Abstract
Opioids are the most effective and widely used treatments for acute and chronic pain in patients with cancer. This review focuses on the impact of opioids and mu-opioid receptors (MORs) on the stages of oncologic metastasis. Studies have shown that opioids can facilitate tumor progression and are related to a poor prognosis in patients with cancer. As the primary receptor for opioids, MORs play a significant role in regulating malignant tumor transformation and are involved in processes, such as proliferation, angiogenesis, epithelial-mesenchymal transition (EMT), circulating tumor cells (CTCs) and the tumor microenvironment (TME). While clinical trials have investigated the relationship between opioids and patient prognosis, further research is needed to clarify the relationship between opioids, MORs and metastasis.
Keywords: Opioids, mu-opioid receptor, metastasis, cancer
Introduction
Cancer is a major public health issue and a significant contributor to the global burden of disease. According to the Centers for Disease Control and Prevention of China, there were 2.3978 million cancer-related deaths in China in 2020 [1]. Many patients with cancer experience severe, debilitating pain, which is categorized as one of the seven types of chronic painful conditions [2]. Pain, a common symptom in cancer patients, has the potential to elicit systemic inflammation and stimulate the sympathetic nervous system. This can lead to alterations within the tumor microenvironment, the stimulation of dormant tumor growth, micro-metastasis, and the advancement of metastatic diseases, highlighting the urgent need to understand the role of opioids in this process [3]. Patients with severe cancer-related pain often require analgesics such as opioids, including fentanyl, morphine, oxycodone, hydromorphone, and tapentadol. These drugs, acting as mu-opioid receptor agonists, play a crucial role in managing the debilitating pain associated with cancer, highlighting the significance of our research in this field.
Cancer cells and tumor microenvironment cells also express mu-opioid receptors (MORs). Recent evidence suggests that the impact of opioids on signal transduction and the behavior of tumor niche cells is significant [4]. Over the past 20 years, numerous studies have examined the relationship between opioid use and MOR use with a potential increase in cancer metastasis. Animal and human studies have suggested that opioid drugs may affect the progression of cancer [5]. In contrast, clinically relevant doses of morphine can induce apoptosis and necrosis in human lung cancer cells [6].
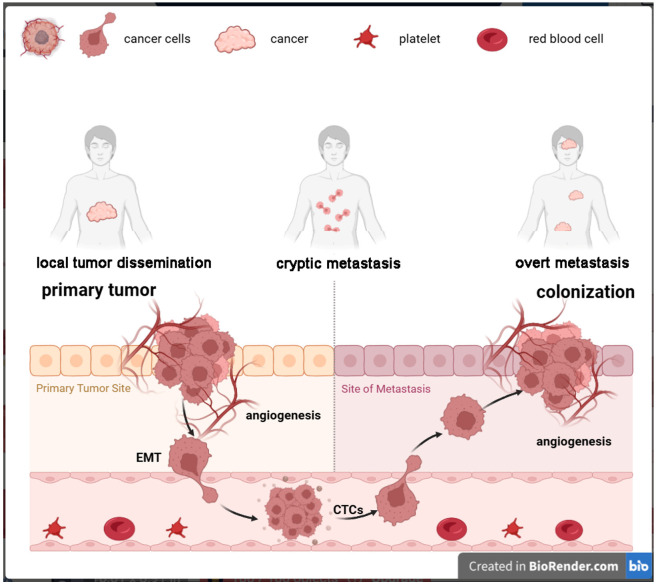
Metastasis, which refers to the proliferation of cancer cells to organs that are situated far away from their original site, represents the most advanced and devastating stage of cancer. Metastasis comprises three stages, namely, dissemination, dormancy, and colonization, which can coexist and overlap over time [7]. Metastasis-initiating cells (MICs) originate from primary tumors and acquire the ability to migrate invasively. During the transmission process, tumor cells with oncogenic mutations invade deeper tissue layers through the basement membrane and survive. Subsequent to this process, intravasation occurs into adjacent blood vessels or lymphatic systems, ultimately leading to extravasation into remote organs via mechanisms such as trans-endothelial migration, capillary disruption, migration along neuronal pathways, or direct dissemination into neighboring spaces, including the peritoneal and pleural cavities [8]. MICs have the capability to migrate either individually or collectively via the bloodstream or lymphatic vessels in the form of circulating tumor cells (CTCs). These CTCs, frequently encased by platelets, tumor-derived stromal cells, or neutrophils, possess the ability to evade immune surveillance and form clusters that exhibit a heightened metastatic potential compared to single cells [9]. Despite the fact that the majority of CTCs are eliminated as a result of physical, biochemical, and immunological pressures, a portion of them become entrapped within the capillary beds of remote organs. These cells can migrate into the organ parenchyma, transforming into disseminated tumor cells (DTCs), and ultimately giving rise to new metastases. While niche-specific or systemic immune defenses eliminate most MICs a few survive, undergo reversible growth arrest, and enter a state of immune escape. These surviving MICs adapt to organ-specific environments and use their tumor microenvironment (TME) to evade immune surveillance. DTCs are often undetectable by clinical imaging, leaving patients unaware of their disease. Clinical metastases arise from successful MICs, which have adapted to enable the growth and colonization of organs by exploiting regenerative, angiogenic, and immune-suppressive programs, resulting in clinically detectable metastases [7]. This leads to uncontrolled tumor growth, resulting in organ dysfunction, systemic failure, and ultimately death (Figure 1).
Figure 1.
Phases of metastasis. Initially, tumor cells undergo genetic mutations that enable them to detach from the primary site. Subsequently, these cells invade surrounding tissues, aided by enzymes that degrade the extracellular matrix. As they migrate, these cells may undergo epithelial to mesenchymal transition and enter blood or lymphatic vessels, as circulating tumor cells. Eventually, they exit the vessels at distant sites, adhere to new tissue, and proliferate, forming secondary tumors. This process is facilitated by interactions with the host immune system and the tumor microenvironment.
This review summarizes the current knowledge regarding the impact of opioids and MORs on cancer metastasis with experimental and clinical evidence.
Opioids and mu-opioid receptors
The development of opioids, a class of potent painkillers, has a rich and complex history spanning millennia. Originating from the opium poppy, opioids such as morphine were first identified and isolated for medical use in the 19th century [10]. These naturally occurring compounds, including morphine and codeine, were followed by a surge of synthetic opioids like heroin, hydrocodone, oxycodone, and fentanyl in the 20th century [10]. Opioid receptors are a large family of receptors including MORs, delta (δ)-opioid receptors (DORs), kappa (κ)-opioid receptors (KORs), and nociceptin receptors (NORs), also referred to as opioid-receptor-like receptor 1 (ORL1) [11]. Opioids exert their effects by binding to these receptors, particularly MORs in the brain, triggering the release of dopamine associated with feelings of pleasure [12], which contributes to both their analgesic and addictive properties.
MORs are key players in the body’s opioid system, which is intricately involved in pain perception, emotional regulation, and addiction. MORs belong to the superfamily of G-protein-coupled receptors (GPCRs) and are predominantly expressed in the central and peripheral nervous systems, as well as in various peripheral tissues such as the gut and liver [11]. MORs initiate G-protein-dependent signaling cascades involving Gi/oα and Gβγ subunits, as well as G-protein-independent pathways which involve essential scaffolding proteins, notably β-arrestins [13]. These signaling events ultimately lead to a decreased pain perception and the promotion of euphoria. In addition to their role in pain management and addiction, MORs are implicated in a diverse range of physiological processes, including stress responses, reward processing, and immune modulation [11].
Opioid receptors in cancer cells
Functional opioid receptors are expressed in various cancer cell lines and patient samples, often showing upregulation in cancer, including esophageal cancer, colon cancer, non-small cell lung cancer (NSCLC), breast, gastric, liver, prostate, and laryngeal cancers [4,14]. In patients with hepatocellular carcinoma or gastric cancer, positive MOR expression is associated with more aggressive tumors and shorter recurrence-free survival and overall survival compared to those without MOR expressions [15]. Studies have reported that MOR expression levels are fourfold higher in prostate cancer tissues and five- to ten-fold higher in NSCLC tissues than in healthy tissues [16,17]. MOR-enriched NSCLC cells showed a 2.5-fold increase in tumor volume and a 20-fold greater metastatic growth in nude mice compared to vector control cells [18]. In breast cancer, compared to the propofol-paravertebral anesthetic technique, the use of general anesthesia combined with opioid analgesia has been associated with raised MOR expression in resected tumor [19]. Clinical studies showed mixed results regarding the impact of opioid exposure on cancer outcomes: some found a correlation between increased opioid use and reduced overall survival (OS) and progression-free survival (PFS), whereas others reported no significant effect [4].
MORs can activate signaling pathways in cancer cells. Activation of MORs promotes crosstalk with the epidermal growth factor receptor (EGFR), leading to increased proliferation in vitro via the phosphorylation of MAPK, ERK, and AKT [20]. MOR agonists also promoted the epithelial-mesenchymal transition (EMT) of bladder cancer cells by activating the MOR/PI3K/AKT/Slug signaling pathway [21]. One research demonstrated that the interaction between MORs and EGFR recruits GAB1, a scaffolding protein, and Src, a tyrosine kinase. Src’s phosphorylation and activation enable GAB1 to serve as a platform for various downstream signaling molecules, including phosphatidylinositol 3-kinase (PI3K) [22]. When MORs are inhibited by an antagonist, the GAB1-Src complex cannot activate PI3K by phosphorylation. This inhibition affects the phosphorylation of PI3K, which in turn regulates AKT and STAT3, proteins crucial for proliferation, migration, and EMT [22]. Opioid receptor levels increase during angiogenesis, decrease vascular endothelial growth factor (VEGF) production, and alter cell-to-cell adhesion upon morphine administration [4]. Overexpression of opioid receptors leads to poor prognosis and a higher incidence of tumors.
Opioids suppress immunity through MORs and interact with tumor microenvironment cells, thereby affecting cancer progression. In vivo, opioid alkaloid (morphine, diamorphine) experiments and in vitro cell culture with these drugs revealed immunosuppressive effects. The specificity of this immunosuppression to MORs has been confirmed using pharmacological antagonists and studies with MOR-deficient mice [23]. Stromal and immune cells within the TME, including macrophages, neutrophils, and lymphocytes, also express of opioid receptors [13]. For instance, Toll-like receptor 4 (TLR4), a key innate immune receptor, enhances cancer invasion and fosters inflammation yet aids in cancer cell elimination post-treatment. Opioids weakly activate TLR4 and modulate its activation by natural ligands, complicating their net effects on cancer progression [24]. Naltrexone, an opioid antagonist, may reduce tumor growth at low doses by interfering with cell signaling and modifying the immune system [25].
How opioids could contribute to cancer metastasis
The role of opioid and mu-opioid receptors in proliferation
Undoubtedly, the fundamental characteristics of cancer cells are their persistence and long-term proliferation ability [26]. For visible tumors to form, cancer cells must have the potential for unlimited proliferation [26]. The increased expression of opioid receptors leads to the hypothesis that these cancers may also take advantage of opioid-induced proliferative signaling [27]. In hepatocellular carcinoma, cell lines overexpressing MORs show enhanced cell growth and metastasis, similar to the effects observed in morphine-treated nontransfected control cells, whereas downregulation of MORs using siRNA or a MOR antagonist suppresses cell proliferation, migration, and invasion [28]. The MORs in breast cancer are indirectly related to Ki-67 in nodal metastasis [29], and MORs antagonists inhibit breast cancer proliferation [30]. Morphine has been shown to increase cisplatin resistance by increasing the Bcl-2/Bax ratio and decreasing caspase-3 activity in nasopharyngeal cancer [31]. Silencing MORs significantly suppress cell proliferation in colorectal cancer (CRC) cell lines [32]. Fentanyl activates ovarian cancer by stimulating EGFR signaling pathways in an opioid mu-receptor-dependent manner [33].
Other studies have reported contrasting effects of distinct opioids. For instance, butorphanol, a partial agonist of the κ-opioid receptor, exerts its inhibitory effect on the proliferation and migration of osteosarcoma cells by enhancing the expression of the piRNA hsa_piR_006613 [34]. Sufentanil inhibits the proliferation, migration, invasion, and EMT of lung cancer cells by regulating the Wnt/β-catenin signaling pathway [35]. Additionally, fentanyl administration decreased the number of cancer stem cells in pancreatic cancer cells, reduced the expression of stem cell markers and increased the expression of apoptosis-related genes [36].
Opioids affect cancer cell EMT
MORs mediate EMT via the PI3K/AKT signaling pathway, whereas silencing MORs significantly suppress EMT, as well as reduce cell proliferation, migration, and invasion [32]. Opioid treatment resulted in the downregulation of E-cadherin and increased expression of EMT markers in breast cancer [37]. Fentanyl upregulated FUT8 expression, which increased α1,6-fucosylation levels through activation of the Wnt/β-catenin signaling pathway and induced stemness and EMT in breast cancer cells [38]. MORs overexpression in human lung cancer cells increases levels of snail, slug and vimentin while decreasing levels of ZO-1 and claudin-1, which are consistent with an EMT phenotype [22]. MORs overexpression in hepatocellular carcinoma cell lines enhance proliferation, migration, and invasion [39]. In addition, morphine increased the expression of RhoA, activated the AMP-activated protein kinase (AMPK) pathway, and induced EMT by upregulating Snail and Slug levels in esophageal carcinoma cells [40]. The low-dose MORs antagonist naltrexone suppressed migration, invasion, proliferation, and promoted apoptosis in HeLa cells in vivo by reducing the number of tumor-associated macrophages [41]. Fentanyl concentrations of 50 and 250 nM significantly increased the migration of NSCLC cell lines [42].
The impact of opioids on cancer cell migration and invasion remains controversial and debatable. Naltrexone, a MOR antagonist, reduces the expression of epithelial markers and increases the expression of mesenchymal markers and EMT-inducing transcription factors, leading to a shift in the morphological phenotype of bladder cancer cells towards a mesenchymal phenotype [43]. In contrast, the combination of sufentanil and parecoxib sodium inhibited the progression of HER2-positive breast cancer cells by affecting cell proliferation, the migration, invasion, cell cycle, and angiogenesis while also regulating EMT [44]. Additionally, sufentanil inhibits EMT in esophageal and breast cancers by modulating the NF-κB and Snail signaling pathways [45,46].
Angiogenesis
Angiogenesis is regulated by various factors, and the role of MORs on tumor angiogenesis is being increasingly recognized. Morphine, fentanyl, and oxycodone exhibit dose-dependent enhancement of endothelial cell tube formation and proliferation, with morphine specifically stimulating angiogenesis through the activation of MAPK pathways [47]. Morphine has been employed as a preferred opioid for eliciting opioid receptor-independent angiogenesis, involving the activation of VEGF receptors (VEGFRs), ultimately facilitating the process of blood vessel formation [48]. Upregulation of MORs produces nitric oxide (NO) by enhancing calcium concentrations within vascular endothelial cells [49]. NO contributes to endothelial cell proliferation, vascular permeability, migration, and protease release [50]. Moreover, it has been demonstrated that morphine stimulates c-Src-dependent VEGFR transactivation in endothelial cells in an opioid receptor-independent manner, promoting cell proliferation and migration [51]. Morphine can inhibit TSP-1 secretion, thereby promoting tumor angiogenesis and metastasis through the PI3K/Akt/c-Myc pathway [52]. In a breast cancer xenograft mouse model, long-term subcutaneous morphine injections enhanced neo angiogenesis [53] and increased tumor angiogenesis through cytokine release and mast cell activation [54]. Fentanyl also stimulates tumor angiogenesis through activation of the early stages of vascular network assembly in human lung tumor-associated endothelial cells [42].
In some reports, opioids have been shown to inhibit angiogenesis. For instance, butorphanol, a synthetic opioid, exerts antiangiogenic and antimetastatic effects on hepatocellular carcinoma and induces the inactivation of MAPK signaling [55]. After chronic systemic application of morphine, the vascularization of Matrigel plugs containing lipopolysaccharide or the angiogenic factors VEGF and FGF is impaired [56]. Morphine inhibits tumor angiogenesis through the HIF-1α-p38-MAPK pathway [57]. The KOR also impedes angiogenesis by suppressing VEGF expression in endothelial cells, thereby delaying tumor-associated blood vessel growth [58]. In melanoma and lung tumor mouse models, κ-receptor knockout mice exhibited increased angiogenesis compared to their wild-type counterparts [59]. In breast cancer, KOR may function as a tumor suppressor by inhibiting angiogenesis [60]. However, the mechanisms underlying KOR’s inhibition of angiogenesis remain to be fully examined, and further research is necessary.
Opioids affect CTCs
CTCs are released from primary and/or metastatic tumors into the bloodstream, with the ultimate goal of seeding metastases at distant sites, and they serve as crucial components in determining cancer prognosis [9]. Despite their importance, research on the impact of opioid therapy on CTCs is limited. Studies have demonstrated that CTCs are independently associated with increased tumor metastasis and reduced OS [61]. Moreover, mu-opioid receptor agonists promoted bladder cancer metastasis by facilitating CTC formation both in vitro and in vivo. This effect is at least partly due to the activation of the MOR/PI3K/AKT/Slug signaling pathway [21].
Opioids affect the tumor microenvironment
The TME encompasses the intricate microenvironment surrounding tumor cells, comprising neighboring blood vessels, immune cells, fibroblasts, bone marrow-derived inflammatory cells, an array of signaling molecules, and the extracellular matrix (ECM) [62]. The TME plays a critical role in promoting tumor cell proliferation and angiogenesis, inhibiting apoptosis and immune system suppression, and contributing to drug resistance [62]. Opioids have consistently been shown to exhibit immunosuppressive effects, compromising both innate immune responses (including neutrophil and macrophage phagocytosis, natural killer (NK) cell cytotoxic activity, chemotaxis, as well as the production of cytokines and chemokines) and adaptive immune responses (including T and B cell proliferative responses to mitogens, cytokine production, modulation of regulatory T cells, and the formation and secretion of antibodies) [63].
MORs are expressed in various immune cells [64]. Opioids predominantly exert immunosuppressive effects on immune cells within the TME [65], including NK cell activity [66], T and B-cell responses to mitogens [67], neutrophil and macrophage phagocytosis, and cytokine expression, all of which contribute to accelerated tumor progression [65]. Morphine can activate the D1 dopamine receptor and stimulate neuropeptide Y secretion, thereby inhibiting splenic NK cell cytotoxicity by binding to peripheral Y1 receptors [68]. Additionally, the proliferation of T lymphocytes was inhibited in mice following 48 to 72 hours of morphine pellet implantation, indicating morphine’s suppressive effect on T lymphocyte function [68]. Morphine also decreases B-cell proliferation through the actions of IgM and interleukin-4 (IL-4) [69], and increases the expression of the inhibitory checkpoint protein PD-L1 in non-small cell lung cancer, mediated via TLR4, thus promoting tumor immune escape [63]. Furthermore, opioids can alter or reduce immune cell infiltration into the TME [65].
Activated inflammatory cells release multiple inflammatory mediators and molecules, such as TNF-α, IL-2, and IL-6, which alter the TME, making it more conducive to malignant tumor progression [70]. Morphine promotes tumor progression by inhibiting the release of IL-4 and macrophage activity in mice [71]. Opioids also stimulate mast cell activation, resulting in the release of inflammatory cytokines, neuropeptides such as substance P (SP), and tryptase [72]. Mast cells play a role in modulating the TME and facilitating metastasis via c-Kit [73]. NF-κB is the key regulator of cancer-associated inflammation in both nonmalignant cells (e.g., tumor-associated macrophages) and cancer cells, and the NF-κB pathway can be sustainably activated by opioids, accelerating the transformation of normal cells into tumor cells [74]. In the clinic, high-dose intraoperative opioid administration has been associated with an increased neutrophil-to-lymphocyte ratio and a decreased lymphocyte-to-monocyte ratio, which are inflammatory biomarkers, in postoperative patients with glioma [75]. In patients who underwent gastric cancer surgery, IL-6, IL-10, and sIL-2R levels increased 24 h post-surgery in the morphine patient-controlled intravenous analgesia group compared to those receiving tramadol alone or tramadol combined with lornoxicam [76].
However, there are distinct opinions regarding this matter. Long-term administration or high doses of morphine may inhibit malignant tumor progression through the cAMP-PKA-NF-κB cascade [77]. For instance, morphine was shown to reduce IL-1α and IL-6 in oral epithelial cells after ionizing radiation, providing protective and anti-inflammatory effects on damaged cells [78]. Additionally, intraperitoneal injection of morphine in mice resulted in decreased levels of matrix metalloproteinase 9 (MMP-9) and inhibited TIMP-1, TIMP-3 and TIMP-4, reduced the invasion and chemotactic potential of endothelial cells [79]. MMPs play a role in matrix degradation, angiogenesis, embryogenesis, wound healing, and tumor progression by promoting tissue remodeling [80].
Clinical
Numerous studies have demonstrated a link between MORs expression and tumor progression. Since these initial studies, many clinical studies have evaluated the effect of opioids on the prognosis of patients with cancer. A pan-cancer genomic analysis including 7274 patients indicates that MORs mRNA overexpression is associated with poor prognosis and poor response to PD-L1 therapy [81]. Moreover, various patient characteristics, such as disease type, opioid agonist type, agonist efficacy, cancer stage, analgesia duration, and disease severity, strongly influence clinical results, as shown in Table 1.
Table 1.
Clinical research about MOR, opioids, and outcome
| Authors | Year | Organ(s) | Patient Sample(s) | Methods | Conclusion |
|---|---|---|---|---|---|
| X Wang, et al. (PMID: 36739595) | 2023 | Bladder | 44 | Two groups: a GA group or a combined GA+E group. GA group received regular GA, which utilized sufentanil and remifentanil for pain relief during surgery and sufentanil for postoperative pain control. GA+E group received a combined epidural-GA, where both intra- and postoperative pain relief was provided by epidural ropivacaine. | On the 3rd day post-surgery, CTCs numbers were significantly lower in the GA+E group than in the GA group, and this effect was still observed at the 1 month after surgery time point. |
| Lili Yu, et al. (PMID: 36585623) | 2022 | Breast | 526 | Patients were recruited and randomized into general anesthesia group and general anesthesia with Pectoral nerve block type II block group. | Pectoral nerve block type II declined the remifentanil consumption and had no significant influence on the OS and RFS of breast cancer patients compared with the general anesthesia group (more remifentanil). |
| Steele GL, et al. (PMID: 32482952) | 2020 | Pancreas | 103 | Patients with metastatic pancreatic adenocarcinoma received chemotherapy, in addition to pain, opioid exposure, survival, and imaging response. Mu-opioid receptor expression was evaluated using an immunohistochemistry assay. | High opioid use is associated with decreased survival, but the severity of baseline pain and mu-opioid receptor expression score in tumor tissue does not correlate with clinical outcomes. |
| H Zhang, et al. (PMID: 32900505) | 2020 | Laryngeal | 207 | Retrospective study. The expression pattern of the MOR protein and OPRM1 gene in tumors and corresponding adjacent non-carcinoma specimens was measured. Propensity score matching was used to minimise bias. The primary endpoints were OS and DFS. | Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. |
| Boudreau DM, et al. (PMID: 30945381) | 2019 | Breast | 4216 | Cohort study of women ≥18 years, diagnosed with early-stage breast cancer. Chronic opioid use was defined as 75+ days of use in any moving 90-day window after breast cancer diagnosis and varied to 150+ days in a 180-day window in a sensitivity analysis. | Breast cancer survivors who consistently utilize opioids for a prolonged duration are at a higher risk of experiencing a recurrence or development of a second breast cancer event. |
| Hasegawa T, et al. (PMID: 29893612) | 2018 | Lung | 150 | OS, opioid requirements, opioid doses, pain levels, and prognostic factors of advanced NSCLC were evaluated. | The opioid dose does not shorten the survival of patients with advanced NSCLC. The opioid requirement is associated with shorter survival when opioids are administered any time during the clinical course, independent of the influence of other key factors. |
| Kim N Du, et al. (PMID: 29757780) | 2018 | Esophageal | 1153 | Records of patients who had undergone esophageal cancer surgery were reviewed. Comparisons were made between patients who received high versus low intraoperative doses of opioids. | The amounts of intraoperative opioids used are associated with recurrence and OS in patients with esophageal squamous cell carcinoma. The association between the dose of intraoperative opioids used and RFS was marginally significant in patients with adenocarcinoma. |
| Akbari M, et al. (PMID: 26317596) | 2015 | Bladder | 198 | Patients and healthy individuals (matched in age, sex and residence (urban/rural)) were investigated. Data about the consumption of opium and its derivatives, tobacco, alcohol and diet were collected using a structured valid and reliable questionnaire. | Opium consumption was associated with an increased risk of bladder cancer. |
| Cronin-Fenton DP, et al. (PMID: 26207518) | 2015 | Breast | 34188 | Opioid prescriptions were ascertained from the authority. Follow-up began on the date of primary surgery for breast cancer and continued until breast cancer recurrence, death, emigration, 10 years, or July 31, 2013, whichever occurred first. | No clinically relevant evidence of an association between opioid prescriptions and breast cancer recurrence. |
| Cata JP, et al. (PMID: 26371714) | 2015 | Laryngeal | 195 | Patients with laryngeal primary or recurrent laryngeal squamous cell carcinoma who had surgery were included. Intravenous opioids and other factors were applied to assess the effects of covariates of interest on OS and RFS. | The association between intraoperative opioid use and cancer recurrence after laryngeal squamous cell carcinoma surgery was weak. |
| Minami S, et al. (PMID: 25564223) | 2015 | Lung | 369 | Compared survival times, calculated from the time of the last hospitalization or the last chemotherapy, between patients who had not used any opioids, those who had used a low dose (<60 mg/day), and those who had used a higher dose (≥60 mg/day). | Opioid use had no significant adverse effect on survival in patients. |
| Forget P, et al. (PMID: 21946823) | 2011 | Prostate | 1111 | Retrospective study includes 1111 consecutive retropubic radical prostatectomies (RRPs) for localized prostate cancer. Median follow-up was 38 months (interquartile range 16-69). | Intraoperative sufentanil administration is associated with an increased risk of cancer relapse, whereas epidural analgesia, with local anesthetic and opioid, was not associated with a significant effect. |
GA: general anesthesia. GA+E: general-epidural anesthesia. CTCs: circulating tumor cells. OS: overall survival. RFS: recurrence free survival. DFS: disease-free survival. NSCLC: non small-cell lung cancer.
In a clinical trial involving patients with bladder cancer patients conducted in June 2020, researchers compared the total epithelial CTCs in two groups: one receiving combined general-epidural anesthesia (GA+E) with only a minimal dose of MORs agonists during anesthesia induction and the other receiving general anesthesia (GA) group. These findings indicated that on the 3rd day after surgery, the GA+E group had significantly fewer CTCs compared to the GA group, and this difference persisted one month after surgery [21]. The decline in CTC numbers at various time points, immediately after surgery, on the 3rd day post-surgery, and 1 month post-surgery, was notably more pronounced in the GA+E group than in the GA group in most cases [21]. This research indicated that perioperative opioid administration was found to stimulate the formation of CTCs in patients with bladder cancer undergoing surgical procedures.
Other articles have provided substantial evidence of the tumor-promoting effect of opioids on cancer biology. MORs have been identified as independent predictors of poor disease-free survival and OS in patients with laryngeal squamous cell carcinoma [28]. A retrospective analysis of patients with advanced prostate cancer showed increased MOR expression, with chronic systemic opioid treatment linked to poor progression-free survival and OS [17]. In another study used 180 paraffin-embedded samples of paired tumors and normal tissues from CRC patients to explore the expression levels of MORs by immunohistochemistry (IHC) and revealed that MORs exhibit significantly higher expression in tumors compared with paired normal tissues. MOR expression level has been associated with tumor differentiation and regional lymph node metastasis [32]. A significant difference was found in OS between patients with low- and high-MORs expression, especially in patients with TNM stage III or IV CRC [32]. In addition, chronic opioid use may increase the risk of secondary breast cancer events in patients with early-stage breast cancer [82].
However, other studies have reported contradictory finding. Patients who underwent esophageal gastrectomy showed improved survival and decreased recurrence time with opioid analgesia for gastroesophageal cancer [83]. Similarly, acute morphine use during surgery significantly reduces cancer recurrence in patients with breast cancer [84]. A systematic review of the literature revealed no conclusive evidence suggesting that avoiding opioids would minimize the risk of recurrence in patients with colorectal cancer [85].
Conclusion and future directions
Although preclinical studies are crucial for modeling complex biological systems and understanding in vivo tumor pathology and further researches are needed to elucidate the effect of opioids and MORs on metastasis. Opioids remain a primary treatment for cancer-related pain and are commonly used during cancer surgery. However, their potential tumor-promoting effects cannot be overlooked. Additional research is needed to clarify the indirect and direct mechanisms through which opioids and MORs influence oncologic metastasis. Clinical trials are essential to assess the effectiveness of targeted MORs therapies in cancer treatment, with the aim of lowering morbidity and mortality rates as well as enhancing overall quality of life.
Acknowledgements
Thanks for Yuliang Ran of Chinese Academy of Medical Sciences and Peking Union Medical College for oncology knowledge. This study was supported by the Star of Anticancer Project, Prof. Hui Zheng, Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 801032241), the National Natural Science Foundation of China, Prof. Gongming Wang, Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University (No. NSFC82171259), the Wu Jie-Ping Medical Foundation, Prof. Gongming Wang, Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University (No. 320.6250.2023-08-4) and the Natural Science Foundation of Shandong Province, Prof. Bomin Wang, Department of Orthopaedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University (No. ZR2021MH301).
Disclosure of conflict of interest
None.
References
- 1.Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943–e955. doi: 10.1016/S2468-2667(23)00211-6. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MI, Kaasa S, Barke A, Korwisi B, Rief W, Treede RD IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain. 2019;160:38–44. doi: 10.1097/j.pain.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 3.Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123:e273–e283. doi: 10.1016/j.bja.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhoir S, Uhelski M, Guerra-Londono JJ, Cata JP. The role of opioid receptors in cancer. Adv Biol (Weinh) 2023;7:e2300102. doi: 10.1002/adbi.202300102. [DOI] [PubMed] [Google Scholar]
- 5.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patino MA, Ramirez RE, Perez CA, Feng L, Kataria P, Myers J, Cata JP. The impact of intraoperative opioid use on survival after oral cancer surgery. Oral Oncol. 2017;74:1–7. doi: 10.1016/j.oraloncology.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Gerstberger S, Jiang Q, Ganesh K. Metastasis. Cell. 2023;186:1564–1579. doi: 10.1016/j.cell.2023.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ring A, Nguyen-Sträuli BD, Wicki A, Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2023;23:95–111. doi: 10.1038/s41568-022-00536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook K, Bennett J, Desai SP. The chemical history of morphine: an 8000-year journey, from resin to de-novo synthesis. J Anesth Hist. 2017;3:50–55. doi: 10.1016/j.janh.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Herman TF, Cascella M, Muzio MR. Mu receptors. StatPearls. Treasure Island (FL) ineligible companies; Disclosure: Marco Cascella declares no relevant financial relationships with ineligible companies. Disclosure: Maria Rosaria Muzio declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024. [Google Scholar]
- 12.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carli M, Donnini S, Pellegrini C, Coppi E, Bocci G. Opioid receptors beyond pain control: the role in cancer pathology and the debated importance of their pharmacological modulation. Pharmacol Res. 2020;159:104938. doi: 10.1016/j.phrs.2020.104938. [DOI] [PubMed] [Google Scholar]
- 14.Lahav Y, Cohen O, Huszar M, Levy I, Cata JP, Halperin D, Shoffel-Havakuk H. Mu-opioid receptor expression in laryngeal cancer. J Voice. 2023;37:433–439. doi: 10.1016/j.jvoice.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Díaz-Cambronero O, Mazzinari G, Giner F, Belltall A, Ruiz-Boluda L, Marqués-Marí A, Sánchez-Guillén L, Eroles P, Cata JP, Argente-Navarro MP. Mu opioid receptor 1 (MOR-1) expression in colorectal cancer and oncological long-term outcomes: a five-year retrospective longitudinal cohort study. Cancers (Basel) 2020;12:134. doi: 10.3390/cancers12010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, Salgia R, Moss J, Singleton PA. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–567. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K, Gupta P. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857–867. doi: 10.1097/ALN.0b013e31824babe2. [DOI] [PubMed] [Google Scholar]
- 19.Levins KJ, Prendeville S, Conlon S, Buggy DJ. The effect of anesthetic technique on μ-opioid receptor expression and immune cell infiltration in breast cancer. J Anesth. 2018;32:792–796. doi: 10.1007/s00540-018-2554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujioka N, Nguyen J, Chen C, Li Y, Pasrija T, Niehans G, Johnson KN, Gupta V, Kratzke RA, Gupta K. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg. 2011;113:1353–1364. doi: 10.1213/ANE.0b013e318232b35a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Zhang S, Jin D, Luo J, Shi Y, Zhang Y, Wu L, Song Y, Su D, Pan Z, Chen H, Cao M, Yang C, Yu W, Tian J. μ-opioid receptor agonist facilitates circulating tumor cell formation in bladder cancer via the MOR/AKT/Slug pathway: a comprehensive study including randomized controlled trial. Cancer Commun (Lond) 2023;43:365–386. doi: 10.1002/cac2.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, Moss J, Singleton PA. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS One. 2014;9:e91577. doi: 10.1371/journal.pone.0091577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019;10:2904. doi: 10.3389/fimmu.2019.02904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scroope CA, Singleton Z, Hollmann MW, Parat MO. Opioid receptor-mediated and non-opioid receptor-mediated roles of opioids in tumour growth and metastasis. Front Oncol. 2021;11:792290. doi: 10.3389/fonc.2021.792290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couto RD, Fernandes BJD. Low doses naltrexone: the potential benefit effects for its use in patients with cancer. Curr Drug Res Rev. 2021;13:86–89. doi: 10.2174/2589977513666210127094222. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Sun M, Zhou D, Gorur A, Sun Z, Zeng W, Cata JP, Chen W, Miao C. Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. Br J Anaesth. 2020;125:722–729. doi: 10.1016/j.bja.2020.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Chen DT, Pan JH, Chen YH, Xing W, Yan Y, Yuan YF, Zeng WA. The mu-opioid receptor is a molecular marker for poor prognosis in hepatocellular carcinoma and represents a potential therapeutic target. Br J Anaesth. 2019;122:e157–e167. doi: 10.1016/j.bja.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 29.De Sousa AM, Dantas TS, Barros Silva PG, Martins CDS, Freire GE, Junior HLR, Brito GAC, Pereira KMA, Leitão RFC. Analysis of the immunoexpression of opioid receptors and their correlation with markers of angiogenesis, cell proliferation and apoptosis in breast cancer. Asian Pac J Cancer Prev. 2021;22:633–640. doi: 10.31557/APJCP.2021.22.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronin-Fenton D. Opioids and breast cancer recurrence. Curr Opin Support Palliat Care. 2019;13:88–93. doi: 10.1097/SPC.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 31.Cao LH, Li HT, Lin WQ, Tan HY, Xie L, Zhong ZJ, Zhou JH. Morphine, a potential antagonist of cisplatin cytotoxicity, inhibits cisplatin-induced apoptosis and suppression of tumor growth in nasopharyngeal carcinoma xenografts. Sci Rep. 2016;6:18706. doi: 10.1038/srep18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, Yang L, He Y, Liu Y, Xu P, Zhang J, Dai S, Luo X, Sun Z. MOR promotes epithelial-mesenchymal transition and proliferation via PI3K/AKT signaling pathway in human colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2022;55:72–80. doi: 10.3724/abbs.2022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao K, Zheng Q, Bao L. Fentanyl activates ovarian cancer and alleviates chemotherapy-induced toxicity via opioid receptor-dependent activation of EGFR. BMC Anesthesiol. 2022;22:268. doi: 10.1186/s12871-022-01812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui P, Xin D, Li F, Deng L, Gao Y. Butorphanol suppresses the proliferation and migration of osteosarcoma by promoting the expression of piRNA hsa_piR_006613. Front Oncol. 2022;12:775132. doi: 10.3389/fonc.2022.775132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan M, Huang Y, Lin X. Sufentanil inhibits the proliferation and epithelial mesenchymal transition of lung cancer cells through Wnt/beta-catenin signaling pathway. Bioengineered. 2022;13:10857–10865. doi: 10.1080/21655979.2022.2066045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celik F, Duran T. Effects of fentanyl on pancreatic cancer cell proliferation and cancer stem cell differentiation. Cell Mol Biol (Noisy-le-grand) 2019;65:21–25. [PubMed] [Google Scholar]
- 37.Tripolt S, Neubauer HA, Knab VM, Elmer DP, Aberger F, Moriggl R, Fux DA. Opioids drive breast cancer metastasis through the δ-opioid receptor and oncogenic STAT3. Neoplasia. 2021;23:270–279. doi: 10.1016/j.neo.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang HF, Yu M, Jin HD, Yao JQ, Lu ZL, Yabasin IB, Yan Q, Wen QP. Fentanyl promotes breast cancer cell stemness and epithelial-mesenchymal transition by upregulating α1, 6-fucosylation via Wnt/β-catenin signaling pathway. Front Physiol. 2017;8:510. doi: 10.3389/fphys.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin ZZ, Bo N, Fan YC, Wu YT, Yao HL, Chen S, Yu HF, Jiang LH. Xanthomicrol suppresses human hepatocellular carcinoma cells migration and invasion ability via Μu-opioid receptor. J Pharm Pharmacol. 2022;74:139–146. doi: 10.1093/jpp/rgab104. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Yao N, Tian S. Morphine stimulates migration and growth and alleviates the effects of chemo drugs via AMPK-dependent induction of epithelial-mesenchymal transition in esophageal carcinoma cells. Biol Pharm Bull. 2020;43:774–781. doi: 10.1248/bpb.b19-00779. [DOI] [PubMed] [Google Scholar]
- 41.Liu N, Ma M, Qu N, Wang R, Chen H, Hu F, Gao S, Shan F. Low-dose naltrexone inhibits the epithelial-mesenchymal transition of cervical cancer cells in vitro and effects indirectly on tumor-associated macrophages in vivo. Int Immunopharmacol. 2020;86:106718. doi: 10.1016/j.intimp.2020.106718. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Chen Y, Xu W, Wang W, Tang L, Xia R, Zhu Q. Fentanyl stimulates tumor angiogenesis via activating multiple pro-angiogenic signaling pathways. Biochem Biophys Res Commun. 2020;532:225–230. doi: 10.1016/j.bbrc.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Zhang R, Wu T, Shi Y, Zhou X, Tang D, Yu W, So EC, Wu X, Pan Z, Tian J. Successive treatment with naltrexone induces epithelial-mesenchymal transition and facilitates the malignant biological behaviors of bladder cancer cells. Acta Biochim Biophys Sin (Shanghai) 2021;53:238–248. doi: 10.1093/abbs/gmaa169. [DOI] [PubMed] [Google Scholar]
- 44.Xu S, Li X, Li W, Ma N, Ma H, Cui J, You X, Chen X. Sufentanil combined with parecoxib sodium inhibits proliferation and metastasis of HER2-positive breast cancer cells and regulates epithelial-mesenchymal transition. Clin Exp Metastasis. 2023;40:149–160. doi: 10.1007/s10585-023-10199-6. [DOI] [PubMed] [Google Scholar]
- 45.Tang H, Li C, Wang Y, Deng L. Sufentanil inhibits the proliferation and metastasis of esophageal cancer by inhibiting the NF-κB and snail signaling pathways. J Oncol. 2021;2021:7586100. doi: 10.1155/2021/7586100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Gu K, Kong Q, Wang G, Gu J. Sufentanil inhibits the metastasis and immune response of breast cancer via mediating the NF-κB pathway. Immunopharmacol Immunotoxicol. 2023;45:663–671. doi: 10.1080/08923973.2023.2228476. [DOI] [PubMed] [Google Scholar]
- 47.Feng T, Zeng S, Ding J, Chen G, Wang B, Wang D, Li X, Wang K. Comparative analysis of the effects of opioids in angiogenesis. BMC Anesthesiol. 2021;21:257. doi: 10.1186/s12871-021-01475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 49.Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256:83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Ziche M, Morbidelli L. Nitric oxide and angiogenesis. J Neurooncol. 2000;50:139–148. doi: 10.1023/a:1006431309841. [DOI] [PubMed] [Google Scholar]
- 51.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Cheng S, Fu G, Ji F, Wang C, Cao M. Postoperative administration of ketorolac averts morphine-induced angiogenesis and metastasis in triple-negative breast cancer. Life Sci. 2020;251:117604. doi: 10.1016/j.lfs.2020.117604. [DOI] [PubMed] [Google Scholar]
- 53.Bimonte S, Barbieri A, Rea D, Palma G, Luciano A, Cuomo A, Arra C, Izzo F. Morphine promotes tumor angiogenesis and increases breast cancer progression. Biomed Res Int. 2015;2015:161508. doi: 10.1155/2015/161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen J, Luk K, Vang D, Soto W, Vincent L, Robiner S, Saavedra R, Li Y, Gupta P, Gupta K. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113(Suppl 1):i4–13. doi: 10.1093/bja/aeu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo P, Hu Q, Wang J, Hai L, Nie X, Zhao Q. Butorphanol inhibits angiogenesis and migration of hepatocellular carcinoma and regulates MAPK pathway. J Antibiot (Tokyo) 2022;75:626–634. doi: 10.1038/s41429-022-00565-z. [DOI] [PubMed] [Google Scholar]
- 56.Martin JL, Charboneau R, Barke RA, Roy S. Chronic morphine treatment inhibits LPS-induced angiogenesis: implications in wound healing. Cell Immunol. 2010;265:139–145. doi: 10.1016/j.cellimm.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, Ramkrishnan S, Roy S. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol. 2014;184:1073–1084. doi: 10.1016/j.ajpath.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamizu K, Hamada Y, Narita M. κ opioid receptor ligands regulate angiogenesis in development and in tumours. Br J Pharmacol. 2015;172:268–276. doi: 10.1111/bph.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamizu K, Furuta S, Hamada Y, Yamashita A, Kuzumaki N, Narita M, Doi K, Katayama S, Nagase H, Yamashita JK, Narita M. κ opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Sci Rep. 2013;3:3213. doi: 10.1038/srep03213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Y, Luo J, Tian J, Zou Q, Wang X. The kappa opioid receptor may be a potential tumor suppressor by regulating angiogenesis in breast cancer. Med Hypotheses. 2021;150:110568. doi: 10.1016/j.mehy.2021.110568. [DOI] [PubMed] [Google Scholar]
- 61.Yang L, Yan X, Chen J, Zhan Q, Hua Y, Xu S, Li Z, Wang Z, Dong Y, Zuo D, Xue M, Tang Y, Herschman HR, Lu S, Shi Q, Wei W. Hexokinase 2 discerns a novel circulating tumor cell population associated with poor prognosis in lung cancer patients. Proc Natl Acad Sci U S A. 2021;118:e2012228118. doi: 10.1073/pnas.2012228118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deepak KGK, Vempati R, Nagaraju GP, Dasari VR, S N, Rao DN, Malla RR. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153:104683. doi: 10.1016/j.phrs.2020.104683. [DOI] [PubMed] [Google Scholar]
- 63.Santoni A, Santoni M, Arcuri E. Chronic cancer pain: opioids within tumor microenvironment affect neuroinflammation, tumor and pain evolution. Cancers (Basel) 2022;14:2253. doi: 10.3390/cancers14092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gavériaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc Natl Acad Sci U S A. 1998;95:6326–6330. doi: 10.1073/pnas.95.11.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Zhou D, Gu J, Qu M, Guo K, Chen W, Miao C. Targeting the mu-opioid receptor for cancer treatment. Curr Oncol Rep. 2021;23:111. doi: 10.1007/s11912-021-01107-w. [DOI] [PubMed] [Google Scholar]
- 66.Shavit Y, Terman GW, Lewis JW, Zane CJ, Gale RP, Liebeskind JC. Effects of footshock stress and morphine on natural killer lymphocytes in rats: studies of tolerance and cross-tolerance. Brain Res. 1986;372:382–385. doi: 10.1016/0006-8993(86)91149-2. [DOI] [PubMed] [Google Scholar]
- 67.Bryant HU, Bernton EW, Holaday JW. Morphine pellet-induced immunomodulation in mice: temporal relationships. J Pharmacol Exp Ther. 1988;245:913–920. [PubMed] [Google Scholar]
- 68.Saurer TB, Ijames SG, Lysle DT. Evidence for the nucleus accumbens as a neural substrate of heroin-induced immune alterations. J Pharmacol Exp Ther. 2009;329:1040–1047. doi: 10.1124/jpet.108.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas PT, Bhargava HN, House RV. Immunomodulatory effects of in vitro exposure to morphine and its metabolites. Pharmacology. 1995;50:51–62. doi: 10.1159/000139266. [DOI] [PubMed] [Google Scholar]
- 70.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73:4965–4977. doi: 10.1158/0008-5472.CAN-13-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khabbazi S, Goumon Y, Parat MO. Morphine modulates interleukin-4- or breast cancer cell-induced pro-metastatic activation of macrophages. Sci Rep. 2015;5:11389. doi: 10.1038/srep11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das Roy L, Curry JM, Sahraei M, Besmer DM, Kidiyoor A, Gruber HE, Mukherjee P. Arthritis augments breast cancer metastasis: role of mast cells and SCF/c-Kit signaling. Breast Cancer Res. 2013;15:R32. doi: 10.1186/bcr3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pacifico F, Leonardi A. NF-kappaB in solid tumors. Biochem Pharmacol. 2006;72:1142–1152. doi: 10.1016/j.bcp.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 75.Liu X, Teng L, Dai J, Shao H, Chen R, Li H, Li J, Zou H. Effect of intraoperative opioid dose on perioperative neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in glioma. J Inflamm Res. 2024;17:2159–2167. doi: 10.2147/JIR.S451455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun HL, Dong YC, Wang CQ, Qian YN, Wang ZY. Effects of postoperative analgesia with the combination of tramadol and lornoxicam on serum inflammatory cytokines in patients with gastric cancer. Int J Clin Pharmacol Ther. 2014;52:1023–1029. doi: 10.5414/CP202190. [DOI] [PubMed] [Google Scholar]
- 77.Börner C, Höllt V, Kraus J. Mechanisms of the inhibition of nuclear factor-κB by morphine in neuronal cells. Mol Pharmacol. 2012;81:587–597. doi: 10.1124/mol.111.076620. [DOI] [PubMed] [Google Scholar]
- 78.Charbaji N, Rosenthal P, Schäfer-Korting M, Küchler S. Cytoprotective effects of opioids on irradiated oral epithelial cells. Wound Repair Regen. 2013;21:883–889. doi: 10.1111/wrr.12115. [DOI] [PubMed] [Google Scholar]
- 79.Xie N, Khabbazi S, Nassar ZD, Gregory K, Vithanage T, Anand-Apte B, Cabot PJ, Sturgess D, Shaw PN, Parat MO. Morphine alters the circulating proteolytic profile in mice: functional consequences on cellular migration and invasion. FASEB J. 2017;31:5208–5216. doi: 10.1096/fj.201700546R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khabbazi S, Hassanshahi M, Hassanshahi A, Peymanfar Y, Su YW, Xian CJ. Opioids and matrix metalloproteinases: the influence of morphine on MMP-9 production and cancer progression. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:123–133. doi: 10.1007/s00210-019-01613-6. [DOI] [PubMed] [Google Scholar]
- 81.Sun W, Zhuang S, Cheng M, Qiu Z. Mu opioid receptor mRNA overexpression predicts poor prognosis among 18 common solid cancers: a pan-cancer analysis. Front Oncol. 2023;13:1134744. doi: 10.3389/fonc.2023.1134744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boudreau DM, Chen L, Yu O, Bowles EJA, Chubak J. Risk of second breast cancer events with chronic opioid use in breast cancer survivors. Pharmacoepidemiol Drug Saf. 2019;28:740–753. doi: 10.1002/pds.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du KN, Feng L, Newhouse A, Mehta J, Lasala J, Mena GE, Hofstetter WL, Cata JP. Effects of intraoperative opioid use on recurrence-free and overall survival in patients with esophageal adenocarcinoma and squamous cell carcinoma. Anesth Analg. 2018;127:210–216. doi: 10.1213/ANE.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 84.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diaz-Cambronero O, Mazzinari G, Cata JP. Perioperative opioids and colorectal cancer recurrence: a systematic review of the literature. Pain Manag. 2018;8:353–361. doi: 10.2217/pmt-2018-0029. [DOI] [PubMed] [Google Scholar]