Abstract
Recent studies have shown that circular RNAs (CircRNAs) have the novel functions and molecular mechanisms in the pathogenesis of malignant diseases. CircRNAs have been found to be associated with the occurrence and development of lymphoproliferative diseases, impacting on lymphocyte proliferation. This article provides a review of the pathogenesis of circRNAs in malignant lymphoproliferative disorders, focusing on conditions such as acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), and lymphoma. Additionally, it discusses the potential value of circRNAs as novel biomarkers or therapeutic targets in these disorders.
Keywords: circRNAs, lymphoproliferative diseases, biomarkers, ceRNAs
Introduction
NcRNAs and circRNAs
The rapid development of high-throughput technologies, such as RNA sequencing (RNA-seq), has provided us the opportunity to explore the true role of non-coding gene product in various types of cancer [1]. Although non-coding RNAs (ncRNAs) do not have encode effects, they have an indispensable role in the regulation of gene expression, including RNA splicing, transcription or translation [2,3]. The role of ncRNAs in the pathogenesis of human diseases was first discovered in chronic lymphocytic leukemia (CLL) [4], and it has been reported to influence the metabolism of many tumors due to its specific biological function [5].
Circular RNA (CircRNAs) has attracted researchers’ attention in recent years as an important subtype of ncRNAs with novel functions and molecular mechanisms in malignant diseases [6]. Various circRNAs appear to be specifically expressed in particular cell type or developmental stage [3], and they are abundantly in various tissues, peripheral blood [7], and even saliva [8]. They derive from precursor RNAs or catalyzed by group I and II ribozyme [9-11]. Initially, considered as byproducts of transcription and regarded as “waste sequences” without specific biological functions, circRNAs were primarily thought to be located in the cytoplasm [12-14]. Characterized by a covalent closed-loop structure joining the upstream splice acceptor site and the downstream splice donor site [3,15], circRNAs lack 5’ to 3’ polarity and a polyadenylated tail to gain the remarkable stability, resisting to RNA enzymes degradation and are shown to have longer half-lives than other linear RNA in vivo [11,16-18]. Based on their formation patterns, circRNAs can be categorized three types: Exonic circRNAs (ecircRNA) [19], exon-intron circRNAs (eicircRNA) [20], and circularized intron RNA (ciRNA) [21]. Moreover, circRNAs are highly conserved in evolution and tissue-specific in expression [11].
CircRNAs and malignant tumors
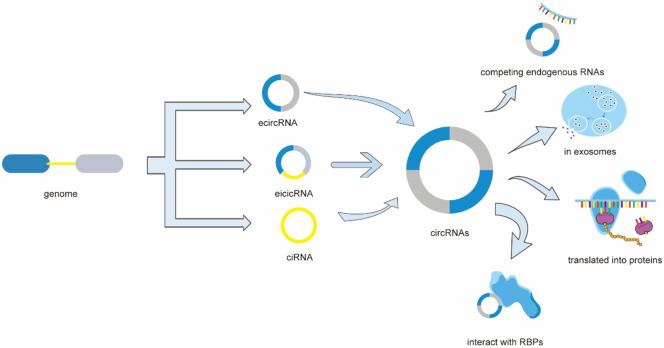
CircRNAs have a significant impact on diverse biological processes such as cell multiplication or migration [22,23], cell cycle progression [24], cell apoptosis [25], mirroring their roles in various neoplasms. CircRNAs can interact with RNA binding proteins (RBPs) [26,27], and a few circRNAs containing short open reading frames (ORF) can be translated into specific functional proteins [28]. In certain cancers, circRNAs have also been found in exosomes [29,30] (Figure 1).
Figure 1.
CircRNAs can be categorized three types: Exonic circRNAs (ecircRNA), exon-intron circRNAs (eicircRNA), and circularized intron RNA (ciRNA). CircRNAs can interact with RNA binding proteins (RBPs), and a few circRNAs containing short open reading frames (ORF) can be translated into specific functional proteins. In certain cancers, circRNAs have also been found in exosomes. Importantly, some circRNAs can indirectly regulate gene expression by acting as competing endogenous RNA (ceRNAs) through sponging microRNAs (miRNAs).
Unlike circRNAs, microRNAs (miRNAs), another linear subtype of ncRNAs, are more unstable [31], and are approximately 18-25 nucleotides in length [32,33]. They are from the purposeful expression of the organism’s own genome, negatively regulating gene expression at the post-transcriptional level by degrading mRNAs or inhibiting translation through interactions with 3’-untranslated regions (UTR) of specific mRNAs, acting as tumor suppressors or oncogenes and serving as potential cancer biomarkers [33-36]. Single-stranded form of miRNA can bind with Argonaute protein (AGO) to form effector assemblies, RNA-induced silencing complex (RISC), and targeting mRNA through complementary base pairing that called microRNA recognition elements (MRE) [36,37], acting mainly in the cytoplasm. With MRE, RNAs can regulate each other, which is known as competing endogenous RNA (ceRNA) hypothesis [37].
Some circRNAs could also indirectly regulate gene expression by acting as ceRNAs through sponging miRNAs [34,38]. CircRNA possess numerous seed-binding sites for miRNAs, thereby enabling them to engage with specific miRNA molecules and form stable complexes. This interaction is highly specific, contingent upon the complementary base pairing between the circRNA and miRNA sequences [39]. Upon adsorption of miRNA by circRNA, the miRNA is sequestered, rendering it incapable of binding to its canonical target mRNAs. This sequestration effectively shields the target mRNAs from miRNA-mediated degradation or translational repression. Consequently, circRNAs enhance the stability of these target mRNAs and/or augment protein expression levels through a mechanism of competitive inhibition [39].
Beyond their established role in modulating gene expression, circRNAs may also engage in protein-level regulation by encoding micro peptides with functional significance [40]. Notably, research has demonstrated that circDIDO1 impedes the progression of gastric cancer. It achieves this by encoding a novel 529-amino acid DIDO1 protein and by modulating the stability of the PRDX2 protein, thereby exerting its tumor-suppressive effects [41].
We have demonstrated the key role of circRNA-miRNA-mRNA network in many malignant disease [42], and numerous studies have highlighted dysregulated levels of circRNAs in various diseases, especially tumors [43]. For example, has_circRNA_100290, significantly upregulated, may act as a sponge of miR-136-5p to promote laryngeal squamous cell carcinoma progression via miR-136-5p/RAP2C axis [44]. Similarly, the absence of circ_BICD2 exerts anti-tumorigenesis and anti-glycolysis in oral squamous cell carcinoma (OSCC) by sponging miR-107 to downregulate HK2 expression [45]. Upregulation of circ-RPL15 in gastric cancer tissues, through the circ-RPL15/miR-502-3p axis, predicts a poorer outcome for gastric cancer patients [46]. Hsa_circ_0001806 can facilitate the stemness of CRC cells by activating the hsa_circ_0001806/miR-193a-5p/COL1A1 axis [47]. Up-regulate expression of circ_0009910 can help chronic myeloid leukemia (CML) cells become imatinib-resistant via the circ_0009910/miR-34a-5p/ULK1 pathway [48].
Recognized as novel potential diagnostic biomarkers and therapeutic targets in blood tumors, circRNAs will play an important role in further elucidating pathological processes and molecular mechanisms.
Normal lymphocyte proliferation process and lymphoproliferative diseases
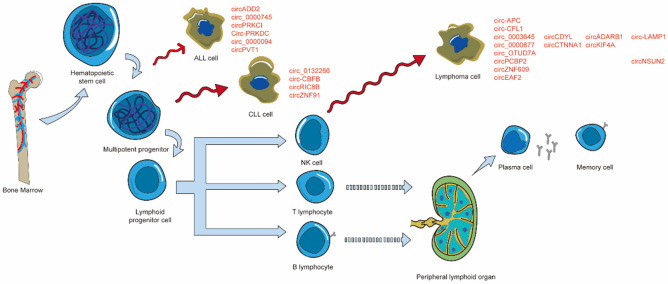
The hematopoietic compartments have responsibility for the function and maintenance of the bone marrow (BM) and blood system [49]. Hematopoietic stem cell (HSC), self-renewing cells from bone marrow with long-term stable multi-lineage regenerative hematopoietic activity [50], can differentiate into all peripheral blood cells [51]. Multipotent progenitor cells arise from three cell type subpopulations of HSCs and differentiated into myeloid progenitor cells or lymphoid progenitor cells, which could establish peripheral effector cell populations of the myeloid and lymphoid lineage [49]. Peripheral lymphocyte clones included natural killer (NK) cells, T lymphocyte, and B lymphocyte [51,52]. There are two main types of T lymphocytes: T helper cells and cytotoxic T cell line [53]. B lymphocytes undergo differentiation and maturation in the bone marrow, then migrate to peripheral lymphoid organs through the blood where they eventually differentiated into plasma cells or memory cells [54,55]. NK cells, like B lymphocytes, mainly develop in the bone marrow and have traditionally been classified as a componen t of the innate immune system; However, they have been shown to possess adaptive immune characteristics [52,56] (Figure 2). Thus far, the participation of circRNAs in the regulation of hematopoiesis in hematopoietic compartments has been proven [57], and it has been found that circRNAs are related to the occurrence and development of lymphoproliferative diseases during the process of lymphocyte proliferation [23]. However, data on the expression and function of these molecules involved in the diseases was still limited [58].
Figure 2.
Hematopoietic stem cell (HSC), self-renewing cells from bone marrow with long-term stable multi-lineage regenerative hematopoietic activity, can differentiate into all peripheral blood cells. Multipotent progenitor cells arise from three cell type subpopulations of HSCs and differentiated into lymphoid progenitor cells, which could establish peripheral effector cell populations of the lymphoid lineage. Peripheral lymphocyte clones included natural killer (NK) cells, T lymphocyte, and B lymphocyte, and functions are exerted by plasma cells and memory cells.
CircRNAs in acute lymphoblastic leukemia
Among the hematological tumors related to children, acute lymphoblastic leukemia (ALL) is the most common type of childhood leukemia [59], characterized by proliferation and accumulation of lymphoid progenitor cells in bone marrow as well as other tissues [60]. Recent studies on ALL in children have shown a significant improvement in overall survival rate (OS) at 5 years [61]. However, ALL in adults has historically had a poor prognosis and limited treatment options [62]. T-cell acute lymphoblastic leukemia (T-ALL) accounts for 15% of pediatric ALL cases and 25% of adult ALL cases [63]. It can be identified as pro-T, pre-T, cortical and mature T-ALL at different stages of differentiation of leukemia clones by flow cytometry [64]. Although the event free survival rate (EFS) of T-ALL has been steadily improving [65], recurrent T-ALL is difficult to cure, and there are relatively few new drugs developed for pediatric T-ALL with drug-resistance [66]. Therefore, exploring the pathological mechanism of circRNAs in ALL or T-ALL could provide new biomarkers and therapeutic targets for future treatment.
Acute lymphoblastic leukemia (ALL)
CircADD2: Zhu et al. conducted a study on circADD2, one of the top three circRNAs that were markedly differentially expressed between the bone marrow samples of children with ALL and non-ALL ones. CircADD2 is a circRNA derived from exon 2-4 of ADD2 gene and it featured potential binding sites for miR-149-5p, this miRNA could act as an oncogene, regulating proliferation, cell cycle and apoptosis in T-cell acute lymphoblastic leukemia (T-ALL) [67,68].
RNA immunoprecipitation assay (RIP) showed a complex interaction between circRNA, AGO2, and miRNA, revealed that the overexpression of circADD2 could sponge miR-149-5p to diminish the expression of AKT2, a signaling molecule in the AKT signaling pathway that promoted angiogenesis, tumor growth, cell migration, invasion, metastasis, and chemotherapy resistance, promoting ALL cell apoptosis in vitro and in vivo of ALL patients as a suppressor [67,69]. The study posited circADD2 as a potential biomarker or therapeutic target for childhood ALL. However, while the research predicted AKT2 as a target gene of miR-149-5p through database and verified that overexpression of circADD2 significantly reduced the protein and mRNA levels of AKT2 in cells, as well as the protein level of p-AKT2, it did not establish whether AKT2 was the direct effector of the circADD2/miR-149-5p/AKT2 axis in the etiopathogenesis of childhood ALL [67].
Circ-0000745: Previous research had demonstrated that circ-0000745 may have a promotional effect in the multiplication of various neoplasms, which are associated with poor prognoses [70-72], including acute lymphoblastic leukemia. Liu et al. traced the assays and gathered information on circ-0000745, revealing its predominant presence in the cytoplasm and significant upregulation in leukemia cells [57]. Subsequent overexpression and knockout experiments revealed that circ-0000745 had the effect of promoting proliferation and inhibiting apoptosis of leukemia cells. Building on prior studies highlighting the cell growth-promoting effect of the ERK signaling pathway, the researchers used a western blot assay to show that the phosphorylation level of ERK was influenced by circ-0000745 overexpressing, but the precise mechanism behind this influence remains to be elucidated [57,73].
In childhood ALL, circ-0000745 also functions as an oncogene. Yang et al. conducted a loss-of-function experiment, revealing that the absence of circ-0000745 suppressed glucose metabolism, causing cell arrest and promoting apoptosis as well as ferroptosis of ALL cells [74]. The researchers predicted, using the Circular RNA Interactome database, that miR-494-3p was a target of circ-0000745, a hypothesis supported by the concurrent enrichment of both circ-0000745 and miR-494-3p by the Ago2 antibody (Anti-Ago2) [74]. Further in vitro experiments confirmed that the oncogenic effect of circ-0000745 was partially mediated through the downregulation of miR-494-3p, which was sponged by circ-0000745 to upregulate the expression of NET1 protein [74]. These findings suggest that circ-0000745 has the potential to serve as a novel biomarker for ALL.
circVRK1: Zhang et al. had reported that in ALL cells, where circVRK1 expression was significantly upregulated, miR-4428 expression was correspondingly downregulated [75]. The overexpression of circVRK1 or the inhibition of miR-4428 was found to diminish the viability and Ki-67 protein expression in ALL cells. Concurrently, there was an observed increase in the rate of apoptosis and in the protein levels of cleaved caspase-3 and cleaved caspase-9. Furthermore, the co-transfection of circVRK1 and miR-4428 was able to counteract the proliferative and apoptotic effects induced by the overexpression of circVRK1 alone [75].
The present study delineated a novel molecular mechanism by which circVRK1 suppressed the expression of miR-4428, consequently attenuating the proliferative capacity of ALL cells [75]. These effects suggested that circVRK1 acted as a potential therapeutic target for ALL, offering a promising avenue for developing targeted interventions.
T-cell acute lymphoblastic leukemia (T-ALL)
circ_0000094: Hou et al. discovered that circ_0000094 was remarkably downregulated in T-ALL tissues. Overexpression of this circular RNA notably suppresses ALL cell viability, migration, and invasion, while also accelerating apoptosis and enhancing the sensitivity of tumor cells to γ-secretase inhibitor (GSIs), which was associated with the NOTCH signaling pathway [76-78]. Subsequent RIP assay showed a direct interaction between circ_0000094 and miR-223-3p [76]. Further experiments indicated that circ_0000094 impeded T-ALL progression through the circ_0000094/miR-223-3p/FBW7 pathway, highlighting its potential as a novel therapeutic target for T-ALL patients [76].
CircPRKCI: CircPRKCI, originating from the amplification of the 3q26.2 locus [79,80], was widely recognized as an oncogene that suggested poor prognosis in various malignancies such as lung adenocarcinoma [81], triple-negative breast cancer [82], hepatocellular carcinoma [82]. Data from studies by Zheng et al., coupled with bioinformatics analysis, had shown that the level of circPRKCI had a positive correlation with that of SOX4, while it exhibited an exactly reverse expression pattern between circPRKCI and miR-20a-5p [83]. Subsequent related research indicated that T-ALL cell survival could be suppressed by the deletion of circPRKCI or SOX4, or by the overexpression of miR-20a-5p in vitro. A poor prognosis of ALL, partly attributed to circPRKCI, suggested its role as a ceRNA in circPRKCI/miR-20a-5p/SOX4 axis in T-ALL, as demonstrated in vitro. Targeting circPRKCI emerged as a promising therapeutic approach for T-ALL [83]. However, additional in vivo animal experiments are required to validate these findings [83].
Circ-PRKDC: Overexpression of circ-PRKDC had been shown to inhibit miR-375, thereby indirectly promoting the expression of FOXM1 and activating the Wnt/β-catenin signaling pathway [84]. This activation contributed to 5-FU resistance in colorectal cancer (CRC). Additionally, circ-PRKDC could function as a ceRNA for miR-198, leading to an increase in discoidin domain receptor 1 (DDR1) levels, enhancing CRC cell proliferation, migration, and invasion [85].
Ling et al. utilized RT-qPCR and western blotting assay, tracing a high level of circ-PRKDC, whose decreased expression could upregulate miR-653-5p but downregulate Reelin (RELN) [86]. Reelin, a secreted extracellular matrix glycoprotein, had been associated with detrimental mutation in ALL [87]. The activation of PI3K/AKT/mTOR signaling pathway was a prevalent phenomenon in a multitude of malignant neoplasms, where it is implicated in the promotion of cell proliferation, survival, and resistance to chemotherapy in T-ALL [88]. Rescue experiments conducted by Ling et al. demonstrated that the downregulation of circ-PRKDC resulted in the suppression of the PI3K/AKT/mTOR signaling pathway’s phosphorylation events, which subsequently led to the enhancement of apoptosis and autophagy in T-ALL cells [86]. These findings suggested that circ-PRKDC may serve as a biomarker in T-ALL and could offer a new treatment pathway for T-ALL patients.
CircPVT1: CircPVT1, a well-studied circRNA [89], had been recognized as an oncogene in various tumor pathological processes, including nasopharyngeal carcinoma [90], gastric cancer [91], clear cell renal cell carcinoma [92]. Notably, it exhibited significantly increased levels in ALL but not in AML samples [93]. Prior research had established a close correlation between PVT-1 and c-MYC expression, with circPVT1 downregulation impacting c-MYC and Bcl-2 expression, leading to cell proliferation arrest and apoptosis in ALL cells [93,94].
Jia et al. further demonstrated that circPVT1 deletion inhibited T-ALL cell line activity and promoted apoptosis [95]. In T-ALL cells, the expression of circPVT1 was markedly elevated relative to that observed in cells derived from healthy individuals, suggesting its potential as a diagnostic biomarker. Acting as a sponge for miR-30e, circPVT1’s high expression in T-ALL cells was linked to poor prognosis, with its overexpression resulting in a high recurrence rate and a low survival rate through the circPVT1/miR-30e/DLL4 axis, which activates the Notch signaling pathway [95]. These findings underscore the role of circPVT1 in T-ALL tumorigenesis via the modulation of miR-30e and DLL4, influencing the Notch signaling cascade, and it could lead to the development of more effective treatments and better prognosis for patients with T-ALL.
Circ-0000745: The high expression of circ-0000745 and Notch receptor 1 (NOTCH1) in T-ALL BM and T-ALL cell lines as well as their effects of promoting cell proliferation and inhibiting cell apoptosis to show a poor prognosis of T-ALL were experimentally validated by Feng et al. [96].
NOTCH1, a class I transmembrane protein in T cell maturation within the NOTCH signaling pathway [97], frequently experiences activating mutation that could either enhance the mTOR signaling pathway by targeting c-myc or elevate the levels of cyclin D3 and CDK4 [88], thereby promoting T-cell progression through the G1/S phase transition of the cell cycle [98]. The mRNA level of NOTCH1 was found to be positively correlated with that of circ-0000745, a relationship mediated by the miR-193b-3p sponge activity of circ-0000745, which in turn targeted NOTCH1 [96]. This discovery provided a new therapeutic strategy targeting circ_0000745 to regulate the proliferation and apoptosis of T-ALL cells.
B-cell acute lymphoblastic leukemia (B-ALL)
CircBCAR3: Zhao et al. had identified CircBCAR3 as a significant sponge for miR-27a-3p, neutralizing the miRNA’s inhibitory effects on SLC7A11, a critical regulator of cellular iron metabolism [99]. The consequent upregulation of SLC7A11, driven by CircBCAR3’s sequestration of miR-27a-3p, led to heightened intracellular iron levels [99]. This increase was instrumental in initiating ferroptosis in B-prolymphocytic leukemia (B-PLL) cells, a regulated cell death mechanism associated with iron metabolism and lipid peroxidation [99]. The onset of ferroptosis was mediated by the intricate balance of iron homeostasis and the generation of reactive oxygen species (ROS) [100,101], which were essential components of the ferroptosis pathway [100]. Therefore, strategies that target CircBCAR3 or modulate its interactions with miR-27a-3p and SLC7A11 could present novel therapeutic opportunities for B-PLL. Such interventions might be particularly effective in inducing ferroptosis in leukemia cells that were unresponsive to standard chemotherapy regimens.
CircRNAs in chronic lymphocytic leukemia (CLL)
Chronic lymphocytic leukemia (CLL) is a prevalent and incurable form of leukemia in Western countries [102], with a mounting incidence rate observed in China [103,104]. Predominantly occurs in elderly patients, CLL is marked by highly variable clinical outcomes [102]. It is characterized by clonal proliferation and accumulation of mature B cells in the blood, bone marrow, lymph nodes, and spleen [105]. Notwithstanding significant advancements in CLL treatment over the past three decades that have prolonged patients’ survival time [106], there remains a critical need for new biomarkers to predicting prognosis and for a deeper understanding of the molecular mechanisms and therapeutic targets of CLL. These insights are crucial for enhancing the future prognosis of CLL patients.
Circ_0132266: Wu et al. observed an up-regulation of miR-337-3p expression in CLL cells in their prior study. Subsequent experiments further substantiated this finding and elucidated the role of miR-337-3p in facilitating cell proliferation and impeding apoptosis [107]. Utilizing bioinformatic analysis, they proposed promyelocytic leukemia (PML) gene, a common tumor suppressor that promoted cell apoptosis and arrested the cell cycle, was the target of miR-337-3p [107]. In patients with acute promyelocytic leukemia (APL), the genetic fusion of PML and retinoic acid receptor alpha (RARA) genes leading to in functional loss of the tumor-suppressive properties of PML and the production of the PML-RARa oncoprotein, disrupting the differentiation of bone marrow progenitor cells [108]. Furthermore, they identified circ_0132266, a novel downregulated circRNA in peripheral blood mononuclear cells (PBMCs) of CLL patients compared to normal individuals, which had the binding sites of miR-337-3p, may act as a tumor suppressor through circ_0132266/miR-337-3p/PML axis [107]. This study underscored the potential of circ_0132266 as both a therapeutic target and a biomarker for CLL, suggesting its utility in improving patient outcomes.
Circ-CBFB: Located at chromosome 16q22.1 and originating from the reverse splicing of CBFB transcripts, hsa_circ_0000707 (circ-CBFB) had a significant overexpression in CLL cells found by Xia et al. [109]. The expression levels of circRNA circ-CBFB served as a robust biomarker, markedly differentiating CLL patient cells from those of healthy individuals. Survival analysis and multivariate cox regression analysis revealed that elevated expression of circCBFB in patients with CLL was associated with reduced survival time, identifying high circCBFB expression as an independent prognostic indicator for CLL [109].
Employing bioinformatics tools for analysis and prediction, the researchers hypothesized that the circular RNA circ-CBFB could directly bound to miR-607, thereby facilitating the restoration of FZD3, which was crucial for the pro-leukemia role of circ-CBFB, as it promoted cell proliferation, regulated cell cycle, and inhibits apoptosis [109]. FZD3, a member of the Frizzled family of receptors, was capable of transducing Wnt signaling, promoting the activation of the Wnt pathway [110]. This pathway acted an effector molecule in the progress of CLL, as demonstrated by the author’s experiment [109,110].
CircRIC8B: Chronically elevated levels of lipoprotein lipase (LPL) in CLL patients usually indicated aggressive disease and were associated with a poor prognosis [111]. In an attempt to explore the role of lipid metabolism-related circRNA in CLL, Wu et al. identified circRIC8B, a highly expressed circRNA implicated in lipid metabolism. The overexpression of circRIC8B was found to promote the proliferation of leukemia cells, which may indicate a poorer prognosis and a shorter survival time. Notably, CLL patients with low level of circRIC8B exhibited a significantly longer time to first treatment time [112].
The authors observed that circRIC8B could act as a sponge for miR-199b-5p, primarily sequestering it in the cytoplasm, thereby upregulating LPL mRNA expression. This mechanism promotes lipid accumulation and suggested that circRIC8B functions as an oncogene in CLL [112]. Furthermore, the study revealed that ezetimibe, a cholesterol-lowering drug, effectively inhibited CLL cells by reducing LPL levels. However, following ezetimibe treatment, an upregulation of circRIC8B levels was observed, which may be attributed to compensatory regulation by other signaling pathways. This finding warrants further investigation to elucidate the underlying mechanisms [112].
CircZNF91: Compared with peripheral blood cells of healthy individuals, circZNF91 had a remarkable elevated level in CLL cells, correlating with a low survival rate and indicating its potential as a dependable diagnostic biomarker, as evidenced by the experimental data from Li et al. [113]. Depletion of circZNF91 resulted in cell cycle arrest and the initiation of apoptosis.
Utilizing bioinformatics tools and databases, the authors predicted the presence of a direct binding site for circZNF91 within miR-1283, which was confirmed through RNA immunoprecipitation (RIP) assays [113]. The overexpression of miR-1283 had been shown to specifically target and inhibit the expression of the WEE1 gene, a pivotal cell cycle regulator that ensured accurate DNA replication and repair by inhibiting CDKs during the G2/M transition [114], thus involving in the progress of CLL. These findings suggested that circZNF91 could serve as a potential neo-target in the treatment of CLL [113] (Table 1).
Table 1.
CircRNAs in leukemia
| CircRNAs | Also Known As | Diseases | Functions | Levels | Pathogenesis | Refs |
|---|---|---|---|---|---|---|
| circADD2 | hsa_circ_0120872 | Childhood ALL | Tumor suppressor | ↓ | circADD2/miR-149-5p/AKT2 | [67] |
| circ_0000745 | hsa_circ_0000745 | Childhood ALL | Oncogene | ↑ | circ_0000745/miR-494-3p/NET1 | [74] |
| circ_0000745 | hsa_circ_0000745 | Childhood ALL | Oncogene | ↑ | circ_0000745/miR-193b-3p/NOTCH1 | [96] |
| circ_0000745 | hsa_circ_0000745 | ALL | Oncogene | ↑ | circ_0000745/ERK | [57] |
| circPRKCI | T-ALL | Oncogene | ↑ | circPRKCI/miR-20a-5p/SOX4 | [83] | |
| Circ-PRKDC | circ_0136666 | T-ALL | Oncogene | ↑ | circPRKDC/miR-653-5p/RELN/PI3K/AKT/mTOR | [86] |
| circ_0000094 | T-ALL | Tumor suppressor | ↓ | circ_0000094/miR-223-3p/FBW7 | [76] | |
| circPVT1 | T-ALL | Oncogene | ↑ | circPVT1/miR-30e/DLL4/Notch | [95] | |
| circ_0132266 | has_circ_0132266 | CLL | Tumor suppressor | ↓ | circ_0132266/miR-337-3p/PML | [107] |
| circ-CBFB | hsa_circ_0000707 | CLL | Oncogene | ↑ | circ-CBFB/miR-607/FZD3/Wnt/β-catenin | [109] |
| circRIC8B | CLL | Oncogene | ↑ | circRIC8B/miR199b-5p/LPL mRNA | [112] | |
| circZNF91 | CLL | Oncogene | ↑ | circZNF91/miR-1283/WEE1 | [113] |
CircRNAs in lymphoma
Diffuse large B-cell lymphoma (DLBCL)
Diffuse large B-cell lymphoma (DLBCL) was the most prevalent type of non-Hodgkin’s lymphoma (NHL) [115], representing approximately one-third of all NHL cases globally [116]. This disease demonstrates significant variability in its clinicopathological and laboratory characteristics [116], leading to diverse subtypes of DLBCL with distinct prognostic implications [117]. Despite this heterogeneity, the specific pathogenesis of DLBCL remained clear [118]. Therefore, there was an urgent need to identify novel biomarkers and therapeutic targets to improve treatment strategies and patient outcomes.
circ-APC: Through an analysis of circRNA microarray expression profiles, Hu et al. identified a novel circRNA associated with DLBCL, designated as circ-APC [119]. This circRNA was found to be significantly downregulated in DLBCL tissue relative to neighboring normal cells. Subsequent qRT-PCR analysis showed that circ-APC was uniformly distributed between the cytoplasm and nucleus. In vitro and in vivo studies revealed that circ-APC functioned as a molecular sponge for miR-888 and also has the capacity to bind to the promoter region of APC, recruiting the DNA demethylase TET1. This interaction enhanced the expression of its host gene APC. The upregulation of APC subsequently facilitated the phosphorylation of β-catenin, leading to the inactivated of the typical Wnt/β-catenin pathway [120]. This regulatory mechanism positioned circ-APC as a potential tumor suppressor in DLBCL [119]. Additionally, the study identified circ-APC expression levels as an independent protective factor for DLBCL, which meant that Lower circ-APC expression in DLBCL was linked to advanced Ann Arbor stage, treatment resistance, and a lower International Prognostic Index (IPI) score [119]. These studies conclusively established circ-APC as a promising biomarker for DLBCL, highlighting its potential in predicting disease progression and therapeutic response.
CircCFL1: In a study conducted by Chen et al., bioinformatics analyses predicted that high mobility group box 1 (HMGB1), a DNA binding protein critical for regulating and maintaining stability, could be directly targeted by miR-107 [121]. This direct interaction was confirmed through the dual-luciferase reporter assay. HMGB1, known for its elevated expression in various malignant disorders [122], played a role in promoting inflammatory responses following tissue damage and is implicated in revascularization, cell proliferation, and even tumor genesis, progress, and metastasis [123].
Further mechanistic insights were provided by the RNA pull-down and the RIP assays, which demonstrated that CircCFL1 interacted with miR-107. The upregulation of CircCFL1 was shown to directly bind to miR-107, alleviating the repression of the target gene HMGB1 and thereby boosting the growth of DLBCL cells [121]. Additionally, CircCFL1 had been shown to activate the AKT/ERK signaling pathway, further promoting DLBCL cell proliferation [121]. These newly elucidated functions and mechanisms of CircCFL1 may provide potential novel molecular targets for the therapeutic intervention of DLBCL.
circ_OTUD7A: Liu et al. identified a circRNA, circ_OTUD7A, originating from OTUD7A, which was showed a demonstrable overexpression in DLBCL [124]. The Forkhead box protein P1 (FOXP1), a member of the FOXP transcription factor sub-family, was essential for the normal development of B cells and served as a prognostic marker for DLBCL in the rituximab era, with elevated expression observed in DLBCL tissues [125]. FOXP1 could foster the growth of B-cell non-Hodgkin lymphoma by enhancing β-catenin-dependent transcription via CREB-binding protein (CBP)’s protein acetylation, which in turn enhanced Wnt signaling [126,127]. The researchers discovered that silencing FOXP1 or circ_OTUD7A resulted in decreased CyclinD1 and Bcl-2 protein levels and elevated Bcl-2-associated X protein (Bax) protein levels, leading to cell cycle arrest and enhanced apoptosis of DLBCL cells [124].
Through bioinformatics analysis, the researchers identified miR-431-5p had been shown to directly bind to both circ_OTUD7A and FOXP1 [124]. By sequestering miR-431-5p, circ_OTUD7A increased FOXP1 expression, thereby promoting the proliferation, migration, and invasion of lymphoma cells [124]. The aforementioned studies suggested that circ_OTUD7A could potentially serve as a therapeutic target for DLBCL.
circPCBP2: Tumor cells had developed a sophisticated mechanism to evade detection and destruction by the immune system, which involved the expression of the protein programmed cell death-ligand 1 (PD-L1) [128]. By overexpression of PD-L1 on their surface, these cells could engage with Programmed Death-1 (PD-1) receptors present on immune cells, particularly T cells [129]. The engagement of PD-1/PD-L1 pathway transmitted an inhibitory signal to the T cells, effectively silencing their ability to mount an effective immune response against the tumor [130]. This strategic manipulation of the immune system by tumor cells was a key factor in immune evasion and contributes to the progression of cancer, and targeting the PD-1/PD-L1 pathway could reactivate the immune system’s capacity to recognize and attack cancer cells [130,131].
Through bioinformatic analysis, Dong et al. discovered a reciprocal relationship between PD-L1 mRNA and a tumor suppressor miR-33a/b [132]. Furthermore, they found a direct binding interaction between miR-33a/b and circPCBP2 [133]. These findings prompted the researchers to propose a novel molecular axis, circPCBP2/miR-33a/b/PD-L1 axis, which may play a critical role in stem-like characteristics and resistance to CHOP chemotherapy observed in DLBCL. This hypothesis was bolstered by subsequent research, which provided corroborating evidence for the involvement of this axis in the disease’s progression and treatment response [133]. This molecular network’s characterization uncovered critical mechanisms in DLBCL’s evolution, suggesting potential targets for future diagnostics and treatments.
circEAF2: Identification of Epstein-Barr virus (EBV) as the first virus to express specific miRNAs, such as miR-BART19-3p from the Bam HI-A region rightward transcript (BART) of the virus [134,135], had illuminated its role of EBV as a potent oncogenic agent. EBV was particularly implicated in cellular transformation and the development of tumors, particularly lymphomas [136].
Using circRNA high-throughput sequencing, Zhao et al. identified significantly reduced expression of circEAF2 in DLBCL tissue with chronic Epstein-Barr virus (EBV) infection, as opposed to EBV-negative DLBCL tissues [137]. The downregulation of circEAF2 was significantly associated with the presence of EBV in DLBCL, yet it appears to mitigate the progression of DLBC [137]. Lower levels of circEAF2 were associated with unfavorable clinical features and a more severe prognosis. In contrast, an elevated circEAF2 expression was indicative of superior progression-free survival (PFS) and overall survival (OS) rates, suggesting that circEAF2 could serve as a biomarker for a more favorable outcome in the progression of DLBCL [137]. Subsequent experiments revealed that circEAF2 had the sponge effect for miR-BART19-3p, which results in the upregulation of APC and the downregulation of β-catenin levels. This mechanism inhibited the progression of EBV-positive DLBCL and enhanced its sensitivity to chemosensitivity, as well as promoting apoptosis [137].
Intriguingly, the researchers also discovered that EBV may disrupt the cyclization process of EAF2, selectively targeting the circular form and not its linear counterpart, thereby affecting the formation of circEAF2 [137]. Understanding circEAF2’s role in EBV-positive DLBCL progression could enhance our knowledge of EBV lymphomagenesis and inform the development of novel treatment strategies for EBV-associated lymphoid malignancies.
Mantle cell lymphoma (MCL)
Mantle cell lymphoma (MCL), a rare type of non-Hodgkin lymphoma (NHL) [138], originated from B-lymphocytes and predominantly affected patients aged over 60 years [139]. Characterized by a median overall survival (OS) of 3-5 years with standard therapies [140,141], MCL poses a significant clinical challenge. Although allogeneic bone marrow or hematopoietic stem cell transplantation during the first remission might offer a potential cure [141], the prognosis remains poor, with less than half of the patients surviving beyond five years post-diagnosis [142]. Consequently, the urgent need to identify novel genetic markers for targeted treatments in MCL was an urgent and formidable task in clinical practice.
circCDYL: Mei et al. conducted a series of experiments to explore the biological significance of circ-chromodomain Y-like (circCDYL), which was reported to be circular spliced from exon 4 of CDYL gene [143,144]. Their findings indicated that the upregulation of circCDYL in MCL cells could serve as a robust biomarker, effectively differentiating MCL patients from healthy individuals [145].
Through comprehensive bioinformatics analyses, the researchers identified a complex co-expression network involving circCDYL, encompassing five miRNAs, three lncRNAs, and five mRNAs. Specifically, the long non-coding RNA (lncRNA) MALAT1, which was found to be overexpressed in MCL tissues, was highlighted as a potential prognostic factor. MALAT1’s role in cell cycle regulation was further elucidated through its interaction with EZH2 and the cyclin-dependent kinase suppressors p21 and p27 [145]. Additionally, the study suggested that circCDYL might regulate NOTCH1 expression, which was associated with poor survival in MCL. However, the detailed mechanisms by which circCDYL modulated these pathways and its potential as a therapeutic target in MCL warrant additional investigation [145].
CircCTNNA1: CircCTNNA1, originating from the CTNNA1 gene, had been demonstrated to be upregulated and to act an oncogenic role by promoting colon cancer progression through the sequestration of miR-149-5p, as established in previous studies [146]. Lu et al. observed a comparable expression pattern of circCTNNA1 in patients with MCL, which was associated with poorer survival outcomes. However, no significant correlation was identified between circCTNNA1 expression levels and clinical characteristics, highlighting its potential as an independent prognostic biomarker [147]. The authors further discovered that circCTNNA1 could physically interact with miR-34a, a finding supported by bioinformatics tool and RNA pull-down assays [147]. Additional studies had unveiled the tumor suppressor role of miR-34a, it was downregulated in diffuse large B-cell lymphoma (DLBCL) and its overexpression increased the chemosensitivity of cancer cells to doxorubicin, thus improving therapeutic efficacy [148]. It was hypothesized that circCTNNA1 might contribute to MCL proliferation by sequestering miR-34a [147]. The circCTNNA1 expression level could be utilized as a supplementary diagnostic marker for MCL, potentially aiding in the extension of patients’ survival [147] (Table 2).
Table 2.
CircRNAs in lymphoma
| CircRNAs | Also Known As | Diseases | Functions | Levels | Pathogenesis | Refs |
|---|---|---|---|---|---|---|
| circ-APC | hsa_circ_0127621 | DLBCL | Tumor suppressor | ↓ | Circ-APC/miR-888 or TET1/APC/Wnt/β-catenin | [119] |
| circCFL1 | DLBCL | Oncogene | ↑ | CircCFL1/miR-107/HMGB1 | [121] | |
| circ_0003645 | DLBCL | Oncogene | ↑ | Circ_0003645/miR-335-5p/NFIB | [168] | |
| circ_0000877 | hsa_circ_0000877 | DLBCL | Oncogene | ↑ | Circ_0000877/miR-370-3p/MAPK4/Hippo | [169] |
| circ_OTUD7A | DLBCL | Oncogene | ↑ | Circ_OTUD7A/miR-431-5p/FOXP1 | [124] | |
| circPCBP2 | DLBCL | Oncogene | ↑ | CircPCBP2/miR-33a/b/PD-L1 | [133] | |
| circZNF609 | DLBCL | Oncogene | ↑ | CircZNF609/miR-153 | [170] | |
| circEAF2 | DLBCL (EBV+) | Tumor suppressor | ↓ | CircEAF2/miR-BART19-3p/APC/Wnt/β-catenin | [137] | |
| circCDYL | MCL | Oncogene | ↑ | CircCDYL/miR-101/EZH2/p21 p27 | [145] | |
| circCTNNA1 | MCL | Oncogene | ↑ | CircCTNNA1/miR-34a | [147] | |
| circADARB1 | hsa_circ_0005037 | NKTCL | Oncogene | ↑ | CircADARB1/miR-214/p-Stat3 | [160] |
| circKIF4A | NKTCL | Oncogene | ↑ | CircKIF4A/miR-1231/PDK1 or BCL11A | [167] | |
| circ-LAMP1 | hsa_circ_101303 | T-LBL | Oncogene | ↑ | Circ-LAMP1/miR-615-5p/DDR2 | [153] |
| circNSUN2 | Lymphoma | Oncogene | ↑ | NRF1/CircNSUN2/HMGA1/Wnt | [171] |
Other lymphomas
Beyond the B-cell-derived lymphomas previously discussed, there were additional, more aggressive forms of lymphoma, including T-cell lymphoblastic lymphoma (T-LBL) and Natural Killer/T-cell lymphoma (NKTCL), which were characterized by higher lethality as the disease progresses [149,150]. Over the years, a plethora of novel and validated biomarkers, along with effective therapeutic targets, had been discovered for these malignancies. Among these, circRNAs stood out as a promising area of focus for targeted therapies, holding the potential to significantly enhance patient outcomes.
T-cell lymphoblastic lymphoma (T-LBL)
T-cell lymphoblastic lymphoma (T-LBL) was an aggressive malignancy that arose from immature T-cell precursors or lymphoblasts, showcasing heterogeneity [149]. This disease was marked by the presence of a localized mass, with minimal or no detectable involvement of blood or bone marrow [149]. Currently, the treatment of T-LBL typically involved chemotherapy regimens designed for leukemia, incorporating a variety of intensified drugs. However, the identification of reliable prognostic factors for T-LBL remained a challenge [151]. Consequently, there was a critical need for the discovery of new, reliable biomarkers and therapeutic targets that could be effectively utilized throughout the disease’s trajectory [152].
Circ-LAMP1: In their investigation into the role of circRNAs in T-LBL, Deng et al. conducted a comparative analysis of circRNAs expression between T-LBL sample and thymic tissue from young children. This study led to the identification of circ-LAMP1, a transcript of LAMP gene, as the most abundantly expressed circRNA in T-LBL tissues [153]. The authors discovered that circ-LAMP1 possessed growth-promoting and apoptotic inhibitory functions in T-LBL cells. Through bioinformatics analysis, they also identified miR-615-5p, a known tumor suppressor across various neoplasms [154-156], as a downstream target of circ-LAMP1 [153]. The research team hypothesized that DDR2, a receptor tyrosine kinase (RTK) family member [157], might be involved in the circ-LAMP1/miR-615-5p/DDR2 regulatory axis, modulated by miR-615-5p [153]. Subsequent research corroborated this hypothesis, underscoring the significance of circ-LAMP1 in T-LBL, suggesting its potential as a therapeutic target and a biomarker in T-LBL patients [153].
Natural Killer/T-cell lymphoma (NKTCL)
Natural Killer/T-cell lymphoma (NKTCL), a rare and highly aggressive subtype of non-Hodgkin’s lymphoma, was frequently associated with Epstein-Barr virus (EBV) [158]. This malignancy was predominantly extra-nodal, often occurring in sites such as the nasal cavity, palate, skin and other soft tissue, and was characterized by atypical early clinical manifestations, including fever, night sweats, and fatigue [159,160]. Accurate diagnosis of NKTCL required immunohistochemical staining that was positive for CD2, CD56, cytoplasmic CD3ε (cCD3ε), and cytotoxic molecules specific to this lymphoma [161]. The standard treatment protocol typically combined chemotherapy with radiotherapy [162], however, these approaches had notable limitations, with a high propensity for disease relapse [158]. In the early stages, when symptoms are atypical, NKTCL required reliable biomarker for precise identification. Despite considerable molecular research, there remained a critical need for effective prediction biomarkers and therapeutic targets for NKTCL.
CircADARB1: After microarray analysis and qRT-PCR assays, Mei et al. identified circADARB1 as one of the top five upregulated circular RNAs in NKTCL, whose expression correlated with treatment efficacy rather than demographic or clinical variables. Notably, higher circADARB1 levels were associated with stable disease (SD) and progressive disease (PD) disease states [160]. The knockdown of circADARB1 enhanced Bax protein expression and inhibited NKTCL cell proliferation in both cellular and animal models [160]. Subsequent researches demonstrated an interaction between circADARB1 and miR-214-3p, as indicated by a significant decrease in luciferase activity in a dual luciferase assay. Bioinformatics analysis, corroborated by reduced p-Stat3 levels upon circADARB1 knockdown, suggested that circADARB1 might regulate the STAT3 pathway in NKTCL through miR-214-3p [160]. To sum up, circADARB1 was notably elevated in the plasma of patients with NKTCL, suggesting its utility as a diagnostic and prognostic biomarker.
CircKIF4A: In accordance with earlier research, circKIF4A had been established as an oncogene, playing a pivotal role in the development of various neoplasms, including papillary thyroid cancer [163], glioma [164], triple-negative breast cancer and NSCLC [165,166]. He et al., through qPCR analysis, documented a remarkable up-regulation of circKIF4A expression in NKTCL cell lines when compared to the normal NK cell clones. This overexpression was further identified as a robust independent prognostic biomarker for NKTCL, inversely associated with both overall survival (OS) and progression-free survival (PFS) of NKTL patients [167]. Notably, the suppression of circKIF4A was found to effectively inhibit the glycolysis activity in NKTCL cells [167]. Further experimental analysis indicated that circKIF4A potentially sponged miR-1231, modulating the expression of BCL11A and PDK1, which are key players in the malignant progression of NKTCL. This interaction delineated a novel circKIF4A/miR-1231/BCL11A or PDK1 axis that could be targeted therapeutically [167] (Figure 2).
Conclusions and perspective
In summary, circRNA had emerged as great molecular entities, particularly in the context of malignant diseases pathogenesis. The advent of precision medicine had intensified the demand for more refined diagnostic and therapeutic modalities for lymphoproliferative disorders. The researches described above had underscored the significant role that circRNA played in the initiation and advancement of these diseases, suggesting their potential to serve as both biomarker for disease detection and progression, as well as targets for therapeutic intervention.
Nonetheless, the journey from bench to bedside for circRNA applications in clinical settings was fraught with challenges that must be surmounted. Many studies had thus far only established the suppressive or oncogenic effects of specific circRNA in vitro, necessitating further validation of their roles and the elucidation of their underlying pathological mechanisms through rigorous in vivo experimentation. Moreover, the landscape of circRNA research was currently dominated by investigations into common lymphoproliferative diseases, with a dearth of studies focusing on rarer conditions. This disparity underscores an urgent need for additional research endeavors to address these knowledge deficits and expand the understanding of circRNA’s role across the full spectrum of lymphoproliferative diseases.
Furthermore, the transition from molecular insights to tangible clinical benefits hinged on the execution of extensive clinical research and experimental validation. Future studies must be designed to not only confirm the diagnostic and prognostic value of circRNAs but also to explore their utility in guiding personalized treatment strategies. This included assessing the efficacy and safety of circRNA-based therapies, as well as their potential to complement or even surpass existing treatment paradigms.
In essence, while the potential of circRNAs in the realm of lymphoproliferative diseases was undeniably promising, the path to clinical application was complex and required a concerted, multidisciplinary effort. The scientific community must continue to delve into the intricacies of circRNA biology, while simultaneously fostering collaboration between researchers, clinicians, and regulatory bodies to ensure that the full potential of circRNAs was realized in the fight against lymphoproliferative diseases.
Acknowledgements
This work was supported by the Department of Education of Jilin Province (grant numbers JJKH20211193KJ, JGJX2020D15, JGJX2021D7, JGJX2023D25).
Disclosure of conflict of interest
None.
References
- 1.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S. Translation of circRNAs. Mol Cell. 2017;66:9–21. e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu G, Yang Z, Peng T, Lv Y. Circular RNAs: rising stars in lipid metabolism and lipid disorders. J Cell Physiol. 2021;236:4797–4806. doi: 10.1002/jcp.30200. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Zhan L, Huang K, Wang X. The functions and clinical significance of circRNAs in hematological malignancies. J Hematol Oncol. 2020;13:138. doi: 10.1186/s13045-020-00976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque S, Ames RM, Moore K, Pilling LC, Peters LL, Bandinelli S, Ferrucci L, Harries LW. circRNAs expressed in human peripheral blood are associated with human aging phenotypes, cellular senescence and mouse lifespan. Geroscience. 2020;42:183–199. doi: 10.1007/s11357-019-00120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafari Ghods F. Circular RNA in saliva. Adv Exp Med Biol. 2018;1087:131–139. doi: 10.1007/978-981-13-1426-1_11. [DOI] [PubMed] [Google Scholar]
- 9.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicens Q, Westhof E. Biogenesis of circular RNAs. Cell. 2014;159:13–14. doi: 10.1016/j.cell.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Aufiero S, Reckman YJ, Pinto YM, Creemers EE. Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol. 2019;16:503–514. doi: 10.1038/s41569-019-0185-2. [DOI] [PubMed] [Google Scholar]
- 12.Cocquerelle C, Mascrez B, Hétuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 13.Wilusz JE. A 360° view of circular RNAs: from biogenesis to functions. Wiley Interdiscip Rev RNA. 2018;9:e1478. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34:e63. doi: 10.1093/nar/gkl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. EMBO J. 2013;32:923–925. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH, Pandolfi PP. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;166:1055–1056. doi: 10.1016/j.cell.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Bonizzato A, Gaffo E, Te Kronnie G, Bortoluzzi S. CircRNAs in hematopoiesis and hematological malignancies. Blood Cancer J. 2016;6:e483. doi: 10.1038/bcj.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Qu R, Yang L, Shi G, Hao S, Hu C. Circular RNA controls tumor occurrence and development via cell cycle regulation. Onco Targets Ther. 2022;15:993–1009. doi: 10.2147/OTT.S371629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Ma W, Xiao J, Dai X, Ling W. CircRNA_0092516 regulates chondrocyte proliferation and apoptosis in osteoarthritis through the miR-337-3p/PTEN axis. J Biochem. 2021;169:467–475. doi: 10.1093/jb/mvaa119. [DOI] [PubMed] [Google Scholar]
- 26.Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: an important part of circRNA maintenance and function. J Neurosci Res. 2020;98:87–97. doi: 10.1002/jnr.24356. [DOI] [PubMed] [Google Scholar]
- 27.Chen RX, Chen X, Xia LP, Zhang JX, Pan ZZ, Ma XD, Han K, Chen JW, Judde JG, Deas O, Wang F, Ma NF, Guan X, Yun JP, Wang FW, Xu RH, Dan Xie. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10:4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, Yu J, Gu X, Ge S, Fan X. Novel insights into exosomal circular RNAs: redefining intercellular communication in cancer biology. Clin Transl Med. 2021;11:e636. doi: 10.1002/ctm2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolha L, Ravnik-Glavač M, Glavač D. Circular RNAs: biogenesis, function, and a role as possible cancer biomarkers. Int J Genomics. 2017;2017:6218353. doi: 10.1155/2017/6218353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15 Spec No 1:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 33.Duda P, Akula SM, Abrams SL, Steelman LS, Gizak A, Rakus D, McCubrey JA. GSK-3 and miRs: master regulators of therapeutic sensitivity of cancer cells. Biochim Biophys Acta Mol Cell Res. 2020;1867:118770. doi: 10.1016/j.bbamcr.2020.118770. [DOI] [PubMed] [Google Scholar]
- 34.Gattolliat CH, Uguen A, Pesson M, Trillet K, Simon B, Doucet L, Robaszkiewicz M, Corcos L. MicroRNA and targeted mRNA expression profiling analysis in human colorectal adenomas and adenocarcinomas. Eur J Cancer. 2015;51:409–420. doi: 10.1016/j.ejca.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17:272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 39.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Liang W, Zhang P, Chen J, Qian H, Zhang X, Xu W. Circular RNAs: emerging cancer biomarkers and targets. J Exp Clin Cancer Res. 2017;36:152. doi: 10.1186/s13046-017-0624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Jiang J, Zhang J, Shen H, Wang M, Guo Z, Zang X, Shi H, Gao J, Cai H, Fang X, Qian H, Xu W, Zhang X. CircDIDO1 inhibits gastric cancer progression by encoding a novel DIDO1-529aa protein and regulating PRDX2 protein stability. Mol Cancer. 2021;20:101. doi: 10.1186/s12943-021-01390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong DD, Dang YW, Lin P, Wen DY, He RQ, Luo DZ, Feng ZB, Chen G. A circRNA-miRNA-mRNA network identification for exploring underlying pathogenesis and therapy strategy of hepatocellular carcinoma. J Transl Med. 2018;16:220. doi: 10.1186/s12967-018-1593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi: 10.1016/j.pharmthera.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Huang C, Zhang A, Lu C, Liu L. Overexpression of circRNA_100290 promotes the progression of laryngeal squamous cell carcinoma through the miR-136-5p/RAP2C axis. Biomed Pharmacother. 2020;125:109874. doi: 10.1016/j.biopha.2020.109874. [DOI] [PubMed] [Google Scholar]
- 45.Zhu LS, Wang YL, Li R, Xu XT, Li KY, Zuo CR. circ_BICD2 acts as a ceRNA to promote tumor progression and Warburg effect in oral squamous cell carcinoma by sponging miR-107 to enhance HK2. Am J Transl Res. 2020;12:3489–3500. [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Gong Y, Ma J, Gong X. Overexpressed circ-RPL15 predicts poor survival and promotes the progression of gastric cancer via regulating miR-502-3p/OLFM4/STAT3 pathway. Biomed Pharmacother. 2020;127:110219. doi: 10.1016/j.biopha.2020.110219. [DOI] [PubMed] [Google Scholar]
- 47.Sun J, Liu J, Zhu Q, Xu F, Kang L, Shi X. Hsa_circ_0001806 acts as a ceRNA to facilitate the stemness of colorectal cancer cells by increasing COL1A1. Onco Targets Ther. 2020;13:6315–6327. doi: 10.2147/OTT.S255485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao HX, Miao CF, Sang LN, Huang YM, Zhang R, Sun L, Jiang ZX. Circ_0009910 promotes imatinib resistance through ULK1-induced autophagy by sponging miR-34a-5p in chronic myeloid leukemia. Life Sci. 2020;243:117255. doi: 10.1016/j.lfs.2020.117255. [DOI] [PubMed] [Google Scholar]
- 49.Carroll D, St Clair DK. Hematopoietic stem cells: normal versus malignant. Antioxid Redox Signal. 2018;29:1612–1632. doi: 10.1089/ars.2017.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dzierzak E, Bigas A. Blood development: hematopoietic stem cell dependence and independence. Cell Stem Cell. 2018;22:639–651. doi: 10.1016/j.stem.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Gunsilius E, Gastl G, Petzer AL. Hematopoietic stem cells. Biomed Pharmacother. 2001;55:186–194. doi: 10.1016/s0753-3322(01)00051-8. [DOI] [PubMed] [Google Scholar]
- 52.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preglej T, Ellmeier W. CD4(+) cytotoxic T cells - phenotype, function and transcriptional networks controlling their differentiation pathways. Immunol Lett. 2022;247:27–42. doi: 10.1016/j.imlet.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Seifert M, Küppers R. Human memory B cells. Leukemia. 2016;30:2283–2292. doi: 10.1038/leu.2016.226. [DOI] [PubMed] [Google Scholar]
- 55.Franchina DG, Grusdat M, Brenner D. B-cell metabolic remodeling and cancer. Trends Cancer. 2018;4:138–150. doi: 10.1016/j.trecan.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Geiger TL, Sun JC. Development and maturation of natural killer cells. Curr Opin Immunol. 2016;39:82–89. doi: 10.1016/j.coi.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Zhou C, Li Y, Deng Y, Lu W, Li J. Upregulation of circ-0000745 in acute lymphoblastic leukemia enhanced cell proliferation by activating ERK pathway. Gene. 2020;751:144726. doi: 10.1016/j.gene.2020.144726. [DOI] [PubMed] [Google Scholar]
- 58.Gaffo E, Boldrin E, Dal Molin A, Bresolin S, Bonizzato A, Trentin L, Frasson C, Debatin KM, Meyer LH, Te Kronnie G, Bortoluzzi S. Circular RNA differential expression in blood cell populations and exploration of circRNA deregulation in pediatric acute lymphoblastic leukemia. Sci Rep. 2019;9:14670. doi: 10.1038/s41598-019-50864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarnas JC, Brown PA, Aoun P, Ballen KK, Bellam N, Blum W, Boyer MW, Carraway HE, Coccia PF, Coutre SE, Cultrera J, Damon LE, DeAngelo DJ, Douer D, Frangoul H, Frankfurt O, Goorha S, Millenson MM, O’Brien S, Petersdorf SH, Rao AV, Terezakis S, Uy G, Wetzler M, Zelenetz AD, Naganuma M, Gregory KM National Comprehensive Cancer Network. Acute lymphoblastic leukemia. J Natl Compr Canc Netw. 2012;10:858–914. doi: 10.6004/jnccn.2012.0089. [DOI] [PubMed] [Google Scholar]
- 60.Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121:2517–2528. doi: 10.1002/cncr.29383. [DOI] [PubMed] [Google Scholar]
- 61.Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105:2524–2539. doi: 10.3324/haematol.2020.247031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, Bueso-Ramos CE, Pierce S, Shan J, Koller C, Beran M, Keating M, Freireich EJ. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 63.Vadillo E, Dorantes-Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T-ALL): new insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32:36–51. doi: 10.1016/j.blre.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Chiaretti S, Foà R. T-cell acute lymphoblastic leukemia. Haematologica. 2009;94:160–162. doi: 10.3324/haematol.2008.004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, Rowntree C, Richards S. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14:199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 66.Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Ma X, Zhang H, Wu Y, Kang M, Fang Y, Xue Y. Mechanism of circADD2 as ceRNA in childhood acute lymphoblastic leukemia. Front Cell Dev Biol. 2021;9:639910. doi: 10.3389/fcell.2021.639910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan SJ, Li HB, Cui G, Kong XL, Sun LL, Zhao YQ, Li YH, Zhou J. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk Res. 2016;41:62–70. doi: 10.1016/j.leukres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Honardoost M, Rad SMAH. Triangle of AKT2, miRNA, and tumorigenesis in different cancers. Appl Biochem Biotechnol. 2018;185:524–540. doi: 10.1007/s12010-017-2657-3. [DOI] [PubMed] [Google Scholar]
- 70.Li K, Fan X, Yan Z, Zhan J, Cao F, Jiang Y. Circ_0000745 strengthens the expression of CCND1 by functioning as miR-488 sponge and interacting with HuR binding protein to facilitate the development of oral squamous cell carcinoma. Cancer Cell Int. 2021;21:271. doi: 10.1186/s12935-021-01884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiao J, Zhang T, Jiao X, Huang T, Zhao L, Ma D, Cui B. hsa_circ_0000745 promotes cervical cancer by increasing cell proliferation, migration, and invasion. J Cell Physiol. 2020;235:1287–1295. doi: 10.1002/jcp.29045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang M, He YR, Liang LC, Huang Q, Zhu ZQ. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol. 2017;23:6330–6338. doi: 10.3748/wjg.v23.i34.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muta Y, Matsuda M, Imajo M. Divergent dynamics and functions of ERK MAP kinase signaling in development, homeostasis and cancer: lessons from fluorescent bioimaging. Cancers (Basel) 2019;11:513. doi: 10.3390/cancers11040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang X, Li Y, Zhang Y, Liu J. Circ_0000745 promotes acute lymphoblastic leukemia progression through mediating miR-494-3p/NET1 axis. Hematology. 2022;27:11–22. doi: 10.1080/16078454.2021.2008590. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Wu B, Wang Y. Molecular mechanism of circVRK1 regulating the proliferation and apoptosis of acute lymphoblastic leukemia KOCL44 cells by targeting miR-4428. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024;55:872–877. doi: 10.12182/20240760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou Y, Sun J, Huang J, Yao F, Chen X, Zhu B, Zhao D. Circular RNA circRNA_0000094 sponges microRNA-223-3p and up-regulate F-box and WD repeat domain containing 7 to restrain T cell acute lymphoblastic leukemia progression. Hum Cell. 2021;34:977–989. doi: 10.1007/s13577-021-00504-4. [DOI] [PubMed] [Google Scholar]
- 77.Kaushik B, Pal D, Saha S. Gamma secretase inhibitor: therapeutic target via NOTCH signaling in T cell acute lymphoblastic leukemia. Curr Drug Targets. 2021;22:1789–1798. doi: 10.2174/1389450122666210203192752. [DOI] [PubMed] [Google Scholar]
- 78.Castro MA, Parson KF, Beg I, Wilkinson MC, Nurmakova K, Levesque I, Voehler MW, Wolfe MS, Ruotolo BT, Sanders CR. Verteporfin is a substrate-selective γ-secretase inhibitor that binds the amyloid precursor protein transmembrane domain. J Biol Chem. 2022;298:101792. doi: 10.1016/j.jbc.2022.101792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu M, Xia W, Chen R, Wang S, Xu Y, Ma Z, Xu W, Zhang E, Wang J, Fang T, Hu J, Dong G, Yin R, Wang J, Xu L. The circular RNA circPRKCI promotes tumor growth in lung adenocarcinoma. Cancer Res. 2018;78:2839–2851. doi: 10.1158/0008-5472.CAN-17-2808. [DOI] [PubMed] [Google Scholar]
- 80.Liu Z, Ren X, Yang Z, Mei L, Li W, Tu C, Li Z. Prognostic and clinical value of circPRKCI expression in diverse human cancers. Chin Med J (Engl) 2024;137:152–161. doi: 10.1097/CM9.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sui MH, Zhang WW, Geng DM, Sun DJ. CircPRKCI regulates proliferation, migration and cycle of lung adenocarcinoma cells by targeting miR-219a-5p-regulated CAMK1D. Eur Rev Med Pharmacol Sci. 2021;25:1899–1909. doi: 10.26355/eurrev_202102_25085. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Song H, Fang L, Wu T. EIF4A3-mediated circPRKCI expression promotes triple-negative breast cancer progression by regulating WBP2 and PI3K/AKT signaling pathway. Cell Death Discov. 2022;8:92. doi: 10.1038/s41420-022-00892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng Y, Niu B, Zhang W, Ru X, Gao Y, Li C, Wu X. Circular RNA circPRKCI contributes to malignant progression of T-cell acute lymphoblastic leukemia by modulating miR-20a-5p/SOX4 axis. Aging (Albany NY) 2021;13:23757–23768. doi: 10.18632/aging.203647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen H, Pei L, Xie P, Guo G. Circ-PRKDC contributes to 5-fluorouracil resistance of colorectal cancer cells by regulating miR-375/FOXM1 axis and Wnt/β-catenin pathway. Onco Targets Ther. 2020;13:5939–5953. doi: 10.2147/OTT.S253468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang G, Li Y, Zhu H, Huo G, Bai J, Gao Z. Circ-PRKDC facilitates the progression of colorectal cancer through miR-198/DDR1 regulatory axis. Cancer Manag Res. 2020;12:12853–12865. doi: 10.2147/CMAR.S273484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ling Z, Fang ZG, Wu JY, Liu JJ. The depletion of circ-PRKDC enhances autophagy and apoptosis in T-cell acute lymphoblastic leukemia via microRNA-653-5p/Reelin mediation of the PI3K/AKT/mTOR signaling pathway. Kaohsiung J Med Sci. 2021;37:392–401. doi: 10.1002/kjm2.12352. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, Ahmadzadeh A, Saki N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72:2337–2347. doi: 10.1007/s00018-015-1867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yehui L, Zhihong L, Fang T, Zixuan Z, Mengyuan Z, Zhifang Y, Jiuhong Z. Bibliometric analysis of global research on circular RNA: current status and future directions. Mol Biotechnol. 2024;66:2064–2077. doi: 10.1007/s12033-023-00830-y. [DOI] [PubMed] [Google Scholar]
- 90.Mo Y, Wang Y, Wang Y, Deng X, Yan Q, Fan C, Zhang S, Zhang S, Gong Z, Shi L, Liao Q, Guo C, Li Y, Li G, Zeng Z, Jiang W, Xiong W, Xiang B. Circular RNA circPVT1 promotes nasopharyngeal carcinoma metastasis via the β-TrCP/c-Myc/SRSF1 positive feedback loop. Mol Cancer. 2022;21:192. doi: 10.1186/s12943-022-01659-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu YY, Zhang LY, Du WZ. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci Rep. 2019;39:BSR20193045. doi: 10.1042/BSR20193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng Z, Chen Z, Zhong Q, Zhu D, Xie Y, Shangguan W, Xie W. CircPVT1 promotes progression in clear cell renal cell carcinoma by sponging miR-145-5p and regulating TBX15 expression. Cancer Sci. 2021;112:1443–1456. doi: 10.1111/cas.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu J, Han Q, Gu Y, Ma J, McGrath M, Qiao F, Chen B, Song C, Ge Z. Circular RNA PVT1 expression and its roles in acute lymphoblastic leukemia. Epigenomics. 2018;10:723–732. doi: 10.2217/epi-2017-0142. [DOI] [PubMed] [Google Scholar]
- 94.Carramusa L, Contino F, Ferro A, Minafra L, Perconti G, Giallongo A, Feo S. The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J Cell Physiol. 2007;213:511–518. doi: 10.1002/jcp.21133. [DOI] [PubMed] [Google Scholar]
- 95.Jia Y, Gu W. Up-regulation of circPVT1 in T cell acute lymphoblastic leukemia promoted cell proliferation via miR-30e/DLL4 induced activating NOTCH signaling. Pathol Res Pract. 2021;224:153536. doi: 10.1016/j.prp.2021.153536. [DOI] [PubMed] [Google Scholar]
- 96.Feng H, Li F, Tang P. Circ_0000745 regulates NOTCH1-mediated cell proliferation and apoptosis in pediatric T-cell acute lymphoblastic leukemia through adsorbing miR-193b-3p. Hematology. 2021;26:885–895. doi: 10.1080/16078454.2021.1997197. [DOI] [PubMed] [Google Scholar]
- 97.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 98.Joshi I, Minter LM, Telfer J, Demarest RM, Capobianco AJ, Aster JC, Sicinski P, Fauq A, Golde TE, Osborne BA. Notch signaling mediates G1/S cell-cycle progression in T cells via cyclin D3 and its dependent kinases. Blood. 2009;113:1689–1698. doi: 10.1182/blood-2008-03-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao G, Chen H, Luo J. CircBCAR3 sponges miR-27a-3p and mediates ferroptosis in human B-prolymphocytic leukaemia cells via SLC7A11. Cell Mol Biol (Noisy-le-grand) 2023;69:181–187. doi: 10.14715/cmb/2023.69.12.29. [DOI] [PubMed] [Google Scholar]
- 100.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92:946–965. doi: 10.1002/ajh.24826. [DOI] [PubMed] [Google Scholar]
- 103.Yang S, Gale RP, Shi H, Liu Y, Lai Y, Lu J, Huang X. Is there an epidemic of chronic lymphocytic leukaemia (CLL) in China? Leuk Res. 2018;73:16–20. doi: 10.1016/j.leukres.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 104.Dong Y, Shi O, Zeng Q, Lu X, Wang W, Li Y, Wang Q. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020;9:14. doi: 10.1186/s40164-020-00170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating M, Montserrat E, Chiorazzi N, Stilgenbauer S, Rai KR, Byrd JC, Eichhorst B, O’Brien S, Robak T, Seymour JF, Kipps TJ. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 106.Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96:1679–1705. doi: 10.1002/ajh.26367. [DOI] [PubMed] [Google Scholar]
- 107.Wu W, Wu Z, Xia Y, Qin S, Li Y, Wu J, Liang J, Wang L, Zhu H, Fan L, Fu J, Xu W, Jin H, Li J. Downregulation of circ_0132266 in chronic lymphocytic leukemia promoted cell viability through miR-337-3p/PML axis. Aging (Albany NY) 2019;11:3561–3573. doi: 10.18632/aging.101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vogiatzoglou AP, Moretto F, Makkou M, Papamatheakis J, Kretsovali A. Promyelocytic leukemia protein (PML) and stem cells: from cancer to pluripotency. Int J Dev Biol. 2022;66:85–95. doi: 10.1387/ijdb.210154av. [DOI] [PubMed] [Google Scholar]
- 109.Xia L, Wu L, Bao J, Li Q, Chen X, Xia H, Xia R. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;503:385–390. doi: 10.1016/j.bbrc.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 110.Qiao GY, Dong BW, Zhu CJ, Yan CY, Chen BL. Deregulation of WNT2/FZD3/β-catenin pathway compromises the estrogen synthesis in cumulus cells from patients with polycystic ovary syndrome. Biochem Biophys Res Commun. 2017;493:847–854. doi: 10.1016/j.bbrc.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 111.Heintel D, Kienle D, Shehata M, Kröber A, Kroemer E, Schwarzinger I, Mitteregger D, Le T, Gleiss A, Mannhalter C, Chott A, Schwarzmeier J, Fonatsch C, Gaiger A, Döhner H, Stilgenbauer S, Jäger U CLL Study Group. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1216–1223. doi: 10.1038/sj.leu.2403748. [DOI] [PubMed] [Google Scholar]
- 112.Wu Z, Gu D, Wang R, Zuo X, Zhu H, Wang L, Lu X, Xia Y, Qin S, Zhang W, Xu W, Fan L, Li J, Jin H. CircRIC8B regulates the lipid metabolism of chronic lymphocytic leukemia through miR199b-5p/LPL axis. Exp Hematol Oncol. 2022;11:51. doi: 10.1186/s40164-022-00302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li S, Chen J, Fan Y, Xu X, Xiong M, Qi Y, Wu W, Zhao Y. circZNF91 promotes the malignant phenotype of chronic lymphocytic leukemia cells by targeting the miR-1283/WEE1 axis. Biomed Res Int. 2022;2022:2855394. doi: 10.1155/2022/2855394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghelli Luserna di Rorà A, Cerchione C, Martinelli G, Simonetti G. A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. J Hematol Oncol. 2020;13:126. doi: 10.1186/s13045-020-00959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin X, Xu A, Fan F, Huang Z, Cheng Q, Zhang L, Sun C, Hu Y. Incidence and mortality trends and risk prediction nomogram for extranodal diffuse large B-cell lymphoma: an analysis of the surveillance, epidemiology, and end results database. Front Oncol. 2019;9:1198. doi: 10.3389/fonc.2019.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 117.Zhao Y, Cui WL, Feng ZY, Xue J, Gulinaer A, Zhang W. Expression of Foxp3 and interleukin-7 receptor and clinicopathological characteristics of patients with diffuse large B-cell lymphoma. Oncol Lett. 2020;19:2755–2764. doi: 10.3892/ol.2020.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lang J, Ma K, Guo J, Zhang J, Wang Q, Sun H. Clinical significance of elevated antinuclear antibodies in patients with diffuse large B-cell lymphoma: a single center study. J Cancer Res Ther. 2018;14:213–219. doi: 10.4103/0973-1482.183559. [DOI] [PubMed] [Google Scholar]
- 119.Hu Y, Zhao Y, Shi C, Ren P, Wei B, Guo Y, Ma J. A circular RNA from APC inhibits the proliferation of diffuse large B-cell lymphoma by inactivating Wnt/β-catenin signaling via interacting with TET1 and miR-888. Aging (Albany NY) 2019;11:8068–8084. doi: 10.18632/aging.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 121.Chen X, Xie X, Zhou W. CircCFL1/MiR-107 axis targeting HMGB1 promotes the malignant progression of diffuse large B-cell lymphoma tumors. Cancer Manag Res. 2020;12:9351–9362. doi: 10.2147/CMAR.S263222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 124.Liu W, Lei L, Liu X, Ye S. CircRNA_OTUD7A upregulates FOXP1 expression to facilitate the progression of diffuse large B-cell lymphoma via acting as a sponge of miR-431-5p. Genes Genomics. 2021;43:653–667. doi: 10.1007/s13258-021-01094-z. [DOI] [PubMed] [Google Scholar]
- 125.Gascoyne DM, Banham AH. The significance of FOXP1 in diffuse large B-cell lymphoma. Leuk Lymphoma. 2017;58:1037–1051. doi: 10.1080/10428194.2016.1228932. [DOI] [PubMed] [Google Scholar]
- 126.Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2007;11:955–965. doi: 10.1517/14728222.11.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walker MP, Stopford CM, Cederlund M, Fang F, Jahn C, Rabinowitz AD, Goldfarb D, Graham DM, Yan F, Deal AM, Fedoriw Y, Richards KL, Davis IJ, Weidinger G, Damania B, Major MB. FOXP1 potentiates Wnt/β-catenin signaling in diffuse large B cell lymphoma. Sci Signal. 2015;8:ra12. doi: 10.1126/scisignal.2005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824–16837. doi: 10.1002/jcp.28358. [DOI] [PubMed] [Google Scholar]
- 129.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 131.Godfrey J, Tumuluru S, Bao R, Leukam M, Venkataraman G, Phillip J, Fitzpatrick C, McElherne J, MacNabb BW, Orlowski R, Smith SM, Kline J. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood. 2019;133:2279–2290. doi: 10.1182/blood-2018-10-879015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song G, Gu L, Li J, Tang Z, Liu H, Chen B, Sun X, He B, Pan Y, Wang S, Cho WC. Serum microRNA expression profiling predict response to R-CHOP treatment in diffuse large B cell lymphoma patients. Ann Hematol. 2014;93:1735–1743. doi: 10.1007/s00277-014-2111-3. [DOI] [PubMed] [Google Scholar]
- 133.Dong L, Huang J, Gao X, Du J, Wang Y, Zhao L. CircPCBP2 promotes the stemness and chemoresistance of DLBCL via targeting miR-33a/b to disinhibit PD-L1. Cancer Sci. 2022;113:2888–2903. doi: 10.1111/cas.15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 135.Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Caetano BFR, Jorge BAS, Müller-Coan BG, Elgui de Oliveira D. Epstein-Barr virus microRNAs in the pathogenesis of human cancers. Cancer Lett. 2021;499:14–23. doi: 10.1016/j.canlet.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 137.Zhao CX, Yan ZX, Wen JJ, Fu D, Xu PP, Wang L, Cheng S, Hu JD, Zhao WL. CircEAF2 counteracts Epstein-Barr virus-positive diffuse large B-cell lymphoma progression via miR-BART19-3p/APC/β-catenin axis. Mol Cancer. 2021;20:153. doi: 10.1186/s12943-021-01458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J. Clin. Oncol. 2016;34:1256–1269. doi: 10.1200/JCO.2015.63.5904. [DOI] [PubMed] [Google Scholar]
- 139.Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94:710–725. doi: 10.1002/ajh.25487. [DOI] [PubMed] [Google Scholar]
- 140.Vose JM. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2017;92:806–813. doi: 10.1002/ajh.24797. [DOI] [PubMed] [Google Scholar]
- 141.Podzolkov VI, Vargina TS, Pokrovskaya AE, Safronova TA, Abramova AA. Mantle cell lymphoma case report. Case Rep Oncol. 2018;11:814–821. doi: 10.1159/000492665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Geisler CH, Trneny M, Stilgenbauer S, Kaiser F, Doorduijn JK, Salles G, Szymczyk M, Tilly H, Kanz L, Schmidt C, Feugier P, Thieblemont C, Zijlstra JM, Ribrag V, Klapper W, Pott C, Unterhalt M, Dreyling MH. Treatment of older patients with mantle cell lymphoma (MCL): long-term follow-up of the randomized European MCL elderly trial. J. Clin. Oncol. 2020;38:248–256. doi: 10.1200/JCO.19.01294. [DOI] [PubMed] [Google Scholar]
- 143.Dahl M, Daugaard I, Andersen MS, Hansen TB, Grønbæk K, Kjems J, Kristensen LS. Enzyme-free digital counting of endogenous circular RNA molecules in B-cell malignancies. Lab Invest. 2018;98:1657–1669. doi: 10.1038/s41374-018-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun J, Zhang H, Tao D, Xie F, Liu F, Gu C, Wang M, Wang L, Jiang G, Wang Z, Xiao X. CircCDYL inhibits the expression of C-MYC to suppress cell growth and migration in bladder cancer. Artif Cells Nanomed Biotechnol. 2019;47:1349–1356. doi: 10.1080/21691401.2019.1596941. [DOI] [PubMed] [Google Scholar]
- 145.Mei M, Wang Y, Wang Q, Liu Y, Song W, Zhang M. CircCDYL serves as a new biomarker in mantle cell lymphoma and promotes cell proliferation. Cancer Manag Res. 2019;11:10215–10221. doi: 10.2147/CMAR.S232075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen P, Yao Y, Yang N, Gong L, Kong Y, Wu A. Circular RNA circCTNNA1 promotes colorectal cancer progression by sponging miR-149-5p and regulating FOXM1 expression. Cell Death Dis. 2020;11:557. doi: 10.1038/s41419-020-02757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu C, Song C, Han Q, Jing X. CircCTNNA1 is upregulated in mantle cell lymphoma and predicts poor survival by sponging miR-34a to increase cell proliferation. Mediterr J Hematol Infect Dis. 2022;14:e2022047. doi: 10.4084/MJHID.2022.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marques SC, Ranjbar B, Laursen MB, Falgreen S, Bilgrau AE, Bødker JS, Jørgensen LK, Primo MN, Schmitz A, Ettrup MS, Johnsen HE, Bøgsted M, Mikkelsen JG, Dybkær K. High miR-34a expression improves response to doxorubicin in diffuse large B-cell lymphoma. Exp Hematol. 2016;44:238–246. e232. doi: 10.1016/j.exphem.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 149.You MJ, Medeiros LJ, Hsi ED. T-lymphoblastic leukemia/lymphoma. Am J Clin Pathol. 2015;144:411–422. doi: 10.1309/AJCPMF03LVSBLHPJ. [DOI] [PubMed] [Google Scholar]
- 150.Chan JY, Lim JQ, Ong CK. Towards next generation biomarkers in Natural Killer/T-cell lymphoma. Life (Basel) 2021;11:838. doi: 10.3390/life11080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cortelazzo S, Ferreri A, Hoelzer D, Ponzoni M. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2017;113:304–317. doi: 10.1016/j.critrevonc.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 152.Al Khabori M, Samiee S, Fung S, Xu W, Brandwein J, Patterson B, Brien W, Chang H. Adult precursor T-lymphoblastic leukemia/lymphoma with myeloid-associated antigen expression is associated with a lower complete remission rate following induction chemotherapy. Acta Haematol. 2008;120:5–10. doi: 10.1159/000146081. [DOI] [PubMed] [Google Scholar]
- 153.Deng L, Liu G, Zheng C, Zhang L, Kang Y, Yang F. Circ-LAMP1 promotes T-cell lymphoblastic lymphoma progression via acting as a ceRNA for miR-615-5p to regulate DDR2 expression. Gene. 2019;701:146–151. doi: 10.1016/j.gene.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 154.Rahmoon MA, Youness RA, Gomaa AI, Hamza MT, Waked I, El Tayebi HM, Abdelaziz AI. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. 2017;35:76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 155.Wu X, Deng L, Tang D, Ying G, Yao X, Liu F, Liang G. miR-615-5p prevents proliferation and migration through negatively regulating serine hydromethyltransferase 2 (SHMT2) in hepatocellular carcinoma. Tumour Biol. 2016;37:6813–6821. doi: 10.1007/s13277-015-4506-8. [DOI] [PubMed] [Google Scholar]
- 156.Jiang Y, Zhang Y, Li F, Du X, Zhang J. CDX2 inhibits pancreatic adenocarcinoma cell proliferation via promoting tumor suppressor miR-615-5p. Tumour Biol. 2016;37:1041–1049. doi: 10.1007/s13277-015-3900-6. [DOI] [PubMed] [Google Scholar]
- 157.Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, Leitinger B, Hohenester E. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. 2009;17:1573–1581. doi: 10.1016/j.str.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xue W, Zhang M. Updating targets for natural killer/T-cell lymphoma immunotherapy. Cancer Biol Med. 2021;18:52–62. doi: 10.20892/j.issn.2095-3941.2020.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de Mel S, Hue SS, Jeyasekharan AD, Chng WJ, Ng SB. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J Hematol Oncol. 2019;12:33. doi: 10.1186/s13045-019-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mei M, Wang Y, Song W, Li Z, Wang Q, Li J, Zhang M. CircADARB1 serves as a new biomarker in natural killer T-cell lymphoma and a potential regulator of p-Stat3. Cancer Cell Int. 2021;21:594. doi: 10.1186/s12935-021-02296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Somasundaram N, Lim JQ, Ong CK, Lim ST. Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL) J Hematol Oncol. 2019;12:28. doi: 10.1186/s13045-019-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumai T, Kobayashi H, Harabuchi Y. Novel targets for natural killer/T-cell lymphoma immunotherapy. Immunotherapy. 2016;8:45–55. doi: 10.2217/imt.15.103. [DOI] [PubMed] [Google Scholar]
- 163.Chen W, Fu J, Chen Y, Li Y, Ning L, Huang D, Yan S, Zhang Q. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR-1231 and upregulating GPX4 in papillary thyroid cancer. Aging (Albany NY) 2021;13:16500–16512. doi: 10.18632/aging.203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huo LW, Wang YF, Bai XB, Zheng HL, Wang MD. circKIF4A promotes tumorogenesis of glioma by targeting miR-139-3p to activate Wnt5a signaling. Mol Med. 2020;26:29. doi: 10.1186/s10020-020-00159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang H, Huang X, Wang J, Yang L, Kong Y, Gao G, Zhang L, Chen ZS, Xie X. circKIF4A acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Mol Cancer. 2019;18:23. doi: 10.1186/s12943-019-0946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luo W, Liu Y, Qu H, Luo X, Xu L, Zhang J, Wang J. CircKIF4A promotes non-small cell lung cancer proliferation and metastasis through MiR-1238/CLDN14 axis. Aging (Albany NY) 2022;14:7408–7415. doi: 10.18632/aging.204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.He R, Wen W, Fu B, Zhu R, Chen G, Bai S, Cao X, Wang H. CircKIF4A is a prognostic factor and modulator of Natural Killer/T-cell lymphoma progression. Cancers (Basel) 2022;14:4950. doi: 10.3390/cancers14194950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin D, Wang Y, Lei L, Lin C. Circ_0003645 serves as miR-335-5p sponge to promote the biological process of diffuse large B-cell lymphoma by upregulating NFIB. Autoimmunity. 2022;55:127–135. doi: 10.1080/08916934.2021.2023863. [DOI] [PubMed] [Google Scholar]
- 169.Zhan C, Zhou H, Zhang W, Si C. Hsa_circ_0000877 facilitates the progression of diffuse large B-cell lymphoma by miR-370-3p/mitogen-activated protein kinase kinase kinase kinase 4/Hippo pathway. Anticancer Drugs. 2022;33:1091–1102. doi: 10.1097/CAD.0000000000001366. [DOI] [PubMed] [Google Scholar]
- 170.Yang CS, Lou Y, Ke QP, Xu XJ, Zhang Y. Mechanism of circZNF609 targeting miR-153 to regulate the proliferation and apoptosis of diffuse large B-cell lymphoma. Zhonghua Zhong Liu Za Zhi. 2022;44:238–245. doi: 10.3760/cma.j.cn112152-20200723-00677. [DOI] [PubMed] [Google Scholar]
- 171.Wang L, Yang B, Xu Z, Song X, Gong Z, Xue S, Kong L. NRF1-regulated circNSUN2 promotes lymphoma progression through activating Wnt signaling pathway via stabilizing HMGA1. Cell Cycle. 2021;20:819–828. doi: 10.1080/15384101.2021.1897272. [DOI] [PMC free article] [PubMed] [Google Scholar]