Abstract
Interstitial pneumonia (IP) is a significant adverse effect of chemotherapy in B-cell non-Hodgkin’s lymphoma (NHL) patients. This study aimed to identify the clinical characteristics, risk factors, and treatment outcomes associated with IP in these patients. A retrospective review of 615 NHL patients treated at the Fourth Hospital of Hebei Medical University from 2016 to 2021 identified 50 patients with IP post-chemotherapy as the case group. A propensity score matched control group of 55 patients without pneumonia was established. Clinical profiles, risk factors, and treatment outcomes were evaluated. The IP incidence was 8.13% (50/615) in B-cell NHL patients. Multivariate analysis revealed liposomes, elevated lactate dehydrogenase (LDH), and erythrocyte sedimentation rate (ESR) as independent risk factors for IP. Receiver Operating Characteristic (ROC) curve analyses suggested that alterations in LDH and ESR could predict IP risk. The conclusion suggests that IP is associated with liposomal doxorubicin-induced lung injury and other cytotoxic chemotherapy, possibly due to Rituximab (RTX)-induced immune imbalance. Given the potential of IP with pulmonary infections, high-risk patients may need prophylactic antibiotics and appropriate corticosteroid therapy.
Keywords: B-cell non-Hodgkin’s lymphoma, interstitial pneumonia, post-chemotherapy, rituximab, risk factors
Introduction
B-cell non-Hodgkin’s lymphoma (NHL) is a highly heterogeneous malignancy that typically requires targeted first-line treatments, such as cyclophosphamide, doxorubicin, vincristine, and prednisone [1]. In China, B-cell NHL is the most prevalent type of lymphoma, comprising 45.8% of all NHL cases [2]. B-cell NHL is an aggressive cancer that often manifests with rapid disease progression, accompanied by local or systemic symptoms [3]. By the time of diagnosis, the disease has often advanced to stages III or IV, posing a significant risk to the patients’ health and lifespan [4]. The aggressive nature and rapid progression of B-cell NHL underscore the necessity for proactive monitoring and timely intervention to effectively manage the condition and mitigate its impact on the patients’ health and well-being.
As a first-line monotherapy or in combination with other regimens, rituximab (RTX) significantly improves the progression-free survival (PFS) and overall survival (OS) of patients with B-cell NHL [5]. With the widespread use of RTX, the incidence of RTX-associated interstitial pneumonitis (IP) is also on the rise [6]. Emerging evidence suggests that the incidence of RTX-related IP ranges from 3.7% to 16.7% [7]. Furthermore, patients often encounter secondary pulmonary infections due to compromised immunity and myeloid inhibition caused by the toxicity of chemotherapeutic drugs, complicating the management of the primary disease [8]. There is an increase in reports of pulmonary adverse reactions following B-cell NHL treatment, with IP being the most prevalent [9,10]. However, the correlated risk factors of IP in B-cell NHL patients following chemotherapy are yet to be fully understood.
Despite RTX, other factors have also been discovered as risk factors for IP in B-cell NHL patients following chemotherapy. However, most of these findings are from retrospective studies with small sample sizes and case reports. The accuracy of these results needs further validation [11,12]. A comprehensive understanding of the risk factors, prevention, and treatment of chemotherapy-related IP in B-cell NHL patients is still lacking. This study retrospectively analyzed the basic characteristics and treatment outcomes of 50 B-cell NHL patients who developed IP after chemotherapy, as well as 55 patients without IP at the Fourth Hospital of Hebei Medical University from January 2016 to October 2021. The objective of this study is to explore the risk factors of B-cell NHL-associated IP, enhancing clinicians’ ability for early identification, prevention, and treatment of IP following B-cell NHL chemotherapy, ultimately improving patient prognosis.
Methods
Study population
We retrospectively evaluated the clinical characteristics of 50 patients with B-cell NHL who developed IP after chemotherapy at the Fourth Hospital of Hebei Medical University from January 2016 to October 2021 (case group) and 55 patients without IP (control group). This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2021KY122). All procedures adhered to the ethical standards set by the committee and were in accordance with the 1975 Helsinki Declaration, as revised in 1983. Informed consent was waived due to the retrospective nature.
Inclusion criteria: (1) Patients with B-cell NHL initially diagnosed in the Department of Pathology at the Fourth Hospital of Hebei Medical University between January 2016 and October 2021; (2) Patients with complete clinical data; (3) Patients over 18 years of age who had received ≥ one cycle of chemotherapy; (4) Patients who developed IP during initial treatment (1 to 8 cycles of chemotherapy, with or without combined targeted therapy) were included in the case group; (5) Patients who did not develop IP during the initial treatment (1 to 8 cycles of chemotherapy, with or without combined targeted therapy) were included in the control group.
Exclusion criteria: (1) Patients whose pneumonia was not caused by chemotherapy-related factors. (2) Patients with recurrent or refractory diseases. (3) Patients with incomplete or misdiagnosed clinical data during the follow-up period. (4) Cases of primary NHL or NHL metastasized to the lung. (5) Patients who experienced a pulmonary infection prior to the onset of IP.
Diagnostic criteria of B cell NHL
The diagnosis of B cell non-Hodgkin’s lymphoma (NHL) in our study was established based on the latest edition of diagnostic criteria provided by the Chinese Clinical Oncology Association (CSCO) “Guidelines for the Diagnosis and Treatment of Lymphoma” [13]. The criteria recommend a multi-modal approach, including a thorough medical history review, physical examination, laboratory tests (e.g., complete blood count, bone marrow biopsy, and immunohistochemistry results), and imaging studies (e.g., ultrasound, CT scans, and MRI). The diagnostic criteria encompassed the following key components: (1) Clinical Presentation: Evaluation of symptoms such as lymphadenopathy, hepatosplenomegaly, weight loss, and fever; (2) Morphological Examination: Microscopic assessment of biopsy specimens from involved lymph nodes or extranodal sites to identify the presence of malignant B cells; (3) Immunophenotyping: Flow cytometric analysis of blood or bone marrow samples to characterize the immunological features of the lymphocytes; (4) Cytogenetic and Molecular Testing: Karyotype analysis and molecular genetic testing for specific genetic markers or mutations associated with B cell NHL. The diagnostic process also took into account the staging of the disease, which was conducted utilizing the Lugano staging standard version 2014.
Chemotherapy regimen
RTX, 375 mg/m2, was diluted and injected intravenously. Subsequently, to prevent infusion reactions to rituximab, 500 mg of acetaminophen, 20 mg of diphenhydramine, and 5 mg of dexamethasone were injected intravenously. For elderly patients presenting with respiratory symptoms or hypotension, 24-hour ECG monitoring was recommended. Liposomal doxorubicin, 25-40 mg/m2, was adjusted based on age and the occurrence of potential toxic reactions during treatment. In cases of leukopenia induced by chemotherapy, recombinant human granulocyte colony-stimulating factor (rhG-CSF) was administered depending on the severity of myeloid suppression. Patients with thrombocytopenia graded I-IV were treated with recombinant human thrombopoietin (RHTPO) and, if necessary, platelet transfusions. In the event of grade IV myelosuppression, antibiotics should be administered to prevent infections, and the patient should be transferred to a laminar flow ward to minimize the risk of serious complications. Symptomatic supportive care, tailored to the patient’s individual condition, was provided throughout the treatment period.
IP diagnostic criteria
The diagnosis of IP mainly relies on a combination of clinical symptoms, laboratory tests, imaging studies, and pathological examination. Common symptoms associated with IP include dyspnea, fever, and cough; additional symptoms such as expectoration may also exist. The onset of IP is typically gradual, although there are instances where patients experience acute hypersensitive pneumonia or conditions akin to acute respiratory distress syndrome. In this study, the diagnostic criteria for IP were mainly based on the findings from chest CT imaging, corroborated by clinical symptoms.
Statistical analysis
SPSS 25.0 software was used for statistical analysis. The χ2 test was employed to compare the two groups. The propensity matching method was used to match the two groups of patients with and without pneumonia. This matching ensured comparabilitiy in key factors such as gender, diagnosis, and tumor staging. It helped minimize the influence of confounding factors, making the comparison between the two groups more precise. The clinical features and hematological indexes were assessed through univariate analysis to identify potential risk factors for the incidence of IP. The independent risk factors of B cell NHL were determined by including the variables with P < 0.05 from the univariate analysis in the multivariate logistic regression equation. The findings from the analysis were integrated into R software to generate a Nomogram chart. ROC curves for changes in lactate dehydrogenase (LDH) and erythrocyte sedimentation rate (ESR) before and after chemotherapy in predicting IP were constructed. Statistical significance was established at P < 0.05.
Results
Patient characteristics
Table 1 provides a comprehensive overview of the demographic and clinical characteristics of the 105 patients diagnosed with B-cell NHL between May 2018 and October 2020. Univariate analysis revealed significant differences between the case group and the control group in the following aspects: age (≥ 55 years vs. < 55 years), presence of cardiopulmonary underlying diseases, and treatment regimens that included or excluded liposome doxorubicin and/or RTX (all P < 0.05). These findings highlight the potential impact of age, cardiopulmonary comorbidities, and specific treatment modalities on the clinical outcomes of B-cell NHL patients. The identified disparities underscore the importance of tailored therapeutic approaches and personalized care strategies for patients with B-cell NHL. These results emphasize the need for further investigation and consideration of these factors in clinical decision-making.
Table 1.
Clinical characteristics of patients in case group and control group
| Characteristics | Case group (n=50) | Control group (n=55) | P value |
|---|---|---|---|
| Age | 0.000 | ||
| > 55 | 28 | 25 | |
| ≤ 55 | 22 | 30 | |
| Gender | 0.249 | ||
| Male | 24 | 27 | |
| Female | 26 | 28 | |
| Basic cardiopulmonary diseases | 0.005 | ||
| Yes | 31 | 29 | |
| No | 19 | 26 | |
| Diagnosis | 0.773 | ||
| DLBCL | 34 | 37 | |
| Non-DLBCL | 16 | 18 | |
| Disease stage | 0.067 | ||
| limited stage | 17 | 19 | |
| Progressive stage | 33 | 36 | |
| Treatment | |||
| Including RTX | 39 | 41 | 0.000 |
| Not including RTX | 11 | 14 | |
| Liposome containing doxorubicin | 30 | 36 | 0.001 |
| Liposome-free doxorubicin | 20 | 19 |
Note: DLBCL: Diffuse large B-cell lymphoma; RTX: Rituximab.
Hematological indicators of patients
Table 2 demonstrates the the changes in hematological indicators for the 105 patients with B-cell NHL before and after chemotherapy. “Increase” indicates that the difference in the index before and after chemotherapy was positive; “Non-increase” means that the difference of the index before and after chemotherapy was negative or zero; “decrease” means that the difference of this index before and after chemotherapy was negative; and non-decrease indicates the difference of this index before and after chemotherapy was negative or zero. The results of univariate analysis showed that there were significant differences between the two groups in the following aspects: LDH change and ESR change of patients before and after chemotherapy (all P < 0.05, Table 2).
Table 2.
Univariate analysis of hematological indexes in patients before and after chemotherapy
| Hematological indicators | Case group (n=50) | Control group (n=55) | P value |
|---|---|---|---|
| LDH | 0.030 | ||
| Increase | 28 | 29 | |
| Non-increase | 22 | 26 | |
| β2-MG | 0.774 | ||
| Increase | 34 | 38 | |
| Non-increase | 16 | 27 | |
| ESR | 0.000 | ||
| Increase | 42 | 38 | |
| Non-increase | 8 | 19 | |
| Plasma albumin | 0.773 | ||
| Decrease | 34 | 37 | |
| Non-decrease | 16 | 18 | |
| Plasma globulin | 0.705 | ||
| Increase | 14 | 17 | |
| Non-increase | 36 | 38 | |
| leukocyte count | 0.668 | ||
| Increase | 29 | 28 | |
| Non-increase | 21 | 27 | |
| Absolute value of lymphocytes | 0.692 | ||
| Increase | 11 | 10 | |
| Non-increase | 39 | 40 | |
| Medullary inhibition | 0.063 | ||
| I-II degree | 32 | 34 | |
| III-IV degree | 18 | 21 | |
| Hemoglobin | 0.860 | ||
| Diminished | 15 | 20 | |
| Undiminished | 35 | 30 |
Note: Increase: the difference of this index before and after chemotherapy was positive; Non-increase/non-decrease: the difference of this index before and after chemotherapy was negative or zero; Decrease: the difference of this index before and after chemotherapy was negative.
Multivariate regression analysis of independent risk factors for IP
Multivariate regression analysis was conducted to further explore the relationship between clinical characteristics, hematological indexes, and the occurrence of immune paresis (IP) in patients diagnosed with B-cell NHL. The results showed that the chemotherapy regimen containing liposome doxorubicin (OR=3.960, 95% CI: 1.104-5.419), increased LDH after chemotherapy (OR=3.114, 95% CI: 1.428-7.595), and increased ESR (OR=5.587, 95% CI: 2.218-13.672) after chemotherapy were independent risk factors of IP in B-cell NHL patients (Table 3). These findings underscore the importance of considering specific chemotherapy regimens and monitoring post-chemotherapy changes in LDH and ESR levels as potential indicators for IP.
Table 3.
Multivariate logistic regression analysis of independent risk factors for IP
| Factors | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| LDH (Increase) | 0.856 | 0.459 | 7.574 | 0.001 | 3.114 | 1.428-7.595 |
| LDH (Non-increase) | 1.339 | 0.451 | 6.055 | 0.527 | 3.672 | 1.985-6.447 |
| β2-MG (Increase/Non-increase) | 0.682 | 0.249 | 4.528 | 0.133 | 4.550 | 2.334-7.958 |
| ESR (Increase) | 1.975 | 0.551 | 4.907 | 0.004 | 5.587 | 2.218-13.672 |
| ESR (Non-increase) | 0.779 | 0.593 | 3.669 | 0.598 | 2.330 | 1.409-5.677 |
| Plasma albumin (Decrease/Non-decrease) | 0.998 | 0.698 | 3.556 | 0.087 | 1.378 | 0.698-4.330 |
| leukocyte count (Increase/Non-increase) | 0.807 | 0.446 | 5.609 | 0.078 | 2.356 | 1.509-5.669 |
| Absolute value of lymphocytes (Increase/Non-increase) | 1.335 | 0.871 | 3.495 | 1.044 | 3.409 | 2.330-6.409 |
| Medullary inhibition (I-II degree/III-IV degree) | 0.648 | 0.450 | 4.597 | 0.948 | 4.559 | 1.449-5.499 |
| Liposome doxorubicin | 1.533 | 0.550 | 7.662 | 0.000 | 3.960 | 1.104-5.419 |
| Hemoglobin (Diminished/Undiminished) | 0.556 | 0.456 | 5.697 | 0.459 | 2.449 | 1.009-5.650 |
| RTX | 1.841 | 0.7883 | 4.558 | 0.032 | 2.558 | 1.456-10.664 |
Note: B: regression coefficient; SE: standard error; OR: odds ratio; 95% CI: 95% confidence interval.
Construction of a nomogram model
In univariate analysis, liposome doxorubicin, LDH and ESR were significantly associated with the risk of IP in patients with B-cell NHL. Subsequently, liposome doxorubicin, LDH and ESR were included in the final nomogram model. The results of the Nomogram are demonstrated in Figure 1.
Figure 1.
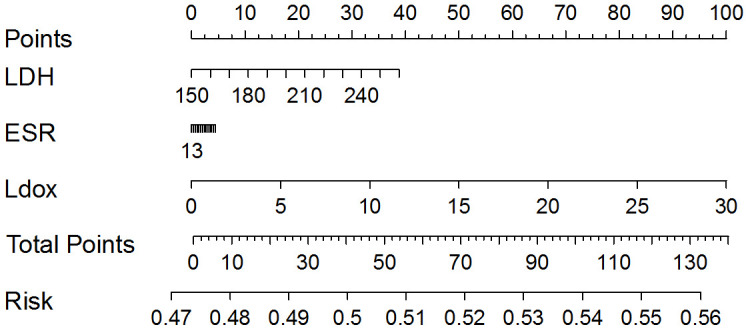
Nomogram for predicting the risk of IP in patients with B-cell non-Hodgkin’s lymphoma. LDH: lactate dehydrogenase; ESR: erythrocyte sedimentation rate; Ldox: liposome doxorubicin.
Cumulative incidence of IP
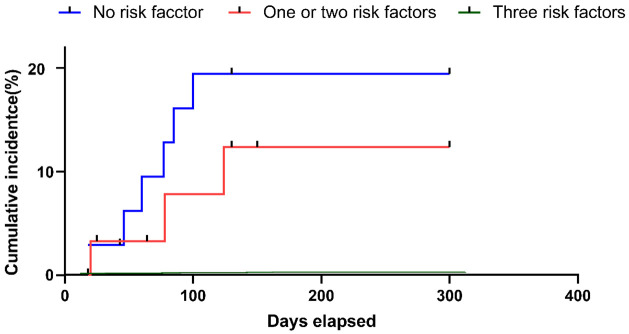
Patients were categorized based on their risk factors: individuals without risk factors were classified as low-risk, those with one to two factors as intermediate-risk, and those with three factors as high-risk. The occurrence of IP differed across the groups, as demonstrated in Figure 2 (low risk: 0%; intermediate risk: 10.3%; high risk: 20.5%; P = 0.059). Further analysis is warranted to validate these risk categories and to explore the potential for incorporating them into clinical decision-making processes. This could aid in the early identification and proactive management of patients at higher risk of developing IP, thereby potentially improving patient outcomes and minimizing the impact of this complication on treatment efficacy.
Figure 2.
The accumulated incidence of IP varied by the count of risk factors. IP, interstitial pneumonia.
Predictive effect of LDH on the occurrence of IP
ROC curve analysis showed that the area under the curve (AUC) for changes in LDH level after chemotherapy in predicting the occurrence of IP was significant (P < 0.05, Table 4 and Figure 3), suggesting that the LDH has certain diagnostic value in predicting the occurrence of IP.
Table 4.
Evaluation of diagnostic value of LDH
| Indicator | AUC | 95% CI | p | Sensitivity (%) | Specificity (%) | Cut-off value |
|---|---|---|---|---|---|---|
| LDH | 0.596 | 0.509-0.679 | 0.036* | 67 | 55.6 | 1.1 |
P < 0.05.
AUC: area under the curve; 95% CI: 95% confidence interval; LDH: lactate dehydrogenase.
Figure 3.
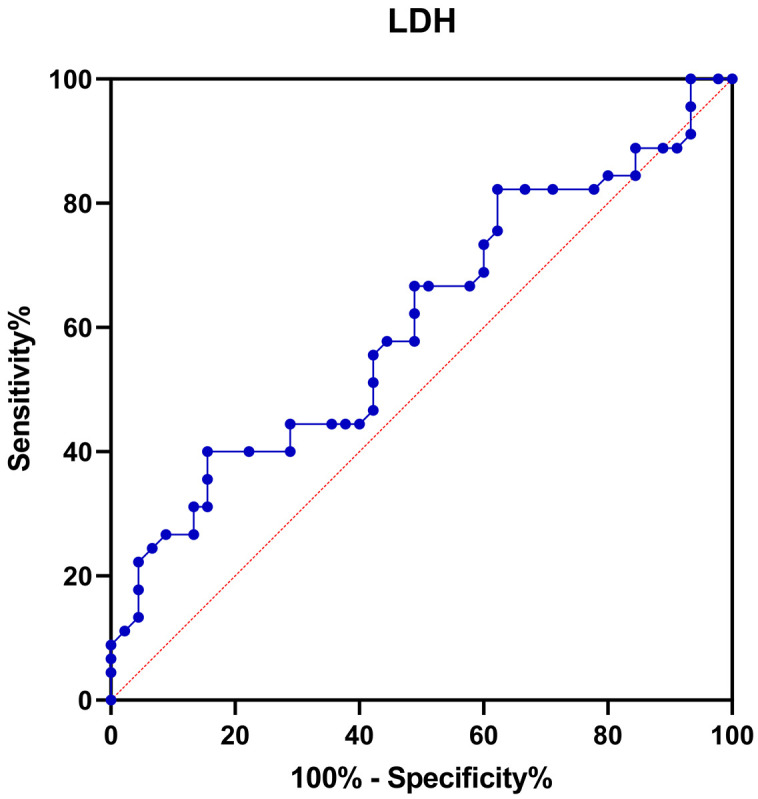
ROC curve for changes in LDH level in predicting the occurrence of IP in B-cell NHL patients after chemotherapy. ROC: receiver operating characteristic; LDH: lactate dehydrogenase; IP: interstitial pneumonia; B-cell NHL: B-cell Non-Hodgkin’s Lymphoma.
Predictive effect of ESR on the occurrence of IP
ROC curve analysis exhibited that the AUC for changes in ESR after chemotherapy in predicting the occurrence of IP was significant (P < 0.05, Table 5 and Figure 4). The diagnostic performance of ESR was superior to that of LDH, with an AUC of 0.789. The cutoff value was 10.5, with a specificity of 83.6% and a sensitivity of 68.0%. This suggests that IP might develop after chemotherapy in cases where the ESR value is ≥ 11.5 mm/h.
Table 5.
Evaluation of diagnostic value of ESR
| Indicator | AUC | 95% CI | p | Sensitivity (%) | Specificity (%) | Cut-off value |
|---|---|---|---|---|---|---|
| ESR | 0.789 | 0.735-0.895 | 0.000* | 68 | 83.6 | 10.5 |
P < 0.05.
AUC: area under the curve; 95% CI: 95% confidence interval; ESR: erythrocyte sedimentation rate.
Figure 4.
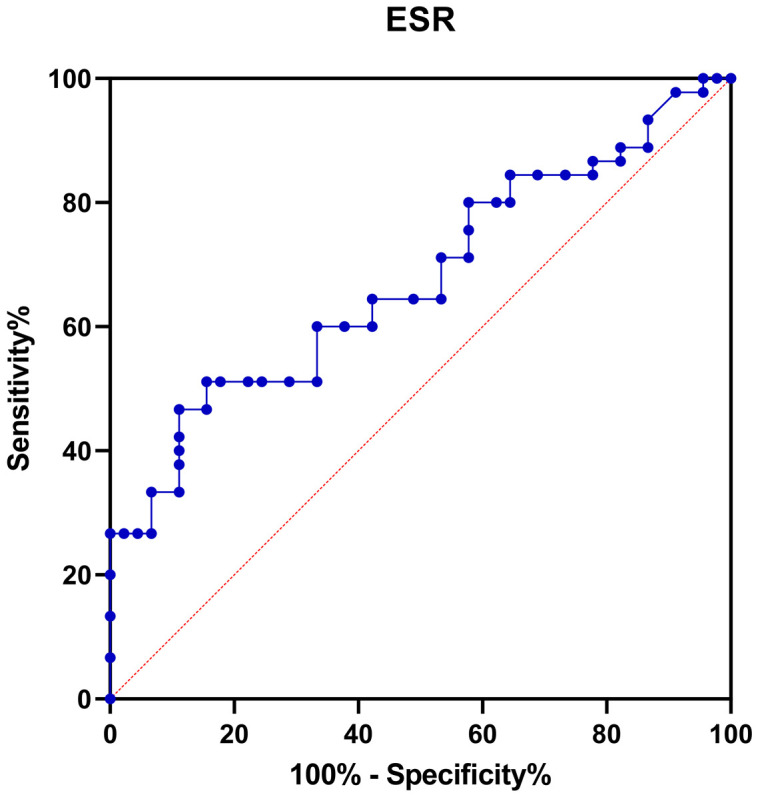
ROC curve for changes in ESR level in predicting the occurrence of IP in B-cell NHL patients after chemotherapy. ESR: erythrocyte sedimentation rate; ROC: receiver operating characteristic; B-cell NHL: B-cell Non-Hodgkin’s Lymphoma; IP: interstitial pneumonia.
Clinical characteristics and treatment outcome of case group
After radiotherapy, sixteen patients (32%) were asymptomatic, and follow-up observation revealed a stable condition in these individuals. Of the patients who underwent IP treatment, treatment approaches varied: 10 cases received antibiotics alone, 15 were treated with glucocorticoids, six cases received a combination of glucocorticoids and antibiotics, and three cases received glucocorticoids exclusively. Following anti-inflammatory treatment, symptoms were significantly alleviated or conditions were controlled in the majority of patients (86%). However, two patients (4%) passed away due to respiratory failure secondary to IP. These patients were elderly males over the age of 65, all categorized as high risk and with a history of smoking for over 20 years. Notably, these patients exhibited apparent symptoms, including cough, expectoration, chest tightness, shortness of breath, and fever, with the highest body temperature reaching up to 39.6°C (Table 6).
Table 6.
Characteristics and treatment outcome of case group
| Hematological indicators | Number of cases |
|---|---|
| Fever | |
| Yes | 28 |
| No | 22 |
| Symptom | |
| Yes | 34 |
| No | 16 |
| The pathogen of co-infection | |
| Fungus | 13 |
| Bacteria | 14 |
| Fungus and Bacteria | 5 |
| Sporotrichosis | 2 |
| IP treatment | |
| Contains glucocorticoids | 15 |
| Glucocorticoid alone | 3 |
| Unused corticosteroids | 11 |
| Antibiotics alone | 10 |
| Glucocorticoids and antibiotics | 6 |
| Untreated | 5 |
| Outcome | |
| Improvement or stable control | 43 |
| Disease progression | 5 |
| Death | 2 |
Note: IP, interstitial pneumonia.
Discussion
The treatment of B-cell NHL varies greatly, depending on the stage of the tumor, the grade and type of lymphoma, and various patient factors [14]. B-cell NHL patients who undergo chemotherapy are at risk of developing various respiratory complications [15]. The literature suggests a significant increase in the incidence of adverse respiratory events following chemotherapy [16]. The direct toxic effects of chemotherapy agents on the lung parenchyma, the systemic immunosuppression induced by these treatments, and the potential exacerbation of pre-existing pulmonary conditions are contributing factors. Moreover, the concurrent use of corticosteroids, often prescribed with chemotherapy to reduce inflammation and tissue damage, can complicate the respiratory situation by suppressing the immune response and potentially increasing the risk of opportunistic infections. In the setting of immune paresis, characterized by an impaired immune response, patients may be at increased risk for developing noninfectious interstitial pneumonias (NIIP) or infectious interstitial pneumonias (IIP), each with distinct clinical presentations and management approaches [17]. Emerging evidence suggests that the incidence of IP in NHL patients receiving chemotherapy is approximately 6.9% [9]. In this study, the incidence of IP in B-cell NHL patients after chemotherapy was 8.13% (50/615), which is consistent with a previous study [10]. This highlights the importance of monitoring for respiratory complications and managing them proactively in patients undergoing chemotherapy for B-cell NHL.
Vicente-Rabaneda et al. demonstrated that RTX-related IP predominantly occurred in male patients and was most frequent in individuals aged 50-60 [18], which aligns with the finding in this study that the majority of the patients with IP were over the age of 55. Vacchi et al. [19] compared the occurrence of IP after treatment with RCHOP and RCDOP regimen for diffuse large B-cell lymphoma (DLBCL), and found that the disease stage before treatment was an independent risk factor for poor prognosis, which was further confirmed by Liossis et al. [20]. However, in this study, there was no significant difference in the distribution of disease stages between the two groups. In conclusion, although our findings are generally consistent with the existing literature on IP in DLBCL patients, the lack of a significant difference in disease stage distribution and the predominance of older patients are noteworthy exceptions. These findings emphasize the complexity of IP and the importance of considering multiple factors, such as age and disease stage, when designing clinical trials and treatment plans for IP patients. Additional research with increased sample sizes and long-term tracking is required to fully grasp the implications of these findings and to enhance the prognosis for patients suffering from IP. Comprehensive, long-term follow-up studies are crucial to confirm these results and to provide guidance for clinical interventions in the treatment of IP associated with DLBCL.
A multivariate analysis showed that low serum albumin levels were associated with pulmonary complications [20]. Sunny et al. retrospectively analyzed the correlation between RTX-related IP and clinical outcomes in 6 patients with CD20+ B cell lymphoma, and the results showed that the increased LDH level was a risk factor of RTX-related IP, but the sample size of this study was small, and the bias was large [21]. The accuracy of the results needs to be verified by large-scale clinical studies. In this study, 50 patients with IP were studied, the sample size was relatively large, and by comparing LDH levels before and after chemotherapy, we concluded that the increase in LDH after chemotherapy was an independent risk factor for the occurrence of IP. The implications of such findings could inform clinical practice by highlighting the potential role of LDH as a predictive biomarker for the occurrence of IP in patients undergoing chemotherapy for CD20+ B cell lymphoma.
The National Comprehensive Cancer Network (NCCN) guidelines recommend that the RCDOP regimen containing liposomal doxorubicin (combined with RTX, cyclophosphamide, vincristine, and prednisone) is the standard first-line treatment for DLBCL patients with left ventricular insufficiency and poor tolerance [22]. Experts have reached a consensus on the use of liposomal doxorubicin in treating lymphoma. Liposomal doxorubicin is a formulation of the chemotherapy drug doxorubicin encapsulated within liposomes, allowing for targeted drug delivery to cancer cells while reducing toxicity to healthy tissues [23]. However, with the wide application of liposome doxorubicin, the occurrence of IP is increasing [24]. Data from a phase II clinical trial showed that the incidence of IP in multiple myeloma patients after liposomal doxorubicin combined chemotherapy was 15%, suggesting that the use of liposomal doxorubicin is a significant risk factor for the occurrence of IP [25]. Patients treated with liposomal doxorubicin need to strengthen the prevention, monitoring, and supportive treatment of IP, which requires special attention of clinicians. In addition, the pulmonary toxicity of targeted drugs commonly used in patients with B-cell NHL, such as rituximab (RTX), is also reported frequently. RTX, a human-mouse chimeric anti-CD20 monoclonal antibody, has demonstrated efficacy and a favorable safety profile in patients with various CD20- expressing lymphoid malignancies [26]. Despite its recognized efficacy and good safety profile in patients with CD20- expressing lymphoid malignancies, RTX is not free of potential for respiratory complications. Clinicians must be vigilant and proactive in managing the pulmonary risks associated with these treatments to ensure the best outcomes for patients with B-cell NHL.
In clinical practice, B-cell NHL patients treated with a combination of RTX or liposomal doxorubicin should be thoroughly assessed for the possibility of IP by monitoring dynamic changes in hematological indexes before and after chemotherapy, particularly LDH and ESR, clinical symptoms such as fever exceeding 38.0°C, cough, chest tightness, and shortness of breath, as well as imaging findings. This approach aims to facilitate early prevention, diagnosis, and treatment of IP, thereby avoiding delays in treating the primary disease and impacting patient survival outcomes. Patients diagnosed with mild IP, based on asymptomatic presentation, physical signs, and imaging findings, can undergo follow-up and continue treatment as per the original plan. Our study revealed that about 68% (34/50) of B-cell NHL patients developed IP complicated by infections post-chemotherapy, indicating a significantly elevated risk of pulmonary infections due to lung injury during IP. Hormone and antibiotic therapies should be administered based on individual’s condition. These findings suggests that a significant proportion of B-cell NHL patients experience complications related to infection after chemotherapy, which is a concerning issue considering its potential impact on treatment outcomes and patient recovery. Post-chemotherapy infections, particularly pulmonary infections, can be a serious concern for patients with NHL, as chemotherapy can compromise the immune system to fight off pathogens. The development of infections in these patients may be exacerbated by lung injury caused by the cancer itself or by the cytotoxic effects of the chemotherapy drugs. The recommendation to administer hormone and antibiotic therapies based on individual’s condition is a key aspect of managing these complications. This personalized approach takes into account the patient’s overall health, the severity of the infection, the potential for antibiotic resistance, and the patient’s previous treatment history. Clinicians should be vigilant to the potential for interstitial pneumonia (IP) in patients with B-cell NHL treated with RTX or liposomal doxorubicin, and should closely monitor hematological parameters, clinical symptoms, and imaging findings to facilitate early detection and management of IP. The development of IP can increase the risk of post-chemotherapy infections, particularly pulmonary infections, highlighting the need for personalized treatment strategies to mitigate these risks and preserve patient outcomes.
Our analysis has several limitations. Firstly, the sample size is small, which hinders our ability to predict the risk factors of IP accurately. Therefore, it is necessary to conduct larger-scale research to address this significant issue. Additionally, this study did not analyze the survival prognosis of patients with IP, making it impossible to determine the impact of IP on the survival and prognosis of patients. However, previous studies have indicated that the occurrence of IP can significantly impact the disease outcome and increase the risk of mortality [27-29]. In this study, 2 out of the 50 patients died, resulting in a mortality rate of 4%, which further validated these findings.
In conclusion, chemotherapy-induced pneumonia is a common complication during the comprehensive treatment of lymphoma patients, potentially impacting and even disrupting further disease management. Our study found that the incidence of IP in B-cell NHL patients after chemotherapy was 8.13% (50/615). Multivariate analysis revealed that IP was more frequently observed B-cell NHL patients receiving treatment regimens that included liposomal doxorubicin, with the use of this medication being identified as an independent risk factor for IP. Additionally, increased LDH and/or ESR levels were also independent risk factors for IP in B-cell NHL patients during treatment, with ROC curve analysis proving their predictive value for the occurrence of IP. Therefore, refining the diagnosis of drug-induced pneumonia, evaluating the impact of drug-induced lung injury on pneumonia, and developing a reliable threshold for predicting pneumonia will be key areas of focus for future investigations.
Acknowledgements
This work was supported by the Hebei Provincial Health Commission Scientific Research Fund Project “Risk Assessment and Prevention of Pneumonia after Chemotherapy for Diffuse Large B-Cell Lymphoma” (No. 20220165).
Disclosure of conflict of interest
None.
References
- 1.Armengol M, Santos JC, Fernández-Serrano M, Profitós-Pelejà N, Ribeiro ML, Roué G. Immune-checkpoint inhibitors in B-cell lymphoma. Cancers (Basel) 2021;13:214. doi: 10.3390/cancers13020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pi M, Kuang H, Yue C, Yang Q, Wu A, Li Y, Assaraf YG, Yang DH, Wu S. Targeting metabolism to overcome cancer drug resistance: a promising therapeutic strategy for diffuse large B cell lymphoma. Drug Resist Updat. 2022;61:100822. doi: 10.1016/j.drup.2022.100822. [DOI] [PubMed] [Google Scholar]
- 3.Wang SS, Vajdic CM, Linet MS, Slager SL, Voutsinas J, Nieters A, Casabonne D, Cerhan JR, Cozen W, Alarcón G, Martínez-Maza O, Brown EE, Bracci PM, Turner J, Hjalgrim H, Bhatti P, Zhang Y, Birmann BM, Flowers CR, Paltiel O, Holly EA, Kane E, Weisenburger DD, Maynadié M, Cocco P, Foretova L, Breen EC, Lan Q, Brooks-Wilson A, De Roos AJ, Smith MT, Roman E, Boffetta P, Kricker A, Zheng T, Skibola CF, Clavel J, Monnereau A, Chanock SJ, Rothman N, Benavente Y, Hartge P, Smedby KE. B-cell NHL subtype risk associated with autoimmune conditions and PRS. Cancer Epidemiol Biomarkers Prev. 2022;31:1103–1110. doi: 10.1158/1055-9965.EPI-21-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu T, Hu L, Zhang Y, Wang Y, Ma S, Li D, Li Z, Xu K. Cytopenia after CAR-T cell therapy: analysis of 63 patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Oncol Lett. 2023;26:338. doi: 10.3892/ol.2023.13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Z, Zhang Y, Wang X. Advances in chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Biomark Res. 2021;9:58. doi: 10.1186/s40364-021-00309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Chai L, Jia J, Su L, Hao Z. Meta-analysis of risk factors and incidence of Interstitial pneumonia with CHOP-Like regimens for Non-Hodgkin lymphoma. Front Oncol. 2022;12:880144. doi: 10.3389/fonc.2022.880144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadura S, Raghu G. Antineutrophil cytoplasmic antibody-associated interstitial lung disease: a review. Eur Respir Rev. 2021;30:210123. doi: 10.1183/16000617.0123-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang L, Li J, Zhao Q, Pan T, Zhong H, Wang W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics. 2021;13:1151. doi: 10.3390/pharmaceutics13081151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Lu F, Lei T, Yu H, Yang H. Clinical features, treatment and risk factors for interstitial pneumonia in B-cell non-Hodgkin lymphoma patients. Transl Cancer Res. 2020;9:5139–5146. doi: 10.21037/tcr-20-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Lu F, Lei T, Yu H, Chen X, Peng S, Han S, Yang H. Prophylactic antibiotic treatment with TMP-SMX decreased the incidence of interstitial pneumonia in patients with B-cell lymphoma on chemotherapy. BMC Cancer. 2020;20:742. doi: 10.1186/s12885-020-07254-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanguedolce F, Zanelli M, Zizzo M, Bisagni A, Soriano A, Cocco G, Palicelli A, Santandrea G, Caprera C, Corsi M, Cerrone G, Sciaccotta R, Martino G, Ricci L, Sollitto F, Loizzi D, Ascani S. Primary pulmonary B-cell lymphoma: a review and update. Cancers (Basel) 2021;13:415. doi: 10.3390/cancers13030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodina Y, Deripapa E, Shvets O, Mukhina A, Roppelt A, Yuhacheva D, Laberko A, Burlakov V, Abramov D, Tereshchenko G, Novichkova G, Shcherbina A. Rituximab and abatacept are effective in differential treatment of interstitial lymphocytic lung disease in children with primary immunodeficiencies. Front Immunol. 2021;12:704261. doi: 10.3389/fimmu.2021.704261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oncology Society of Chinese Medical Association; Chinese Medical Association Publishing House. Oncology Society of Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2021 edition) Zhonghua Zhong Liu Za Zhi. 2021;43:591–621. doi: 10.3760/cma.j.cn112152-20210207-00118. [DOI] [PubMed] [Google Scholar]
- 14.Moleti ML, Testi AM, Foà R. Treatment of relapsed/refractory paediatric aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2020;189:826–843. doi: 10.1111/bjh.16461. [DOI] [PubMed] [Google Scholar]
- 15.Marofi F, Rahman HS, Achmad MH, Sergeevna KN, Suksatan W, Abdelbasset WK, Mikhailova MV, Shomali N, Yazdanifar M, Hassanzadeh A, Ahmadi M, Motavalli R, Pathak Y, Izadi S, Jarahian M. A deep insight into CAR-T cell therapy in non-hodgkin lymphoma: application, opportunities, and future directions. Front Immunol. 2021;12:681984. doi: 10.3389/fimmu.2021.681984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Singh R, Shaik S, Negi BS, Rajguru JP, Patil PB, Parihar AS, Sharma U. Non-Hodgkin’s lymphoma: a review. J Family Med Prim Care. 2020;9:1834–1840. doi: 10.4103/jfmpc.jfmpc_1037_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackintosh JA, Wells AU, Cottin V, Nicholson AG, Renzoni EA. Interstitial pneumonia with autoimmune features: challenges and controversies. Eur Respir Rev. 2021;30:210177. doi: 10.1183/16000617.0177-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente-Rabaneda EF, Serra López-Matencio JM, Ancochea J, Blanco R, González-Gay M, Castañeda S. Efficacy and safety of biological drugs in interstitial lung disease associated with connective tissue diseases. Expert Opin Drug Saf. 2022;21:311–333. doi: 10.1080/14740338.2021.1973428. [DOI] [PubMed] [Google Scholar]
- 19.Vacchi C, Manfredi A, Cassone G, Erre GL, Salvarani C, Sebastiani M. Efficacy and safety of rituximab in the treatment of connective tissue disease-related interstitial lung disease. Drugs Context. 2021;10 doi: 10.7573/dic.2020-8-7. 2020-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liossis SC, Bounia CA. Treating autoimmune-related interstitial lung disease with B cell depletion. Front Med (Lausanne) 2022;9:937561. doi: 10.3389/fmed.2022.937561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chisari CG, Sgarlata E, Arena S, Toscano S, Luca M, Patti F. Rituximab for the treatment of multiple sclerosis: a review. J Neurol. 2022;269:159–183. doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Chen R, Zhou D, Sun J, Wang L, Zhu L, Shen H, Xie W, Ye X. The efficacy and cardiac toxicity of different-dose pegylated liposomal doxorubicin in elderly patients with diffuse large B lymphoma. Cancer Med. 2023;12:4184–4194. doi: 10.1002/cam4.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szebeni J, Kiss B, Bozó T, Turjeman K, Levi-Kalisman Y, Barenholz Y, Kellermayer M. Insights into the structure of comirnaty covid-19 vaccine: a theory on soft, partially bilayer-covered nanoparticles with hydrogen bond-stabilized mRNA-lipid complexes. ACS Nano. 2023;17:13147–13157. doi: 10.1021/acsnano.2c11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nara K, Taguchi A, Yamamoto T, Tsuruga T, Tojima Y, Miyamoto Y, Tanikawa M, Sone K, Mori M, Takada T, Suzuki H, Osuga Y. Efficacy of regional cooling + oral dexamethasone for primary prevention of hand-foot syndrome associated with pegylated liposomal doxorubicin. Support Care Cancer. 2023;31:283. doi: 10.1007/s00520-023-07718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Zhu Y, Tang J, Xu C, Li J, He S, Zhang Z, Wu P, Luo L, Guo Q, Li F, Ren Y, Yu S, Li R, Li L. Not all bad: drug-induced interstitial pneumonia in DLBCL patients is potentially fatal but could be linked to better survival. Leuk Res. 2021;111:106688. doi: 10.1016/j.leukres.2021.106688. [DOI] [PubMed] [Google Scholar]
- 26.Terheggen-Lagro SWJ, Haarman EG, Rutjes NW, van den Berg JM, Schonenberg-Meinema D. Rituximab in idiopathic pulmonary hemosiderosis in children: a novel and less toxic treatment option. Pharmaceuticals (Basel) 2022;15:1549. doi: 10.3390/ph15121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jo JC, Jeon Y, Kim D, Yang DH, Lee WS, Choi YS, Yi JH, Yoon DH, Kong JH, Choe JY, Kim S, Ahn K, Park T, Ju H, Kwon S, Cho SG. A non-interventional, post-marketing surveillance study evaluating the safety and effectiveness of biosimilar rituximab (CT-P10) during routine clinical practice in the Republic of Korea. Expert Opin Biol Ther. 2023;23:737–747. doi: 10.1080/14712598.2023.2177101. [DOI] [PubMed] [Google Scholar]
- 28.Xia F, Liu H, Zhang H, Ping N, Wang P, Jin Z, Zhu J, Qu C. Multiple blood parameters may serve as a warning to immunochemotherapy-related interstitial lung disease in B-cell lymphoma. Ann Palliat Med. 2021;10:9660–9668. doi: 10.21037/apm-21-2027. [DOI] [PubMed] [Google Scholar]
- 29.Oguri T, Sasada S, Aramaki-Sumii Y, Tsuchiya Y, Ishioka K, Takahashi S, Kunieda H, Kimura Y, Seki R, Hirose S, Nakamura M. A case of intravascular diffuse large B-cell lymphoma initially suspected as interstitial pneumonia associated with systemic scleroderma. J Investig Med High Impact Case Rep. 2021;9:2324709621999226. doi: 10.1177/2324709621999226. [DOI] [PMC free article] [PubMed] [Google Scholar]