Abstract
Depression is a common co-morbidity among cancer cases, which has a detrimental influence on cancer treatment and prognosis. Recent advancements in the neurobiology of depression and cancer pathophysiology have revealed several shared biobehavioral mechanisms and introduced new therapeutic strategies. In this review, we summarize the biological mechanisms driving cancer-related depression, including psychosocial factors, immuno-inflammatory processes, chronic stress, dysbiosis of gut microbiota, and medically-induced factors. Interventions used for cancer-related depression may include psychosocial therapies, pharmacological therapies, immunotherapies, psychobiological medications, and dietary strategies. This review could inspire the elucidation of possible co-occurring mechanisms and complex interactions between cancer and depression, provide an opportunity to propose faster and more effective therapies for cancer-related depression, and well as new strategies for cancer in the future.
Keywords: Cancer, depression, pathogenesis, treatment
Introduction
It is widely recognized that both cancer and depression are significant reasons of disability and death across the globally. The occurrence of depression has been rising steadily each year, and data from 1990 to 2017 indicate a significant rise in its prevalence [1]. Worldwide, the incidence of depression has risen by 49.86% [1]. Major depression is highly debilitating, with a disability rate reaching 37.3%, making it the primary cause of disability among psychiatric disorders [2]. WHO statistics suggest that about 4.2% of China’s population suffers from depression [3]. It is anticipated that by 2030, major depression will be the key reason of global disability [3]. Cancer represents a significant challenge in contemporary medicine and public health. Cancer-related deaths account for approximately 15-20% of annual global deaths, and this proportion is increasing significantly each year [4]. Worse still, when these two diseases occur together, it will exacerbate the burden on the patients and society.
A cancer diagnosis can be psychologically and emotionally stressful for patients, and when it exceeds their coping mechanisms, it may lead to major depression. While the diagnosis of cancer can trigger a rapid onset of psychological and emotional stress, the significant incidence of depression in cancer patients may extend beyond the realm of psychological stress, suggesting the involvement of other contributing factors. Cancer-related depression (CRD) is a complex condition influenced by psychosocial, biological, and medical factors. Recent advancements in understanding the neurobiology of depression and the pathophysiology of cancer have uncovered several shared biobehavioral mechanisms.
This review explores the biological mechanisms of underlying CRD, including tissue damage, inflammatory mediators, chronic stress responses, gut microbial pathways, and possible medical causes. Additionally, it will briefly discuss the adverse effects of CRD and therapeutic strategies aimed at addressing this condition.
Correlations between cancer and depression
Depression is more prevalent among cancer patients [5]. Research has shown that depression occurrence rate is obvious higher among cancer cases, with rates varying from 1.5% to 50%, and an average prevalence of 15% to 29% [6]. In addition, patients diagnosed with cancer experience more significant distress than those diagnosed with non-oncological diseases [7]. Prolonged high-level mental distress may cause cancer patients to develop anxiety, depression, or both [8]. Age and gender are also essential factors in the incidence of depression [8]. There is evidence that age is negatively correlated with depression in certain cancers in adults, but with no significant correlation in adolescents; Female patients are three times more prone to be depressed than males in specific cancer types. Moreover, the primary site of cancer can significantly affect the occurrence of depression. Pancreatic and lung cancers are associated with the highest incidences, while invasive skin cancers have the lowest rates [8].
Conversely, mood disorders was found to accelerate the progression of chronic inflammatory diseases, such as cancer [9]. Depression can drastically diminish patients’ quality of life (QOL) and contribute to higher cancer mortality rates [10,11]. A meta-analysis has shown that individuals with mild depressive symptoms may experience a 25% higher risk of mortality compared to those without depression, while severe depression raises the mortality rate among cancer patients by 39% [12]. These result indicate a bidirectional association in cancer and depression, yet the specific biological mechanisms underlying this connection are not completely clear.
A glance at pathogenic theories of depression
Depression, which a complex disorder with a multifactorial origin, and its pathogenesis is still not fully determined. Current theories include the psychosocial and genetic hypothesis, the monoamine hypothesis, the HPA axis dysfunction model, as well as the neurotrophic and inflammatory hypotheses. Additionally, the microbiota-gut-brain axis is increasingly being recognized as a key factor in understanding depression. However, no single hypothesis fully explains the pathological mechanisms of depression, as many of these mechanisms interact with each other. Therefore, the etiology of depression cannot be explained from a singular perspective.
Genetic and psychosocial hypothesis
Although the etiology of Major Depressive Disorder (MDD) remains unclear, considerable research utilizing diverse models has explored the genetic and environmental factors, which are associated with the disorder [13]. In recent years, certain research has highlighted the significant influence of genetic factors in contributing to depression [14]. MDD has a heritability ranging from 30 to 50% [15]. Genomic research has identified over 100 genetic loci and neuronal calcium signaling pathways related to higher risk of MDD [16]. Although numerous genetic researches have been conducted, there has not been a definitive identification of a single genetic variant that significantly elevates the risk for MDD [15]. It is proposed that genetic variants alone have a limited impact on disease risk, and the combination of multiple genetic factors with environmental influences, like stress, may be key in developing major depressive disorder (MDD) [17].
Traumatic or stressful life experiences are significant environmental risk factors for the onset MDD. The exact pathomechanism linking social stress to depression remains unclear. The uncertainty mainly arises from the challenge of distinguishing social factors from genetic ones in cases and the impracticality of using animal models to study relevant environmental stressors [16]. Research into microglia and astrocyte damage has underscored the function of glial cells in the emergence of depressive-like behaviors induced by environmental factors [18]. Furthermore, depression may cause various comorbidities, including neurodegenerative diseases, cardiovascular diseases, metabolic disorders, and endocrine disorders [16]. The association in depression onset and different diseases is complex, with the two often influencing each other. The coexistence of additional conditions amplifies the social and economic consequences of depression [19].
The monoamine theory
Tricyclic antidepressants (TCAs) block the reuptake of synaptic serotonin (5-HT) and norepinephrine (NE) by inhibiting the serotonin transporter (SERT) and norepinephrine transporter (NET), thereby raising the concentrations of 5-HT and NE in the synaptic cleft [20]. Despite structural differences and varying sites of action among antidepressants, the monoamine theory suggests that shortage in monoamine neurotransmitters, like 5-HT, dopamine (DA), and NE, are the fundamental causes of clinical depression [21].
Specifically, 5-HT is crucial in the pathophysiology of depression, and depletion of the 5-HT precursor tryptophan leads to decreased levels of 5-HT and reduced functional connectivity in specific brain zones associated with cognitive processes [22]. This hypothesis, however, is primarily based on the serendipitously discovered drugs with antidepressant effects. Monoamine oxidase inhibitors (MAOIs) exert their antidepressant effects by inhibiting the degradation of monoamine neurotransmitters in the synaptic cleft, they all acutely elevate synaptic monoamine levels. Consequently, the observation that depression can be alleviated by both MAOIs and monoamine reuptake provides robust support for the monoamine hypothesis.
The HPA axis theory
The HPA axis is intricately connected to depression; stress activation of it can lead to changes to cognition and mood [23]. Elevated HPA activity is frequently observed as a neurobiological change in individuals with depression. The HPA axis serves as the main system for stress response, orchestrating the production of glucocorticoids (GC). When stress triggers the HPA axis, the paraventricular nucleus (PVN) of the hypothalamus quickly releases corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) [24]. This activation induce the anterior pituitary gland to release adrenocorticotropic hormone (ACTH), which then promotes the production and release of glucocorticoids (GCs) into the bloodstream, ultimately leading to elevated levels of these stress hormones in circulation.
GCs, including cortisol, bind to glucocorticoid receptors (GRs) found in the pituitary gland, hypothalamus, and different regions in limbic system, including the amygdala, hippocampus. These regions are essential for regulating emotional responses and cognitive functions, highlighting the significant role GCs play in both emotional and mental processes. GCs also negative regulate the HPA axis. Some scholars found that HPA axis dysregulation and elevated GC levels are central to the development of depression, making GR a potential target for depression therapy [25].
Neurotrophic theory
Recent research has established a strong association in the onset of depression and abnormal activities within the prefrontal cortex (PFC) and limbic system [26]. These brain regions are related to neuroplasticity, and irregularities in their function have been connected to deficits in neuroplasticity, which is a key factor in the development of depression. This suggests that neurons can restructure in response to environmental changes. Neurotrophic factors that bind to tyrosine kinase receptors are a crucial factor influencing this reorganization.
The most well-known and essential factor in neuroplasticity across the nervous system is brain-derived neurotrophic factor (BDNF) [27,28]. It is now understood that BDNF regulates neuronal activity and is produced by neurons and astrocytes [29]. BDNF, produced by astrocytes after extended antidepressant treatment, may improve synaptic plasticity at presynaptic terminals by facilitating neurotransmitter release [30]. Additionally, BDNF secreted by astrocytes can stimulate neurogenesis in the adult hippocampus, influencing the long-term behavioral effects of antidepressants [30].
Another neurotrophic factor, glial cell-derived neurotrophic factor (GDNF), is crucial for nervous system development and regeneration. Its dysregulation may contribute to various neurological disorders, including depression [31]. Recent research indicates that diminished neurogenesis in the hippocampus of individuals with depression correlates with decreased levels of GDNF [31].
Inflammation theory
Declining physical well-being and psychological health could potentially be related to the excessive activation of pro-inflammatory factors [32]. Several meta-analyses have demonstrated elevated levels of such factors, including IL-6, IL-8, IL-1β, TNF-α, soluble IL-2 receptor (sIL-2R), and C-reactive protein (CRP), in the peripheral blood of MDD case [33,34]. Longitudinal studies have found that higher CRP levels can predispose individuals to future depressive symptoms, supporting the theory that immune activation may cause the onset of depression [35].
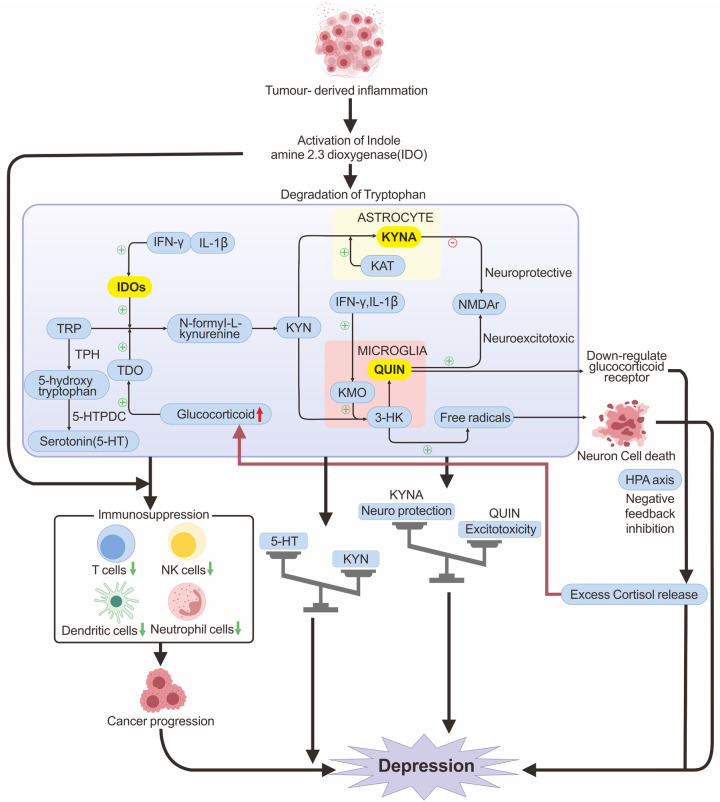
Proinflammatory cytokines can activate indoleamine-2,3-dioxygenase (IDO), diverting tryptophan from the 5-HT pathway to the kynurenine (KYN) pathway (Figure 1), thus reducing 5-HT production [36]. In microglia, KYN can be further metabolized into quinolinic acid (QUIN), a strong agonist of the N-methyl-D-aspartate (NMDA) receptor, which stimulates glutamate release, significantly increasing Ca2+ efflux and inhibiting glutamate reabsorption by glial cells. Hyperexcitability of NMDA receptors, caused by inadequate synaptic clearance, results in neuronal death through excitotoxicity [37].
Figure 1.
The immunoinflammatory mechanisms of CRD occurrence. TRP is metabolized in the body mainly to 5-HT and TRP. TRP generates 5-HT by the enzyme TPH, and 5-HTPDC. TRP is metabolized to KYN in response to IDO and TDO, with subsequent metabolism broadly categorized into two pathways: the TRP-KYN-3-HK-QUIN pathway located in microglia and the TRP-KYN-KYNA pathway located in astrocytes. QUIN is a potent NMDA receptor agonist that causes neuroexcitotoxicity and neurodegeneration associated with depression. Intermediate product 3-HK induces neuron cell death through oxidative stress mediated by free radicals. KYNA is the only endogenous antagonist of NMDA receptors and can attenuate QUIN-induced excitotoxicity. Cancer-induced inflammation and the excessive accumulation of pro-inflammatory cytokines (e.g., IFN-γ, IL-1β) can enhance the activity of IDO and KMO. This promotes tryptophan metabolism in favor of the TRP-KYN-3-HK-QA excitotoxic pathway, which causes an increase in QUIN and neuron cell death. Overproduction of QUIN causes an imbalance between excitotoxicity and neuro-protection, resulting in depression. Elevated QUIN also down-regulates glucocorticoid receptors, causing negative feedback dysregulation of the HPA axis, leading to an excessive release of glucocorticoids, which not only causes depression but also further activates TDO. IDO also exhibits immunosuppressive properties, reducing the number of immune cells and impairing their functions, which can facilitate cancer progression.
The glutamatergic system is very closely related to the depression [38]. Glutamine synthetase converts glutamate into glutamine, which subsequently enters GABAergic neurons and is metabolized into GABA. QUIN also blocks the expression of glutamine synthetase, which leads to reduced production of GABA, the primary inhibitory neurotransmitter in the developed brain. Numerous studies indicate that a disruption in GABAergic transmission may significantly impact the pathogenesis process of depression [39]. QUIN and other tryptophan degradation products amplify the release of inflammatory cytokines, caused stronger production of these signaling molecules [40]. They also exacerbate both oxidative and nitrosative stress, which promote the cellular damage [40]. This process results in lipid peroxidation, impairing the integrity of cell membranes, as well as mitochondrial dysfunction, which disrupts cellular energy production, and DNA damage, potentially leading to mutations and impaired cellular functions [40]. The human brain is highly vulnerable to oxidative stress for lower antioxidant defenses and high oxygen usage, making it more prone to oxidative damage.
Increased pro-inflammatory cytokines, combined with reduced signals from anti-inflammatory mediators, can shift T cell differentiation towards the Th1/Th17 helper T cell phenotype instead of regulatory T (Treg) cells. This shift further enhances the generation of pro-inflammatory mediators (e.g., interferon, IL-2), thereby creating a vicious cycle [41,42]. Additionally, inflammasomes present in microglia have the capacity to be triggered by reactive oxygen species (ROS), which initiates a cascade that caused the increased expression of inflammatory cytokines, including TNF-α, IL-1β, and IFN-γ [43].
Microbiota-gut-brain axis theory
Recently, observations have noted disturbances in the microbiota-gut-brain axis among patients suffering from depression [44]. Stressful stimuli can significantly impact the function of the gut microbiota, leading to positive influence in the production of inflammatory mediators, particularly IL-6 and IFN-γ [45]. Additionally, these stressors contribute to a reduction in the levels of short-chain fatty acids (SCFAs), which is a key factor in maintaining overall homeostasis [45]. Increased inflammatory cytokine levels may result from microbiota disruption, which can also compromise the intestinal barrier [46].
Toxic byproducts generated by proinflammatory cytokines or microbiota alterations may breach the blood-brain barrier, elevating cytokine levels in brain-resident cells [47,48]. Specifically, with these inflammatory cytokines, microglia and astrocytes are activated, and altered in their interactions. Alterations in glial cells affect brain networks that are crucial for learning, memory, and the regulation of emotions, and mood modulation, potentially leading to depressive symptoms or anxiety episodes [49].
Pathogenic mechanisms of cancer-related depression
A cancer diagnosis can be very stressful psychologically and emotionally for the patient. While sadness is a common response to a cancer diagnosis, excessive stress that overwhelms a patient’s coping abilities can result in significant depression. Depression induced by cancer is a multifaceted condition that encompasses psychosocial, biological, and medical elements. Although a cancer diagnosis can lead to a rapid onset of psychological and emotional stress, the elevated prevalence of depression in cancer cases may partly due to the psychological stress of the illness alone. Recent research into the neurobiological aspects of depression and the underlying pathophysiology of cancer has revealed overlapping biobehavioral mechanisms.
Psychosocial factors
The progression of cancer-related depression is a continuous process that begins with non-pathological sadness, advances to adjustment disorder, evolves ultimately into major depression [50]. Stressors are general factors that cause the organism to adapt to various internal and external stimuli. Cancer, as a life-threatening event, acts as a stressor for patients upon diagnosis. Once diagnosed with cancer, patients will inevitably develop fear due to the uncertainty of the poor prognosis [51]. Thus, effective cancer control is cited as an important factor influencing their emotions [52]. Moreover, cancer pain is a major stressor, with depression occurring more frequently in cancer cases who experience pain than that do not [53]. Of the elderly cancer patients with pain, 79.6% experienced depression [54].
Families dealing with cancer face significant financial burdens due to expensive treatment costs and extensive nursing care. Therefore, the health status of the family and the cost of medical care significantly impacts the stress levels of cancer patients. Research shows a strong link between depression rates in cancer patients and the amount of social support they receive, both objective and subjective. Higher levels of support are associated with a lower likelihood of experiencing depression [55]. Emotional support from friends and family, along with a positive outlook, are significant protective factors that can help prevent the depression in cancer patients [8].
Immunoinflammatory mechanisms
Beyond socio-psychological factors, biological mechanisms are crucial to the onset of cancer-related depression. Evidence suggests that inflammation could promote the progression of both cancer and depression [56]. The presence of tumors leads to the release of various proinflammatory cytokines, causing neuroinflammation [57]. Immune mechanisms are implicated in the pathogenesis process of depression and the heightened mortality observed in cancer patients [58]. Both preclinical and clinical data indicate that elevated levels of proinflammatory cytokines are linked to various “disease syndromes” characterized by symptoms like anhedonia, irritability, cognitive impairment, fatigue, loss of appetite, sleep disturbances, and heightened pain sensitivity [59]. These symptoms, which partly overlap with those of depression, show some response to antidepressant treatment. Thus, immune crosstalk with nervous system is thought to be a significant promote the progression of somatic and affective symptoms of depression in cancer patients and may also impact the progression of cancer [60].
It is well-established that tumor-associated inflammation is initiated by hematopoietic growth factors, cytokines, resulting from oncogene expression triggered by driver mutations [61]. An additional mechanism that contributes to tumor-associated inflammation is the release of damage-associated molecular patterns (DAMPs), which result from rapid tumor cell replication, apoptosis, nutrient deprivation, and hypoxia [61]. DAMPs are recognized by pattern recognition receptors (PRRs), triggering a pro-inflammatory cascade.
Inflammation resulting from cancer can trigger psychological stress through complex interactions between the central nervous system (CNS) and the peripheral nervous system (PNS) [5]. These interactions highlight how systemic inflammation can influence mental health [5]. Peripheral cytokine production amplifies the pro-inflammatory response within the central nervous system based on enhancing the blood-brain barrier permeability and via vagal fibers linked to the solitary tract nucleus [62]. Furthermore, growing evidence indicates that perivascular mononuclear cells might enter the brain and promote the spread of inflammatory signals to astrocytes, which are key producers of immunoinflammatory mediators in the brain [63].
Proinflammatory cytokines, both centrally and peripherally released, can impact brain neurotransmitter systems, particularly the monoamine system, which is crucial in depression. Inflammatory cytokines activate p38 activity and increase the expression of norepinephrine and 5-HT reuptake transporter protein [64], causing lower synaptic concentrations of 5-HT and NE, which are associated with depressive behaviors in cancer patients [65]. These cytokines also diminish the availability of crucial nerve growth factors. For instance, brain-derived neurotrophic factor (BDNF), is crucial in neurogenesis. Reduced BDNF levels, combined with impaired neurogenesis, are significantly related to the progression of depression [66].
Tryptophan (TRP) can be metabolized in the body mainly to 5-HT, kynurenine (KYN), and other substances. TRP acts as a precursor of 5-HT, generating 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase (TPH), which is then synthesized into 5-HT by the enzyme 5-HT decarboxylase (5-HTPDC) (Figure 1). Under cancer-induced inflammatory conditions, the tryptophan-kynurenine metabolic pathway will be over-activated, resulting in excessive metabolism of TRP to KYN, which reduces 5-HT production [67]. Several proinflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IFN-γ, have been shown to increase the activity of IDO, an enzyme involved in TRP catalysis. TRP is first metabolized to the intermediate product KYN by IDO and tryptophan-2,3-dioxygenase (TDO) [59,68,69]. The subsequent kynurenine pathway can be broadly categorized into two distinct branches (Figure 1). One is the excitotoxic pathway, primarily characterized by the action of kynurenine 3-monooxygenase (KMO), which is located in microglia. This enzyme acts on 3-hydroxykynurenine (3-HK) to produce QUIN. QUIN is a potent NMDA receptor agonist that causes neuroexcitotoxicity and neurodegeneration associated with depression by interfering with the dynamic equilibrium of the glutamate-glutamine-GABA cycle [36]. The other is the neuroprotective pathway (Figure 1), which focuses on the synthesis of kynurenic acid (KYNA) catalyzed by the enzyme kynurenine aminotransferase (KAT) located in astrocytes. KYNA is the sole endogenous antagonist of NMDA receptors and serves as a potential endogenous antioxidant. The primary function of KYNA is to mitigate excitotoxic neurotoxicity induced by QUIN.
Under normal conditions, the production of KYNA and QUIN maintains a dynamic balance. However, in the presence of foreign inflammation or when the organism is under stress, there has promoted the release of pro-inflammatory cytokines (e.g., IFN-γ, IL-1β). This may result in the activation of microglial cells, along with the activation of IDO and KMO [67,70], ultimately resulting in a predisposition of the kynurenine pathway towards the excitotoxic TRP-KYN-3-HK-QUIN pathway. Consequently, this creates an imbalance between excitotoxicity and neuroprotection. Meanwhile, the overproduction of 3-HK can lead to neuron cell death via oxidative stress damage induced by free radicals, which might also be pertinent to the pathology of neurodegenerative diseases [71]. Moreover, elevated levels of QUIN have been shown to damage the medial prefrontal cortex, leading to the downregulation of glucocorticoid receptors and causing excessive release of glucocorticoid [72] (Figure 1). This, in turn, activates TDO, leading to excessive metabolism of TRP to KYN. Excessive metabolism of TRP to KYN will result in reduced levels of 5-HT production, this results in diminished functional connectivity within certain brain regions related to emotional and cognitive processes [22].
Additionally, IDOs in the tryptophan-kynurenine pathway exert immunosuppressive effects by impairing the function of immune cells, such as T cells, natural killer cells, dendritic cells, and neutrophils, which further drive cancer progression [73] (Figure 1). These mechanisms might collectively exert a crucial function in the evolution of CRD.
Chronic stress and the HPA axis
For patients, the diagnosis and treatment of cancer impose chronic stress on both the body and mind. Stress activates the sympathetic nervous system (SNS), inhibits the parasympathetic nervous system (PSNS), and sets off the hypothalamic-pituitary-adrenal (HPA) axis response [74-78]. The activation of the HPA axis in response to stress orchestrates a unified reaction from the immune, endocrine. This coordination is crucial for restoring biochemical and physiological equilibrium and preserving the body’s overall balance. However, HPA axis hyperactivity caused by chronic stress is now considered to be a hallmark of major depression [79-82].
In the HPA axis, higher cortisol levels and their feedback suppression of additional ACTH and CRH release are crucial in mood regulation within the hippocampus [83]. Over-activation of that axis caused excessive release of stress-related cortisol, leading to desensitization of glucocorticoid receptors [84]. This desensitization can negatively feedback-regulate the HPA axis, exacerbating the post-stress cortisol overload [85]. It is suggested that this desensitization may be caused by tumor-released proinflammatory cytokines interfering with the translocation or normal function of glucocorticoid receptors [74].
Endogenous glucocorticoids, which act as powerful anti-inflammatory agents, generally suppress the production of proinflammatory cytokines. However, due to rapid tumor replication, metabolism, and apoptosis, large amounts of DAMP were produced. DAMPs activate the PRR, which leads to the inactivation of the intraleukocytic glucocorticoid receptor by p38MAPK, weakening the anti-inflammatory effects of glucocorticoids, and increasing inflammatory cytokine expression [86,87]. Furthermore, chronic stimulation of SNS increases the release of NE, which binds to adrenergic receptors on macrophages, leading to increased activation of NF-κB and elevated cytokine expression [75,88]. Proinflammatory cytokines decrease the sensitivity of the remaining activated glucocorticoid receptors, subsequently diminishing the anti-inflammatory efficacy of glucocorticoids and further elevating cytokine production [89], forming a positive feedback circle. Excessive cortisol levels diminish the function of the hippocampal 5-HT system and reduce the levels of various neurotrophic factors [90], which leads to the degeneration of hippocampal neurons and ultimately results in depression.
Intestinal microbial pathway
Microorganisms with intricate functions have been identified as potential components of the tumor microenvironment, originating from mucosal sites and normal adjacent tissues [91]. Various events, such as diet, potential stress, tumors, and certain illnesses, can disrupt the balance of intestinal flora, resulting in dysbiosis. Recent research suggests that gut microbiota can affect an organism’s stress levels and behaviors via the brain-gut axis [92]. When the gut flora from depressed cases is transplanted into germ-free mice, the mice exhibit physiological characteristics resembling those of depressed individuals, including symptoms such as pleasure deprivation and anxiety-like behaviors [93]. Therefore, dysregulation of the gut microbiota in cancer cases may be a possible pathway contributing to cancer-related depression.
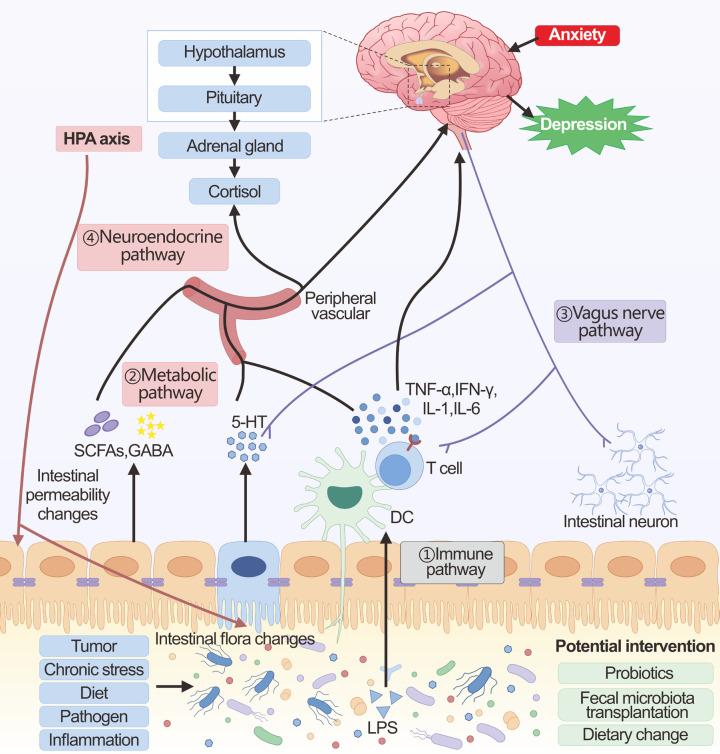
The gut microbiota influences central nervous system function with the help of the brain-gut axis mainly through four pathways: immune, metabolic, vagus nerve, and neuroendocrine (Figure 2). In immune pathways, chronic stress can lead to dysbiosis of the intestinal flora, subsequently triggering the release of inflammatory mediators, primarily IL-6 and IFN-γ [45]. Disruption of the intestinal flora, along with altered levels of inflammatory cytokines, can weaken the integrity of the intestinal barrier. This weakening may allow gut bacteria and microbial patterns to enter circulation, triggering inflammasome activation and a heightened pro-inflammatory response [94,95]. Furthermore, under inflammatory conditions, the blood-brain barrier permeability is reduced, and cytokines or toxic by-products caused by altered microbiota may cross the barrier of blood-brain and enter the brain, causing activation of microglia and atrophy of astrocytes [96]. These alterations in glial cells can influence the brain networks responsible for mood regulation, potentially resulting in depressive symptoms or anxiety attacks. Furthermore, when the intestinal barrier is compromised, it allows for the movement of Gram-negative bacterial lipopolysaccharide (LPS) into circulation, which then activates the immune system [97]. This activation triggers the release of IL-6, IFN-γ, CRP, and TNF-α [97]. These cytokines exert a critical function in perpetuating the chronic activation of the immune-inflammatory pathway.
Figure 2.
The brain-gut axis in cancer-related depression. The main communication pathways between the brain-gut axis in the context of gut microbiota homeostasis include immune, metabolic, vagus nerve, and neuroendocrine pathways. ① Immune pathway: Alteration of the gut microbiota and translocation of LPS activates the immune system. The induced pro-inflammatory cytokines can then affect brain function either directly through the blood-brain barrier or using the vagal pathway. ② Metabolic pathway: The intestinal microbiota produces a large number of metabolites (SCFAs, GABA, 5-HT, etc.), which can enter the brain through the peripheral circulation, affecting the glial cells and neural plasticity. In addition, these metabolites also affect brain function by affecting both the vagus nerve and the HPA axis. ③ Vagus nerve pathway: Changes in the gut microbiota, microbial products, and microbiota-dependent immune mediators can directly interact with intestinal neurons and the vagus nerve, which can be transmitted via sensory circuits to brain regions involved in cognition and mood. ④ Neuroendocrine pathway: The neuroendocrine pathway is mainly regulated through the HPA axis. The HPA axis can be activated by chronic stress, as well as by the immune-inflammatory response induced by microbial alterations in the gut and by metabolites. The large amounts of cortisol released by over-activation of the HPA axis cause shifts in the composition of the gut microbiota as well as changes in the permeability of the gut barrier.
In the metabolic pathway, the mechanism of action of gut microbes may be associated with their secretion of metabolites [98]. Gut flora secretions cross the gut epidermis and enter the internal circulatory system. Microbial metabolites have the potential to influence brain function, either directly or indirectly [99,100]. Among these, SCFAs are considered crucial in influencing the signaling intermediate to the gut and the brain [101]. Increasing SCFAs ameliorates neuroinflammation and stimulates the production of BDNF, which is associated with neuroplasticity in the brain [102]. Gut probiotics, Lactobacillus and Bifidobacterium, which produce the neurotransmitter GABA, can inhibit tumor development by reversing chronic stress in the body through the inhibition of the HPA axis and the nervous system [103]. However, GABA metabolism may be decreased when the intestinal flora is dysbiotic [104]. These changes in metabolites affect CNS function through various mechanisms, which may contribute to CRD.
The vagus nerve stretches from the brainstem down to the stomach and intestines, acting as a conduit between the gut and the brain’s neural functions. Various microbial products, hormones regulated by the microbiota, and immune mediators dependent on the microbiota can directly engage with the enteric neurons and the vagus nerve within the gut. It has been shown that Lactobacilli can rely on the vagus nerve to regulate GABA receptor mRNA expression, thereby influencing the production of depressive-like behaviors [105]. Research indicates that the application of a vagus nerve stimulator activates hippocampal 5-HT1B receptors and enhances BDNF expression in mice subjected to chronic restraint stress, which contributes to the reduction of depressive behaviors [106]. Changes in gut-metabolized neurotransmitters such as GABA and 5-HT can also activate the vagus nerve and affect synaptic transmission function, which in turn can contribute to mood problems such as depression. Pro-inflammatory molecules can also induce apoptosis of vagal neurons and alter synaptic function [107]. Result in highlight the vagus nerve’s role in microbiota-linked depression.
In the neuroendocrine pathway, the central component of the neuroendocrine pathway is the HPA axis. The gut microbiota is crucial for the normal development of the HPA axis, with proper gut colonization being essential for the formation of this stress-related signaling pathway [108]. Alterations in the gut microbiota can result in the enhanced production and secretion of cytokines, including IL-1β, IL-6, and TNF-α [97]. These cytokines are capable of crossing the blood-brain barrier and triggering the activation of the HPA axis. Certain intestinal metabolites like SCFAs, GABA, 5-HT, and acetylcholine are also involved in the HPA axis [109]. Gut microbiota is linked to neuropsychiatric disorders through its influence on the gene expression pattern of the HPA axis and subsequent HPA response [110]. When the HPA axis is activated, it triggers a substantial release of cortisol from the adrenal glands, which subsequently contributes to the onset of depression. Additionally, during chronic stress, the overactivation of the HPA axis results in alterations in gut motility and further disrupts both the composition and stability of the gut microbiota, along with modifying the permeability of the intestinal barrier [111], which will result in a vicious cycle of CRD formation.
Medical causes
It should be noted that conventional chemotherapeutic agents, hormone deprivation therapies, immunotherapies, and other cancer treatments may cause adverse neurological effects on the psyche [112]. For example, in studies investigating hormone suppression therapy for prostate cancer, the occurrence of depression and anxiety was notably higher compared to baseline levels throughout the nine-month treatment period [113]. Mood disorders affect 37% of patients with solid tumors receiving buparlisib-targeted therapy [114]. Furthermore, approximately half of patients with advanced-stage non-small cell lung carcinoma experience sleep disturbances and persistent fatigue while undergoing treatment with PD-1/PDL1 inhibitors [115]. CAR-T therapy has been linked to various neurological complications, such as encephalopathy, agitation, movement disorders, aphasia, and ataxia [116].
The alteration of cytokine levels in tumor patients due to tumor therapy may contribute to the development of psychiatric side effects [117]. The tissue and cellular damage resulting from treatments like chemotherapy, radiation therapy, and surgical procedures in cancer patients post-diagnosis triggers the release of DAMPs. These molecules bind to PRRs on leukocytes, particularly macrophages, which then initiate the production of various pro-inflammatory cytokines, including IL-1, IFN-α, IL-6, and TNF-α [87]. Ionizing radiation and specific chemotherapy drugs can independently trigger NF-κB activation, bypassing the need for tissue damage, thereby this process further amplifies the production of inflammatory mediators [118]. Immunotherapy stimulates the body’s immune cells, such as T cells, NK cells, and dendritic cells, resulting in the generation of substantial levels of cytokines. Some immunotherapy components bind to Toll-like receptors, elevating levels of pro-inflammatory cytokines [119]. Some of the exogenous cytokines used in immunotherapy, including IFN-α, studies have shown that up to 50% of patients may experience depression as a result of these treatments [75,78,88].
Certain medications have been linked to triggering cancer patients’ depressive symptoms by directly influencing neurotransmitter regulation in the brain. For example, haloperidol, a dopamine receptor-2 antagonist used to manage chemotherapy-induced nausea, can reduce dopamine activity and contribute to depressive symptoms [120].
Adverse effects of cancer-related depression
The phenomenon of depression and anxiety in oncology patients, characterized by feelings of helplessness, despair, and panic, not only complicates treatment and reduces patient compliance, but also suppresses the patient’s immune function, hastening the advancement of the disease, affecting the patient’s overall well-being, survival, and even influencing their choice of treatment [121].
Depressed patients exhibit a greater mortality risk than non-depressed individuals with cancer [11]. Firstly, depression impacts treatment participation, leads to reduced adherence, and is associated with higher mortality rates [10,11]. A study focusing on breast cancer outcomes revealed that only 51% of patients suffering from depression initiated chemotherapy, in stark contrast to the 92% of non-depressed patients who accepted the treatment [10]. This distress as well as psychological burden may result in decreased treatment engagement and higher suicidal tendencies in depressed cancer patients compared to regular depressed patients [122].
Patients with cancer-related depression are also in a chronic stress response for a long time. Research in animals and humans indicates that chronic stress reactions in cancer patients may play a role in advancing cancer and promoting metastasis [50]. Chronic stress is thought to increase mortality risk in depressed patients relative to those without depression [12]. Chronic stress can remodel the tumor’s immune microenvironment and suppress the antitumor immune response [123,124]. Research on UV-sensitive mice under stress and non-stress conditions revealed a higher incidence of squamous cell carcinoma in the chronically stressed group [125]. The stress group exhibited decreased IFN-γ expression, higher infiltration and circulating levels of regulatory T cells, lower infiltration rates of Th cells, and a shorter latency period before tumor development [125]. Depression has been demonstrated to reduce the count of circulating NK cells in healthy adults, which are crucial for tumor surveillance [126]. Surgery, a primary treatment method for tumors, can induce chronic stress that may result in an elevated number of myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment [127]. Chronic stress results in ongoing HPA axis activity, leading to glucocorticoid resistance and an overproduction of pro-inflammatory cytokines. This process impairs the adaptive immune response and increases immunosuppression, thereby, in turn, diminishing the body’s capacity to fight cancer effectively [128].
In addition, chronic stress responses can directly impact tumor cells through gene regulation. This stress response upregulates the expression of transforming growth TGF-β1, vascular endothelial growth factor (VEGF), and IL-10, thereby inducing the epithelial-mesenchymal transition (EMT) of tumor cells, which subsequently promotes tumor metastasis [127]. Another mouse study observed that chronic sympathetic nervous system stimulation during chronic stress interfered with the P53 tumor suppressor gene, reducing its critical tumor suppressor effects [129]. Activation of the HPA axis also leads to elevated glucocorticoids and increased expression of the P53 suppressor (i.e., MDM2) [129]. Catecholamines have also driven tumor growth by phosphorylating and inactivating pro-apoptotic molecules in a model of prostate cancer [130]. Chronic stress can also stimulate angiogenesis, increasing the invasiveness of certain tumors. In ovarian cancer models, chronic stress results in elevated catecholamine levels in tissues, increased expression of angiogenic factors (including VEGF), and increased formation of preinvasive enzymes through protein kinase A stimulation [130].
Clinical treatment strategies for CRD
Effective treatment for depression is crucial for patients suffering from cancer-related depression, as it significantly enhances their quality of life and chances of survival [10,11]. Unlike patients with common depression, individuals with CRD experience a more complex etiology and may endure greater psychological stress. Additionally, certain drugs or therapies used in cancer treatment can exacerbate the development of depression and interact with antidepressant medications, thereby affecting the management of depressive symptoms. Studies have shown that patients with metastatic breast cancer who receive treatment for depression experience a significantly longer median survival of 28.5 months compared to those who don’t receive treatment [131]. While psychosocial and pharmacological approaches are both successful in addressing depression among cancer patients, the optimal way to combine and apply these therapies remains undetermined [132]. Given that advanced cancers often have a limited survival prognosis, it is crucial to consider the onset and duration of anti-depressive medications.
Psychological intervention in CRD
Meta-analyses suggest that psychological interventions offer substantial relief for symptoms of anxiety and depression in individuals with cancer [133]. The commonly used psychological intervention techniques include cognitive behavioral therapy (CBT), group psychotherapy, reading therapy, music therapy, individual psychotherapy, and interpersonal therapy. As the incidence of CRD is closely related to the social support received by cancer patients, it is also essential to provide patients with psychosocial supportive therapy on top of CBT. A clinical practice has shown that CBT combined with psychosocial support treatment for patients with depression can effectively alleviate their psychological pressure of patients and eliminate the psychological barriers, thus promoting the recovery of the patient’s cognitive function and ultimately achieving a curative effect [134]. Additionally, it has been reported that psychological intervention can also reduce cancer mortality [135].
Medication in CRD
The main medications for managing cancer-related depression are tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) [136]. Although their exact mechanisms aren’t fully understood, both are known to trigger brain adaptations that enhance 5-HT neurotransmission, thereby improving mood [78,137]. However, CRD patients are different from normal depressed patients due to the more complex treatment they receive. Therefore, it is essential to use antidepressants cautiously, since SSRIs can often exacerbate nausea and vomiting [132], and the anticholinergic effects of TCAs may exacerbate delusions linked with chemotherapy [78,132].
Antidepressants can interact with chemotherapy drugs, potentially causing neurological toxicity by either reducing the metabolism of the antidepressant or due to the combined effects of the cancer treatments. Certain antidepressants might diminish the effectiveness of chemotherapy drugs, for instance, the interaction between specific SSRIs like fluoxetine and paroxetine. Concomitant prescriptions are found in 20-30% of breast cancer patients taking tamoxifen, which reduces its metabolism by inhibiting the CYP2D6 enzyme, significantly lowering the drug’s effectiveness and increasing the risk of breast cancer recurrence [138,139]. Currently prescribed antidepressants may have a minimal impact on alleviating depression in advanced cancer patients due to their delayed onset of action [140]. Thus, it is essential to identify new alternative or complementary treatment strategies for CRD patients.
Immunotherapy in CRD
Some immunotherapies administered to tumor patients may induce psychiatric side effects by altering cytokine levels. If cytokine imbalances can be effectively managed, immunotherapy could result in improved prognosis and a reduction in tumor-associated depression. IL-2 can promote the production of 5-HT through the STAT5-TPH1 pathway [141] and has been found to be reduced in depressed patients [142]. Clinical data on the use of IL-2 in treating depression are limited, and IL-2 injections may lead to multiple side effects, including vascular leak syndrome and pulmonary edema [143]. These complications limit its broader application in clinical settings. However, if these adverse effects could be reduced, IL-2 could potentially be beneficial in managing depression among cancer patients. Elevated levels of TNF-α and IL-6 have been associated with the severity of depression. Infliximab, which inhibits TNF-α, and tocilizumab, an inhibitor of IL-6, may help in reducing symptoms of depression [144,145]. However, some studies have reported contrary findings, as tocilizumab, which block the IL-6 receptor has been linked to an increase in depressive symptoms [146].
Though some evidence connects cytokines with depression and antitumor effects, but developing antidepressants is really challenging. The dual nature of cytokines makes inhibiting specific ones potentially harmful. Thus, selectively targeting central inflammation associated with depression, while avoiding effects on peripheral immune responses, is a significant challenge.
The critical role of IDO in the development of tumors and depression has heightened interest in IDO-inhibiting compounds within the field of immuno-oncology. Since cancers sustain an immunosuppressive microenvironment through activation of the IDO and kynurenine pathways [147], inhibition of IDO may be beneficial in increasing response rates to anticancer immunotherapy, while also affecting the neuronal activity in the brain. While the effectiveness of IDO inhibitors for treating cancer and depression is still largely theoretical, they could offer new therapeutic targets for enhancing depression management in cancer patients.
Targeting the brain-gut axis in CRD
Increasing research highlights the role of microbiota, gut permeability, and immune-inflammatory processes in tumor-associated depression. Microbes and their metabolites can influence the blood-brain barrier and overall brain health [148]. Additionally, gut dysbiosis is also crucial in cancer through dynamic regulation of cancer gene expression and immune surveillance [149]. Thus, regulating the gut-microbe-brain axis could offer a potential approach for both treating and preventing tumor-associated depression.
The developing discipline of psychobiology explores how certain probiotics might regulate the brain-gut connection and impact mental well-being. The enhancement of HPA axis functioning has been cited as the primary rationale for the use of probiotics [150]. Findings from randomized controlled trials involving probiotic use in patients with depression indicate that psychobiotic supplements can moderately yet significantly reduce depressive symptoms [108]. Psychotropic probiotics strengthen the intestinal barrier, modulate immune activity within the gut-associated lymphoid tissue (GALT), and contribute to inflammation, while also impacting vagal stimulation [151].
Moreover, adopting a gluten-free diet can impact the communication between the gut, microbiota, and brain by altering the gut microbiota and modifying gut permeability [152-154]. Research suggests that combining a gluten-free diet with probiotic supplements can suppress the immune-inflammatory response in depression, reducing inflammation, strengthening the intestinal barrier, and easing depressive symptoms [153]. In a similar manner, dietary fiber has been demonstrated to enhance immune function and prevent depressive disorder by regulating intestinal flora [155], a process attributed to the suppression of oxidative stress and inflammation. Overall, the findings suggest that behavioral interventions, particularly those involving dietary modifications, might effectively prevent the development of depression [156,157].
As an innovative method for addressing depression, psychobiology needs further research to better understand how psychobiotics work, identify the most effective strains and dosages, and assess their long-term impact. This research will pave the way for personalized psychobiotic treatments tailored to individual needs. Additionally, future studies may develop fecal transplants targeting depression after identifying the specific pathogenic bacteria [158].
Summary and perspective
Cancer-related depression is a complex and multifactorial psychological condition that often goes unrecognized. Understanding the immunoinflammatory mechanisms, HPA axis mechanisms, and gut microbial pathways, as well as their relationship with cancer prognosis, is crucial. New therapeutic strategies aimed at targeting cancer-related depression by inhibiting pro-inflammatory signaling and neuroendocrine pathway transmission could be promising approaches that warrant considerable attention. Additionally, further identification of the most effective combinations of pharmacological and psychosocial treatments for cancer-related depression is essential.
In summary, a deeper exploration of the common and interactive mechanisms between cancer and depression will provide clearer causal inferences and inform potential new treatment options. An expanded could also provide new insights into designing preventive strategies for managing depression in cancer survivors, ultimately easing the suffering of cancer patients and reducing the health burden on healthcare systems and society as a whole.
Acknowledgements
We thank Ms. Jie Chen (National Key Laboratory of Immunity and Inflammation) for her technical assistance. This work was funded by the National Natural Science Foundation of China (82071789, 31870910), Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai.
Disclosure of conflict of interest
The author declare the following potential conflicts of interest: Briefly describe any potential conflicts, such as financial interests, personal relationships, or affiliations that could be perceived as influencing the work.
References
- 1.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Li R, Hu C, He Y, Zhang X, Jin L. Global, regional, and national incidence trends of depressive disorder, 1990-2019: an age-period-cohort analysis based on the Global Burden of Disease 2019 study. Gen Hosp Psychiatry. 2024;88:51–60. doi: 10.1016/j.genhosppsych.2024.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, Cui R. The effects of psychological stress on depression. Curr Neuropharmacol. 2015;13:494–504. doi: 10.2174/1570159X1304150831150507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, Kubera M, Kohler CA, Fernandes BS, Stubbs B, Pavlidis N, Carvalho AF. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Venkateswaran C, Purkayastha D, Nayar K, Unnikrishnan UG. Prevalence of depression in breast cancer patients and its association with their quality of life: a cross-sectional observational study. Indian J Palliat Care. 2017;23:268–273. doi: 10.4103/IJPC.IJPC_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishel MH, Hostetter T, King B, Graham V. Predictors of psychosocial adjustment in patients newly diagnosed with gynecological cancer. Cancer Nurs. 1984;7:291–299. [PubMed] [Google Scholar]
- 8.Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Balon R. Mood, anxiety, and physical illness: body and mind, or mind and body? Depress Anxiety. 2006;23:377–387. doi: 10.1002/da.20217. [DOI] [PubMed] [Google Scholar]
- 10.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356:1326–1327. doi: 10.1016/S0140-6736(00)02821-X. [DOI] [PubMed] [Google Scholar]
- 11.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 13.Tsuang MT, Taylor L, Faraone SV. An overview of the genetics of psychotic mood disorders. J Psychiatr Res. 2004;38:3–15. doi: 10.1016/s0022-3956(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 14.Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall KM, Van Assche E, Andlauer TFM, Choi KW, Luykx JJ, Schulte EC, Lu Y. The genetic basis of major depression. Psychol Med. 2021;51:2217–2230. doi: 10.1017/S0033291721000441. [DOI] [PubMed] [Google Scholar]
- 16.Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, Wang Y, Tang Y, Xia M, Li B. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 2024;9:30. doi: 10.1038/s41392-024-01738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen-Woods S, Craig IW, McGuffin P. The current state of play on the molecular genetics of depression. Psychol Med. 2013;43:673–687. doi: 10.1017/S0033291712001286. [DOI] [PubMed] [Google Scholar]
- 18.Dong L, Li B, Verkhratsky A, Peng L. Cell type-specific in vivo expression of genes encoding signalling molecules in the brain in response to chronic mild stress and chronic treatment with fluoxetine. Psychopharmacology (Berl) 2015;232:2827–2835. doi: 10.1007/s00213-015-3921-2. [DOI] [PubMed] [Google Scholar]
- 19.Arnaud AM, Brister TS, Duckworth K, Foxworth P, Fulwider T, Suthoff ED, Werneburg B, Aleksanderek I, Reinhart ML. Impact of major depressive disorder on comorbidities: a systematic literature review. J Clin Psychiatry. 2022;83:21r14328. doi: 10.4088/JCP.21r14328. [DOI] [PubMed] [Google Scholar]
- 20.Sun N, Qin YJ, Xu C, Xia T, Du ZW, Zheng LP, Li AA, Meng F, Zhang Y, Zhang J, Liu X, Li TY, Zhu DY, Zhou QG. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science. 2022;378:390–398. doi: 10.1126/science.abo3566. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt S, Devadoss T, Manjula SN, Rajangam J. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr Neuropharmacol. 2021;19:1545–1559. doi: 10.2174/1570159X18666201015155816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar KJ, Kohler S, Cruz F, Schumann A, Zepf FD, Wagner G. Functional consequences of acute tryptophan depletion on raphe nuclei connectivity and network organization in healthy women. Neuroimage. 2020;207:116362. doi: 10.1016/j.neuroimage.2019.116362. [DOI] [PubMed] [Google Scholar]
- 23.Dostal CR, Carson Sulzer M, Kelley KW, Freund GG, McCusker RH. Glial and tissue-specific regulation of Kynurenine Pathway dioxygenases by acute stress of mice. Neurobiol Stress. 2017;7:1–15. doi: 10.1016/j.ynstr.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Li H, Cheng Z, Lu Y, Li W, Feng J, Wang L, Cheng J. Dex modulates the balance of water-electrolyte metabolism by depressing the expression of AVP in PVN. Front Pharmacol. 2022;13:919032. doi: 10.3389/fphar.2022.919032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbert J, Lucassen PJ. Depression as a risk factor for Alzheimer’s disease: genes, steroids, cytokines and neurogenesis - What do we need to know? Front Neuroendocrinol. 2016;41:153–171. doi: 10.1016/j.yfrne.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology. 2022;47:225–246. doi: 10.1038/s41386-021-01101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo JW, Chaudhury D, Han MH, Nestler EJ. Role of mesolimbic brain-derived neurotrophic factor in depression. Biol Psychiatry. 2019;86:738–748. doi: 10.1016/j.biopsych.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl) 2018;235:2195–2220. doi: 10.1007/s00213-018-4950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang Q, Zheng Z, Sun Y, Niu Y, Li D, Wang S, Meng W. BDNF enhances electrophysiological activity and excitatory synaptic transmission of RA projection neurons in adult male zebra finches. Brain Res. 2023;1801:148208. doi: 10.1016/j.brainres.2022.148208. [DOI] [PubMed] [Google Scholar]
- 30.Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, Nicolas V, Auregan G, David I, Dranovsky A, Hantraye P, Hen R, Gardier AM, Déglon N, Guiard BP. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3:e253. doi: 10.1038/tp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:321–338. doi: 10.1038/s41380-019-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, Schneider HG, Leonard BE, Berk M. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 33.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30:1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- 37.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarawagi A, Soni ND, Patel AB. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front Psychiatry. 2021;12:637863. doi: 10.3389/fpsyt.2021.637863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Slyepchenko A, Maes M, Kohler CA, Anderson G, Quevedo J, Alves GS, Berk M, Fernandes BS, Carvalho AF. T helper 17 cells may drive neuroprogression in major depressive disorder: proposal of an integrative model. Neurosci Biobehav Rev. 2016;64:83–100. doi: 10.1016/j.neubiorev.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM, Angiari S, Ryan DG, Corcoran SE, Timmons G, Geiger SS, Fitzpatrick DJ, O’Connell D, Xavier RJ, Hokamp K, O’Neill LAJ, Curtis AM. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A. 2018;115:E8460–E8468. doi: 10.1073/pnas.1800431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Z, Huang J, Sun G, He S, Luo Z, Zhang L, Li L, Yao M, Du C, Yu W, Feng Y, Yang D, Zhang J, Ge C, Li H, Geng M. Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. 2024;334:115804. doi: 10.1016/j.psychres.2024.115804. [DOI] [PubMed] [Google Scholar]
- 45.Skonieczna-Żydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, Łoniewski I, Kaczmarczyk M, Marlicz W, Czerwińska-Rogowska M, Pełka-Wysiecka J, Dec K, Stachowska E. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 2018;10:1939. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480. e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Hashimoto K. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry. 2017;7:e1138. doi: 10.1038/tp.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Woodburn SC, Bollinger JL, Wohleb ES. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress. 2021;14:100312. doi: 10.1016/j.ynstr.2021.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith HR. Depression in cancer patients: pathogenesis, implications and treatment (Review) Oncol Lett. 2015;9:1509–1514. doi: 10.3892/ol.2015.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin J, Zheng XL, Zheng M, Gao Zl. An analysis on related factors of psychological status in 74 inpatients with cancer. Bulletin of Chinese Cancer. 2006;10:707–708. [Google Scholar]
- 52.Zhang ZJ. Analysis of stressors associated with anxiety and depression in hospitalised cancer patients. Tianjin Journal of Nursing. 2010;3:151–152. [Google Scholar]
- 53.Zhang TY, An B, Yang J, Shi XL, Chen L, Liu XL. A survey and analysis of depression patients with cancer pain. Chinese Journal of Pain Medicine. 2012;6:350–353. [Google Scholar]
- 54.Shen LD, Yang J, Li YX, Yang JL, Long TF. Correlation between pain and depression and their effect on immune function in elderly cancer patients. Journal of Kunming Medical University. 2011;32:34–37. [Google Scholar]
- 55.Xu LX, Shen SS, He JJ, Fu Y, Xue XD, Liang Y, Lin Y, Zhang XH, He JC. Relationship among anxiety, depression, well-being index and social support in breast cancer patients. Chinese Mental Health Journal. 2013;27:473–478. [Google Scholar]
- 56.Irwin MR. Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep. 2013;15:404. doi: 10.1007/s11920-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 58.Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, Leung J, Ravindran AV, Chen WQ, Qiao YL, Shi J, Lu L, Bao YP. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25:1487–1499. doi: 10.1038/s41380-019-0595-x. [DOI] [PubMed] [Google Scholar]
- 59.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–1156. doi: 10.1038/s41590-022-01267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Schwamborn R, Brown E, Haase J. Elevation of cortical serotonin transporter activity upon peripheral immune challenge is regulated independently of p38 mitogen-activated protein kinase activation and transporter phosphorylation. J Neurochem. 2016;137:423–435. doi: 10.1111/jnc.13596. [DOI] [PubMed] [Google Scholar]
- 65.Jeon SW, Kim YK. Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J Psychiatry. 2016;6:283–293. doi: 10.5498/wjp.v6.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Murakami Y, Ishibashi T, Tomita E, Imamura Y, Tashiro T, Watcharanurak K, Nishikawa M, Takahashi Y, Takakura Y, Mitani S, Fujigaki H, Ohta Y, Kubo H, Mamiya T, Nabeshima T, Kim HC, Yamamoto Y, Saito K. Depressive symptoms as a side effect of Interferon-alpha therapy induced by induction of indoleamine 2,3-dioxygenase 1. Sci Rep. 2016;6:29920. doi: 10.1038/srep29920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 69.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 70.Badawy AA, Dawood S, Bano S. Kynurenine pathway of tryptophan metabolism in pathophysiology and therapy of major depressive disorder. World J Psychiatry. 2023;13:141–148. doi: 10.5498/wjp.v13.i4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 72.Shi XW, Zhou MM, Zhu HY. Research progress of traditional Chinese medicine in the treatment of depression through tryptophan-kynurenine metabolic pathway. Acta Chin Med Pharm. 2017;45:98–102. [Google Scholar]
- 73.AIT-Belkacem R, Bol V, Gomes B, Hamm G, Linehan S, Stauber J. Biomarker monitoring by quantitative maldi imaging: application to the tryptophan-kynurenine pathway in immuno-oncology. Drug Metab Pharmacok. 2018;33:S26. [Google Scholar]
- 74.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 77.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 78.Szabo ST, Gold TD, Manji HK. The American Psychiatric Publishing textbook of psychopharmacology. 3rd edition. New York, NY, USA: American Psychoanalytic Association; 2004. Neurotransmitters, receptors, signal transduction, and second messengers in psychiatric disorders; pp. 3–52. [Google Scholar]
- 79.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Varghese FP, Brown ES. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 2001;3:151–155. doi: 10.4088/pcc.v03n0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clin Child Fam Psychol Rev. 2011;14:135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 85.Tseilikman V, Dremencov E, Tseilikman O, Pavlovicova M, Lacinova L, Jezova D. Role of glucocorticoid- and monoamine-metabolizing enzymes in stress-related psychopathological processes. Stress. 2020;23:1–12. doi: 10.1080/10253890.2019.1641080. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- 87.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 88.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 89.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 90.Braquehais MD, Picouto MD, Casas M, Sher L. Hypothalamic-pituitary-adrenal axis dysfunction as a neurobiological correlate of emotion dysregulation in adolescent suicide. World J Pediatr. 2012;8:197–206. doi: 10.1007/s12519-012-0358-0. [DOI] [PubMed] [Google Scholar]
- 91.Xie Y, Xie F, Zhou X, Zhang L, Yang B, Huang J, Wang F, Yan H, Zeng L, Zhang L, Zhou F. Microbiota in tumors: from understanding to application. Adv Sci (Weinh) 2022;9:e2200470. doi: 10.1002/advs.202200470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 93.Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 94.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Beninson L, Ursell L, Greenwood BN, Knight R, Fleshner M. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1β and IL-18 but not IL-6, IL-10 or MCP-1. PLoS One. 2012;7:e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang K, Liu R, Gao Y, Ma W, Shen W. Electroacupuncture relieves LPS-induced depression-like behaviour in rats through IDO-mediated tryptophan-degrading pathway. Neuropsychiatr Dis Treat. 2020;16:2257–2266. doi: 10.2147/NDT.S274778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lucas K, Morris G, Anderson G, Maes M. The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets. 2015;14:838–854. doi: 10.2174/1871527314666150317224645. [DOI] [PubMed] [Google Scholar]
- 98.Shen G, Wu J, Ye BC, Qi N. Gut microbiota-derived metabolites in the development of diseases. Can J Infect Dis Med Microbiol. 2021;2021:6658674. doi: 10.1155/2021/6658674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry. 2019;10:34. doi: 10.3389/fpsyt.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2020;11:709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 102.Church JS, Bannish JAM, Adrian LA, Rojas Martinez K, Henshaw A, Schwartzer JJ. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front Neurosci. 2023;17:1134080. doi: 10.3389/fnins.2023.1134080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stephenson M, Rowatt E. The production of acetylcholine by a strain of Lactobacillus plantarum. J Gen Microbiol. 1947;1:279–298. doi: 10.1099/00221287-1-3-279. [DOI] [PubMed] [Google Scholar]
- 104.Zhao N, Shu Y, Jian C, Zhou Z, Bao H, Li X, Cheng X, Zhao Y, Jin S, Shu X. Lactobacillus ameliorates SD-induced stress responses and gut dysbiosis by increasing the absorption of gut-derived GABA in rhesus monkeys. Front Immunol. 2022;13:915393. doi: 10.3389/fimmu.2022.915393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin HC, Jo BG, Lee CY, Lee KW, Namgung U. Hippocampal activation of 5-HT1B receptors and BDNF production by vagus nerve stimulation in rats under chronic restraint stress. Eur J Neurosci. 2019;50:1820–1830. doi: 10.1111/ejn.14368. [DOI] [PubMed] [Google Scholar]
- 107.Kim JS, Kirkland RA, Lee SH, Cawthon CR, Rzepka KW, Minaya DM, de Lartigue G, Czaja K, de La Serre CB. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiol Behav. 2020;225:113082. doi: 10.1016/j.physbeh.2020.113082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Misiak B, Loniewski I, Marlicz W, Frydecka D, Szulc A, Rudzki L, Samochowiec J. The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog Neuropsychopharmacol Biol Psychiatry. 2020;102:109951. doi: 10.1016/j.pnpbp.2020.109951. [DOI] [PubMed] [Google Scholar]
- 110.Ge T, Yao X, Zhao H, Yang W, Zou X, Peng F, Li B, Cui R. Gut microbiota and neuropsychiatric disorders: implications for neuroendocrine-immune regulation. Pharmacol Res. 2021;173:105909. doi: 10.1016/j.phrs.2021.105909. [DOI] [PubMed] [Google Scholar]
- 111.Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733-e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wick W, Hertenstein A, Platten M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 2016;17:e529–e541. doi: 10.1016/S1470-2045(16)30571-X. [DOI] [PubMed] [Google Scholar]
- 113.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi: 10.1136/bmj.k1415. [DOI] [PubMed] [Google Scholar]
- 115.Steffen McLouth LE, Lycan TW Jr, Levine BJ, Gabbard J, Ruiz J, Farris M, Grant SC, Pajewski NM, Weaver KE, Petty WJ. Patient-reported outcomes from patients receiving immunotherapy or chemoimmunotherapy for metastatic non-small-cell lung cancer in clinical practice. Clin Lung Cancer. 2020;21:255–263. e254. doi: 10.1016/j.cllc.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rubin DB, Vaitkevicius H. Neurological complications of cancer immunotherapy (CAR T cells) J Neurol Sci. 2021;424:117405. doi: 10.1016/j.jns.2021.117405. [DOI] [PubMed] [Google Scholar]
- 117.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 119.Xun Y, Yang H, Kaminska B, You H. Toll-like receptors and toll-like receptor-targeted immunotherapy against glioma. J Hematol Oncol. 2021;14:176. doi: 10.1186/s13045-021-01191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patten SB, Barbui C. Drug-induced depression: a systematic review to inform clinical practice. Psychother Psychosom. 2004;73:207–215. doi: 10.1159/000077739. [DOI] [PubMed] [Google Scholar]
- 121.Hu ZQ, Xiong H, Zhu YH, Tang QC. Research progress on anxiety and depression in patients with colorectal cancer. Chinese Journal of Colorectal Diseases (Electronic Edition) 2021;10:520–523. [Google Scholar]
- 122.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J. Clin. Oncol. 2008;26:4731–4738. doi: 10.1200/JCO.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang Y, Nagaraja AS, Armaiz-Pena GN, Dorniak PL, Hu W, Rupaimoole R, Liu T, Gharpure KM, Previs RA, Hansen JM, Rodriguez-Aguayo C, Ivan C, Ram P, Sehgal V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin Cancer Res. 2016;22:1713–1724. doi: 10.1158/1078-0432.CCR-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muthuswamy R, Okada NJ, Jenkins FJ, McGuire K, McAuliffe PF, Zeh HJ, Bartlett DL, Wallace C, Watkins S, Henning JD, Bovbjerg DH, Kalinski P. Epinephrine promotes COX-2-dependent immune suppression in myeloid cells and cancer tissues. Brain Behav Immun. 2017;62:78–86. doi: 10.1016/j.bbi.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 125.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maes M, Meltzer HY, Stevens W, Calabrese J, Cosyns P. Natural killer cell activity in major depression: relation to circulating natural killer cells, cellular indices of the immune response, and depressive phenomenology. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:717–730. doi: 10.1016/0278-5846(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 127.Ma X, Wang M, Yin T, Zhao Y, Wei X. Myeloid-derived suppressor cells promote metastasis in breast cancer after the stress of operative removal of the primary cancer. Front Oncol. 2019;9:855. doi: 10.3389/fonc.2019.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rudak PT, Choi J, Parkins KM, Summers KL, Jackson DN, Foster PJ, Skaro AI, Leslie K, McAlister VC, Kuchroo VK, Inoue W, Lantz O, Haeryfar SMM. Chronic stress physically spares but functionally impairs innate-like invariant T cells. Cell Rep. 2021;35:108979. doi: 10.1016/j.celrep.2021.108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, Zhao Y, Wang X, Levine AJ, Hu W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:7013–7018. doi: 10.1073/pnas.1203930109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R Jr, Danial N, Datta SR, Kulik G. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123:874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J. Clin. Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li M, Fitzgerald P, Rodin G. Evidence-based treatment of depression in patients with cancer. J. Clin. Oncol. 2012;30:1187–1196. doi: 10.1200/JCO.2011.39.7372. [DOI] [PubMed] [Google Scholar]
- 133.Wang LJ, Liu W. Effect of psychological intervention on anxiety and depression symptoms of patients with lung cancer: a meta-analysis. Chinese General Practice. 2013;16:808–810. [Google Scholar]
- 134.Sun BM. Clinical effect by drug therapy and cognitive behavior therapy combined with psychological and social support treatment for depression. China Practical Medicine. 2017;12:25–27. [Google Scholar]
- 135.Abrahams HJG, Gielissen MFM, Verhagen CAHHVM, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev. 2018;63:1–11. doi: 10.1016/j.cpr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Ng CG, Boks MP, Zainal NZ, de Wit NJ. The prevalence and pharmacotherapy of depression in cancer patients. J Affect Disord. 2011;131:1–7. doi: 10.1016/j.jad.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 137.Norman TR. The new antidepressants - Mechanisms of action. Australian Prescriber. 1999;22:106–108. [Google Scholar]
- 138.Caraci F, Crupi R, Drago F, Spina E. Metabolic drug interactions between antidepressants and anticancer drugs: focus on selective serotonin reuptake inhibitors and hypericum extract. Curr Drug Metab. 2011;12:570–577. doi: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
- 139.Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 140.Lloyd-Williams M, Payne S, Reeve J, Kolamunnage Dona R. Antidepressant medication in patients with advanced cancer--an observational study. QJM. 2013;106:995–1001. doi: 10.1093/qjmed/hct133. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Zhou N, Zhou L, Wang J, Zhou Y, Zhang T, Fang Y, Deng J, Gao Y, Liang X, Lv J, Wang Z, Xie J, Xue Y, Zhang H, Ma J, Tang K, Fang Y, Cheng F, Zhang C, Dong B, Zhao Y, Yuan P, Gao Q, Zhang H, Xiao-Feng Qin F, Huang B. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. 2021;22:358–369. doi: 10.1038/s41590-020-00850-9. [DOI] [PubMed] [Google Scholar]
- 142.Rybka J, Korte SM, Czajkowska-Malinowska M, Wiese M, Kedziora-Kornatowska K, Kedziora J. The links between chronic obstructive pulmonary disease and comorbid depressive symptoms: role of IL-2 and IFN-gamma. Clin Exp Med. 2016;16:493–502. doi: 10.1007/s10238-015-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim J, Kang S, Kim KW, Heo MG, Park DI, Lee JH, Lim NJ, Min DH, Won C. Nanoparticle delivery of recombinant IL-2 (BALLkine-2) achieves durable tumor control with less systemic adverse effects in cancer immunotherapy. Biomaterials. 2022;280:121257. doi: 10.1016/j.biomaterials.2021.121257. [DOI] [PubMed] [Google Scholar]
- 144.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tiosano S, Yavne Y, Watad A, Langevitz P, Lidar M, Feld J, Tishler M, Aamar S, Elkayam O, Balbir-Gurman A, Molad Y, Ehrlich S, Abu-Shakra M, Amital D, Amital H. The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. Eur J Clin Invest. 2020;50:e13268. doi: 10.1111/eci.13268. [DOI] [PubMed] [Google Scholar]
- 146.Knight JM, Costanzo ES, Singh S, Yin Z, Szabo A, Pawar DS, Hillard CJ, Rizzo JD, D’Souza A, Pasquini M, Coe CL, Irwin MR, Raison CL, Drobyski WR. The IL-6 antagonist tocilizumab is associated with worse depression and related symptoms in the medically ill. Transl Psychiatry. 2021;11:58. doi: 10.1038/s41398-020-01164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S, Grellety T, Ryckewaert T, Bessede A, Ghiringhelli F, Pulido M, Italiano A. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol. 2018;4:93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paul B, Barnes S, Demark-Wahnefried W, Morrow C, Salvador C, Skibola C, Tollefsbol TO. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics. 2015;7:112. doi: 10.1186/s13148-015-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Trzeciak P, Herbet M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients. 2021;13:927. doi: 10.3390/nu13030927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mohan M, Chow CT, Ryan CN, Chan LS, Dufour J, Aye PP, Blanchard J, Moehs CP, Sestak K. Dietary gluten-induced gut dysbiosis is accompanied by selective upregulation of microRNAs with intestinal tight junction and bacteria-binding motifs in rhesus macaque model of celiac disease. Nutrients. 2016;8:684. doi: 10.3390/nu8110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Karakula-Juchnowicz H, Rog J, Juchnowicz D, Loniewski I, Skonieczna-Zydecka K, Krukow P, Futyma-Jedrzejewska M, Kaczmarczyk M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr J. 2019;18:50. doi: 10.1186/s12937-019-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bonder MJ, Tigchelaar EF, Cai X, Trynka G, Cenit MC, Hrdlickova B, Zhong H, Vatanen T, Gevers D, Wijmenga C, Wang Y, Zhernakova A. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8:45. doi: 10.1186/s13073-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khosravi M, Sotoudeh G, Amini M, Raisi F, Mansoori A, Hosseinzadeh M. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatry. 2020;20:63. doi: 10.1186/s12888-020-2455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bamber DJ, Stokes CS, Stephen AM. The role of diet in the prevention and management of adolescent depression. Nutrition Bulletin. 2007;32:90–99. [Google Scholar]
- 157.Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, Borsini A, Firth J, Pariante CM, Berding K, Cryan JF, Clarke G, Craig JM, Su KP, Mischoulon D, Gomez-Pinilla F, Foster JA, Cani PD, Thuret S, Staudacher HM, Sanchez-Villegas A, Arshad H, Akbaraly T, O’Neil A, Segasby T, Jacka FN. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 2021;26:134–150. doi: 10.1038/s41380-020-00925-x. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Q, Bi Y, Zhang B, Jiang Q, Mou CK, Lei L, Deng Y, Li Y, Yu J, Liu W, Zhao J. Current landscape of fecal microbiota transplantation in treating depression. Front Immunol. 2024;15:1416961. doi: 10.3389/fimmu.2024.1416961. [DOI] [PMC free article] [PubMed] [Google Scholar]