitate the progression of prostate [13,14]. Recently, RNASE4 is reported to share promoter with ANG [15] and is co-expressed with ANG to protect hypothermia-induced neurodegeneration [16]. Nevertheless, oncogenic role of RNASE4 still remains largely elusive except for its ability to stimulate AKT and S6 phosphorylation in prostate cancer cells [8] that suggests that RNASE4 may contribute to oncogenic processes by enhancing cancer growth and survival.
In present study, we unprecedentedly uncovered the functional association between RNASE4 and GBM clinicopathogenesis, by elucidating influences of RNASE4 on oncogenic and stemness attributes including regulation of cell signaling networks composed of AXL/AKT and NF-κB/cIAP2/SURVIVIN under hypoxia using various in vitro and in vivo analyses. Current study thus posits RNASE4 as a potent prognostic biomarker and therapeutic target within the regulatory circuitry propagating aggressive GBM phenotypes.
Materials and methods
Cell lines and culture conditions
The human GBM cell lines U87MG and DBTRG-05MG were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in RPMI-1640 medium (Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a humidified incubator containing 5% CO2. Cells were routinely sub-cultured at 70 to 90% confluence using phosphate-buffered saline containing 0.05% trypsin-EDTA. Short tandem repeat profiling (Promega, Madison, WI, USA) was utilized for cell line authentication. Cells were cultured for no more than three months and periodically tested for mycoplasma contamination. GBM cancer cell lines were cultured as mammospheres as previously described [17,18] to enrich for stem-like subpopulations. Treatments with RNASE4 recombinant protein (at 1 µg/mL, PME100678, DIMA Biotech, Wuhan, Hubei, China) were carried out in serum-free culture medium for 16 hours prior to subsequent western blotting or luciferase reporter analysis, respectively. All experiments were performed in triplicates and data are presented as mean ± standard error of mean (SEM).
Analysis of cell proliferation and clonogenicity
To assess cell proliferation, GBM cells were seeded in RPMI-1640 supplemented with 10% FBS at a density of 5 × 103 cells/well in triplicate. At indicated timepoints, cells were harvested, and viable cells quantified by trypan blue exclusion assay using a LUNA Automated Cell Counter (Logos Biosystems, Villeneuve-d’Ascq, France). For clonogenic assay, 1 × 105 GBM cells were seeded per well and cultured with regular medium changes for seven days. Resulting colonies were fixed with cold methanol, stained with crystal violet solution, and imaged after overnight air-drying.
RNA interference and overexpression experiments
Lentiviral particles were generated by co-transfecting short hairpin RNA (shRNA), packaging, and envelope plasmids into 293FT cells using Lipofectamine 3000 reagent (ThermoFisher Scientific, Rockville, MD, USA). Two RNASE4-specific shRNA sequences were utilized to specifically knockdown expression of RNASE4 (ShRNASE4-1: CCGGGAGCACTAGACGTGTTGTCATCTCGAGATGACAACACGTCTAGTGCTCTTTTTG; ShRNASE4-2: CCGGTGATCGCTACTGCAACTTGATCTCGAGATCAAGTTGCAGTAGCGATCATTTTTG). Viral supernatants were collected to infect target cells in the presence of polybrene (Sigma-Aldrich). RNASE4 over-expression was achieved by transfecting a Flag-tagged expression plasmid (OriGene, Rockville, MD, USA) and selecting with neomycin (Sigma-Aldrich). Successful over-expression and knockdown were verified by western blot analysis.
Cell migration and invasion assays
Transfected cells were harvested, resuspended in serum-free medium containing 0.1% bovine serum albumin (Sigma-Aldrich), and seeded into Transwell chambers at 1 × 105 cells/chamber. For the invasion assay, the upper chambers were pre-coated with Matrigel, and the bottom chambers contained RPMI-1640 with 20% FBS for chemoattractant purposes. After 24 hours, non-migrated cells were removed using cotton swabs. Migrated cells on the underside were fixed with methanol, stained with crystal violet, and imaged in three random fields per chamber. Cell numbers were quantified and normalized to control. Experiments were performed in triplicates.
Immunoblot analysis
Total cell lysates and conditioned media, collected and concentrated using Amicon Ultra-4 Centrifugal Filter Devices (Merck Millipore Ltd.), were analyzed using western blot as described previously [19]. The following primary antibodies were used at the recommended dilutions: anti-RNASE4 (Invitrogen, PA5-121640), anti-ACTIN (Abcam, Waltham, MA, ab8227). anti-AKT (Cell signaling technology, Rockville, MD, USA, #9272), anti-phospho-Akt (Ser473) (Cell signaling technology, Rockville, MD, USA, #9271), anti-AXL (Cell signaling technology, Rockville, MD, USA, #8661), anti-Phospho-AXL (Tyr702) (Cell signaling technology, Rockville, MD, USA, #5724), anti-HIF1A (Genetex, Irvine, CA, USA, GTX127309), anti-phospho-IκBα (Ser32/36) (Cell signaling technology, Rockville, MD, USA, #9246), anti-IκBα (Cell signaling technology, Rockville, MD, USA, #4821), anti-cIAP1 (Proteintech, Chicago, IL, USA, 10022-1-AP), anti-cIAP2 (Proteintech, Chicago, IL, USA, 24304-1-AP) and anti SURVIVIN (Proteintech, Chicago, IL, USA, 10508-1-AP).
In vivo xenograft experiments
Xenograft experiments were performed by subcutaneous injection of RNASE4-overexpressing GBM cells into the flanks of 6 to 8 weeks old male NOD/SCID mice (n=4 per group). Mice were randomly assigned to experimental groups. Tumor dimensions were measured once weekly using digital calipers. Tumor volumes were calculated as length × width2 × 0.5. After four weeks, mice were euthanized, and excised tumors were weighed. All procedures were approved by the Institutional Animal Care and Use Committee of China Medical University, Taiwan (CMUIACUC-2022-095) and performed in accordance with established guidelines. Experiments were conducted in a blinded randomized fashion.
Amido black staining
Due to the complexity of individual secreted proteins in cell culture and their tendency to fluctuate under varying cellular conditions, Amido Black staining of total secreted proteins is considered an acceptable internal control for assessing the expression of target secreted proteins [20]. Total protein staining with Amido Black dye (Sigma-Aldrich) was employed to detect and visualize secreted proteins from GBM cells, serving as a loading control. Protein samples (10 μg) from conditioned medium of GBM cells were first separated by SDS-PAGE and subsequently transferred onto the PVDF membrane. Staining was conducted by incubating the membrane with 0.1% amino black dye in 10% acetic acid and 25% isopropanol for up to 10 minutes. The membranes were then destained by rinsing with a solution of 10% acetic acid and 25% isopropanol until clear protein bands are visible.
Gene set enrichment analysis (GSEA)
Gene expression data from 154 glioblastoma (GBM) patients were retrieved from The Cancer Genome Atlas (TCGA) database. Pearson correlation coefficients were calculated between RNASE4 expression and the expression of all other genes in the dataset, using RNASE4 as the target gene. Gene set enrichment analysis (GSEA) was subsequently performed, with the ranked list based on Pearson correlation coefficients serving as the input and the HALLMARK gene set as the reference for enrichment. The significance of each gene set associated with RNASE4 was evaluated by calculating enrichment scores from the ranked list, followed by permutation testing to assess significance levels. This analysis identified gene sets that are significantly correlated with RNASE4 in GBM.
Statistical analysis
All in vitro experiments were performed in triplicates and data are presented as mean ± standard error of the mean (SEM). Statistical comparisons between groups were conducted using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Based on data characteristics, differences between two groups were determined using unpaired two-tailed Student’s t-test or non-parametric Mann-Whitney U test. Paired t-test was used for paired sample analysis. P Values less than 0.05 were considered statistically significant. The Kaplan-Meier survival analysis was conducted to compare the survival rates of two patient groups with GBM from TCGA dataset, categorized based on differing levels of RNASE4 expression.
Results
Augmented expression of RNASE4 is correlated to GBM progression and poor prognosis
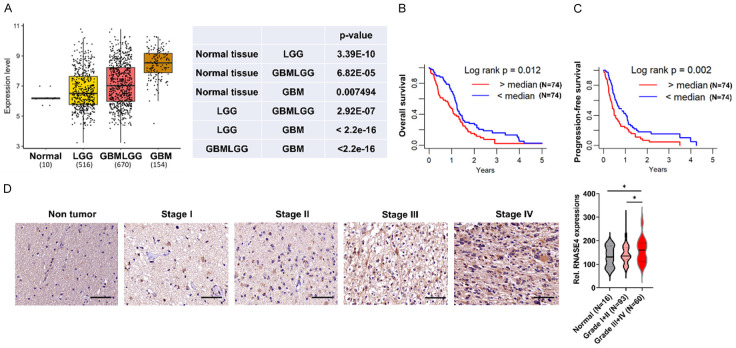
The Cancer Genome Atlas (TCGA) has significantly advanced brain cancer research by serving as an invaluable repository that facilitates comprehensive bioinformatic analyses of molecular alterations implicated in tumorigenesis [21,22]. Utilization of the TCGA datasets can be instrumental in uncovering pivotal genes potentially associated with oncogenic development and exacerbated malignancy of GBM. Our analysis thus encompassed TCGA cohorts comprising normal brain tissue (n=10), lower-grade glioma (LGG, n=516), a combined dataset of lower-grade glioma and GBM (GBMLGG, n=670), and GBM (n=154), unveiling significantly escalating upregulations in RNASE4 expression when comparing normal cerebral tissue through LGG and GBMLGG to GBM (Figure 1A). This observation is supported by Kaplan-Meier survival analysis of the TCGA GBM cohort, which demonstrated inverse correlation of elevated RNASE4 expression to mitigated overall survival (OS) rate and progression-free survival (PFS) rate (Figure 1B, 1C). Subsequent immunohistochemical (IHC) assessments of human brain tumor specimens spanning low-grade (grade I to II) and high-grade (grade III to IV) along with adjacent normal tissues corroborated our TCGA analyses, showing highest level of RNASE 4 in grade III+IV tumors relative to grade I+II tumors or normal brain sections (Figure 1D). These marked increases in RNASE4 protein levels in high-grade tumors compared to low-grade sections thus suggest that progressively elevated RNASE4 expression is closely associated with advancing pathological stages.
Figure 1.
RNASE4 expression correlates positively with glioblastoma malignancy grade. A. TCGA analysis illustrating a progressive increase in RNASE4 mRNA levels from normal brain tissue (n=10) to lower-grade glioma (LGG, n=516), a combination of lower-grade glioma and glioblastoma (GBMLGG, n=670) and glioblastoma (GBM, n=154). B, C. Kaplan-Meier plots depicting significantly reduced overall and progression-free survival in TCGA GBM patients with high versus low RNASE4 expression. D. Immunohistochemistry images and quantification revealing elevated RNASE4 protein levels in high-grade (III-IV) compared to low-grade (I-II) glioma tissues and normal brain. Scale bar, 50 μm. The right panel shows RNASE4 immunohistochemistry scores, indicating a progressive upregulation with advancing tumor grade. Data are presented as mean ± SEM. *P<0.05.
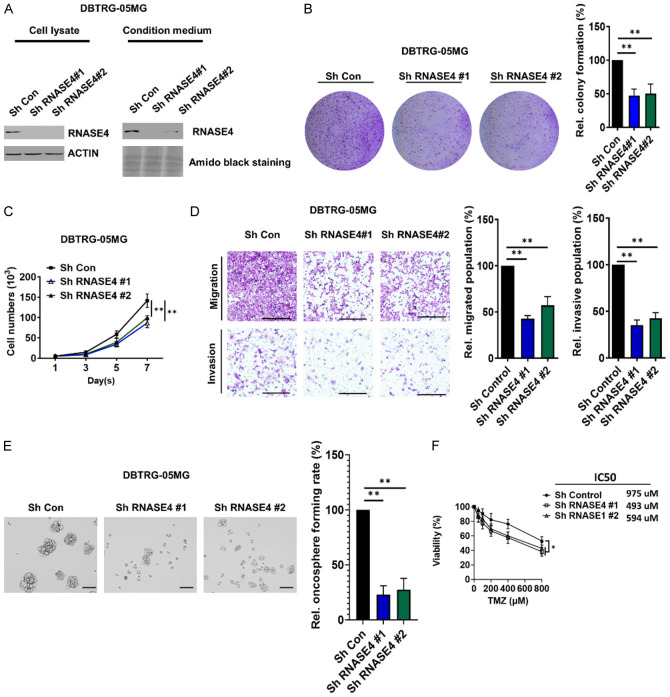
Knockdown of RNASE4 suppresses clonogenicity, proliferation, migration, chemoresistance and stemness of GBM cells
To elucidate the functional role of RNASE4 in GBM cellular dynamics, our study employed targeted knockdown of RNASE4 expression in DBTRG-05MG cells using shRNA vector-mediated silencing (Figure 2A). Functional assessment using clonogenic assay revealed a notable diminution in clonogenic potential from RNASE4-knocked down DBTRG-05MG cells with two distinct shRNA when compared to control shRNA-transduced counterparts (Figure 2B). Of note, a marked reduction in the cell proliferation rate was observed following RNASE4 depletion (Figure 2C), while transwell migration and invasion assays confirmed substantial attenuations in the migratory and invasive capacities of RNASE4-silenced DBTRG-05MG cells compared to control cells (Figure 2D). Further, in light of accumulating evidence indicating the role of cancer stem cells (CSCs) in GBM initiation, progression, therapeutic resistance and recurrence [23,24], we next assessed previously unexamined impact of RNASE4 on GBM stem cell properties. Oncosphere formation assays demonstrated a significant reduction in oncosphere formation capacities in RNASE4-silenced DBTRG-05MG cells, suggesting the ability of RNASE4 in stemness regulation (Figure 2E). Subsequently, we investigated the effect of RNASE4 inhibition on the sensitivity of GBM cells to temozolomide (TMZ), the frontline chemotherapeutic agent for brain cancer. Our results showed that RNASE4 knockdown led to significantly enhanced sensitivity of GBM cells to TMZ (Figure 2F). These findings implicate RNASE4 as a pivotal modulator of GBM progression and chemoresistance, impacting fundamental oncogenic attributes such as clonogenicity, proliferation, migration, invasion and CSC properties, stressing the potential role of RNASE4 in advanced cancer stages as unraveled in Figure 1.
Figure 2.
RNASE4 knockdown suppresses oncogenic phenotypes of GBM. A. Western blot analysis was employed to confirm RNASE4 knockdown using two RNASE4-specific shRNAs in the cell lysates and conditioned medium of DBTRG-05MG cells. ACTIN served as the internal control for cellular lysates, while Amido Black staining of total secreted proteins served as the loading control for proteins in the conditioned medium. B, C. Colony formation and proliferation assays was evaluated in DBTRG-05MG cells with or without RNASE4 knockdown (**P<0.01). D. Transwell migration and Matrigel invasion assays were performed to evaluate changes in cell motility following RNASE4 knockdown. The right panels display statistical results from each assay, with significance indicated as P<0.01. E. The sphere formation ability of RNASE4-depleted DBTRG-05MG cells, achieved using two different shRNAs, was assessed to determine the impact of RNASE4 knockdown. Scale bar, 100 μm. Statistical significance is indicated as P<0.01. F. MTS assays were conducted to evaluate the effect of RNASE4 on cellular chemoresistance to TMZ in control versus RNASE4-KD DBTRG-05MG cells over a period of 3 days. Data are presented as mean ± SEM from three independent experiments. Data are mean ± SEM of three independent experiments (**P<0.01).
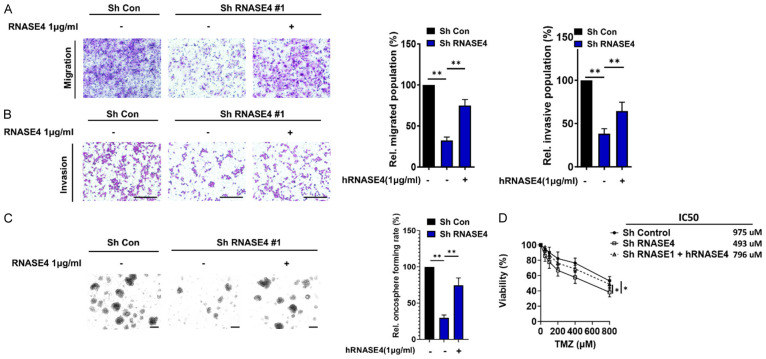
Extracellular (secreted) RNASE4 enhances the oncogenic properties of GBMR
Members of the RNase A family, including RNASE1, RNASE2, RNASE3, and RNASE5, have been shown to be oncogenic secreted proteins that contribute to cancer and tumorigenesis through the activation of key signaling pathways such as PI3K/AKT, NF-κB and MAPK [9,11,25,26]. To elucidate the potential oncogenic role of extracellular RNASE4, gain-of-function strategy utilizing RNASE4-knockdown model and recombinant hRNASE4 protein (hRNASE4) was employed. Firstly, cellular migration and invasion assays were conducted using the engineered RNASE4 stable knockdown (RNASE4-KD) DBTRG-05MG cells, which were treated with exogenous hRNASE4 protein. The results demonstrated that the mitigated migration and invasion populations in the RNASE4-KD cells were significantly restored upon exogenous addition of hRNASE4 (Figure 3A, 3B). The functional impact of extracellular RNASE4 was next evaluated by oncosphere-formation assays. The data consistently revealed significantly restored oncosphere formation rate in the exogenous RNASE4 group as compared to RNASE4-KD group (Figure 3C). Similarly, the significantly reduced viability of RNASE4-KD cells was restored upon treatment with recombinant hRNASE4. Additionally, the IC50 for cellular resistance to TMZ in RNASE4-KD cells treated with hRNASE4 was significantly increased compared to cells without RNASE4 treatment (Figure 3D). These findings suggest the oncogenic properties of secreted RNASE4 in mediating migration, cancer stemness, and chemoresistance in GBM.
Figure 3.
Recombinant human RNASE4 protein potently restores the oncogenic properties mitigated by RNASE4 knockdown in DBTRG-05MG cells. Cellular migration (A) and invasion (B) abilities of DBTRG-05MG cells, significantly reduced by RNASE4 knockdown, were restored upon treatment with recombinant human RNASE4 protein (hRNASE4) for 16 h (**P<0.01). (C) Oncosphere formation in RNASE4-KD cells was restored by treatment with hRNASE4 (oncosphere culture medium replaced every three days with or without hRNASE4 supplement), demonstrating the extracellular effects of RNASE4 on this oncogenic property. (D) Cellular viability following TMZ treatment for 3 days in control or RNASE4-knockdown DBTRG-05MG cells, with or without recombinant human RNASE4 treatment, was assessed. P<0.05. Data are mean ± SEM from at least three independent experiments.
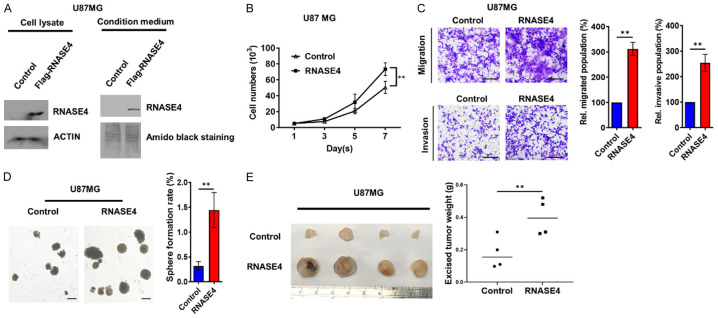
In vivo tumor growth and oncogenesis of GBM U87MG cells is augmented by RNASE4 overexpression
To substantiate the oncogenic role of RNASE4, we engineered U87MG cells to stably overexpress Flag-tagged RNASE4 (Figure 4A). This modification yielded a significant proliferation surge in RNASE4-overexpressing U87MG cells compared to controls (Figure 4B), alongside marked enhancements in cellular migration and invasion capacities (Figure 4C). Considering the renowned role of CSCs in GBM progression and therapeutic resistance [27], we evaluated the impact of RNASE4 overexpression on stemness characteristics in GBM. Under stem cell-selective culture conditions, RNASE4 overexpression facilitated a significant elevation in oncosphere numbers, denoting heightened cancer stemness in GBM (Figure 4D). These in vitro findings were corroborated by in vivo experiments employing a xenograft mouse model, where RNASE4 overexpression significantly augmented the tumor growth of U87MG xenograft (Figure 4E). Collectively, these in vitro and in vivo outcomes from engineered RNASE4-overexpressing cells affirmed the role of RNASE4 as a novel GBM oncogenesis and tumorigenesis facilitator.
Figure 4.
RNASE4 overexpression promotes glioblastoma progression in vitro and in vivo. A. The efficiency of RNASE4 overexpression in U87MG cells was confirmed by Western blotting, with ACTIN serving as the internal control for cellular lysates, and Amido Black staining of total secreted proteins serving as the loading control for proteins in the conditioned medium. B. Cell proliferation curve based on viable cell counts from control or RNASE4-overexpressing U87MG cells for a period of 7 days was plotted (**P<0.01). C. Transwell migration and matrigel invasion assays were used to assess changes in cell motility of RNASE4-overexpressed cells. Graphs on the right depict the statistical results (**P<0.01). D. The effects of RNASE4 overexpression on U87MG cells were evaluated using a sphere formation assay under stem cell-selective culture conditions. The right panel displays the statistical results for the number of oncospheres (greater than 100 μm in size) in control versus RNASE4-overexpressing cells. Data are presented as mean ± SEM from three independent experiments (**P<0.01). Scale bar, 100 μm. E. Control or RNASE4-overexpessing U87MG cells (2 × 106) were subcutaneously injected into NOD/SCID mice for monitoring of tumor growth. Tumors from the tumor-bearing mice (n=4 for each group) were collected as shown in the left panel image. Weights of the excised tumors were measured and plotted in the right panel, and statistical differences between the control group and the RNASE4-overexpression group were determined using Student’s t-test (**P<0.01).
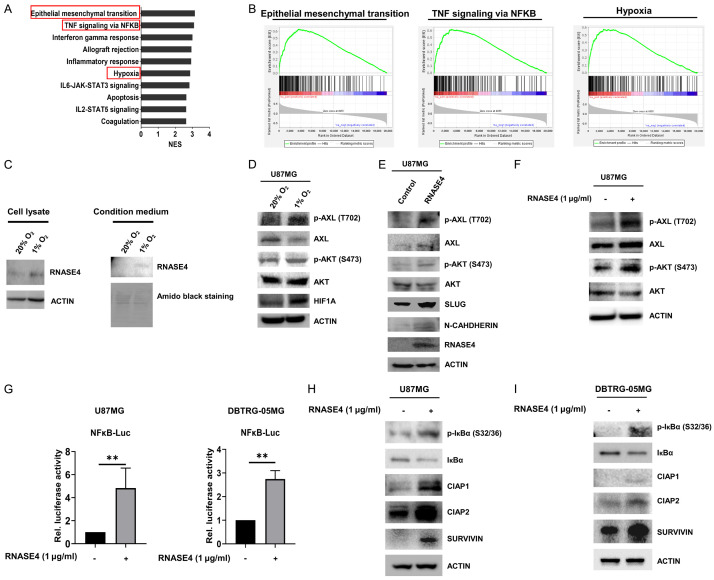
RNASE4 activates AXL/AKT and NF-κB/cIAP1/cIAP2/SURVIVIN signaling pathways under hypoxic conditions
To uncover genes and pathways linked to RNASE4 activity in GBM, we conducted Gene Set Enrichment Analysis (GSEA) using TCGA datasets. The GSEA demonstrated a positive correlation between RNASE4 expression and gene signatures characteristic of EMT, TNF-mediated NF-κB signaling and hypoxia (Figure 5A, 5B). Echoing the identification of EMT as a RNASE4-associated pathway in GBM progression, our in vitro and in vivo functional findings presented in Figures 2 to 4 that encompassed cellular proliferation, migration, invasion, clonogenic potential, oncosphere formation and tumorigenesis attested the involvement of RNASE4 in facilitating EMT during GBM progression. Since GBM is demonstrated to exhibit hypoxic microenvironment that promotes cancer progression through elevated EMT [28,29], we next investigated whether RNASE4 could be induced in hypoxic environment of GBM. Our data revealed that both intracellular and extracellular levels of RNASE4 were significantly increased in U87MG cells under hypoxic conditions (1% O2) (Figure 5C). In hypoxic conditions, hypoxia-inducible factor 1 (HIF1A) is reported to be involved in upregulating the expression of receptor tyrosine kinase AXL and promoting metastasis in neoplasms such as renal cell carcinoma and prostate cancer [30,31]. In this study, activation of AXL phosphorylation (T702) and AKT phosphorylation (S473) was observed in U87MG cells where HIF1A was induced under hypoxic condition (Figure 5D). Further, RNASE4-overexpressing model of U87MG also elicited augmented phosphorylation levels of AXL (T702) and AKT (S473) as compared to control cells, alongside upregulation of EMT markers including SLUG and N-CADHERIN (Figure 5E). In line with this observation, exogenous hRNASE4 treatment resulted in signaling activation of AXL and AKT phosphorylation (Figure 5F). Moreover, since RNASE4 expression in GBM specimens was predictively associated with NF-κB (Figure 5A, 5B), and NF-κB signaling is known to be a canonical downstream effector of EMT under hypoxia [32]. We next employed an NF-κB reporter system to ascertain the influence of RNASE4 on NF-κB activity in our GBM models. The results demonstrated that hRNASE4 treatment significantly enhanced luciferase activity when compared to vehicle group in NF-κB-Luc-transfected U87MG cells and DBTRG-05MG cells (Figure 5G), suggesting a prominent role of RNASE4 in modulating NF-κB signaling activity. To corroborate the molecular mechanism underlying RNASE4-mediated activation of NF-κB signaling, we next examined the phosphorylation of IκBα at serine residues 32 and 36 (S32/36). This post-translational modification is critical for the proteasomal degradation of IκBα and the subsequent nuclear translocation of NF-κB induced by hypoxia [32]. The data revealed that treatment with recombinant hRNASE4 protein in U87MG cells led to a marked increase in IκBα phosphorylation at serine residues 32 and 36 (S32/36) (Figure 5H). Similar results of IκBα S32/36 phosphorylation was also observed in the RNASE4-treated DBTRG-05MG cells (Figure 5I). Furthermore, other relevant downstream effectors of the NF-κB signaling axis, such as members of the inhibitors of apoptosis (IAPs), were investigated, with a focus on their primary function as inhibitors of apoptotic cell death. Our results revealed that IAP family members including cIAP1, cIAP2, and SURVIVIN were significantly increased in RNASE4-treated GBM U87MG and DBTRG-05MG cells (Figure 5H, 5I). These findings collectively suggest the predominant influence of RNASE4 on AXL/AKT and NF-κB-cIAP1/cIAP2/SURVIVIN signaling axes in hypoxic GBM.
Figure 5.
Hypoxia-induced RNASE4 expression activates AXL/AKT and NF-κB/cIAP1/cIAP2/SURVIVIN signaling in GBM. A, B. GSEA was conducted to reveal the correlation of RNASE4 expression to potential biological pathways. The enrichment plots indicated positive correlation with epithelial-mesenchymal transition (EMT), TNF signaling via NF-κB and hypoxia signatures in TCGA GBM cohort. C. RNASE4 expressions in the cell lysates and conditioned medium of U87MG cells under normoxia and hypoxia conditions were assessed using Western blot analysis. ACTIN served as the internal control for cellular lysates, while Amido Black staining of total secreted proteins was used as the loading control for proteins in the conditioned medium. D. Expression levels of the indicated proteins in U87MG cells cultured under normoxia and hypoxia for 16 hours were assessed by Western blot analysis. E. In addition to the unphosphorylated and phosphorylated levels of AXL and AKT, the protein expressions of EMT markers, including SLUG and N-CADHERIN, in RNASE4-overexpressing U87MG cells were assessed by Western blotting. F. Expressions of unphosphorylated and phosphorylated AXL and AKT in U87MG cells treated with recombinant human RNASE4 protein (hRNASE4) were detected using Western blotting. G. The impact of RNASE4 on NF-κB activation was evaluated by transfecting U87MG and DBTRG-05MG cells with the luciferase reporter construct containing NF-κB-responsive elements. Cells were treated with either vehicle or recombinant human RNASE4 for 16 hours before harvest for reporter activity analysis. Data are presented as mean ± SEM of three independent experiments (**P<0.01). H, I. U87MG and DBTRG-05MG cells treated with recombinant human RNASE4 for 16 hours were subjected to immunoblotting using antibodies specific for phosphorylated IκBα at serine residues 32 and 36 (S32 and S36), unphosphorylated IκBα, cIAP1, cIAP2, and SURVIVIN. ACTIN was used as the loading control.
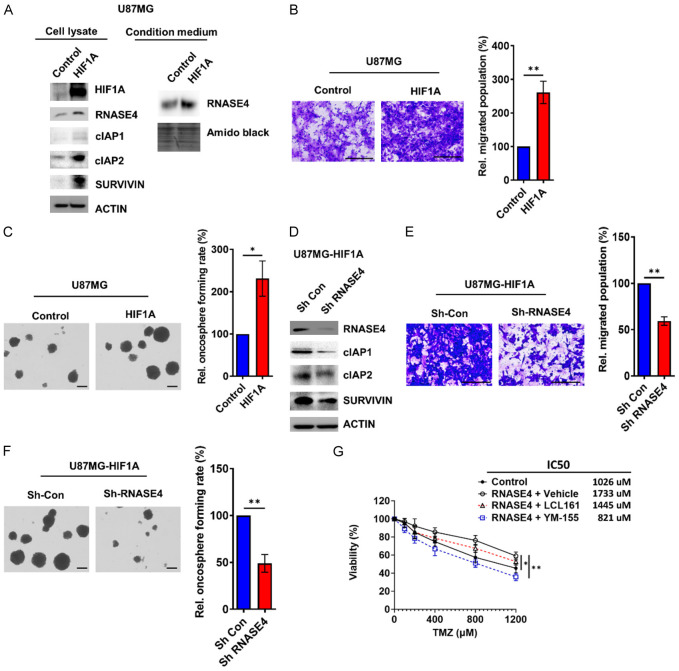
RNASE4 activates signaling network composed of AXL/AKT and NF-κB-cIAP1/cIAP2/SURVIVIN to promote GBM oncogenesis
The aforementioned effects of exogenous RNASE4 on downstream effectors of the IκBα/NF-κB pathway, including cIAP1, cIAP2, and SURVIVIN, were further investigated to determine whether this signaling network regulated by RNASE4 was mediated through HIF1A during hypoxia. As shown in Figure 6A, overexpression of HIF1A in U87MG cells resulted in increased protein levels of not only RNASE4, but also cIAP1, cIAP2 and SURVIVIN. Of note, secreted protein level of RNASE4 was augmented in the condition medium of HIF1A-overexpressed cells (Figure 6A). Under HIF1A-overexpression, significantly increased population of migrated cells and oncospheres were observed as compared to that of control cells (Figure 6B and 6C). To verify whether HIF1A-mediated GBM oncogenic progression is mediated through RNASE4, RNASE4 knockdown was performed in HIF1A-overexpressing cells (Figure 6D). Analysis of NF-κB downstream effectors revealed that RNASE4 knockdown reduced the protein expression of cIAP1, cIAP2 and SURVIVIN, which were elevated in HIF1A-overexpressing U87MG cells (Figure 6A, 6D). Cell migration and oncosphere formation, which were elevated in HIF1A-overexpressing cells, were significantly reduced when RNASE4 expression was abrogated (Figure 6E, 6F), suggesting that RNASE4 was responsible for modulating the effects of HIF1A during GBM progression. Since our data in Figure 3D suggested an association between RNASE4 and TMZ chemoresistance, and IAPs are known for promoting chemoresistance [33,34], we next used small molecule inhibitors against IAPs and SURVIVIN to further investigate the role of these IAPs in RNASE4-mediated chemoresistance in GBM. Our data showed that the TMZ chemoresistance significantly enhanced by RNASE4 overexpression was notably reduced by treatment with the SURVIVIN inhibitor YM-155 (Figure 6G). Nonetheless, treatment with cIAP1/2 inhibitor LCL161 only exhibited a mild suppressive effect on RNASE4-induced chemoresistance to TMZ, suggesting that RNASE4-mediated chemoresistance could be specifically targeted by inhibiting the RNASE4-NF-κB-SURVIVIN signaling axis.
Figure 6.
RNASE4 mediates oncogenic effects and cIAPs induction by HIF1A in U87MG cells. (A) Effects of HIF1A overexpression on RNASE4, cIAP1, cIAP2, and SURVIVIN protein levels in U87MG cells were assessed by Western blot analysis. (B) Transwell migration assays and oncosphere formation assays (C) were conducted to evaluate changes in cell motility and cancer stemness following HIF1A overexpression in U87MG cells. The right panel displays the statistical quantification results (**P<0.01). (D) The effects of RNASE4 knockdown on the expression levels of cIAP1, cIAP2, and SURVIVIN in HIF1A-overexpressing U87MG cells were assessed by Western blot analysis. (E, F) The effects of RNASE4 knockdown on the migration and oncosphere formation capacities of HIF1A-overexpressing U87MG cells were evaluated using transwell migration and oncosphere formation assays. Data are presented as mean ± SEM from three independent experiments (**P<0.01). (G) MTS assay was conducted to evaluate the effects of LCL-161 (cIAP1/2 inhibitor, 10 µM) and YM-155 (SURVIVIN inhibitor, 5 nM) on control and RNASE4-overexpressing U87MG cells treated with Temozolomide (TMZ) for 3 days (*P<0.05, **P<0.01).
Discussion
Despite advances in discovering reliable prognostic and predictive biomarkers for GBM, providing therapeutic guidance and tracking disease progression remain unresolved clinical challenges for this most prevalent and aggressive malignancy of the central nervous system [35,36]. Aberrations in RNase activity within the RNase A superfamily have been linked to cancer development through the modulation of receptor tyrosine kinases (RTKs) [6], even though RNases and RTKs were traditionally considered distinct families. Recently, RNASE5 has emerged as an EGFR ligand and a prognostic serum marker for breast cancer [13], while RNASE1 interacts with the ephrin A4 receptor (EphA4) and contributes to the tumorigenesis of breast cancer [37]. Consistent with these observations, activation of AXL signaling was indicated by elevated tyrosine phosphorylation (T702) in RNASE4-overexpressing and hRNASE4-treated GBM cells (Figure 5E, 5F). In addition to AXL phosphorylation, concurrent phosphorylation of AKT (S473) in RNASE4-overexpressing GBM cells was similar to what has been observed in prostate cancer cells [8].
Although RNASE4 has recently been reported to activate AXL signaling and is suggested as a biomarker to differentiate prostate cancer from benign prostatic hyperplasia [8], pathophysiological roles underlined by RNASE4 in driving GBM progression remains largely obscure. In this study, our findings revealed a significant correlation between elevated RNASE4 expression and advanced clinicopathological stages, as well as reduced survival rates in GBM (Figure 1). This suggests that RNASE4 could serve as a novel prognostic biomarker for GBM. The prognostic value of RNASE4 was further supported by experiments manipulating RNASE4 expression in GBM cells, which demonstrated its substantial role in regulating cellular migration, invasion, oncosphere formation, and chemoresistance (Figure 2). Notably, extracellular RNASE4 exerted significant influence on the oncogenic features of GBM. The addition of recombinant hRNASE4 significantly restored migration, invasion, oncosphere formation, and TMZ chemoresistance in RNASE4-KD cells (Figure 3). It is anticipated that extracellular RNASE4 may interact with the AXL and/or PI3K complex, initiating downstream signaling cascades in a manner similar to how secretory RNASE1 binds to EphA4 to promote breast tumorigenesis [9]. This notion is further supported by the observation that RNASE4 overexpression greatly increased secretory RNASE4 in the conditioned medium and significantly enhanced cellular migration, invasion, oncosphere formation, as well as the growth of GBM xenografts (Figure 4).
Furthermore, although RNASE4 shared transcriptional promoter with RNASE5 [15], our data indicate that RNASE4 promotes oncogenesis via the AXL/AKT and NF-κB signaling axes (Figure 5A, 5B, 5D-F) but not through EGFR. Supporting our findings that the AXL/AKT signaling axis contributes to GBM oncogenesis, the previous study also underscore its role in enhancing cell migration and invasion through the induction of EMT genes in various cancer types, including GBM [38]. In addition to AKT activation, AXL has been shown to activate NF-κB signaling in esophageal adenocarcinoma cells [39]. In the present study, we identified inhibitors of apoptosis (IAP) members, including cIAP1, cIAP2, and SURVIVIN, as key downstream effectors of RNASE4 under hypoxic conditions (Figure 5C, 5G, 5H). These results are consistent with the literature, which reports that cIAP2 and SURVIVIN are upregulated during the activation of NF-κB signaling [40]. Furthermore, given the established role of IAPs in conferring resistance to apoptosis and modulating drug and immune responses in various cancers [41,42], our subsequent experiments on RNASE4-mediated TMZ chemoresistance were corroborated by employing inhibitors LCL-161 and YM-155, which specifically target cIAP1/2 and SURVIVIN, respectively. Notably, our results demonstrated that the SURVIVIN inhibitor YM-155 was more effective, significantly mitigating RNASE4-induced chemoresistance to TMZ (Figure 6G). Importantly, TMZ is currently the first-line chemotherapeutic agent capable of effectively crossing the blood-brain barrier for GBM treatment. Our data suggest that hypoxia-induced RNASE4-mediated upregulation of cIAPs could represent a promising target for enhancing GBM chemotherapy. Indeed, targeting AXL and cIAPs in cancer therapy has shown significant potential in improving immunotherapy and angiogenesis [33,43]. RNASE4-induced AXL activation and cIAPs induction in GBM progression thus warrants further exploration as potential therapeutic strategies for GBM treatment. Our findings advocate for the clinical translation of RNASE4 and warrant novel development of tailored therapies against this deadly malignancy.
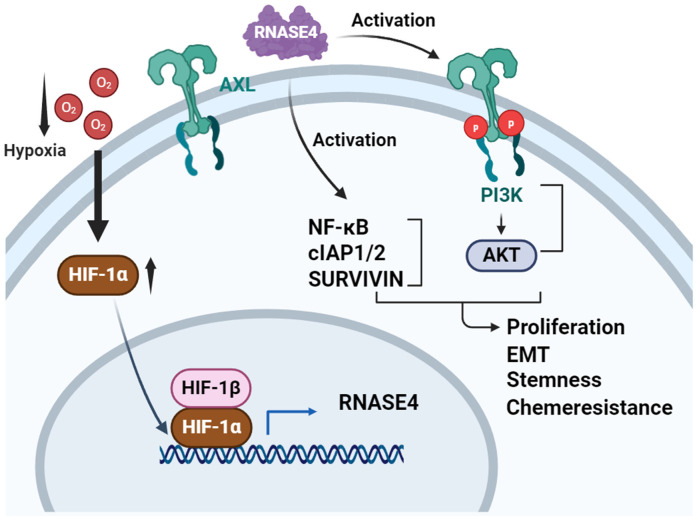
Moreover, exogenous ribonucleases have recently attracted increasing research attention as RNASEs derived from non-mammalian sources such as Bacillus pumilus have exhibited significant antitumor activity by reorganizing the microRNA (miRNA) network within tumor cells [44], while engineered RNases can control cellular levels of specific miRNA within tumor cells and lead to p53-mediated apoptosis [45,46]. Present findings position RNASE4 as a potential biomarker and therapeutic target through a novel mechanism, where extracellular RNASE4 mediates GBM progression via activation of AXL/AKT and IκBα/NF-κB signaling pathways. This mechanism can be targeted by inhibiting elevated levels of cIAP1, cIAP2, and SURVIVIN. Collectively, this study reveals that RNASE4 acts as a novel hypoxia-responsive mediator of AXL/AKT and NF-κB/cIAPs signaling, thus promoting GBM progression and chemoresistance (Figure 7). These findings implicate potential avenues for non-invasive diagnostics and the exploration of RNASE4-driven TMZ chemoresistance to enhance targeted therapies for advanced GBM.
Figure 7.
Proposed model of RNASE4-mediated promotion of GBM oncogenesis through AXL/AKT and NF-κB/cIAPs signaling activation under hypoxia. RNASE4 expression is induced under hypoxic conditions, leading to the activation of the AXL/AKT and NF-κB/cIAP1/cIAP2/SURVIVIN signaling pathways. These cascades, in turn, promote glioblastoma proliferation, epithelial-to-mesenchymal transition (EMT), stemness, and chemoresistance.
Acknowledgements
The Authors would like to thank Institute of Biochemistry and Molecular Biology & Chinese Medicine Research Center of China Medical University and the Instrumentation Center of China Medical University for their support and assistance in this work. An Nan Hospital, China Medical University, Tainan, Taiwan (ANHRF111-01) for providing us with materials to conduct the experiments in the present study. This work was supported by the National Science and Technology Council (NSTC 112-2326-B-039-002-MY3, NSTC 112-2314-B-182A-046-, NSTC 112-2320-B-442-001-), China Medical University, Taiwan (CMU113-MF-72), Taipei Medical University (TMU110-AE1-B08), the An Nan Hospital, China Medical University, Tainan, Taiwan (ANHRF111-01), Chang Gung Memorial Hospital, Taoyuan, Taiwan (CMRPD1M0363 to KHL) and the National Science and Technology Council (112-2320-B-182-004 to KHL).
Disclosure of conflict of interest
None.
Abbreviations
- GBM
Glioblastoma
- RNASE4
Ribonuclease 4
- TCGA
The Cancer Genome Atlas
- TMZ
Temozolomide
- GSEA
Gene Set Enrichment Analysis
- EMT
Epithelial-mesenchymal transition
- ANG
Angiogenin
- EGFR
Epidermal growth factor receptor
- HIF1A
Hypoxia-inducible factor 1A
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- cIAPs
cellular inhibitor of apoptosis proteins
- ATCC
American Type Culture Collection
- shRNA
short hairpin RNA
- LGG
Lower grade glioma
- GBMLGG
Lower grade glioma and GBM
- IHC
Immunohistochemical
- CSCs
Cancer stem cells
References
- 1.Rabab’h O, Al-Ramadan A, Shah J, Lopez-Negrete H, Gharaibeh A. Twenty years after glioblastoma multiforme diagnosis: a case of long-term survival. Cureus. 2021;13:e16061. doi: 10.7759/cureus.16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nizamutdinov D, Stock EM, Dandashi JA, Vasquez EA, Mao Y, Dayawansa S, Zhang J, Wu E, Fonkem E, Huang JH. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67–e74. doi: 10.1016/j.wneu.2017.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer K, Saini S, Bhadra S, Kulavi S, Bandyopadhyay J. Precision medicine advancements in glioblastoma: a systematic review. Biomedicine (Taipei) 2023;13:1–13. doi: 10.37796/2211-8039.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3–9. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, Day L, Lam D, Jette N. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17:776–783. doi: 10.1093/neuonc/nou283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HH, Wang YN, Hung MC. Functional roles of the human ribonuclease A superfamily in RNA metabolism and membrane receptor biology. Mol Aspects Med. 2019;70:106–116. doi: 10.1016/j.mam.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berezovsky AD, Poisson LM, Cherba D, Webb CP, Transou AD, Lemke NW, Hong X, Hasselbach LA, Irtenkauf SM, Mikkelsen T, deCarvalho AC. Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia. 2014;16:193–206. 206.e19–25. doi: 10.1016/j.neo.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanli N, Sheng J, Li S, Xu Z, Hu GF. Ribonuclease 4 is associated with aggressiveness and progression of prostate cancer. Commun Biol. 2022;5:625. doi: 10.1038/s42003-022-03597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HH, Wang YN, Yang WH, Xia W, Wei Y, Chan LC, Wang YH, Jiang Z, Xu S, Yao J, Qiu Y, Hsu YH, Hwang WL, Yan M, Cha JH, Hsu JL, Shen J, Ye Y, Wu X, Hou MF, Tseng LM, Wang SC, Pan MR, Yang CH, Wang YL, Yamaguchi H, Pang D, Hortobagyi GN, Yu D, Hung MC. Human ribonuclease 1 serves as a secretory ligand of ephrin A4 receptor and induces breast tumor initiation. Nat Commun. 2021;12:2788. doi: 10.1038/s41467-021-23075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrabes S, Pages-Pons L, Radcliffe CM, Tabares G, Fort E, Royle L, Harvey DJ, Moenner M, Dwek RA, Rudd PM, De Llorens R, Peracaula R. Glycosylation of serum ribonuclease 1 indicates a major endothelial origin and reveals an increase in core fucosylation in pancreatic cancer. Glycobiology. 2007;17:388–400. doi: 10.1093/glycob/cwm002. [DOI] [PubMed] [Google Scholar]
- 11.Wu T, Chen Y, Yang L, Wang X, Chen K, Xu D. Ribonuclease a family member 2 promotes the malignant progression of glioma through the PI3K/Akt signaling pathway. Front Oncol. 2022;12:921083. doi: 10.3389/fonc.2022.921083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Han B, Zhang R, Su Y, Hosseini DK, Wu H, Yang M, Sun H. Development and validation of a RNA binding protein-associated prognostic model for head and neck squamous cell carcinoma. Aging (Albany NY) 2021;13:7975–7997. doi: 10.18632/aging.202848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YN, Lee HH, Chou CK, Yang WH, Wei Y, Chen CT, Yao J, Hsu JL, Zhu C, Ying H, Ye Y, Wang WJ, Lim SO, Xia W, Ko HW, Liu X, Liu CG, Wu X, Wang H, Li D, Prakash LR, Katz MH, Kang Y, Kim M, Fleming JB, Fogelman D, Javle M, Maitra A, Hung MC. Angiogenin/ribonuclease 5 is an EGFR ligand and a serum biomarker for erlotinib sensitivity in pancreatic cancer. Cancer Cell. 2018;33:752–769. e8. doi: 10.1016/j.ccell.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Han C, Sheng J. The role of human ribonuclease A family in health and diseases: a systematic review. iScience. 2022;25:105284. doi: 10.1016/j.isci.2022.105284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng J, Luo C, Jiang Y, Hinds PW, Xu Z, Hu GF. Transcription of angiogenin and ribonuclease 4 is regulated by RNA polymerase III elements and a CCCTC binding factor (CTCF)-dependent intragenic chromatin loop. J Biol Chem. 2014;289:12520–12534. doi: 10.1074/jbc.M114.551762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Sheng J, Hu JK, Yu W, Kishikawa H, Hu MG, Shima K, Wu D, Xu Z, Xin W, Sims KB, Landers JE, Brown RH Jr, Hu GF. Ribonuclease 4 protects neuron degeneration by promoting angiogenesis, neurogenesis, and neuronal survival under stress. Angiogenesis. 2013;16:387–404. doi: 10.1007/s10456-012-9322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi HC, Tsai CY, Wang CS, Yang HY, Lo CH, Wang WJ, Lee KF, Lai LY, Hong JH, Chang YF, Tsai MM, Yeh CT, Wu CH, Hsieh CC, Wang LH, Chen WJ, Lin KH. DOCK6 promotes chemo- and radioresistance of gastric cancer by modulating WNT/beta-catenin signaling and cancer stem cell traits. Oncogene. 2020;39:5933–5949. doi: 10.1038/s41388-020-01390-0. [DOI] [PubMed] [Google Scholar]
- 18.Chu PY, Huang WC, Tung SL, Tsai CY, Chen CJ, Liu YC, Lee CW, Lin YH, Lin HY, Chen CY, Yeh CT, Lin KH, Chi HC. IFITM3 promotes malignant progression, cancer stemness and chemoresistance of gastric cancer by targeting MET/AKT/FOXO3/c-MYC axis. Cell Biosci. 2022;12:124. doi: 10.1186/s13578-022-00858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH, Lin KH. Interleukin-32 increases human gastric cancer cell invasion associated with tumor progression and metastasis. Clin Cancer Res. 2014;20:2276–2288. doi: 10.1158/1078-0432.CCR-13-1221. [DOI] [PubMed] [Google Scholar]
- 20.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badkas A, De Landtsheer S, Sauter T. Expanding the disease network of glioblastoma multiforme via topological analysis. Int J Mol Sci. 2023;24:3075. doi: 10.3390/ijms24043075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti CG Jr, Tirapelli DP, Rao A, Mikkelsen T, Lau CC, Yung WK, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH TCGA Research Network. Noushmehr H, Iavarone A, Verhaak RG. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimple RC, Bhargava S, Dixit D, Rich JN. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019;33:591–609. doi: 10.1101/gad.324301.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie XP, Laks DR, Sun D, Ganbold M, Wang Z, Pedraza AM, Bale T, Tabar V, Brennan C, Zhou X, Parada LF. Quiescent human glioblastoma cancer stem cells drive tumor initiation, expansion, and recurrence following chemotherapy. Dev Cell. 2022;57:32–46. e8. doi: 10.1016/j.devcel.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YN, Lee HH, Jiang Z, Chan LC, Hortobágyi GN, Yu D, Hung MC. Ribonuclease 1 enhances antitumor immunity against breast cancer by boosting T cell activation. Int J Biol Sci. 2023;19:2957–2973. doi: 10.7150/ijbs.84592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao Y, Zhang H, Su B, Wang J, Quan W, Li Q, Mi D. Construction and validation of an RNA-binding protein-associated prognostic model for colorectal cancer. PeerJ. 2021;9:e11219. doi: 10.7717/peerj.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam SY, Wu VWC, Law HKW. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1alpha and beyond. Front Oncol. 2020;10:486. doi: 10.3389/fonc.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jawhari S, Ratinaud MH, Verdier M. Glioblastoma, hypoxia and autophagy: a survival-prone ‘menage-a-trois’. Cell Death Dis. 2016;7:e2434. doi: 10.1038/cddis.2016.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, LaGory EL, Kariolis MS, Chan A, Lindgren D, Axelson H, Miao YR, Krieg AJ, Giaccia AJ. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111:13373–13378. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra A, Wang J, Shiozawa Y, McGee S, Kim J, Jung Y, Joseph J, Berry JE, Havens A, Pienta KJ, Taichman RS. Hypoxia stabilizes GAS6/Axl signaling in metastatic prostate cancer. Mol Cancer Res. 2012;10:703–712. doi: 10.1158/1541-7786.MCR-11-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Ignazio L, Rocha S. Hypoxia Induced NF-kappaB. Cells. 2016;5:10. doi: 10.3390/cells5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silke J, Meier P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb Perspect Biol. 2013;5:a008730. doi: 10.1101/cshperspect.a008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathore R, McCallum JE, Varghese E, Florea AM, Busselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs) Apoptosis. 2017;22:898–919. doi: 10.1007/s10495-017-1375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sledzinska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. 2021;22:10373. doi: 10.3390/ijms221910373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 37.Li YC, Yamaguchi H, Liu YY, Hsu KC, Sun TH, Sun PC, Hung MC. Structural insights into EphA4 unconventional activation from prediction of the EphA4 and its complex with ribonuclease 1. Am J Cancer Res. 2022;12:4865–4878. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18:153. doi: 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maacha S, Hong J, von Lersner A, Zijlstra A, Belkhiri A. AXL mediates esophageal adenocarcinoma cell invasion through regulation of extracellular acidification and lysosome trafficking. Neoplasia. 2018;20:1008–1022. doi: 10.1016/j.neo.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Laver T, Hong SW, Twitty GB Jr, DeVos A, DeVos M, Benveniste EN, Nozell SE. An NF-κB P65-cIAP2 link is necessary for mediating resistance to TNF-α induced cell death in gliomas. J Neurooncol. 2011;102:367–381. doi: 10.1007/s11060-010-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rada M, Nallanthighal S, Cha J, Ryan K, Sage J, Eldred C, Ullo M, Orsulic S, Cheon DJ. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene. 2018;37:4809–4820. doi: 10.1038/s41388-018-0297-x. [DOI] [PubMed] [Google Scholar]
- 42.Samanta D, Huang TY, Shah R, Yang Y, Pan F, Semenza GL. BIRC2 expression impairs anti-cancer immunity and immunotherapy efficacy. Cell Rep. 2020;32:108073. doi: 10.1016/j.celrep.2020.108073. [DOI] [PubMed] [Google Scholar]
- 43.Goyette MA, Cote JF. AXL receptor tyrosine kinase as a promising therapeutic target directing multiple aspects of cancer progression and metastasis. Cancers (Basel) 2022;14:466. doi: 10.3390/cancers14030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamed ISE, Sen’kova AV, Nadyrova AI, Savin IA, Markov AV, Mitkevich VA, Makarov АА, Ilinskaya ОN, Мironova NL, Zenkova MA. Antitumour activity of the ribonuclease binase from bacillus pumilus in the RLS40 tumour model is associated with the reorganisation of the mirna network and reversion of cancer-related cascades to normal functioning. Biomolecules. 2020;10:1509. doi: 10.3390/biom10111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Galindo G, Castro J, Mates J, Bravo M, Ribó M, Vilanova M, Benito A. The selectivity for tumor cells of nuclear-directed cytotoxic rnases is mediated by the nuclear/cytoplasmic distribution of P27KIP1. Molecules. 2021;26:1319. doi: 10.3390/molecules26051319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla S, Bjerke GA, Muhlrad D, Yi R, Parker R. The RNase PARN controls the levels of specific miRNAs that contribute to p53 regulation. Mol Cell. 2019;73:1204–1216. e1204. doi: 10.1016/j.molcel.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]