Abstract
This study aims to construct a Nomogram model to predict the risk of developing castration-resistant prostate cancer (CRPC) in patients with high tumor burden (HTB) and osseous metastatic prostate cancer (PCa), and to identify key prognostic factors. A retrospective analysis was conducted on patients with HTB and osseous metastatic PCa treated at The Sixth Affiliated Hospital, School of Medicine, South China University of Technology and the Second Affiliated Hospital of Guangzhou Medical University from January 2018 to February 2022. Patients’ baseline data and laboratory indexes were collected. Cox regression analysis identified neural invasion (NI; P<0.001, HR: 2.371, 95% CI: 1.569-3.582), Gleason score (P=0.002, HR: 1.787, 95% CI: 1.241-2.573), initial PSA (P=0.004, HR: 1.677, 95% CI: 1.174-2.396), and lactate dehydrogenase (LDH; P<0.001, HR: 2.729, 95% CI: 1.855-4.014) as significant prognostic factors for progression to CRPC. The constructed Nomogram model exhibited high accuracy in predicting one- and two-year progression to CRPC, with external validation confirming its predictive performance. Time-dependent receiver operating characteristic (ROC) curves indicated that the areas under the curves (AUCs) of the model for one- and two-year progression to CRPC were 0.81 and 0.76, respectively. This model demonstrates high predictive performance, aiding clinical decision-making and providing personalized treatment strategies for patients with HTB and osseous metastatic PCa.
Keywords: Prostate cancer, castration-resistant prostate cancer, nomogram model, prognostic factors, neural invasion
Introduction
Prostate cancer (PCa) remains one of the most prevalent malignancies among men worldwide, showing a continuously rising incidence and mortality across countries and regions and presenting as a grave public health issue [1]. Statistics indicate over 160,000 new cases of PCa in the United States annually, making PCa the third leading cause of cancer death in males [2]. As the population ages and lifestyle changes continue, the burden of PCa is expected to rise in the coming decades [3]. Additionally, the disease often lacks early symptoms, resulting in a diagnosis in advanced stages among most patients when seeking medical advice [4].
High tumor burden (HTB) and osseous metastatic PCa, a manifestation of advanced PCa, is characterized by tumor cells spreading to the bones, leading to bone destruction and related complications, seriously impairing patients’ quality of life and survival [5]. While this type of PCa may initially respond to hormone therapy, many patients eventually develop castration-resistant prostate cancer (CRPC), where tumor cells are no longer sensitive to hormone therapy, leading to rapid disease progression, limited treatment options, and adverse outcomes [6].
The therapeutic goal for CRPC focuses on prolonging survival and enhancing quality of life [7]. However, due to the high heterogeneity and complex molecular mechanisms of CRPC, there is currently a lack of precise biomarkers and effective treatment approaches. Research has shown that among patients with first-onset metastatic PCa, patients with HTB are more susceptible to progression to CRPC than those with a low tumor burden, accompanied by shorter overall survival [8]. Clinically, while single photon emission computed tomography (SPECT) and, if necessary, contrast-enhanced MRI, for patients undergoing whole-body bone scintigraphy (BS) can provide a comprehensive detection and diagnosis of osseous metastasis, they do not account for other risk factors such as Gleason scores and clinical staging, which also influence disease progression [9]. Hence, identifying and validating prognostic factors that can predict progression to CRPC is of great significance for the early identification of high-risk patients, development of personalized treatment plans, optimization of resource allocation, and improvement of patient prognosis.
Analyzing prognostic factors and constructing prediction models are crucial for achieving precision medicine and improving the management of CRPC patients [10]. By analyzing the clinical characteristics, biomarkers, and genetic information of patients, key factors associated with disease progression can be identified [11], which helps doctors better evaluate patient outcomes and treatment responses, guide the design of clinical trials, and develop of new drugs.
This retrospective study comprehensively analyzed the clinical data of patients with HTB and osseous metastatic PCa, aiming at revealing the potential prognostic factors for the progression from HTB and osseous metastatic PCa to CRPC and constructing an innovative prediction model. Given the limited research on prognostic factors for this specific patient population, the results of this study are expected to fill the knowledge gap in this field and provide clinicians with a novel and more accurate assessment tool for predicting disease progression and devising personalized treatment plans.
Data and methods
Clinical data
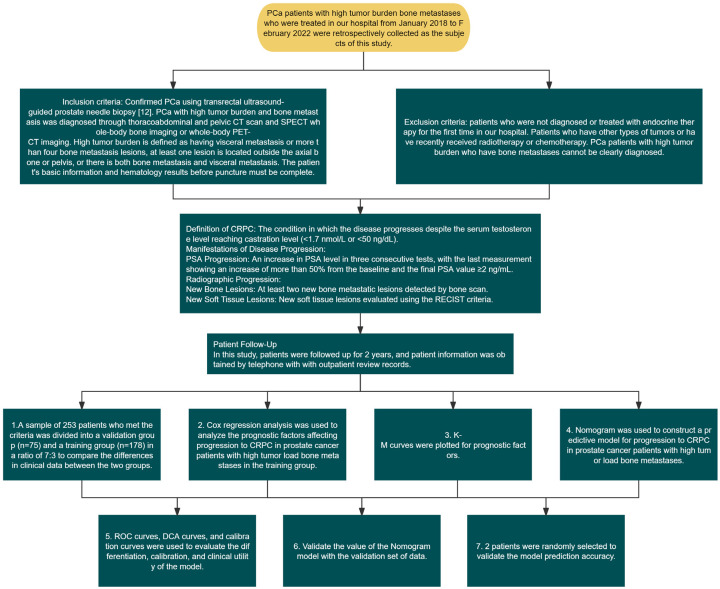
The clinical data from patients with HTB and osseous metastatic PCa treated in The Sixth Affiliated Hospital, School of Medicine, South China University of Technology and the Second Affiliated Hospital of Guangzhou Medical University between January 2018 and February 2022 were retrospectively collected for this study. The study was approved by The Sixth Affiliated Hospital, School of Medicine, South China University of Technology. Due to the retrospective nature of the study, informed consent was waived. To understand this, we drew a flow chart (Figure 1).
Figure 1.
Research flowchart.
Eligibility criteria
Inclusion criteria: PCa was confirmed by transrectal ultrasound-guided prostate biopsy [12]; The diagnosis of PCa with HTB and osseous metastasis was based on thoracic, abdominal, and pelvic CT scans, as well as SPECT whole-body bone scans or whole-body PET-CT imaging; HTB was defined as having visceral metastases or more than four osseous metastases, with at least one located outside the axial skeleton or pelvis, or the presence of both osseous metastases and visceral metastases; Patient had complete basic data and hematological results before the biopsy, and a confirmed diagnosis at the time of inclusion; Availability of complete clinical data.
Exclusion criteria: Patients who were either not diagnosed for the first time or received initial endocrine therapy at other institutions; Patients with other types of tumors or who had recently undergone radiotherapy or chemotherapy; HTB PCa patients without clear diagnosis of osseous metastases.
CRPC stage definition
For PCa patients who received treatment, disease progression was defined as the presence of progressive disease even if the serum testosterone level reached the castration level (<1.7 nmol/L or <50 ng/dL), according to the Chinese Guidelines for the Diagnosis and Treatment of Urology and Andrology Diseases (2022 edition) [13]. Disease progression can be either prostate-specific antigen (PSA) progression or radiographic progression.
PSA progression is specifically defined as three consecutive PSA tests showing an increase in PSA levels over a period of at least 1 week, with the last test indicating a >50% increase compared to the basal value and the final PSA value reaching more than 2 ng/mL. The sustained increase in PSA levels is indicative of persistent disease activity and progression, despite achieving the goal of drug therapy (castration levels of testosterone) [14].
The specific definition of radiographic progression is the identification of new lesions in imaging examinations, including at least 2 new osseous metastases indicated by bone scans, or new soft tissue lesions evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.
Information acquisition
Patient data were acquired through electronic medical records, outpatient review records, and follow-up records. Baseline data included age, body mass index (BMI), ethnicity, marital status, educational level, family history of cancer, intraductal carcinoma of the prostate, neural invasion (NI), hypertension, diabetes mellitus, T-staging, N-staging, and Gleason score. The above data were the first records of patients after admission. Laboratory indexes included PSA, testosterone, hemoglobin (HGB), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and albumin (ALB). Laboratory indicators were grouped using X-tile software.
Patient follow-up
In this study, patients were followed up for 2 years. Follow-up data were obtained by telephone and outpatient review records. Specifically, follow-up was scheduled at 3, 6, 9, and 12 months in the first year after diagnosis and initiation of treatment, and at 16, 20, and 24 months in the second year.
Outcome measures
1. A total of 253 eligible cases were screened out according to the inclusion and exclusion criteria and CRPC definition. These cases were then assigned to a validation group (n=75) and a training group (n=178) in a 7:3 ratio to compare their differences in clinical data. 2. Prognostic factors affecting the progression to CRPC in patients with HTB and osseous metastatic PCa in the training group were identified by Cox regression analysis. 3. Kaplan-Meier (K-M) curves were plotted for the prognostic factors identified. 4. A Nomogram was used to construct a prediction model for the progression to CRPC in patients with HTB and osseous metastatic PCa. 5. Receiver operating characteristic (ROC) curves, decision curve analysis (DCA), and calibration curves were used to evaluate the discrimination, calibration, and clinical practical value of the model. 6. The value of the Nomogram model was validated with data from validation group. 7. The prediction accuracy of the model was verified by randomly selecting two patients.
Statistical analysis
Data processing and analysis were performed using SPSS 26.0 and R software. First, a chi-square test was performed on the baseline data (count data) between different groups. Cox regression analyses were used to identify prognostic factors affecting progression to CRPC in the training group. K-M survival curves were used to demonstrate the value of different prognostic factors, and differences in the survival distributions were compared by Log-rank tests. A Nomogram model was constructed based on the prognostic factors screened by Cox regression analysis. The accuracy of the model in predicting progression to CRPC in patients with HTB bone metastases PCa at 1 and 2 years was analyzed using time-dependent ROC curves, and the discriminative power, calibration, and clinical practical value of the model were assessed by DCA and calibration curves. Internal validation was performed using the Bootstrap method to ensure the stability and reliability of the model. Meanwhile, external validation was carried out using data from validation group. The predictive performance of the model was further confirmed by time-dependent ROC curves, DCA, and calibration curves. P<0.05 was considered statistically significant.
Results
Baseline data
Based on the data collected, patients were divided into validation and training groups in a 7:3 ratio. Further comparison of baseline data found no statistical inter-group differences in age, BMI, ethnicity, marital status, education level, family history of cancer, intraductal carcinoma of the prostate, NI, hypertension, diabetes mellitus, T-staging, N-staging, Gleason score, initial PSA, testosterone, HGB, ALP, LDH, and ALB (all P>0.05, Table 1), indicating that the two groups were comparable.
Table 1.
Comparison of baseline data between the two groups
| Variables | Validation group (n=75) | Training group (n=178) | χ2 | P |
|---|---|---|---|---|
| Age (years old) | ||||
| ≥65 | 24 | 64 | 0.364 | 0.546 |
| <65 | 51 | 114 | ||
| Body mass index | ||||
| ≥25 kg/m2 | 17 | 29 | 1.441 | 0.230 |
| <25 kg/m2 | 58 | 149 | ||
| Ethnicity | ||||
| Han | 61 | 129 | 2.216 | 0.137 |
| Others | 14 | 49 | ||
| Marital status | ||||
| Married | 71 | 171 | 0.249 | 0.618 |
| Divorced | 4 | 7 | ||
| Educational level | ||||
| ≥ High school | 48 | 112 | 0.026 | 0.871 |
| < High school | 27 | 66 | ||
| Family history of cancer | ||||
| With | 4 | 14 | 0.512 | 0.474 |
| Without | 71 | 164 | ||
| Intraductal carcinoma of the prostate | ||||
| With | 8 | 20 | 0.017 | 0.895 |
| Without | 67 | 158 | ||
| Neural invasion | ||||
| With | 45 | 121 | 1.488 | 0.222 |
| Without | 30 | 57 | ||
| Hypertension | ||||
| With | 35 | 93 | 0.657 | 0.417 |
| Without | 40 | 85 | ||
| Diabetes mellitus | ||||
| With | 26 | 45 | 2.303 | 0.129 |
| Without | 49 | 133 | ||
| T-staging | ||||
| T4 | 51 | 118 | 0.069 | 0.792 |
| Below T4 | 24 | 60 | ||
| N-staging | ||||
| N1 | 45 | 116 | 0.609 | 0.435 |
| N0 | 30 | 62 | ||
| Gleason score | ||||
| ≤8 | 52 | 110 | 1.301 | 0.254 |
| >8 | 23 | 68 | ||
| Initial PSA (ng/mL) | ||||
| ≥182.76 | 27 | 62 | 0.032 | 0.859 |
| <182.76 | 48 | 116 | ||
| Testosterone (nmol/L) | ||||
| ≥21.59 | 26 | 61 | 0.004 | 0.952 |
| <21.59 | 49 | 117 | ||
| HGB (g/L) | ||||
| ≥103 | 67 | 153 | 0.531 | 0.466 |
| <103 | 8 | 25 | ||
| ALP (U/L) | ||||
| ≥185.7 | 13 | 24 | 0.626 | 0.429 |
| <185.7 | 62 | 154 | ||
| LDH (U/L) | ||||
| ≥237.05 | 21 | 48 | 0.028 | 0.866 |
| <237.05 | 54 | 130 | ||
| ALB (g/L) | ||||
| ≥48 | 10 | 32 | 0.822 | 0.365 |
| <48 | 65 | 146 |
Note: PSA: Prostate-specific antigen, HGB: Hemoglobin, ALP: Alkaline phosphatase, LDH: Lactate dehydrogenase, ALB: Albumin.
Prognostic factors affecting the progression to CRPC
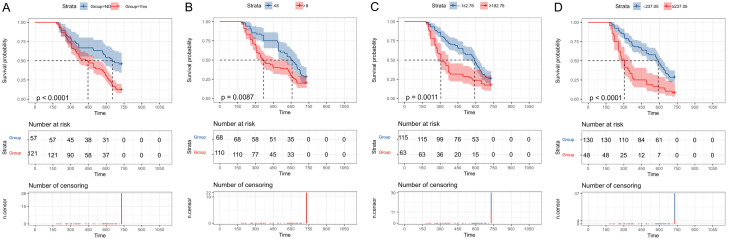
Cox regression analysis was performed in the training group to identify the prognostic factors affecting the progression of PCa patients with HTB and osseous metastasis to CRPC. NI (P<0.001, HR: 2.371, 95% CI: 1.569-3.582), Gleason score (P=0.002, HR: 1.787, 95% CI: 1.241-2.573), initial PSA (P=0.004, HR: 1.677, 95% CI: 1.174-2.396), and LDH (P<0.001, HR: 2.729, 95% CI: 1.855-4.014) were identified as prognostic factors leading to the progression of PCa patients with HTB and osseous metastasis to CRPC (Table 2 and Figure 2).
Table 2.
Analysis of prognostic factors
| Factor | P | HR | 95% CI | P | HR | 95% CI |
|---|---|---|---|---|---|---|
| Age (years old) | 0.809 | 1.044 | 0.736-1.481 | |||
| Body mass index | 0.519 | 0.86 | 0.545-1.358 | |||
| Ethnicity | 0.079 | 1.422 | 0.96-2.105 | |||
| Marital status | 0.749 | 1.157 | 0.473-2.826 | |||
| Educational level | 0.645 | 0.922 | 0.653-1.302 | |||
| Family history of cancer | 0.514 | 1.228 | 0.663-2.275 | |||
| Intraductal carcinoma of the prostate | 0.833 | 1.06 | 0.619-1.813 | |||
| Neural invasion | <0.001 | 2.309 | 1.54-3.462 | <0.001 | 2.371 | 1.569-3.582 |
| Hypertension | 0.240 | 1.224 | 0.873-1.716 | |||
| Diabetes mellitus | 0.903 | 0.976 | 0.66-1.444 | |||
| T-staging | 0.372 | 1.179 | 0.821-1.692 | |||
| N-staging | 0.245 | 1.236 | 0.865-1.766 | |||
| Gleason score | 0.009 | 1.592 | 1.121-2.261 | 0.002 | 1.787 | 1.241-2.573 |
| Initial PSA | 0.001 | 1.777 | 1.256-2.515 | 0.004 | 1.677 | 1.174-2.396 |
| Testosterone | 0.123 | 0.751 | 0.522-1.081 | |||
| HGB | 0.467 | 1.197 | 0.737-1.944 | |||
| ALP | 0.345 | 1.251 | 0.786-1.993 | |||
| LDH | <0.001 | 2.57 | 1.785-3.7 | <0.001 | 2.729 | 1.855-4.014 |
| ALB | 0.294 | 1.257 | 0.82-1.928 |
Note: PSA: Prostate-specific antigen, HGB: Hemoglobin, ALP: Alkaline phosphatase, LDH: Lactate dehydrogenase, ALB: Albumin.
Figure 2.
Cox regression analysis and curves of prognostic factors for disease progression. A. K-M curve analysis of neural invasion; B. K-M curve analysis of Gleason score; C. K-M curve analysis of initial PSA; D. K-M curve analysis of LDH. Note: K-M: Kaplan-Meier, PSA: Prostate-specific antigen, LDH: Lactate dehydrogenase.
Construction of a Nomogram model for the progression to CRPC in PCa patients with HTB and osseous metastases
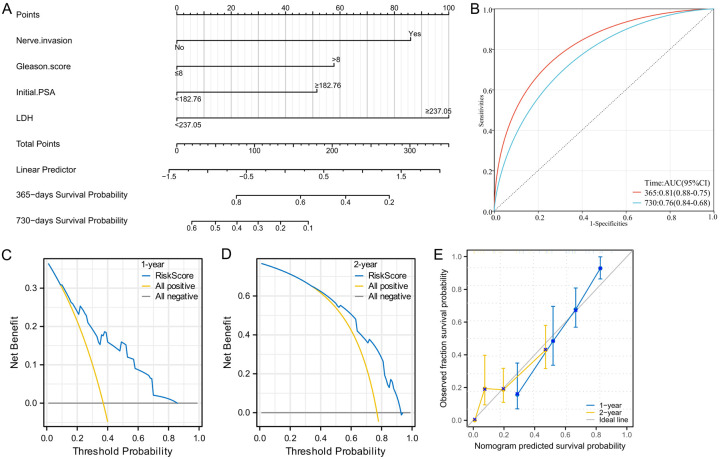
A Nomogram model was constructed based on the prognostic factors screened by Cox regression in the training group. As shown by the Nomogram, LDH had the strongest effect on the progression to CRPC, followed by NI, Gleason score, and initial PSA (Figure 3A). ROC curves, DCA, and calibration curves were used to evaluate the discrimination, calibration, and clinical practical value of the model. According to time-dependent ROC curve analysis, the risk score in predicting the progression of HTB osseous metastasis PCa patients to CRPC was 0.81 and 0.76 at 1 and 2 years, respectively, indicating certain accuracy of the model in predicting the progression to CRPC (Figure 3B). DCA indicated that for the 1-year (Figure 3C) and 2-year (Figure 3D) risk prediction, this Nomogram model showed higher net benefits compared to the scenario where all patient prognoses were assumed positive, demonstrating the potential practical value of the model in clinical decision support, highlighting the model’s potential clinical utility. Furthermore, the calibration curve (Figure 3E) revealed that the 1-year and 2-year progression probability predicted by the Nomogram was in good agreement with the actual observed progression probability at multiple prediction points, demonstrating that the model has good predictive accuracy and reliability.
Figure 3.
Nomogram model construction and internal validation. A. Nomogram for predicting the progression to CRPC in patients with osseous metastatic PCa and HTB; B. ROC analysis for Nomogram in predicting the 1-year and 2-year risk of progression to CRPC in patients with HTB and osseous metastatic PCa; C. Decision curve analysis for Nomogram in predicting 1-year risk of progression to CRPC in patients HTB and osseous metastatic PCa; D. Decision curve analysis for evaluating the 2-year risk of progression to CRPC in patients with HTB and osseous metastatic PCa; E. Calibrated curves of the Nomogram model for predicting the probability of progression to CRPC at 1 and 2 years versus the actual observed progression probability in patients with high-tumor burden and bone metastatic PCa. Note: PCa: Prostate cancer, HTB: high tumor burden, CRPC: castration-resistant prostate cancer, DCA: decision curve analysis.
External validation of the Nomogram for progression to CRPC in patients with PCa with HTB osseous metastases
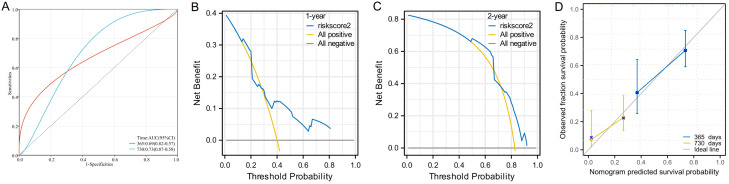
External validation of the Nomogram for predicting progression to CRPC in patients with HTB and osseous metastases showed favorable predictive accuracy. According to time-dependent ROC curve analysis, the AUC values of the model for predicting progression to CRPC at 1 year and 2 years were 0.81 and 0.76, respectively, indicating the reliable predictive power of the model for short-term disease progression (Figure 4A). DCA revealed a high net benefit of prediction by the model, especially in 1-year and 2-year risk prediction; moreover, compared to a scenario where a positive prognosis for all patients was assumed, the Nomogram provided better decision support (Figure 4B, 4C). The calibration curve also confirmed that the predicted progression probability by the model matched well with the observed progression probability, demonstrating the model’s accuracy and reliability (Figure 4D). These validation results underscore the practical value of the Nomogram as a clinical tool in improving the management and treatment strategies of PCa patients with HTB and osseous metastases.
Figure 4.
External validation of the Nomogram model for progression to CRPC in patients with osseous metastatic PCa and high tumor burden (HTB). A. ROC curve analysis for Nomogram in predicting 1-year and 2-year risk of progression to CRPC in patients with osseous metastatic PCa and HTB using validation group data; B. Decision curve analysis for Nomogram in predicting the 1-year risk of progression to CRPC in patients with HTB and osseous metastatic PCa using validation group data; C. Decision curve analysis for Nomogram in predicting the 2-year risk of progression to CRPC in patients with HTB and osseous metastatic PCa using validation group data; D. Calibration curve of the Nomogram model to predict the 1-year and 2-year progression probability to CRPC in patients with HTB and osseous metastatic PCa. Note: PCa: Prostate cancer, HTB: high tumor burden, CRPC: castration-resistant prostate cancer, DCA: decision curve analysis.
Case analysis
We randomly selected two patients for detailed case analysis. Patient 1 progressed to castration-resistant prostate cancer (CRPC) in 278 days, while Patient 2 progressed in 671 days. Computationally, we found that Patient 1 scored 294.5 points, with an 11% chance of progression to CRPC within 1 year and 0% within 2 years, while the Patient 2 scored 51 points, with an 85% chance of progression to CRPC within 1 year and 53% progression within 2 years (Table 3).
Table 3.
Information of two selected patients
| Variables | Basic information of patient 1 | Score | Basic information of patients 2 | Score |
|---|---|---|---|---|
| Neural invasion | With | 86 | Without | 0 |
| Gleason score | >8 | 57.5 | ≤8 | 0 |
| Initial PSA (ng/mL) | 189.05 | 51 | 189.23 | 51 |
| LDH (U/L) | 240.39 | 100 | 177.3 | 0 |
| Total score | 294.5 | 51 | ||
| 1-year progression | 11% | 85% | ||
| 2-year progression | 0% | 53% | ||
Note: LDH: Lactate dehydrogenase.
Discussion
Prostate cancer (PCa) is one of the most common malignancies in elderly men. Localized PCa can be treated by radical prostatectomy or radiotherapy, which usually contributes to a good survival rate and favorable patient outcomes [14]. In China, the proportion of patients with advanced PCa is high, especially those with HTB and osseous metastases associated with adverse prognoses, imposing a heavy psychological and economic burden on patients and their families [15]. Therefore, it is crucial to establish a model that can predict the progression of PCa to CRPC in patients with HTB and osseous metastases.
Our retrospective analysis identified NI, Gleason score, initial PSA, and LDH as important prognostic factors affecting the progression of HTB and osseous metastatic PCa to CRPC. The statistical significance and clinical correlation analysis of these factors offer insights into the mechanism of disease progression and assist clinicians in making more accurate treatment decisions. NI, as an important factor for a bleak prognosis, may be linked to the biological behavior of tumor cell distant metastases through neural pathways in patients with HTB and osseous metastatic PCa [16]. The invasion of tumor cells along nerve bundles may accelerate disease spread to other organs, hastening progression to CRPC [17]. Besides, NI may interact with the tumor microenvironment, where nerve growth factors and signaling molecules may promote tumor cell proliferation and survival. Studies have shown that tumors with NI features exhibit higher invasiveness and local expansion risks, potentially increasing the risk of postoperative positive surgical margins and thereby affecting patient prognosis and treatment choices [16]. In addition, the presence of tumor and perineural invasion has been identified as an independent risk factor for biochemical recurrence [18,19]. Therefore, the detection of NI is crucial for evaluating patient outcomes and formulating treatment strategies.
Patients with high Gleason scores often exhibit characteristics such as low tumor cell differentiation, high tumor heterogeneity, and rapid tumor growth rate, all of which contribute to increased resistance to hormone therapy, accelerating the transition to CRPC [20]. Tumors with high Gleason scores may have stronger invasiveness and metastatic potentials, as well as higher angiogenesis and microvascular density, which may promote tumor growth and spread [21]. A report shows that patients with Gleason scores ≤8 benefit less from androgen deprivation therapy than those with Gleason scores >8 [22]. Additionally, Meynard et al. [23] found that localized PCa patients with a Gleason score ≥7 who received iodine particles had a worse prognosis. Therefore, the Gleason score is not only an important index to evaluate the biological characteristics of tumors, but also a key factor in predicting disease progression.
The initial PSA level reflects the tumor’s biological activity and tumor burden of PCa at the initial diagnosis. A higher initial PSA level may imply a wider tumor cell distribution and higher tumor activity, which may lead to increased resistance to initial treatment, increasing the risk of progression to CRPC [24]. Fujita et al. [25] proposed that an initial PSA ≥500 ng/mL and a Gleason score ≥8 were prognostic factors affecting overall survival (OS) in patients with metastatic CRPC. Kodama et al. [26] found that initial PSA <100 ng/mL had a significant impact on the post-CRPC OS, and that PSA <100 ng/mL may be an adverse prognostic factor in mCNPC patients after developing CRPC. These studies suggest that the initial PSA level is related to the sensitivity of tumor cells to androgens, and high levels of PSA may indicate poor response of tumors to androgen deprivation therapy, requiring early consideration of the use of novel endocrine therapies or chemotherapy.
An increase in LDH, an indicator of cellular metabolism, in patients with HTB and osseous metastatic PCa may be indicative of hightened metabolic activity and rapid proliferation of tumor cells [27]. Elevated LDH levels are associated with enhanced glycolytic pathway of tumor cells, providing the energy and biosynthetic precursors required for rapid tumor cell growth [28]. The elevation of LDH may reflect inflammatory reactions and tissue destruction within the tumor microenvironment, which together enhance tumor invasiveness and metastasis, thereby accelerating disease progression. Katharina et al. [29] showed that LDH levels were related to PSMA mRNA expression and tumor volume in patients with metastatic CRPC. A recent report by Pisano et al. [30] suggests that elevated LDH levels are a poor prognostic indicator in patients with HTB and osseous metastatic PCa, closely related to shorter progression-free survival and OS. LDH, as an independent prognostic factor, reflects the high metabolic activity and rapid proliferation of tumors, aiding in the identification of patients who may benefit from early docetaxel treatment. This study identified NI, Gleason score, initial PSA, and LDH as important prognostic factors for progression to CRPC in patients with HTB and osseous metastatic PCa, providing valuable insights for physicians to assess the risk of disease progression and formulate treatment strategies.
To translate the identified factors into clinical practice, we constructed a comprehensive prediction model designed to provide clinicians with a tool to more accurately predict the risk of progression to CRPC in patients with HTB and osseous metastatic PCa. By combining key prognostic factors such as NI, Gleason score, initial PSA, and LDH, this model can provide a personalized risk assessment for each patient, thus assisting physicians in developing more accurate treatment plans. Developed using extensive clinical data and rigorous statistical analysis, the model ensures high applicability and accuracy across diverse patient populations. Through internal and external validation, the model has demonstrated good predictive performance and provides a powerful decision-support tool for doctors in clinical practice.
Limitations of this study include the small sample size that may affect the generalizability of the results, the retrospective design that may bias data collection, the singularity of the data source that may limit the broad applicability of the results, and the relatively short follow-up time that may not be sufficient to comprehensively assess long-term prognosis. In view of these limitations, future research should consider conducting prospective, multicenter clinical trials in a larger patient population and extending follow-up time to more accurately evaluate long-term treatment efficacy and patient prognosis. Meanwhile, more clinical and biological parameters, such as molecular biomarkers, should be explored to improve the accuracy and clinical application value of the prediction model.
Conclusion
In summary, this study successfully constructed a Nomogram model to predict the progression to CRPC in patients with HTB and osseous metastatic PCa. The model identified NI, Gleason score, initial PSA, and LDH as the key prognostic factors. With demonstrated predictive efficiency, the model provides valuable support for clinical decision-making in managing patients with HTB and osseous metastatic PCa.
Acknowledgements
The Grants from the National Natural Science Foundation of China (82160577; 82073762); Key Program for Regional Joint Funds of Natural Science Foundation of Guangdong Province (No. 2023A1515030091; 2020B1515120094; 2021B1515120053) and The Program for Science and Technology Plan of Guangzhou (No. 2024A03J0204; 2023A03J0405; 202102010071). Guangdong Medical Science and Technology Research Fund (No. A2022306). The Program for Science and Technology Plan Medical and Health Project of Zhuhai (No. 20191210E030075).
Disclosure of conflict of interest
None.
References
- 1.Pinsky PF, Parnes H. Screening for prostate cancer. N Engl J Med. 2023;388:1405–1414. doi: 10.1056/NEJMcp2209151. [DOI] [PubMed] [Google Scholar]
- 2.Patel N, Benipal B. Incidence of neuroendocrine tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11:e4322. doi: 10.7759/cureus.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SH, Patel N, Du M, Liang PS. Trends in early-onset vs late-onset colorectal cancer incidence by race/ethnicity in the United States cancer statistics database. Clin Gastroenterol Hepatol. 2022;20:e1365–e1377. doi: 10.1016/j.cgh.2021.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgo KS, Rumble RB, Talcott J. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J. Clin. Oncol. 2023;41:3652–3656. doi: 10.1200/JCO.23.00155. [DOI] [PubMed] [Google Scholar]
- 5.Koo KC, Park SU, Kim KH, Rha KH, Hong SJ, Yang SC, Chung BH. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015;3:10–15. doi: 10.1016/j.prnil.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Roubaud G, Özgüroğlu M, Kang J, Burgents J, Gresty C, Corcoran C, Adelman CA, de Bono J PROfound Trial Investigators. Survival with Olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383:2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal N, Azad AA, Carles J, Fay AP, Matsubara N, Heinrich D, Szczylik C, De Giorgi U, Young Joung J, Fong PCC, Voog E, Jones RJ, Shore ND, Dunshee C, Zschäbitz S, Oldenburg J, Lin X, Healy CG, Di Santo N, Zohren F, Fizazi K. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402:291–303. doi: 10.1016/S0140-6736(23)01055-3. [DOI] [PubMed] [Google Scholar]
- 8.Das M. Androgen deprivation therapy for prostate cancer. Lancet Oncol. 2017;18:e567. doi: 10.1016/S1470-2045(17)30670-8. [DOI] [PubMed] [Google Scholar]
- 9.Cook GJ, Azad G, Padhani AR. Bone imaging in prostate cancer: the evolving roles of nuclear medicine and radiology. Clin Transl Imaging. 2016;4:439–447. doi: 10.1007/s40336-016-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semba R, Uchida K, Hirokawa Y, Shiraishi T, Onishi T, Sasaki T, Inoue T, Watanabe M. Short-term prognosis of low-risk prostate cancer patients is favorable despite the presence of pathological prognostic factors: a retrospective study. BMC Urol. 2023;23:174. doi: 10.1186/s12894-023-01345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer K, Moris L, Lardas M, Haire A, Barletta F, Scuderi S, Molnar M, Herrera R, Rauf A, Campi R, Greco I, Shiranov K, Dabestani S, van den Broeck T, Arun S, Gacci M, Gandaglia G, Omar MI, MacLennan S, Roobol MJ, Farahmand B, Vradi E, Devecseri Z, Asiimwe A, Zong J, Maclennan SJ, Collette L, NDow J, Briganti A, Bjartell A, Van Hemelrijck M the PIONEER Consortium. Diagnostic and prognostic factors in patients with prostate cancer: a systematic review. BMJ Open. 2022;12:e058267. doi: 10.1136/bmjopen-2021-058267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abudoubari S, Bu K, Mei Y, Maimaitiyiming A, An H, Tao N. Prostate cancer epidemiology and prognostic factors in the United States. Front Oncol. 2023;13:1142976. doi: 10.3389/fonc.2023.1142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quality control index for standardized diagnosis and treatment of prostate cancer in China (2022 edition) Zhonghua Zhong Liu Za Zhi. 2022;44:1011–1016. doi: 10.3760/cma.j.cn112152-20220803-00530. [DOI] [PubMed] [Google Scholar]
- 14.Pollack A, Karrison TG, Balogh AG, Gomella LG, Low DA, Bruner DW, Wefel JS, Martin AG, Michalski JM, Angyalfi SJ, Lukka H, Faria SL, Rodrigues GB, Beauchemin MC, Lee RJ, Seaward SA, Allen AM, Monitto DC, Seiferheld W, Sartor O, Feng F, Sandler HM. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet. 2022;399:1886–1901. doi: 10.1016/S0140-6736(21)01790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.van der Slot MA, Remmers S, Kweldam CF, den Bakker MA, Nieboer D, Busstra MB, Gan M, Klaver S, Rietbergen JBW, van Leenders GJLH Anser Prostate Cancer Network. Biopsy prostate cancer perineural invasion and tumour load are associated with positive posterolateral margins at radical prostatectomy: implications for planning of nerve-sparing surgery. Histopathology. 2023;83:348–356. doi: 10.1111/his.14934. [DOI] [PubMed] [Google Scholar]
- 17.Sigorski D, Gulczyński J, Sejda A, Rogowski W, Iżycka-Świeszewska E. Investigation of neural microenvironment in prostate cancer in context of neural density, perineural invasion, and neuroendocrine profile of tumors. Front Oncol. 2021;11:710899. doi: 10.3389/fonc.2021.710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu Y, Förster S, Muders M. The role of perineural invasion in prostate cancer and its prognostic significance. Cancers (Basel) 2022;14:4065. doi: 10.3390/cancers14174065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves FA, Battye S, Roth H, Peters JS, Hovens C, Costello AJ, Corcoran NM. Prostatic nerve subtypes independently predict biochemical recurrence in prostate cancer. J Clin Neurosci. 2019;63:213–219. doi: 10.1016/j.jocn.2019.01.052. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Pham H, Abreu A, Amin MB, Sherrod AE, Xiao GQ, Aron M. Prognostic value of cribriform size, percentage, and intraductal carcinoma in Gleason score 7 prostate cancer with cribriform Gleason pattern 4. Hum Pathol. 2021;118:18–29. doi: 10.1016/j.humpath.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 21.van Leenders GJLH, Verhoef EI, Hollemans E. Prostate cancer growth patterns beyond the Gleason score: entering a new era of comprehensive tumour grading. Histopathology. 2020;77:850–861. doi: 10.1111/his.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang DD, Mahal BA, Muralidhar V, Martin NE, Orio PF, Mouw KW, King MT, Choueiri TK, Trinh QD, Hoffman KE, Spratt DE, Feng FY, Nguyen PL. Androgen deprivation therapy and overall survival for Gleason 8 versus Gleason 9-10 prostate cancer. Eur Urol. 2019;75:35–41. doi: 10.1016/j.eururo.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Meynard C, Huertas A, Dariane C, Toublanc S, Dubourg Q, Urien S, Timsit MO, Méjean A, Thiounn N, Giraud P. Tumor burden and location as prognostic factors in patients treated by iodine seed implant brachytherapy for localized prostate cancers. Radiat Oncol. 2019;15:1. doi: 10.1186/s13014-019-1449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferraro S, Biganzoli D, Rossi RS, Palmisano F, Bussetti M, Verzotti E, Gregori A, Bianchi F, Maggioni M, Ceriotti F, Cereda C, Zuccotti G, Kavsak P, Plebani M, Marano G, Biganzoli EM. Individual risk prediction of high grade prostate cancer based on the combination between total prostate-specific antigen (PSA) and free to total PSA ratio. Clin Chem Lab Med. 2023;61:1327–1334. doi: 10.1515/cclm-2023-0008. [DOI] [PubMed] [Google Scholar]
- 25.Fujita N, Hatakeyama S, Momota M, Tobisawa Y, Yoneyama T, Yamamoto H, Imai A, Ito H, Yoneyama T, Hashimoto Y, Yoshikawa K, Mariya Y, Ohyama C. Safety and feasibility of radiation therapy to the primary tumor in patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18:e523–e530. doi: 10.1016/j.clgc.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Kodama H, Hatakeyama S, Narita S, Takahashi M, Sakurai T, Kawamura S, Hoshi S, Ishida M, Kawaguchi T, Ishidoya S, Shimoda J, Narita T, Sato H, Mitsuzuka K, Tochigi T, Tsuchiya N, Arai Y, Habuchi T, Ohyama C. Clinical characterization of low prostate-specific antigen on prognosis in patients with metastatic castration-naive prostate cancer. Clin Genitourin Cancer. 2019;17:e1091–e1098. doi: 10.1016/j.clgc.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Chen G, Liu Z, Liu S, Cai Z, You P, Ke Y, Lai L, Huang Y, Gao H, Zhao L, Pelicano H, Huang P, McKeehan WL, Wu CL, Wang C, Zhong W, Wang F. Aberrant FGFR tyrosine kinase signaling enhances the warburg effect by reprogramming LDH isoform expression and activity in prostate cancer. Cancer Res. 2018;78:4459–4470. doi: 10.1158/0008-5472.CAN-17-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascardo F, Anselmino N, Páez A, Labanca E, Sanchis P, Antico-Arciuch V, Navone N, Gueron G, Vázquez E, Cotignola J. HO-1 modulates aerobic glycolysis through LDH in prostate cancer cells. Antioxidants (Basel) 2021;10:966. doi: 10.3390/antiox10060966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessel K, Seifert R, Weckesser M, Roll W, Humberg V, Schlack K, Bögemann M, Bernemann C, Rahbar K. Molecular analysis of circulating tumor cells of metastatic castration-resistant prostate cancer patients receiving (177)Lu-PSMA-617 radioligand therapy. Theranostics. 2020;10:7645–7655. doi: 10.7150/thno.44556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisano C, Turco F, Arnaudo E, Fea E, Vanella P, Ruatta F, Filippi R, Brusa F, Prati V, Vana F, Mennitto A, Cattrini C, Vignani F, Dionisio R, Icardi M, Guglielmini P, Buosi R, Stevani I, Vormola R, Numico G, Depetris I, Comandone A, Gennari A, Airoldi M, Rossi M, Vellani G, Ortega C, Tucci M, Maio MD, Buttigliero C. TEAM study: upfront docetaxel treatment in patients with metastatic hormone-sensitive prostate cancer: a real-world, multicenter, retrospective analysis. Clin Genitourin Cancer. 2024;22:56–67. e16. doi: 10.1016/j.clgc.2023.08.006. [DOI] [PubMed] [Google Scholar]