Abstract
Induction chemotherapy followed by concomitant chemoradiation is the standard therapy for patients with locoregionally advanced NPC. There is a limitation of clinical studies that compare different induction regimens. The purpose of this work is to analyze the efficacy of two distinct chemotherapy regimens, docetaxel, cisplatin, and 5-fluorouracil (TPF) and gemcitabine/cisplatin (GP), in treating patients with loco-regionally advanced nasopharyngeal carcinoma (NPC). We analyzed 81 patients initially presented with stage III-IVA NPC from January 2019 to June 2023. Participants were randomized in 1:1 ratio to obtain GP regimen or TPF regimen followed by concurrent CRT. The overall response rate was 97.5% after induction chemotherapy in both groups (In GP arm, 78% of patients achieved complete remission compared to 70% of patients treated with TPF regimen). The satisfactory tumor response to induction chemotherapy was linked with significant enhanced progression free survival [CI (3.37-13.92), HR=2.16, P=0.001] and overall survival [CI (3.717-9.443), HR=1.873, P=0.001]. The GP regimen was both efficacious and well-tolerated. Leucopenia and neutropenia (Grade 3-4) were significantly lower in GP group contrasted to in TPF group. There was no significant difference in the 3-year DFS and OS between GP and TPF protocols.
Keywords: Induction chemotherapy, nasopharyngeal carcinoma, TPF regimen, GP regimen
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor that develops from the epithelial cells lining of the nasopharynx. It is various from other types of head and neck malignancies in terms of its epidemiology, natural progression, histology, and responses to therapy.
NPC is common in Asia particularly Southern China. The Middle East and North Africa are areas with a moderate risk. The nonkeratinizing, undifferentiated squamous cell carcinoma is considered the most abundant histopathological type in these regions [1]. The geographical variety of NPC indicates that it has several causes. The primary risk factors involve Epstein-Barr virus (EBV) infections, environmental variables, and genetic vulnerability [2].
Radiotherapy (RT) is the backbone treatment in early and locoregionally advanced NPC disease owing to its radiosensitivity and anatomical position. RT alone is very effective in achieving local and regional control for those with the early stage (stage I) and eliminates the toxicity associated with concurrent chemoradiation [3].
The treatment of stage II disease depends on the risk of reoccurrence. For low-risk stage II disease, radiation therapy alone rather than concurrent chemoradiation is recommended [4]. For high-risk stage II disease, concurrent chemoradiation demonstrated enhanced overall survival (OS) and reduced the likelihood of distant metastases [5].
Induction chemotherapy (IC) added to concurrent chemoradiation is the recommended therapy for stage III and IVA NPC. This approach has been shown to enhance OS in many randomized studies [6-8].
Induction chemotherapy have the ability to decrease the burden of the tumor, increase locoregional control, reduce risk of distant metastases, and reduce the high dose radiation volume during concurrent chemoradiation [3,9].
Available Induction regimens that may be used in locally advanced NPC consists of GP, TPF, cisplatin with fluorouracil and cisplatin with paclitaxel. The GP regimen improves OS with manageable toxicity than other chemotherapy regimens [10,11].
There is a limitation of clinical studies that explicitly compare different induction chemotherapy regimens.
Patients and methods
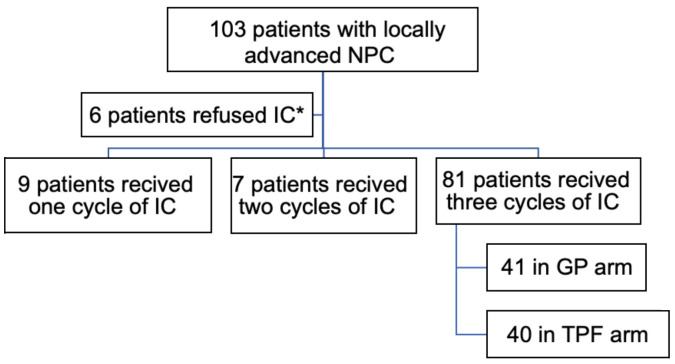
This study is an open label randomized trial. A total of 103 NPC participants had been managed in our hospital from January 2019 to June 2023. The Participants were randomized in a 1:1 ratio to receive GP regimen or TPF regimen followed by concurrent CRT. Twenty-two patients were excluded from the study because 6 patients refused to receive induction chemotherapy (IC), 9 patients received only one cycle of IC and the remaining 7 patients received 2 cycles (Figure 1).
Figure 1.
Study design and enrollment. *IC, induction chemotherapy.
This trial was permitted by the Research Ethical Committee of Faculty of medicine, Tanta University (Approval code number: 36264PR561/2/24). Each participant submitted a well-informed written consent.
Inclusion and exclusion criteria
The eligibility criteria included: Stage III-IV NPC verified through histopathological examination (except T3N0); performance status scores 0-2, and patients aged ≥18 years old. Individuals with evidence of second malignancy or distant metastases were excluded.
Pre-treatment evaluation
A confirmed diagnosing is established by endoscope-guided biopsy of the primary tumor. A comprehensive physical examination of the head and neck was done to all participants, as well as neck MRI, and CT scans of the chest. A complete blood picture and biochemical profile were conducted.
The 8th edition of the UICC/AJCC manual had been utilized for staging all included participants.
Treatment details
Induction chemotherapy
1. The GP regimen consisted of 3 cycles of 80 mg/m2 of cisplatin on D1 and 1000 mg/m2 of gemcitabine on D1 and 8 of a 21-day cycle. 2. The TPF regimen consisted of 3 cycles of (60 mg/m2 of docetaxel on D1), (60 mg/m2 of cisplatin on D1), and (600 mg/m2 of fluorouracil per day/continuous infusion on days 1 to 5) every three weeks.
Concurrent chemoradiation
All patients had been managed with concurrent chemoradiation utilizing cisplatin (40 mg/m2 weekly). Chemoradiotherapy started within 21-day following the 1st day of the 3rd cycle of IC.
Radiotherapy
Intensity modulated radiotherapy (IMRT) with 6 MV photon was applied to all participants, commencing 3 weeks following the beginning of the previous cycle of IC. The CT simulation had been conducted using consecutive slices with a thickness ranging from 3 to 5 mm. We were adherent to the international protocol for clinical target volumes delineation for NPC [12].
Gross target volume (GTV) involved all gross lesions on physical assessment and imaging. The high-risk primary tumor (CTVp1) included GTV + 5 mm margin. The intermediate risk volume (CTVp2) included GTV + 10 mm and whole nasopharynx with proper coverage of soft palate inferiorly, posterior maxillary sinuses, posterior nasal cavity, pterygoid fossa, parapharyngeal spaces, posterior ethmoid sinus, skull base. The coverage of cavernous sinus, sphenoid sinus and clivus depended on T stage.
The high-risk nodal volume (CTVn1) included all grossly involved LN + 5 mm (we Consider 10 mm expansion if extracapsular extension exists). The Intermediate risk nodal volume (CTVn2) included intermediate risk nodal regions.
Follow up
The median follow-up time had been 36-month period (range: 12-60). All the patients were followed for at least one year.
Clinical endpoints
The primary endpoint is the response rate and acute toxicity profile after IC. The secondary endpoints were PFS, that was determined from the initial day of therapy to the date of failure of therapy or death due to any reason and OS that was determined from the onset of diagnosing to death. Toxicity profile events were evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
Statistical analysis
The baseline features of the participants had been compared by t-tests and chi-square tests. The OS and PFS were determined with the Kaplan-Meier approach. The variations among the groups had been contrasted by Log-rank tests. The 95% confidence intervals (CIs), hazard ratios (HRs), and independent prognostic factors were evaluated by A multivariate Cox proportional hazard model. The results of the main parameters had been contrasted with the chi-square test or Fisher’s exact test employing SPSS IBM 22 software (SPSS Inc., Chicago, IL, USA).
Results
A total of 103 NPC participants had been managed in our hospital from January 2019 to June 2023. Eighty-one patients received 3 cycles of IC, involving 41 patients in the GP group and 40 patients in the TPF group (Figure 1).
The baseline features of all participants are demonstrated in Table 1.
Table 1.
Patient characteristics
| Patient characteristics | GP (n=41) | TPF (n=40) | Total =81 | p |
|---|---|---|---|---|
| Age | ||||
| >50 | 21 (51.2) | 22 (55) | 43 (53.1) | 0.733 |
| <50 | 20 (48.8) | 18 (45) | 38 (46.9) | |
| Sex | ||||
| Male | 25 (61) | 24 (60) | 49 (60.5) | 0.928 |
| Female | 16 (39) | 16 (40) | 32 (39.5) | |
| Smoking | ||||
| Smoker | 16 (39) | 11 (27.5) | 27 (33.3) | 0.167 |
| Non | 24 (58.5) | 24 (60) | 48 (59.3) | |
| Ex | 1 (2.4) | 5 (12.5) | 6 (7.4) | |
| T stage | ||||
| T1 | 8 (19.5) | 4 (10) | 12 (14.8) | 0.060 |
| T2 | 16 (39) | 24 (60) | 40 (49.4) | |
| T3 | 1 (2.4) | 4 (10) | 5 (6.2) | |
| T4 | 16 (39) | 8 (20) | 24 (29.6) | |
| N stage | ||||
| N1 | 14 (34.1) | 6 (15) | 20 (24.7) | 0.128 |
| N2 | 23 (56.1) | 30 (75) | 53 (65.4) | |
| N3 | 4 (9.8) | 4 (10) | 8 (9.9) | |
| Stage | ||||
| III | 21 (51.2) | 28 (70) | 49 (60.5) | 0.084 |
| IVA | 20 (48.8) | 12 (30) | 32 (39.5) | |
| Who | ||||
| I | 0 | 0 | 0.127 | |
| II | 8 (19.5) | 16 (40) | 24 (29.6) | |
| III | 21 (51.2) | 16 (40) | 37 (45.7) | |
| IV | 12 (29.3) | 8 (20) | 20 (24.7) |
Sex, age, T stage, N stage and clinical stage, were comparable among the two groups.
Tumor response after IC
We had assessed the response in two times:
The 1st time of evaluation was 4-8 weeks after 3 cycles of induction chemotherapy and before starting concurrent chemoradiation. The overall response rate was 97.5% after induction chemotherapy in both GP group and TPF group. In the GP group, 32 (78%) patients achieved complete response (CR), and 9 (22%) participants had a partial response (PR). This was numerically better compared to the TPF group, where 28 (70%) patients achieved (CR), 11 (27.5%) achieved PR, and one patient had stable disease (Table 2).
Table 2.
Tumor response in both treatment groups
| Response | GP group (n=41) | TPF group (n=40) | P value |
|---|---|---|---|
| No (%) | No (%) | ||
| After induction chemotherapy | |||
| CR | 32 (78) | 28 (70) | 0.483 |
| PR | 9 (22) | 11 (27.5) | |
| SD | 0 | 1 (2.5) | |
| After IC + CCRT | |||
| CR | 38 (92.7) | 34 (85) | 0.271 |
| PR | 3 (7.3) | 6 (15) |
The 2nd time of evaluation was 12-16 weeks after completion of full course of treatment (3 cycles induction chemotherapy followed by concurrent chemoradiation). Thirty-eight (92.7%) patients obtained CR and 3 (7.3%) patients obtained PR in GP group compared to 34 (85%) patients obtained CR and 6 (15%) obtained PR in TPF group (P=0.271).
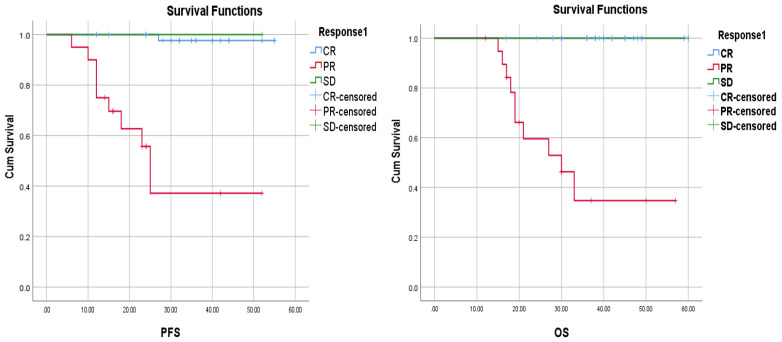
The tumor response after induction chemotherapy was relevant to the survival status. The 3-year PFS rates was 97.7% with CR versus 37.1% with PR and OS rates was 100% with CR vs 34.7% with PR (P<0.001) (Figure 3).
Figure 3.
Kaplan-Meier Analysis of PFS and OS curves based on treatment response to induction chemotherapy.
Acute toxicity during induction chemotherapy
During induction chemotherapy, the most prevalent side effect was leucopenia. The grade 3-4 leucopenia had been significantly higher in TPF group contrasted to GP [12 (30%) in TPF versus 5 (12.2%) in GP group, P=0.040]. Also, a significantly more febrile neutropenia had existed in TPF group than in GP group [8 (20%) versus 2 (4.9%), P=0.039].
Grade 3-4 anemia was higher in TPF group [6 (15%)] compared to GP group [3 (7.3%)]. While ≥G3 thrombocytopenia was reported in GP arm only.
Seven patients in both groups developed ≥G3 mucositis which was significantly greater in TPF group contrasted to GP group [6 (15%) versus 1 (2.4%), P=0.044].
Non hematological toxicity involving Grade 3-4 nausea/vomiting and diarrhea were higher in TPF group compared to GP group [7 (17.5%) versus 4 (9.8%), P=0.309 and 4 (10%) versus 1 (2.4%) respectively] with no significant correlation (Table 3).
Table 3.
Acute toxicity during induction chemotherapy
| Toxicity | GP group (n=41) | TPF group (n=40) | P value |
|---|---|---|---|
| No (%) | No (%) | ||
| Anemia | |||
| G1-2 | 38 (92.7) | 34 (85) | 0.271 |
| ≥G3 | 3 (7.3) | 6 (15) | |
| Leucopenia | |||
| G1-2 | 36 (87.8) | 28 (70) | 0.040 |
| ≥G3 | 5 (12.2) | 12 (30) | |
| Febrile neutropenia | |||
| No | 39 (95.1) | 32 (80) | 0.039 |
| Yes | 2 (4.9) | 8 (20) | |
| Thrombocytopenia | |||
| G1-2 | 38 (92.7) | 40 (100) | 0.081 |
| ≥G3 | 3 (7.3) | 0 (0.0) | |
| Nausea/vomiting | |||
| G1-2 | 37 (90.2) | 33 (82.5) | 0.309 |
| ≥G3 | 4 (9.8) | 7 (17.5) | |
| Mucositis | |||
| G1-2 | 40 (97.6) | 34 (85) | 0.044 |
| ≥G3 | 1 (2.4) | 6 (15) | |
| Diarrhea | |||
| G1-2 | 40 (97.6) | 36 (90) | 0.157 |
| ≥G3 | 1 (2.4) | 4 (10) |
Progression free survival (PFS)
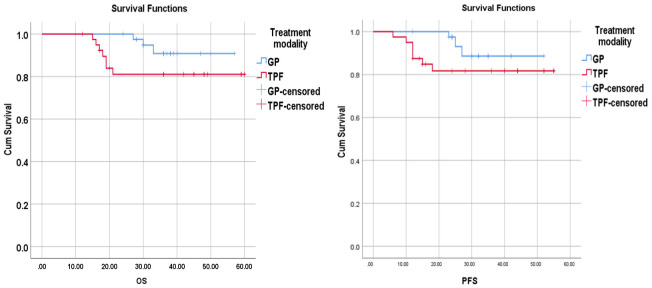
The 3-year PFS rate was 88.6% vs 81.7% in the GP group and TPF group, correspondingly. No variations had existed in the 3-year PFS rate among the GP and the TPF group (P=0.151) (Figure 2).
Figure 2.
Kaplan-Meier Analysis of OS and PFS curves for 81 patients received GP and TPF as induction chemotherapy.
In total, 10 patients out of 81 (12.3%) had treatment failure, including 7 patients (17.5%) in the TPF group and three patients (7.3%) in the GP arm.
The outcomes from the univariate analysis showed that N stage (N1-2 vs N3), T stage, overall stage and response (CR vs PR) are prognostic factors for DFS.
The multivariate regression analysis showed that individuals who achieved CR had significantly better PFS [CI (3.37-13.92), HR=2.16, P=0.001)] (Table 4).
Table 4.
Multivariate prognostic factors and survival
| Variables | CI | HR | P value | |
|---|---|---|---|---|
| PFS | T stage | 0.46-10.60 | 2.23 | 0.313 |
| N stage | 0.76-4.42 | 1.84 | 0.172 | |
| Stage | 0.16-9.08 | 0.380 | 0.551 | |
| Response | 3.37-13.92 | 2.16 | 0.001 | |
| OS | Median age | 0.018-0.739 | 0.114 | 0.023 |
| N stage | 0.238-1.69 | 0.635 | 0.365 | |
| Response | 3.717-9.443 | 1.873 | 0.001 |
Overall survival (OS)
The 3-year OS rate was 90.8% in the GP group, and 81% in TPF group. No statistical variation had existed in the 3-year OS rate across the treatment groups (P=0.121) (Figure 2).
The 3-year OS had been significantly better in the individuals younger than 50 years (P=0.043), N1-2 stage (P=0.002) and the patients who achieved complete remission (P≤0.001).
The multivariate analysis suggested that participants younger than 50 years and those who achieved CR had significantly better OS [CI (0.018-0.739), HR=0.114, P=0.023], and [CI (3.717-9.443), HR=1.873, P=0.001] respectively (Table 4).
Discussion
The combined approach of chemo-radiotherapy is the backbone for the standard treatment of individuals with stages III and IVA NPC. Induction chemotherapy followed by chemoradiation provides an OS advantage [13,14]. At a median follow up of 7.6 years of the MAC-NPC meta-analysis, the addition of induction chemotherapy improved the OS contrasted with concurrent chemoradiation only (HR 0.75, 95% 0.59-0.96) [15].
The available IC regimens that may be used in locally advanced NPC include TPF, GP, cisplatin/paclitaxel and cisplatin/fluorouracil. The TPF regimen proved to be superior to cisplatin with fluorouracil and cisplatin with paclitaxel. The incidence of neutropenia and leucopenia (grade 3-4), are higher with TPF chemotherapy. In addition, pretreatment use of steroids before TPF regimen may increase the side effects for diabetic and hypertensive patients [16,17].
For induction chemotherapy, the GP regimen proved to be effective and tolerable with mild toxicity [10,11]. In the current work, we assess the efficacy of GP as an IC regimen and compare treatment response, treatment related toxicity, and survival outcomes versus the TPF regimen.
Yau and colleagues clarified that 3 cycles of GP as induction treatment for locoregionally advanced NPC resulted in overall clinical response rates above 90% [18]. In our study the overall response was 97.5% after IC in both groups (78% of patients achieved CR with GP regimen compared to 70% of patients with TPF regimen). By the end of the whole therapy, 92% of patients in the GP arm achieved CR, whereas 85% of patients in the TPF arm achieved CR.
The response to IC may possess a potential clinical significance [19]. The current research demonstrated that favorable tumor responses to IC were significantly linked to a notable increase in both PFS and OS. This finding aligns with the work performed by Liu et al., which shown that an unsatisfactory tumor responses during IC is linked to a poor prognosis among individuals diagnosed with stage III and IV NPC (3-year PFS 71.1% vs 85.9%, P=0.005 and 3-year LRFS 82.7% vs 93.5%, P=0.012) [20].
In our study, the incidence of grade 3-4 toxicity with the GP regimen was lower compared to the TPF regimen. The grade 3-4 leucopenia was 30% in TPF group compared to 12.2% in GP group matched with Zhu J study, where grade 3 or 4 leucopenia occurred in 34.5% and 14.1 in TPF and GP respectively [21]. Febrile neutropenia was also substantially greater in TPF group contrasted to in GP group [8 (20%) versus 2 (4.9%), P=0.039]. In consistent with our results, Zhu and colleagues reported non-hematological adverse events including vomiting (14.1% vs 13.8%), stomatitis (mucositis) (14.1% vs 17.2%) and diarrhea (1.4 vs 2.3) in TPF and GP correspondingly [21].
The study carried out by Zhang et al. revealed significant improvement in failure-free survival and OS with induction GP plus concurrent chemoradiation versus concurrent chemoradiation alone in locoregionally advanced NPC. The recipients had a high level of tolerance to GP regimen, with 97% of them successfully completing all three cycles of GP treatment [10]. In the current work, the 3-year PFS rate was 88.6% in the GP group compared to 81.7% in TPF and the 3-year OS was 90.8% in the GP group versus 81% in TPF group. The 3-year rates of survival was better with GP regimen but without significant difference. Our results were consistent with Zeng et al., the 3-year DFS had been 83.1% versus 81.6% and the OS had been 94.4% versus 92.0% in the GP group and TPF group correspondingly [22].
The multivariate analysis explained that those who achieved CR had significantly better OS [CI (3.717-9.443), HR=1.873, P=0.001]. In agreement with Beng et al., the 4-year DFS rates for CR, PR and SD had been 90.0%, 79.0% and 58.2% correspondingly and the 4-year OS rates were 95.7%, 88.7%, and 70.2% for CR, PR and SD correspondingly. Through multivariate analysis, it was shown that the tumor’s responses to IC is an independent and crucial factor in predicting both OS and DFS [19].
Conclusion
This manuscript clarified that the GP regimen has a comparable efficacy to TPF as induction chemotherapy for locally advanced NPC, but it has much lower incidence of grade 3-4 acute toxicity.
Acknowledgements
We acknowledge the work of Head and Neck surgery department, the otorhinolaryngologist involved in the diagnosis of the disease (Drs. Mostafa Ammar and Ahmed S Elhamshary).
Disclosure of conflict of interest
None.
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–24. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 3.Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, Halámková J, Mattheis S, Baujat B, Hardillo J, Smeele L, van Herpen C, Castro A, Machiels JP ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; EURACAN. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(†) Ann Oncol. 2021;32:452–65. doi: 10.1016/j.annonc.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Tang LL, Guo R, Zhang N, Deng B, Chen L, Cheng ZB, Huang J, Hu WH, Huang SH, Luo WJ, Liang JH, Zheng YM, Zhang F, Mao YP, Li WF, Zhou GQ, Liu X, Chen YP, Xu C, Lin L, Liu Q, Du XJ, Zhang Y, Sun Y, Ma J. Effect of radiotherapy alone vs radiotherapy with concurrent chemoradiotherapy on survival without disease relapse in patients with low-risk nasopharyngeal carcinoma: a randomized clinical trial. JAMA. 2022;328:728–36. doi: 10.1001/jama.2022.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li XY, Chen QY, Sun XS, Liu SL, Yan JJ, Guo SS, Liu LT, Xie HJ, Tang QN, Liang YJ, Wen YF, Guo L, Mo HY, Chen MY, Sun Y, Ma J, Tang LQ, Mai HQ. Ten-year outcomes of survival and toxicity for a phase III randomised trial of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma. Eur J Cancer. 2019;110:24–31. doi: 10.1016/j.ejca.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, Qian CN, Zhao C, Xiang YQ, Zhang XP, Lin ZX, Li WX, Liu Q, Qiu F, Sun R, Chen QY, Huang PY, Luo DH, Hua YJ, Wu YS, Lv X, Wang L, Xia WX, Tang LQ, Ye YF, Chen MY, Guo X, Hong MH. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. doi: 10.1016/j.ejca.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, Lin M, You R, Zou X, Liu YP, Xie YL, Wang ZQ, Mai HQ, Chen QY, Tang LQ, Mo HY, Cao KJ, Qian CN, Zhao C, Xiang YQ, Zhang XP, Lin ZX, Li WX, Liu Q, Li JB, Ling L, Guo X, Hong MH, Chen MY. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. doi: 10.1016/j.ejca.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Hong RL, Hsiao CF, Ting LL, Ko JY, Wang CW, Chang JTC, Lou PJ, Wang HM, Tsai MH, Lai SC, Liu TW. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol. 2018;29:1972–9. doi: 10.1093/annonc/mdy249. [DOI] [PubMed] [Google Scholar]
- 9.Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, Wee JTS, Whitley AC, Yi JL, Yom SS, Chan ATC, Hu CS, Lang JY, Le QT, Lee AWM, Lee N, Lin JC, Ma B, Morgan TJ, Shah J, Sun Y, Ma J. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J. Clin. Oncol. 2021;39:840–59. doi: 10.1200/JCO.20.03237. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, Jin F, Shi M, Chen YP, Hu WH, Cheng ZB, Wang SY, Tian Y, Wang XC, Sun Y, Li JG, Li WF, Li YH, Tang LL, Mao YP, Zhou GQ, Sun R, Liu X, Guo R, Long GX, Liang SQ, Li L, Huang J, Long JH, Zang J, Liu QD, Zou L, Su QF, Zheng BM, Xiao Y, Guo Y, Han F, Mo HY, Lv JW, Du XJ, Xu C, Liu N, Li YQ, Chua MLK, Xie FY, Sun Y, Ma J. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381:1124–35. doi: 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, Jin F, Shi M, Chen YP, Hu WH, Cheng ZB, Wang SY, Tian Y, Wang XC, Sun Y, Li JG, Li WF, Li YH, Mao YP, Zhou GQ, Sun R, Liu X, Guo R, Long GX, Liang SQ, Li L, Huang J, Long JH, Zang J, Liu QD, Zou L, Su QF, Zheng BM, Xiao Y, Guo Y, Han F, Mo HY, Lv JW, Du XJ, Xu C, Liu N, Li YQ, Xie FY, Sun Y, Ma J, Tang LL. Final overall survival analysis of gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma: a multicenter, randomized phase III trial. J. Clin. Oncol. 2022;40:2420–5. doi: 10.1200/JCO.22.00327. [DOI] [PubMed] [Google Scholar]
- 12.Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, Corry J, Grau C, Grégoire V, Harrington KJ, Hu CS, Kwong DL, Langendijk JA, Le QT, Lee NY, Lin JC, Lu TX, Mendenhall WM, O’Sullivan B, Ozyar E, Peters LJ, Rosenthal DI, Soong YL, Tao Y, Yom SS, Wee JT. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol. 2018;126:25–36. doi: 10.1016/j.radonc.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, Chan I, Ahuja AT, Zee BC, Chan AT. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2009;27:242–9. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, Hu CS, Xu XY, Chen YY, Hu WH, Guo L, Mo HY, Chen L, Mao YP, Sun R, Ai P, Liang SB, Long GX, Zheng BM, Feng XL, Gong XC, Li L, Shen CY, Xu JY, Guo Y, Chen YM, Zhang F, Lin L, Tang LL, Liu MZ, Ma J. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–20. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 15.Petit C, Lee A, Ma J, Lacas B, Ng WT, Chan ATC, Hong RL, Chen MY, Chen L, Li WF, Huang PY, Tan T, Ngan RKC, Zhu G, Mai HQ, Hui EP, Fountzilas G, Zhang L, Carmel A, Kwong DLW, Moon J, Bourhis J, Auperin A, Pignon JP, Blanchard P MAC-NPC Collaborative Group. Role of chemotherapy in patients with nasopharynx carcinoma treated with radiotherapy (MAC-NPC): an updated individual patient data network meta-analysis. Lancet Oncol. 2023;24:611–23. doi: 10.1016/S1470-2045(23)00163-8. [DOI] [PubMed] [Google Scholar]
- 16.Peng H, Tang LL, Chen BB, Chen L, Li WF, Mao YP, Liu X, Zhang Y, Liu LZ, Tian L, Guo Y, Sun Y, Ma J. Optimizing the induction chemotherapy regimen for patients with locoregionally advanced nasopharyngeal carcinoma: a big-data intelligence platform-based analysis. Oral Oncol. 2018;79:40–6. doi: 10.1016/j.oraloncology.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Lee AW, Tung SY, Ngan RK, Chappell R, Chua DT, Lu TX, Siu L, Tan T, Chan LK, Ng WT, Leung TW, Fu YT, Au GK, Zhao C, O’Sullivan B, Tan EH, Lau WH. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 trials. Eur J Cancer. 2011;47:656–66. doi: 10.1016/j.ejca.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Yau TK, Lee AW, Wong DH, Yeung RM, Chan EW, Ng WT, Tong M, Soong IS. Induction chemotherapy with cisplatin and gemcitabine followed by accelerated radiotherapy and concurrent cisplatin in patients with stage IV(A-B) nasopharyngeal carcinoma. Head Neck. 2006;28:880–7. doi: 10.1002/hed.20421. [DOI] [PubMed] [Google Scholar]
- 19.Peng H, Chen L, Zhang J, Li WF, Mao YP, Zhang Y, Liu LZ, Tian L, Lin AH, Sun Y, Ma J. Induction chemotherapy improved long-term outcomes of patients with locoregionally advanced nasopharyngeal carcinoma: a propensity matched analysis of 5-year survival outcomes in the era of intensity-modulated radiotherapy. J Cancer. 2017;8:371–7. doi: 10.7150/jca.16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu LT, Tang LQ, Chen QY, Zhang L, Guo SS, Guo L, Mo HY, Zhao C, Guo X, Cao KJ, Qian CN, Zeng MS, Bei JX, Hong MH, Shao JY, Sun Y, Ma J, Mai HQ. The prognostic value of plasma Epstein-Barr viral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:862–9. doi: 10.1016/j.ijrobp.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Duan B, Shi H, Li Y, Ai P, Tian J, Chen N. Comparison of GP and TPF induction chemotherapy for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2019;97:37–43. doi: 10.1016/j.oraloncology.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Z, Yan RN, Tu L, Wang YY, Chen PR, Luo F, Liu L. Assessment of concurrent chemoradiotherapy plus induction chemotherapy in advanced nasopharyngeal carcinoma: cisplatin, fluorouracil, and docetaxel versus gemcitabine and cisplatin. Sci Rep. 2018;8:15581. doi: 10.1038/s41598-018-33614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]