Abstract
Gallbladder cancer (GBC) is a malignancy with a bleak prognosis, and radical surgery remains the primary treatment option. However, the high postoperative recurrence rate and the lack of individualized risk assessment tools limit the effectiveness of current treatment strategies. This study aims to identify risk factors affecting the short-term disease-free survival (DFS) of GBC patients using machine learning methods and to build a prediction model. A retrospective analysis was conducted on the clinical data from 328 GBC patients treated at the First Affiliated Hospital of Huzhou University from 2008 to 2021. Patients were randomly divided into a training set (n=230) and a validation set (n=98). Clinical data, laboratory indexes, and follow-up data were collected. Univariate Cox regression analysis identified age, tumor T-staging, lymph node metastasis, differentiation degree, and CA199 level as prognostic factors affecting DFS (all P<0.05). A prediction model constructed using the LASSO regression achieved AUCs of 0.827 and 0.801 for predicting 1-year and 3-year DFS, respectively. Notably, the XGBoost regression model showed higher prediction accuracy with AUCs of 0.922 and 0.947, respectively. The Delong test confirmed that the XGBoost model had significantly higher AUC values compared to the LASSO model (all P<0.001). In the validation set, the XGBoost model demonstrated AUCs of 0.764 and 0.761 for predicting 1-year and 3-year DFS, respectively. Overall, the XGBoost regression model demonstrates high accuracy and clinical value in predicting short-term DFS in GBC patients after radical surgery, offering a valuable tool for personalized treatment.
Keywords: Gallbladder carcinoma, disease-free survival, machine learning, LASSO regression, XGBoost regression
Introduction
Gallbladder cancer (GBC), a gastrointestinal malignancy originating from the gallbladder mucous membrane, is highly occult and malignant, often metastasizing at an early stage [1]. The five-year survival rate for GBC is less than 5%, with radical surgery being the only potential cure [2]. In China, the incidence of GBC is approximately 1.00 to 1.30 per 100,000 people, making it the sixth most common gastrointestinal cancers and accounting for 0.4 to 3.8% of biliary tract diseases [3]. Globally, the incidence of GBC is higher in Asia and Latin America compared to other regions, with notable gender differences (higher incidence in females while higher mortality rate in males) [4]. Major risk factors for GBC include gallbladder stones, chronic cholecystitis, gallbladder polyps, and gallbladder-preserving cholecystolithotomy [5]. Calculi and chronic inflammation can cause long-term irritation of the mucosa, potentially leading to malignancy [6]. Although research into the molecular mechanisms of GBC is limited, the disease is believed to be associated with various genetic changes.
Early-stage GBC usually presents no obvious or specific symptoms, with some patients developing gallstones or cholecystitis symptoms, such as right upper quadrant pain and postprandial nausea and vomiting [7]. GBC diagnosis relies primarily on imaging, with ultrasound being the preferred option, while contrast-enhanced computed tomography (CT) is more valuable in evaluating tumor invasion and lymph node metastasis (LNM) [8]. Magnetic resonance imaging (MRI) has high accuracy in diagnosing early GBC, while positron emission tomography-CT (PET-CT), although highly accurate, is less commonly used in clinical practice due to cost. At present, the treatment options for GBC mainly include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy, with surgery being the only potential radical cure [9]. However, the postoperative recurrence rate of GBC is as high, ranging from 40-60% [10]. The specific reason for this high recurrence rate remains elusive, but some studies suggest it may be related to the biological behavior of tumors, individual differences in patients, and specific surgical procedures. Although some research has been conducted on the recurrence and long-term prognosis of GBC following radical surgery, the number of related studies is limited, and the results are inconsistent. An international multicenter study by Margonis et al. [11] found that approximately 35.0% of patients experienced postoperative recurrence, with a median recurrence-free survival (RFS) time of 9.5 months; and their analysis identified that T3 GBC, tumor remnants, and lymphovascular invasion were independent risk factors for postoperative recurrence. A retrospective study [12] involving 117 patients found that about 73.5% (86) of patients relapsed within two years of postoperative follow-up.
Despite these findings, existing studies have several limitations. Many of them rely on traditional statistical methods, which may not fully capture the complexity and interactions among multiple prognostic factors. Additionally, these studies often have small sample sizes, limiting the generalizability of their findings. Moreover, the predictive models used in previous research lack the precision and individualized approach required for effective risk assessment and personalized treatment planning.
To address these gaps, our study employed advanced machine learning techniques, specifically the least absolute shrinkage and selection operator (LASSO) regression and XGBoost regression, to identify characteristic factors affecting disease-free survival (DFS), and a highly accurate predictive model was constructed based on the idented risk factors. These findings provide more reliable tools for clinicians to develop targeted treatment plans and follow-up strategies, significantly improving patient outcomes and quality of life. Furthermore, the validation of our model with external datasets confirms its robustness and practical applicability, promoting the advancement of personalized healthcare in GBC treatment.
Methods and data
Clinical data
A retrospective analysis was conducted on GBC patients who received treatment at the First affiliated Hospital of Huzhou University from March 2008 to March 2021. This study was approved by the Medical Ethics Committee of the First affiliated Hospital of Huzhou University. Due to the retrospective nature of this study, informed consent was waived.
Inclusion and exclusion criterion
Inclusion criteria: (1) Confirmed primary GBC by postoperative pathological results; (2) Pathological TNM stage of the tumor: T1b to T3; (3) No preoperative chemotherapy or other related treatment; (4) Complete postoperative follow-up records, including at least one postoperative image examination data.
Exclusion criteria: (1) Patients diagnosed with secondary GBC; (2) Patients who did not receive surgical treatment or underwent only palliative surgery with positive surgical margins, or those who only received cholecystectomy; (3) Patients who had received neoadjuvant therapy or conversion therapy before surgery; (4) Patients with other malignancies or diseases (e.g., heart, lung, or cerebrovascular diseases) that seriously affected their survival, or those with active inflammation and abnormal coagulation function; (5) Patients who tool non-steroidal anti-inflammatory drugs or intravenous albumin after admission; (6) Patients who died within 30 days after surgery; (7) Patients who were lost to follow-up.
Sample information
According to the above inclusion and exclusion criteria, we identified 328 cases that met the requirements. Subsequently, the patients were divided into a training set (n=230) and a validation set (n=98) at a 7:3 ratio.
Clinical data acquisition
Patient-related information was collected through outpatient follow-up records, electronic pathology reports, and the hospital follow-up system. The baseline data included age, sex, body mass index (BMI), surgical approach, pathological type, tumor size, T-staging, lymph node metastasis (LNM), perineural invasion, vascular invasion, and differentiation degree. The laboratory indexes were collected, including carbohydrate antigen 199 (CA199), alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), and albumin (Alb).
XGBoost regression
We utilized the XGBoost algorithm to model the survival analysis data, using Cox regression as the objective function and evaluating the model performance through negative log-likelihood. To prevent over-fitting, we set the maximum tree depth to 6 and the learning rate to 0.01, applying random sampling of both samples and features. We evaluated the model’s performance through 10-fold cross-validation, with 400 iterations per tree, and used the early stopping strategy to halt the training after 50 rounds without improvement in performance. In addition, stratified sampling was implemented to maintain consistency in data distribution, and the training results were regularly output to monitor progress. These steps collectively ensured the generalization ability and prediction accuracy of the model [13].
LASSO regression
We used the LASSO method in the glmnet package to perform Cox regression analysis. The glmnet function was used to fit a Cox regression model, where survival time and survival status were considered as response variables, and covariate matrices as predictors. To determine the optimal regularization strength, we used the cv.glmnet function for cross-validation, which helps to select the optimal model complexity while preventing over-fitting. Cross-validation results provided two important lambda values: lambda_min that minimizes the cross-validation error, and lambda_1se which lies within the standard error range of 1, offering a more lenient regularization option. In this study, we selected lambda_min for further study [14].
DFS definition
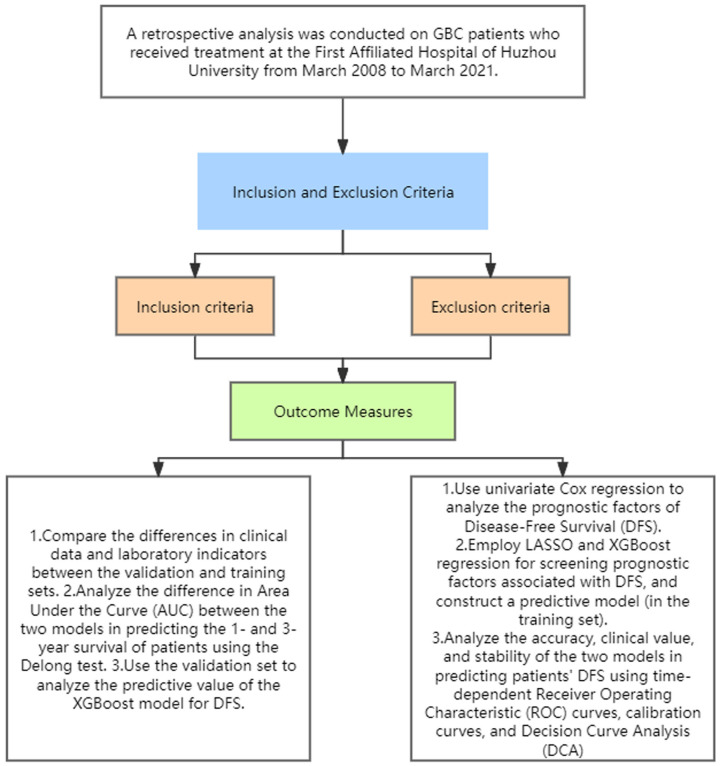
Disease-free survival (DFS) is defined as the period following primary treatment for gallbladder cancer (GBC) during which the patient remains free from any signs or symptoms of the cancer. For our study, DFS was determined using the following criteria: (1) No Recurrence of Gallbladder Cancer: DFS was determined by the absence of local, regional, or distant recurrence of gallbladder cancer. This was confirmed through follow-up imaging studies, such as ultrasound, contrast-enhanced computed tomography (CT), or magnetic resonance imaging (MRI). (2) No New Primary Cancer: Patients were monitored for the development of any new primary malignancies. The occurrence of a new, unrelated primary cancer was considered as an event impacting DFS (Figure 1).
Figure 1.
Study flowchart.
Outcome measures
Primary outcome measures
1. Univariate Cox regression was used to identify prognostic factors of DFS. 2. LASSO and XGBoost regression were employed for the screening of DFS-associated prognostic factors, and a prediction model (training set) was constructed. 3. Time-dependent receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA) were used to analyze the accuracy, clinical value, and stability of the two models in predicting patients’ DFS on the training set.
Secondary outcome measures
1. Differences in clinical data and laboratory indicators between the validation and training sets were compared. 2. The area under the curve (AUC) difference between the two models in predicting the 1- and 3-year survival of patients was analyzed by the Delong test. 3. The validation set was used to evaluate the predictive value of the XGBoost model in determining DFS.
Statistical analysis
SPSS 26.0 software was first used to pre-process the data and perform basic statistical analysis. Count data were expressed in rate (%) and analyzed using chi-square tests. For measurement data, the data distribution was first evaluated by the Kolmogorov-Smirnov test. Normally distributed data were expressed as mean ± standard deviation (Mean ± SD) and analyzed by independent samples t-test. Non-normally distributed data were represented by quartiles P50 (P25, P75). Further statistical analysis was carried out by R language (version 4.3.3). The R packages used for these analyses included rms (version 6.4.0), ResourceSelection (version 0.3-5), survival, survminer, openxlsx, Matrix, rms, XGBoost, rpart, data.table, ggplot2, and pROC. A P-value less than 0.05 indicated a statistically significant difference.
Results
Patient baseline data
By comparing patients’ baseline data, it was found that the two groups did not differ significantly in age, sex, BMI (kg/m2), operation mode, pathological type, tumor size, T-staging, LNM, perineural invasion, vascular invasion, or differentiation degree (P>0.05, Table 1).
Table 1.
Comparison of baseline data of patients between the two sets
| Training set (n=230) | Validation set (n=98) | χ2 | P | |
|---|---|---|---|---|
| Age (years) | ||||
| ≥65 | 133 | 51 | 0.934 | 0.334 |
| <65 | 97 | 47 | ||
| Sex | ||||
| Male | 53 | 24 | 0.080 | 0.777 |
| Female | 177 | 74 | ||
| BMI (kg/m2) | ||||
| ≥23 | 179 | 80 | 0.599 | 0.439 |
| <23 | 51 | 18 | ||
| Operation mode | ||||
| Open surgery | 80 | 37 | 0.265 | 0.607 |
| Laparoscopic surgery | 150 | 61 | ||
| Pathological type | ||||
| Adenocarcinoma | 207 | 85 | 0.75 | 0.387 |
| Squamous carcinoma | 23 | 13 | ||
| Tumor size | ||||
| ≥3 cm | 127 | 57 | 0.242 | 0.623 |
| <3 cm | 103 | 41 | ||
| T-staging | ||||
| T1b | 41 | 17 | 0.833 | 0.659 |
| T2 | 115 | 54 | ||
| T3 | 74 | 27 | ||
| Lymph node metastasis | ||||
| With | 92 | 43 | 0.427 | 0.514 |
| Without | 138 | 55 | ||
| Perineural invasion | ||||
| With | 97 | 44 | 0.208 | 0.648 |
| Without | 133 | 54 | ||
| Vascular invasion | ||||
| With | 78 | 29 | 0.584 | 0.445 |
| Without | 152 | 69 | ||
| Differentiation degree | ||||
| Poorly differentiated | 78 | 37 | 0.446 | 0.504 |
| Moderately or well differentiated | 152 | 61 |
Note: BMI, body mass index.
Comparison of laboratory indexes between training and validation sets
The inter-group comparison of laboratory indexes revealed no statistical differences in CA199 levels, as well as preoperative albumin, ALT, AST, and TBIL levels between the training and validation sets (all P>0.05, Table 2).
Table 2.
Comparison of laboratory indexes between the two sets
| Variable | Training set (n=230) | Validation set (n=98) | statistic | P |
|---|---|---|---|---|
| CA199 (U/ml) | 128.10 [69.88, 435.68] | 167.85 [47.55, 436.03] | -0.610 | 0.542 |
| Preoperative ALT (U/L) | 26.30 [19.70, 33.48] | 27.60 [18.00, 35.55] | 0.052 | 0.959 |
| Preoperative AST (U/L) | 27.00 [23.00, 31.75] | 26.50 [21.00, 33.00] | -0.793 | 0.427 |
| Preoperative TBIL (μmol/L) | 18.00 [14.00, 22.00] | 18.00 [14.85, 22.75] | 0.235 | 0.815 |
| Preoperative albumin (g/L) | 39.80 [34.42, 44.88] | 38.50 [33.62, 42.27] | -1.357 | 0.175 |
Note: CA199, carbohydrate antigen 199; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin.
Screening for factors influencing 3-year DFS in the training set
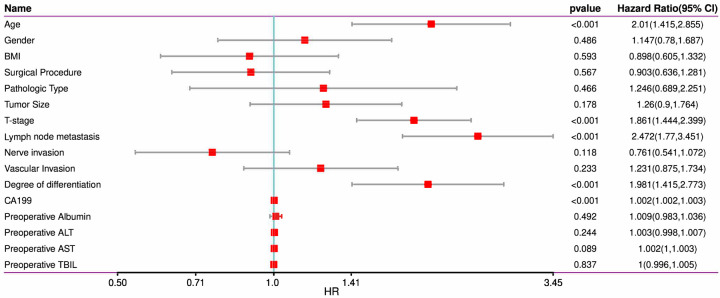
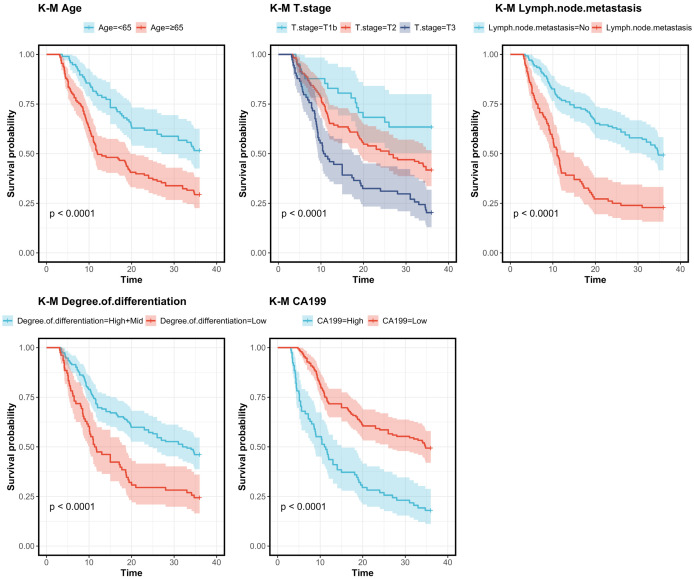
Univariate Cox regression analysis was conducted to screen factors affecting 3-year DFS in patients after radical surgery. The analysis identified age, T-staging, LNM, differentiation degree, and CA199 as prognostic factors for 3-year DFS (Figures 2, 3).
Figure 2.
Univariate analysis of 3-year DFS in the training set. Note: BMI, body mass index; CA199, carbohydrate antigen 199; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin.
Figure 3.
K-M survival curves based on various prognostic factors. Note: CA199, carbohydrate Antigen 199.
Construction of a 3-year DFS model using LASSO regression
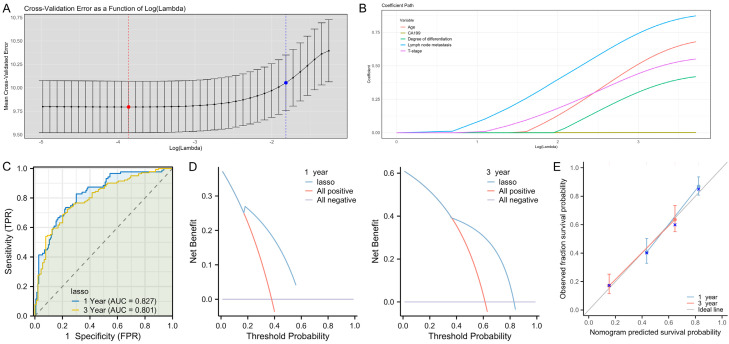
LASSO regression was used to construct a 3-year DFS prediction model for GBC patients. All the 5 factors identified in the feature selection phase were incorporated into the model (Figure 4A, 4B). Subsequently, the LASSO regression model was used to plot the time-dependent ROC curves for predicting 1- and 3-year DFS, with the AUCs of 0.827 and 0.801, respectively (Figure 4C). The DCA for 1- and 3-year DFS also demonstrated clinical net benefits (Figure 4D). Moreover, the calibration curve showed a high degree of agreement between the predicted 1-year and 3-year outcomes and the ideal gray reference line, indicating that the model has good calibration and fitting accuracy (Figure 4E).
Figure 4.
A prediction model for 1- and 3-year DFS in GBC patients after surgery based on LASSO regression. A, B. Regularization coefficients and feature factor screening. C. ROC curve analysis of the model’s predictive accuracy for 1- and 3-year DFS after GBC surgery. D. DCA of the clinical value of the model in predicting 1- and 3-year DFS after GBC surgery. E. Calibration curve analysis of the model’s stability in predicting 1- and 3-year DFS after GBC surgery. Note: CA199, carbohydrate antigen 199; LASSO, Least Absolute Shrinkage and Selection Operator; ROC, receiver operating characteristic; DCA, decision curve analysis; DFS, disease-free survival.
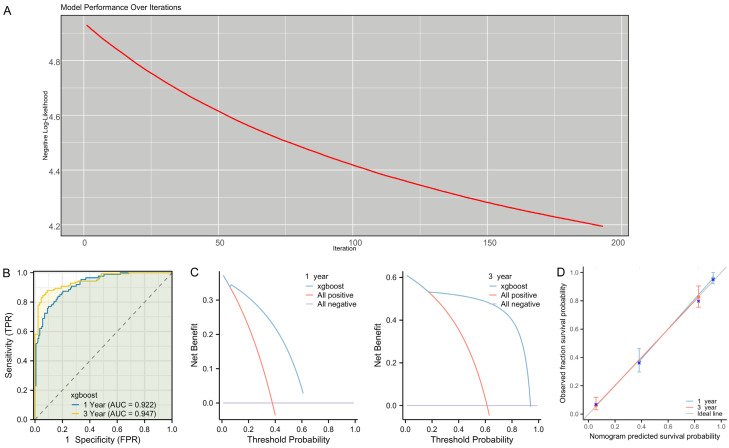
Construction of a 3-year DFS model using XGBoost regression
XGBoost regression was used to construct a 3-year DFS prediction model for patients. The modeling process involved 200 iterations, with the optimal number of iterations determined to be 143, which was subsequently used for training the model (Figure 5A). Time-dependent ROC curves for the XGBoost regression model were plotted, with the AUCs of 0.922 for 1-year DFS and 0.947 for 3-year DFS (Figure 5B). Then, the clinical net benefit of the model for predicting patients’ 1-year and 3-year DFS was demonstrated by DCA (Figure 5C). In addition, the calibration curves showed a high degree of alignment with the ideal gray reference line, indicating that the model had a good fit for both 1-year and 3-year DFS predictions (Figure 5D).
Figure 5.
A prediction model for 1- and 3-year DFS in GBC patients after surgery based on XGBoost regression. A. Regularization coefficients and feature factor screening. B. ROC curve analysis of the model’s predictive accuracy for 1- and 3-year DFS after GBC surgery. C. DCA of the clinical value of the model in predicting 1- and 3-year DFS after GBC surgery. D. Calibration curve analysis of the model’s stability in predicting 1- and 3-year DFS after GBC surgery. Note: XGBoost, Extreme Gradient Boosting; ROC, receiver operating characteristic; DCA, decision curve analysis; DFS, disease-free survival.
Comparison between LASSO and XGBoost models
The Delong test was used to compare the predictive accuracy of the two models for 1- and 3-year DFS. The results showed that the XGBoost model had a significantly higher AUC in predicting both the 1-year and 3-year DFS than the LASSO model (Table 3, P<0.001).
Table 3.
Comparison of the predictive accuracy between the two models in training set by Delong test
| Marker 1 | Marker 2 | Z_value | P_value | AUC difference | 95% CI |
|---|---|---|---|---|---|
| 1 year-XGBoost | 1 year-LASSO | 5.359 | <0.001 | 0.095 | 0.060-0.129 |
| 3-year-XGBoost | 3-year-LASSO | 6.895 | <0.001 | 0.146 | 0.105-0.188 |
Note: LASSO, Least Absolute Shrinkage and Selection Operator; XGBoost, Extreme Gradient Boosting.
External data validation of the Xboost model
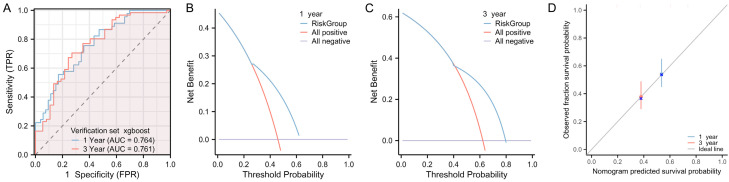
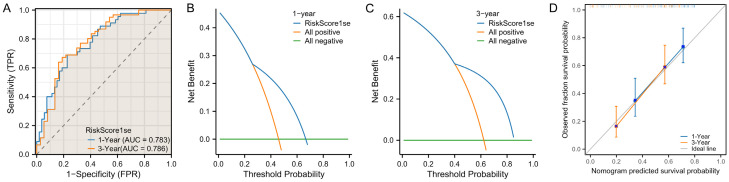
At the end of the study, we validated the model using the data from validation set. The results showed that, when applied to the validation dataset, the XGBoost model achieved AUCs of 0.764 for predicting 1-year DFS and 0.761 for predicting 3-year DFS (Figure 6A). In addition, the DCA revealed that the model was clinically beneficial in predicting both 1- and 3-year DFS (Figure 6B, 6C). Moreover, the calibration curves showed a high degree of alignment with the ideal gray line, indicating a good fitting of the model for both 1-year and 3-year DFS predictions (Figure 6D). Based on the results of the Lasso model, the AUCs for predicting 1-year and 3-year DFS was 0.783 and 0.786, respectively (Figure 7A). In addition, DCA also confirmed that clinical benefit of the LASSO model in predicting patient 1- and 3- year DFS (Figure 7B, 7C). Furthermore, the calibration curve for the LASSO model similarly showed a high overlap with the ideal gray line, indicating a good model fit (Figure 7D). No significant difference was observed in the ROC curves for 1-year and 3-year DFS between the XGBoost and LASSO models in the validation set (Table 4).
Figure 6.
Validation of the XGBoost prediction model using validation dataset. A. ROC curve analysis of the model’s predictive accuracy for 1- and 3-year DFS after GBC surgery. B, C. DCA of the clinical value of the model in predicting 1- and 3-year DFS after GBC surgery. D. Calibration curve analysis of the model’s stability in predicting 1- and 3-year DFS after GBC surgery. Note: XGBoost, Extreme Gradient Boosting; ROC, receiver operating characteristic; DCA, decision curve analysis; DFS, disease-free survival.
Figure 7.
Validation of the Lasso prediction model using validation dataset. A. ROC curve analysis of the model’s predictive accuracy for 1- and 3-year DFS after GBC surgery. B, C. DCA of the clinical value of the model in predicting 1- and 3-year DFS after GBC surgery. D. Calibration curve analysis of the model’s stability in predicting 1- and 3-year DFS after GBC surgery. Note: LASSO, Least Absolute Shrinkage and Selection Operator; ROC, receiver operating characteristic; DCA, decision curve analysis; DFS, disease-free survival.
Table 4.
Comparison of the predictive accuracy between the two models in validation set by Delong test
| Marker 1 | Marker 2 | Z_value | P_value | AUC difference | 95% CI |
|---|---|---|---|---|---|
| 1 year-XGBoost | 1 year-LASSO | -0.661 | 0.509 | -0.019 | -0.058-0.029 |
| 3-year-XGBoost | 3-year-LASSO | -1.438 | 0.15 | -0.025 | -0.067-0.010 |
Note: LASSO, Least Absolute Shrinkage and Selection Operator; XGBoost, Extreme Gradient Boosting.
Discussion
GBC is primarily treated with surgery. Although there is some controversy over surgical approaches, radical excision remains the accepted mainstay of treatment for GBC [15]. However, due to the characteristics of GBC, most patients are diagnosed at an advanced stage, resulting in a low percentage of patients feasible for radical surgery, usually not exceeding 10% [16]. Additionally, the risk of postoperative recurrence remains high, significantly affecting long-term survival and quality of life.
The therapeutic effect for GBC is usually evaluated by recurrence-free survival and overall survival, providing straightforward indicators for clinical outcomes. Clinical studies by Thorsten [17] and Uzun [18] have identified radical surgical resection as the most critical factor affecting the prognosis of GBC. Further research has highlighted that urban living background, the presence of cholelithiasis, N-staging, and M-staging also play significant roles in determining prognosis for GBC patients [19]. Additionally, multivariate analyses have shown that tumor differentiation, perineural invasion, T-staging, and N-staging are significant predictors of patient prognosis [20]. Currently, there is a lack of uniform criteria for predicting early recurrence of GBC. A previous multicenter study [21] involving 309 post-surgical GBC patients found that 33.3% of patients relapsed at a median follow-up of 15.1 months, suggesting that 12 months may be a critical threshold for early recurrence. The study also noted that T3/4 GBC and poor tumor differentiation were independent risk factors for early recurrence.
Age, T-staging, LNM, differentiation degree, and CA199 level were identified as prognostic factors affecting 3-year DFS in patients through univariate Cox regression analysis. Further analysis using LASSO and XGBoost regression confirmed these factors as independent prognostic factors affecting DFS of GBC patients. Age is related to the decline of physiological functions and may affect patients’ tolerance and response to treatment [20]. Older patients may have more comorbidities, limiting their likelihood of receiving more aggressive treatment, thereby affecting treatment efficacy and increasing recurrence risk [18]. T-staging is a key indicator for evaluating tumor invasiveness. T3-T4 tumors may have penetrated the gallbladder wall and invaded adjacent tissues or organs, increasing the complexity of surgery and the risk of postoperative recurrence [22]. Higher T-staging tumors may require more extensive surgical resection, impacting patient recovery and quality of life [23]. A multicenter study by Kim et al. [24] reported that the 2-year RFS of GBC patients was about 41% and that stage T3-T4 was an independent risk factor for recurrence within 2 years after radical surgery. As T-staging increases, there is a higher likelihood of microsatellite tumor metastases in the liver or peritoneum, resulting in occult tumor remnants during surgical resection. Patients with T3 GBC are more likely to develop micrometastases in the liver or adjacent organs than those with T1b-T2 tumors, contributing to higher risk of early postoperative recurrence.
The presence of LNM is an important marker of tumor spread. In GBC, LNM may indicate a wider spread of the disease, leading to more complicated postoperative management and treatment strategies [25]. Effective identification and treatment of LNM are essential to reduce recurrence and improve survival. As the N stage increases, the probability of LNM in patients also rises, significantly worsening patient prognosis [26]. The differentiation of tumors is an important indicator of their biological characteristics. Low-differentiated tumors usually have a higher proliferation rate and aggressiveness, leading to faster disease progression and a higher recurrence risk [27]. Poorly differentiated tumors often have a poorer response to chemotherapy and radiotherapy, complicating treatment. Prak et al. [28] identified poorly differentiated GBC as an independent risk factor for postoperative recurrence. Elevated levels of CA199, a tumor marker, are often associated with the presence and progression of GBC and indicate a higher tumor burden, adversely affecting patient outcomes [29]. Previous research by Li et al. [30] found that the long-term survival outcome of GBC patients with abnormal CA19-9 levels after radical surgery was poor. Yang et al. [31] found that adjuvant therapy after radical surgery did not significantly improve the overall survival and DFS of patients with T3 GBC, and CA19-9>39 U/ml was an independent risk factor for both overall survival and DFS. By combining these factors, we can understand how they affect the prognosis of GBC patients through different biological pathways and responses to treatment. In clinical practice, identifying and evaluating these prognostic factors are essential for developing personalized treatment plans and making accurate prognostic predictions.
Building predictive models for DFS in GBC patients is essential for advancing personalized medical care. Such models enable doctors to develop more precise treatment plans tailored to the specific conditions of each patient. Predictive models are instrumental in identifying high-risk patients at an early stage, allowing for timely medical intervention that can improve treatment outcomes [32]. When constructing a prediction model, it is important to consider the strengths of different algorithms. For example, univariate Cox regression analysis can identify prognostic factors affecting DFS, while LASSO and XGBoost regression can further refine these to determine the independent prognostic factors [33]. By comprehensively comparing these methods, a model with high prediction accuracy, strong generalization ability, and high computational efficiency can be selected to optimally predict patient DFS and provide strong decision support for clinical treatment. In this study, we found that the prediction model constructed using XGBoost regression is superior to that built on LASSO regression in terms of clinical benefit and stability. Moreover, the Delong test confirmed that the XGBoost-based prediction model had superior predictive ability for both 1-year and 3-year DFS compared to the LASSO-based model. These results illustrate the effectiveness of advanced machine learning techniques such as XGBoost regression in analyzing complex clinical data and making accurate prognostic prediction. The XGBoost model is a powerful tool for predicting DFS in patients with GBC due to its ability to process a large number of features and control for overfitting.
However, this study has several limitations, including a small sample size, a limited scope, and a short follow-up time, all of which necessitate external validation. As a retrospective analysis from a single medical center, the sample size is relatively small, potentially limiting the generalizability and stability of the results. Additionally, the short-term follow-up data may not fully capture long-term survival outcomes, restricting the evaluation of the prediction model’s long-term effectiveness. Although the model performs well internally, external validation is crucial to confirm its applicability and effectiveness across different clinical settings. These limitations underscore the need for more rigorous validation with larger sample sizes and long-term data in future studies before applying these findings to clinical practice.
Conclusion
To sum up, age, T-staging, LNM, differentiation degree, and CA199 are independent prognostic factors for 3-year DFS in GBC patients. Moreover, the XGBoost regression model shows high accuracy and clinical value in predicting short-term DFS in patients after radical GBC surgery, providing a valuable tool for individualized treatment.
Disclosure of conflict of interest
None.
References
- 1.Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. doi: 10.1038/s41572-022-00398-y. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby H, Rayman S, Oliphant U, Nelson D, Ross S, Rosemurgy A, Sucandy I. Current operative approaches to the diseased gallbladder. Diagnosis and management updates for general surgeons. Am Surg. 2024;90:122–129. doi: 10.1177/00031348231198107. [DOI] [PubMed] [Google Scholar]
- 3.Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943–e955. doi: 10.1016/S2468-2667(23)00211-6. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Lu S, Huo C, Fan L, Lin M, Huang J. Non cancer causes of death after gallbladder cancer diagnosis: a population-based analysis. Sci Rep. 2023;13:13746. doi: 10.1038/s41598-023-40134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Qin L, Wu P, Liu Y, Zhang Y, Mao B, Yan Y, Yan S, Tan F, Yue X, Liu H, Xue H. RNA sequencing revealed the multi-stage transcriptome transformations during the development of gallbladder cancer associated with chronic inflammation. PLoS One. 2023;18:e0283770. doi: 10.1371/journal.pone.0283770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlidis ET, Galanis IN, Pavlidis TE. New trends in diagnosis and management of gallbladder carcinoma. World J Gastrointest Oncol. 2024;16:13–29. doi: 10.4251/wjgo.v16.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara K, Abe A, Masatsugu T, Hirano T, Sada M. Effect of gallbladder polyp size on the prediction and detection of gallbladder cancer. Surg Endosc. 2021;35:5179–5185. doi: 10.1007/s00464-020-08010-8. [DOI] [PubMed] [Google Scholar]
- 9.Munir MM, Ruff SM, Endo Y, Lima HA, Alaimo L, Moazzam Z, Shaikh C, Pawlik TM. Does adjuvant therapy benefit low-risk resectable cholangiocarcinoma? An evaluation of the NCCN guidelines. J Gastrointest Surg. 2023;27:511–520. doi: 10.1007/s11605-022-05558-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Chen YW, Liu C, Wu YT, Zhao WC, Zhu JY, An Y, Xia NX. Development and validation of a nomogram model for predicting the risk of gallstone recurrence after gallbladder-preserving surgery. Hepatobiliary Pancreat Dis Int. 2024;23:288–292. doi: 10.1016/j.hbpd.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Margonis GA, Gani F, Buettner S, Amini N, Sasaki K, Andreatos N, Ethun CG, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick B, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Maithel SK, Pawlik TM. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:872–878. doi: 10.1016/j.hpb.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedmutha AS, Agrawal A, Rangarajan V, Goel M, Patkar S, Puranik AD, Ramadwar M, Purandare NC, Shah S, Choudhury S. Diagnostic performance of F-18 FDG PET/CT in recurrent adenocarcinoma gallbladder and its impact on post-recurrence survival. Jpn J Radiol. 2023;41:201–208. doi: 10.1007/s11604-022-01340-8. [DOI] [PubMed] [Google Scholar]
- 13.Han JW, Lee SK, Kwon JH, Nam SW, Yang H, Bae SH, Kim JH, Nam H, Kim CW, Lee HL, Kim HY, Lee SW, Lee A, Chang UI, Song DS, Kim SH, Song MJ, Sung PS, Choi JY, Yoon SK, Jang JW. A machine learning algorithm facilitates prognosis prediction and treatment selection for barcelona clinic liver cancer stage C hepatocellular carcinoma. Clin Cancer Res. 2024;30:2812–2821. doi: 10.1158/1078-0432.CCR-23-3978. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang W, Liu Y, Huang H, Tan Y, Huang Z, Jia X, Yu Y, Yao H. Unraveling the unfolded protein response signature: implications for tumor immune microenvironment heterogeneity and clinical prognosis in stomach cancer. Aging (Albany NY) 2024;16:7818–7844. doi: 10.18632/aging.205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Gong J, Li Z, Han L, Sun D. Gallbladder cancer: surgical treatment, immunotherapy, and targeted therapy. Postgrad Med. 2024;136:278–291. doi: 10.1080/00325481.2024.2345585. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, Wang Z, Wang F, Liu C, Li Q, Zhang Q, Li Q, Shan X, Zhou J. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. doi: 10.3389/fphar.2023.1183619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol. 2015;21:12211–12217. doi: 10.3748/wjg.v21.i43.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uzun MA, Tilki M, Alkan Kayaoğlu S, Çiçek Okuyan G, Kılıçoğlu ZG, Gönültaş A. Long-term results and prognostic factors after surgical treatment for gallbladder cancer. Turk J Surg. 2022;38:334–344. doi: 10.47717/turkjsurg.2022.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feroz Z, Gautam P, Tiwari S, Shukla GC, Kumar M. Survival analysis and prognostic factors of the carcinoma of gallbladder. World J Surg Oncol. 2022;20:403. doi: 10.1186/s12957-022-02857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D, Jin W, Zhang Y, An Y, Chen X, Chen W. Insights from the analysis of clinicopathological and prognostic factors in patients with gallbladder cancer. Front Oncol. 2022;12:889334. doi: 10.3389/fonc.2022.889334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu Y, Ashida R, Sugiura T, Okamura Y, Ohgi K, Yamada M, Otsuka S, Aramaki T, Notsu A, Uesaka K. Early recurrence in resected gallbladder carcinoma: clinical impact and its preoperative predictive score. Ann Surg Oncol. 2022;29:5447–5457. doi: 10.1245/s10434-022-11937-y. [DOI] [PubMed] [Google Scholar]
- 22.Bilgin B, Bilgin MK, Erol S, Celik G, Ozdemir Kumbasar O. Prognosis of sarcoidosis and factors affecting prognosis. Sarcoidosis Vasc Diffuse Lung Dis. 2023;40:e2023054. doi: 10.36141/svdld.v40i4.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, He M, Wang H, Zhan M, Yang L. Development and validation of a prognostic nomogram for gallbladder cancer patients after surgery. BMC Gastroenterol. 2022;22:200. doi: 10.1186/s12876-022-02281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WS, Choi DW, You DD, Ho CY, Heo JS, Choi SH. Risk factors influencing recurrence, patterns of recurrence, and the efficacy of adjuvant therapy after radical resection for gallbladder carcinoma. J Gastrointest Surg. 2010;14:679–687. doi: 10.1007/s11605-009-1140-z. [DOI] [PubMed] [Google Scholar]
- 25.Choi SY, Kim JH, Lim S, Lee JE, Park HJ, Lee B. CT-based nomogram for predicting survival after R0 resection in patients with gallbladder cancer: a retrospective multicenter analysis. Eur Radiol. 2021;31:3336–3346. doi: 10.1007/s00330-020-07402-7. [DOI] [PubMed] [Google Scholar]
- 26.Goel M, Pandrowala S, Patel P, Patkar S. Node positivity in T1b gallbladder cancer: a high volume centre experience. Eur J Surg Oncol. 2022;48:1585–1589. doi: 10.1016/j.ejso.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Liu H, Huang Y, Wang J, Li J, Liu L, Huang M. Comparative analysis of postoperative curative effect of liver wedge resection and liver IVb + V segment resection in patients with T2b gallbladder cancer. Front Surg. 2023;10:1139947. doi: 10.3389/fsurg.2023.1139947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, Kim BR. Actual recurrence patterns and risk factors influencing recurrence after curative resection with stage II gallbladder carcinoma. J Gastrointest Surg. 2007;11:631–637. doi: 10.1007/s11605-007-0109-z. [DOI] [PubMed] [Google Scholar]
- 29.Lv TR, Wang JK, Li FY, Hu HJ. Prognostic factors for resected cases with gallbladder carcinoma: a systematic review and meta-analysis. Int J Surg. 2024;110:4342–4355. doi: 10.1097/JS9.0000000000001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XL, Liu ZP, Su XX, Gong Y, Yang YS, Zhao XL, Li ZM, Ding JJ, Zhu Y, Yin DL, Yu C, Zhou JX, Zhang D, Ding R, Chen W, Cheng Y, Yue P, Wang ZR, Zhang YQ, Jiang Y, Yin XY, Bai J, Dai HS, Lau WY, Chen ZY Biliary Surgery Branch of Elite Group of Chinese Digestive Surgery (EGCDS) Long-term prognosis of patients with gallbladder carcinoma after curative-intent resection based on changes in the ratio of carbohydrate antigen 19-9 to total bilirubin (CA19-9/TB): a multicenter retrospective cohort study. Int J Surg. 2024;110:3580–3590. doi: 10.1097/JS9.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang FC, Hu J, Su TH, Geng ZM, Zhang K, Ding J, Lei ZQ, Yi B, Li JD, Tang ZH, Cheng ZJ, Qiu YH. Efficacy analysis of surgical combined with postoperative adjuvant therapy for T3 gallbladder carcinoma: a multicenter retrospective study. Zhonghua Wai Ke Za Zhi. 2023;61:863–870. doi: 10.3760/cma.j.cn112139-20230202-00047. [DOI] [PubMed] [Google Scholar]
- 32.Swanson K, Wu E, Zhang A, Alizadeh AA, Zou J. From patterns to patients: advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell. 2023;186:1772–1791. doi: 10.1016/j.cell.2023.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, Zhao L, Wang S, Zhang H, Jiang K, Ji J, Chen S, Wang C, Wei C, Zhou R, Wang Z, Li X, Wang F, Wei X, Hou W. Explainable and visualizable machine learning models to predict biochemical recurrence of prostate cancer. Clin Transl Oncol. 2024;26:2369–2379. doi: 10.1007/s12094-024-03480-x. [DOI] [PubMed] [Google Scholar]