Abstract
Treatment options are limited for tumors after failure of standard therapies. Utidelone (UTD1), a novel microtubule stabilizer, given via 5 days intermittent infusion, has demonstrated high activity in heavily pretreated metastatic breast cancer, while its efficacy in other cancers was unclear. Peripheral neuropathy is a common and severe adverse event (AE) of UTD1. We performed a prospective, multicenter, single-arm trial (ChiCTR2300074299) to evaluate the efficacy and safety of UTD1 with a changed administration mode in patients with advanced or metastatic solid tumors after failure of standard therapies. UTD1 (150 mg/m2, alone or in combination with other anticancer agents) was administrated via 120 h continuous intravenous infusion every 21 days until disease progression or intolerable toxicity. A total of 50 patients were enrolled and analyzed, including 20 breast cancer patients, 11 gynecological cancer patients, 8 gastrointestinal cancer patients, 6 lung cancer patients, and 5 patients with other solid tumors. The overall median progression-free survival (PFS) was 4 months, the overall objective response rate and disease control rate were 20% and 66%, respectively, and the median overall survival was not reached. Most of the AEs were grade 1 or 2 and were manageable and reversible, the rate of grade ≥3 AEs including peripheral neuropathy was 4%. This study demonstrated a promising anti-tumor activity of UTD1 in patients with advanced or metastatic solid tumors after failure of the standard therapies. Moreover, 120 h continuous intravenous infusion was a more tolerable administration mode than 5 days intermittent infusion, and worthy of further study.
Keywords: Utidelone, metastatic cancer, drug resistance, progression-free survival
Introduction
Drug resistance continues to be the main obstacle to cure cancers. The reasons of resistance to therapy include tumor burden, tumor heterogeneity and growth kinetics, immune system and microenvironment and therapeutic pressures et al. [1]. Epothilones, including subtypes A, B, C, D, E and F, are a class of naturally existing microtubule inhibitors produced by the metabolism of the cellulose-degrading myxobacterium Sorangium cellulosum [2], which have shown anticancer activities similar to paclitaxel via competing for binding sites on microtubules [3]. Utidelone (UTD1), a novel genetically modified epothilone analogue, has been approved by National Medical Products Administration of China for the treatment of metastatic breast cancer, especially for patients who have treated with anthracyclines and taxanes [4,5]. Moreover, UTD1 has also shown potential antitumor activities in other solid tumors including colorectal cancer in preclinical or phase I studies [6,7]. However, the exact clinical efficacy of UTD1 in other tumors, especially that are refractory to standard therapy, remains largely unknown. This study aimed to evaluate the efficacy and safety of UTD1 in solid tumors after failure of standard therapy.
The currently recommended usage for UTD1 is 30 mg/m2 intravenously over 90 minutes, once per day on days 1-5, every 3 weeks. The main side effect is severe peripheral neuropathy (PN). It can cause severe numbness in hands and feet, sore limbs, and even render the patients unable to walk [8]. Furthermore, severe PN of UTD1 may cause drug reduction or withdrawal and affect clinical efficacy. It was reported that continuous infusion 5-fluorouracil showed an improvement in overall survival and toxicity profile over bolus infusion 5-fluorouracil in cancer treatment [9]. In view of this, we designed this multi-center trial to evaluate the efficacy and safety of UTD1 alone or in combination with other anticancer agents in solid tumors with changed usage, UTD1 was administrated via 120 h continuous intravenous infusion every 21 days. The primary endpoint was progression-free survival (PFS), secondary endpoints included objective response rate (ORR), disease control rate (DCR), overall survival (OS) as well as safety.
Methods
Study design and participants
We performed an open-label, prospective, multi-center, single-arm trial in seven hospitals in China to evaluate the efficacy and safety of UTD1 (alone or in combination with other anticancer agents) in advanced or metastatic solid tumor after failure of standard therapies from January 2022 to June 2023. The study was approved by the local research ethical committee (number 2022-010-01) and registered at Chinese Clinical Trial Registry (No. ChiCTR2300074299). This trial was done in compliance with Good Clinical Practice and the Declaration of Helsinki.
Eligible patients were at least 18 years of old; had pathologically confirmed advanced/metastatic solid tumor; had progressed on at least one line of standard anti-tumor therapy; had at least one measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; had a life expectancy of at least 3 months; had adequate cardio-pulmonary, hematologic, hepatic, and renal functions. Main exclusion criteria included anti-tumor therapies such as chemotherapy, radiation therapy, or targeted therapy within 4 weeks prior to study entry; peripheral neuropathy within 4 weeks before enrollment of greater than grade 2 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0; active brain metastases; a history of human immunodeficiency virus infection or other active infection; and being pregnant or planning to be pregnant. Informed consent was obtained from each patient before initiating study procedures.
Procedures
The patients received UTD1 (150 mg/m2, 120 h continuous intravenous infusion every 21 days) as monotherapy or in combination with other anticancer agents at the discretion of the physician until disease progression, intolerable toxicity, at the request of the patient or investigator to discontinue. Dose reductions (reducing the original measurement of 20%) were permitted to manage toxic effects. The tumor response was assessed by computed tomography or magnetic resonance imaging scan every two cycles according to RECIST version 1.1. Survival follow-up was done every 6 weeks until the end of the study or death. Safety assessments, including monitoring for adverse events (AEs), were done from the signing of informed consent until 4 weeks after the last administration of study treatment. AEs were graded according to the CTCAE version 5.0.
Outcomes
The primary endpoint was PFS, defined as the time from the signing of informed consent to progressive disease or death due to any cause, whichever occurred first, according to RECIST version 1.1. Secondary endpoints included ORR (defined as the proportion of patients who achieved complete response or partial response), DCR (defined as the proportion of all non-progressive patients at the end of follow-up), OS (defined as the time from the signing of informed consent to death due to any cause), and safety profile.
Statistical analysis
All analyses were performed using Statistical Package for the Social Sciences (SPSS), version 20.0 (Chicago, Illinois, USA). Figures were created by Graphpad 7.0. The efficacy and safety variables were summarized using descriptive statistics. The χ2 or Fisher’s exact test was used to compare proportions. Kaplan-Meier survival curves were used to estimate proportion surviving and the log-rank test was used to compare differences among subgroups. Cox regression models were used to identify independent prognostic factors. If variables were significant at the 0.2 level on univariate analysis, then they were included in the multiple regression. 95% confidence intervals were calculated using the Clopper Pearson method. A P value <0.05 was considered significant.
Results
Patient characteristics
Between January 28, 2022 and June 30, 2023, we enrolled and assigned 58 patients to treatment. Of the patients, five did not undergo efficacy assessment, three had missing data from more than two assessments. Therefore, 50 patients with a broad variety of advanced or metastatic solid tumors after failure of standard therapies were included in the efficacy and safety analyses (Table 1). There were 20 patients with breast cancer, 11 patients with gynecological cancers, eight patients with gastrointestinal cancers, six patients with lung cancer, five patients with other solid tumors. At baseline, 23 (46%) patients had received one or two lines of previous therapies for recurrent or metastatic disease, 27 (54%) patients had received at least three lines of previous therapies. 38 (76%) patients had taxane-refractory diseases. 18 (36%) patients had no more than two metastatic sites including two patients with only lymph node metastasis and one patient with only liver metastasis, while the majority of patients (64%) had more than two metastatic sites. During the study, eight patients received UTD1 as monotherapy, while the remaining 42 patients received UTD1 in combination with other anticancer agents. The combination therapies included targeted therapy in 19 patients, immunotherapy in 12 patients, other cytotoxic drug in five patients, targeted therapy and immunotherapy in six patients.
Table 1.
Patient characteristics (n=50)
| Patient characteristics | n (%) |
|---|---|
| Age (years) | |
| ≤60 | 27 (54%) |
| >60 | 23 (46%) |
| Sex | |
| Men | 14 (28%) |
| Women | 36 (72%) |
| ECOG performance status | |
| 0-1 | 31 (62%) |
| 2 | 19 (38%) |
| Previous lines of therapies for recurrent or metastatic disease | |
| ≤2 | 23 (46%) |
| >2 | 27 (54%) |
| Previous therapeutic regimen | |
| Including taxanes | 38 (76%) |
| Excluding taxanes | 12 (24%) |
| Trial regimen | |
| UTD1 monotherapy | 8 (16%) |
| Combination therapy | 42 (84%) |
| Metastatic sites | |
| ≤2 | 18 (36%) |
| >2 | 32 (64%) |
| Pathological type | |
| Adenocarcinoma | 38 (76%) |
| Squamous cell carcinoma | 8 (16%) |
| Sarcoma | 3 (6%) |
| Urothelial carcinoma | 1 (2%) |
| Tumor entities | |
| Breast cancer | 20 (40%) |
| Gynecological cancers | 11 (22%) |
| Gastrointestinal cancers | 8 (16%) |
| Lung cancer | 6 (12%) |
| Other solid tumors | 5 (10%) |
ECOG, Eastern Cooperative Oncology Group; UTD1, utidelone.
Clinical outcomes
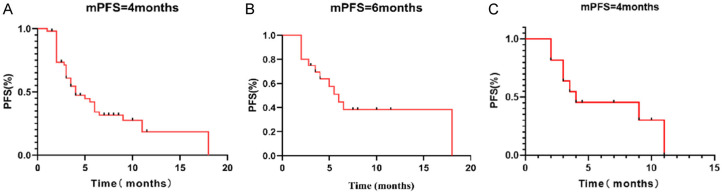
Ten patients achieved the best response of partial response, 23 patients had stable disease and 17 had progressive disease (PD) as the best response by RECIST 1.1. As a result, the overall ORR and DCR were 20% and 66%, respectively. The overall median PFS was 4 months (95% CI 2.23-5.77) (Figure 1A), and the median OS was not reached. For breast cancer patients, the median PFS was 6 months (95% CI 4.15-7.85) (Figure 1B), the ORR and DCR was 40% and 75%, respectively. For gynecological tumor patients, the median PFS was 4 months (95% CI 0.00-9.3) (Figure 1C), the ORR and DCR were 9% and 64%, respectively. For lung cancer patients, the median PFS was 3 months (95% CI 1.46-4.54), the ORR and DCR were 17% and 50%, respectively. For gastrointestinal cancers patients, the median PFS was 3 months (95% CI 0.92-5.08), the ORR and DCR were 0% and 50%, respectively. For patients who received UTD1 alone, the median PFS was 3.5 months (95% CI 0.73-6.27), the ORR and DCR were 25% and 50%, respectively. Patients who had received at least three lines of previous therapies still had an ORR of 19% after the UTD1-based therapy. Patients who had received previous taxanes-containing regimens achieved an ORR of 24%.
Figure 1.
Kaplan-Meier survival curves of progression-free survival (PFS). A. Survival curve of PFS of all the patients. B. Survival curve of PFS of breast cancer patients. C. Survival curve of PFS of gynecological cancer patients.
Safety
Overall, the incidence of treatment-related AEs of any grade were as follows: fatigue (26%), anemia (42%), diarrhea (16%), decreased appetite (12%), increased alanine aminotransferase (10%), alopecia (28%), dizzy (24%), leukopenia (14%) and peripheral neurotoxicity (68%) (Table 2). The incidence of peripheral neurotoxicity was only 37.5% (3/8) in the patients who received UTD1 monotherapy. Most of the AEs were grade 1 or 2 and were manageable and reversible. The rate of grade ≥3 AEs including peripheral neuropathy was 4%. No treatment-related discontinuation or deaths occurred.
Table 2.
Treatment-related adverse events according to monotherapy or combination therapy
| Preferred term | All grades | Grade 1-2 | Grade 3 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Fatigue | 13 (26%) | 13 (26%) | 0 |
| UTD1 monotherapy | 2 (4%) | 2 (4%) | 0 |
| Combination therapy | 11 (22%) | 11 (22%) | 0 |
| Diarrhea | 8 (16%) | 8 (16%) | 0 |
| UTD1 monotherapy | 3 (6%) | 3 (6%) | 0 |
| Combination therapy | 5 (10%) | 5 (10%) | 0 |
| Decreased appetite | 6 (12%) | 6 (12%) | 0 |
| UTD1 monotherapy | 0 | 0 | 0 |
| Combination therapy | 6 (12%) | 6 (12%) | 0 |
| Anemia | 21 (42%) | 21 (42%) | 0 |
| UTD1 monotherapy | 0 | 0 | 0 |
| Combination therapy | 21 (42%) | 21 (42%) | 0 |
| Peripheral neurotoxicity | 34 (68%) | 32 (64%) | 2 (4%) |
| UTD1 monotherapy | 3 (6%) | 2 (4%) | 1 (2%) |
| Combination therapy | 31 (62%) | 30 (60%) | 1 (2%) |
| Alopecia | 14 (28%) | 14 (28%) | 0 |
| UTD1 monotherapy | 4 (8%) | 4 (8%) | 0 |
| Combination therapy | 10 (20%) | 10 (20%) | 0 |
| Dizzy | 12 (24%) | 12 (24%) | 0 |
| UTD1 monotherapy | 3 (6%) | 3 (6%) | 0 |
| Combination therapy | 9 (18%) | 9 (18%) | 0 |
| Leukopenia | 7 (14%) | 7 (14%) | 0 |
| UTD1 monotherapy | 0 | 0 | 0 |
| Combination therapy | 7 (14%) | 7 (14%) | 0 |
| ALT increased | 5 (10%) | 5 (10%) | 0 |
| UTD1 monotherapy | 0 | 0 | 0 |
| Combination therapy | 5 (10%) | 5 (10%) | 0 |
UTD1, utidelone.
Analysis of prognostic factors of PFS
Kaplan-Meier survival curves showed that and ECOG performance status (P=0.036) was significantly associated with PFS, patients with better performance status had longer PFS. Univariable analysis showed that ECOG performance status (P=0.056), previous therapeutic regimen containing taxanes or not (P=0.140), previous lines of therapies for recurrent or metastatic disease (P=0.138) and sex (P=0.11) showed a trend toward significance for PFS. These above-mentioned factors were submitted to multivariable analysis. However, the results showed none of these four factors was independent predictor of PFS (Table 3).
Table 3.
Univariable and multivariable Cox proportional hazard regression analyses of PFS
| Factor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.818 (0.874-3.783) | 0.110 | 0.719 (0.323-1.601) | 0.419 |
| Age (years) | ||||
| ≤60 | Reference | |||
| >60 | 1.201 (0.591-2.440) | 0.613 | ||
| ECOG performance status | ||||
| 0-1 | Reference | Reference | ||
| 2 | 1.983 (0.983-4.001) | 0.056 | 1.399 (0.598-3.271) | 0.438 |
| Previous lines of therapies for recurrent or metastatic disease | ||||
| ≤2 | Reference | Reference | ||
| >2 | 1.703 (0.842-3.442) | 0.138 | 1.459 (0.640-3.328) | 0.369 |
| Previous therapeutic regimen | ||||
| Excluding taxanes | Reference | Reference | ||
| Including taxanes | 0.554 (0.253-1.215) | 0.140 | 0.658 (0.276-1.569) | 0.345 |
| Metastatic sites | ||||
| ≤2 | Reference | |||
| >2 | 0.977 (0.477-2.003) | 0.950 | ||
| Pathological type | ||||
| Adenocarcinoma | Reference | |||
| Squamous cell carcinoma | 0.807 (0.307-2.122) | 0.664 | ||
| Sarcoma | 1.126 (0.265-4.789) | 0.872 | ||
| Urothelial carcinoma | 0.983 (0.132-7.331) | 0.986 | ||
| Tumor entities | ||||
| Breast cancer | Reference | |||
| Gynecological cancers | 1.351 (0.543-3.361) | 0.517 | ||
| Gastrointestinal cancers | 2.124 (0.77-5.858) | 0.146 | ||
| Lung cancer | 2.901 (0.906-9.282) | 0.073 | ||
| Other solid tumors | 1.808 (0.571-5.719) | 0.314 | ||
HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group.
Discussion
Cancer is the second leading cause of death globally, with 10 million deaths in 2020 [10]. Despite the fact that there are many different methods of cancer therapy, including surgery, immunotherapy, radiation therapy, targeted therapy and endocrine therapy, chemotherapy still remains the most common method of cancer healing [11].
Currently, comprehensive and individualized treatment is regarded as the most common and effective measure for cancer therapy. Chemotherapy drugs can effectively eliminate rapidly growing tumor cells, which are widely used in the therapy of various cancer [12]. However, there are still high mortality rate of tumors. The main reason for therapy failure is that the tumors will ultimately become resistant to former treatments, which leads to disease recurrence or progression [13]. There is an urgent need for treatment protocols that can overcome drug resistance in cancer patients after failure of standard therapies.
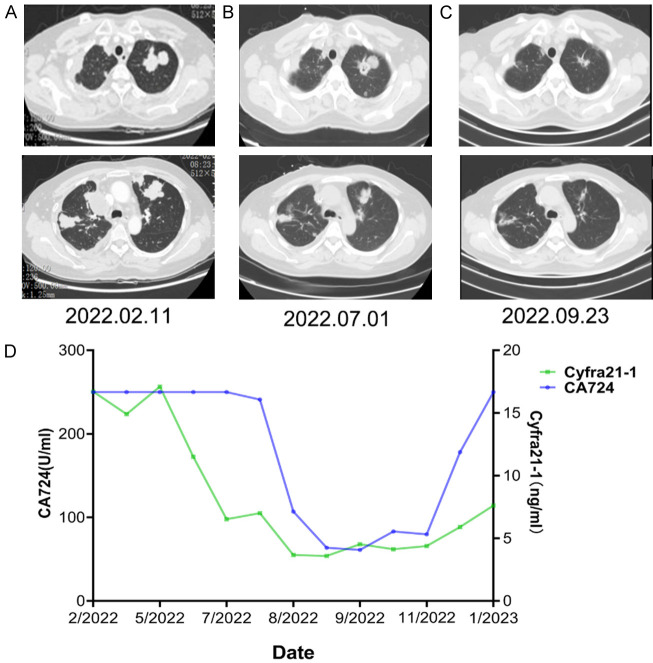
UTD1 is a new microtubule stabilizing agent which is mainly used to treat recurrent or metastatic breast cancer, especially in patients with heavily-pretreated disease that was resistant to anthracyclines and taxanes [14]. The results of phase III study NCT02253459 revealed that, UTD1 plus capecitabine significantly improved PFS (8.0 vs 3.5 m, P<0.0001), increased the proportion of patients with ORR (45.6% vs 23.7%, P<0.0001), and prolonged OS (20.9 vs 15.7 m, P=0.0032) in metastatic breast cancer compare to capecitabine alone [4]. However, the data on efficacy of UTD1 in other solid tumors are very limited except several preclinical studies and a few cases in phase I clinical trial [6,7]. In this study, we enrolled a wide range of advanced or metastatic solid tumors including breast cancer after failure of standard therapies. The patients received continuous intravenous infusion UTD1 alone or in combination with other anticancer agents. The overall median PFS was 4 months (95% CI 2.23-5.77), the overall ORR and DCR were 20% and 66%, respectively, and the median OS was not reached. As mentioned above, the median PFS and ORR in breast cancer patients were 6 months and 40%, respectively, a little lower than the results in NCT02253459 [4,5]. This may be explained by the fact that our study enrolled higher proportion of patients with ECOG performance status 2 and patients who had received at least three lines of previous therapies compared to NCT02253459 [5]. Even so, the efficacy of UTD1-based therapy in this study was superior to that in the capecitabine control group in NCT02253459 [4,5]. In the patients with gynecological tumors including ovarian and cervical cancer et al., UTD1-based therapy demonstrated a promising anti-tumor activity with a median PFS of 4 months, an ORR of 9% and a DCR of 64%, respectively. In an ovarian cancer patient with multiple lung metastases experienced progression after undergoing six lines of treatment including chemotherapy, targeted therapy, immunotherapy alone or combination within 5 years, the lung metastases achieved partial response after combination therapy of UTD1 and capecitabine and the PFS was up to 11 months (Figure 2). To the best of our knowledge, this is the first report of the clinical efficacy of UTD1 in gynecological tumors. In other tumors such as lung and gastrointestinal cancer refractory to standard therapies, UTD1 monotherapy or combination therapy also exhibited a promising anti-tumor activity. Importantly, tumors refractory to taxanes, another type of microtubule stabilizer, could achieve an ORR of 24% from UTD1-based therapy.
Figure 2.
A heavily-pretreated ovarian cancer patient achieved partial response after combination therapy of utidelone (UTD1) and capecitabine. A. Computed tomography of the chest showed bilateral lung metastatic lesions before enrollment. B. Lung metastatic lesions showed partial response after seven cycles chemotherapy with UTD1 and capecitabine. C. Computed tomography showed continued shrinkage of lung metastatic lesions after 11 cycles chemotherapy. D. Curves of serum tumor markers.
The reasons why UTD1 are effective in taxanes-resistant tumors remain unclear. As a novel microtubule stabilizer, UTD1 can cause the arrest of mitosis by promoting microtubule polymerization in the absence of GTP or microtubule-associated proteins [2]. The microtubule stabilized by UTD1 has a reduced number of protofilaments, and it is dysfunctional during the M phase [15], leading to cell cycle arrest at metaphase/anaphase transition and cell death [16]. After UTD1 exposure, both mitochondrial fission and fusion protein, Drp1 and mitofusin2 changed, indicating that it might affect mitochondrial dynamics, which could trigger cell death [7]. Moreover, UTD1 was less influenced by P-glycoprotein which could structurally and functionally affect diverse chemotherapeutics [17]. These may partially explain why UTD1 was still effective in tumors refractory to standard therapies including taxanes-containing regimens. These mechanisms supported the results of the univariable and multivariable analyses of prognostic factors of PFS, which indicated that UTD1 had a broad spectrum of anticancer activity in different subgroups of age, sex, previous lines of therapies, previous therapeutic regimen et al.
PN is a common adverse reaction of chemotherapeutic drugs, including microtubule stabilizers, platinum derivatives, vinca alkaloids and proteasome inhibitors [18]. Risk factors for PN include chemotherapeutic agent, cumulative dose, number of cycles, combination therapies, severity of acute symptoms and chronic alcohol consumption amongst others [19,20]. In the meta-analysis, PN was found in 68.1% of patients at the first month after chemotherapy, 60% at 3 months, and 30% at 6 months and beyond [21]. It is mainly manifested as limb numbness of abnormal sensation, even loss of sensation or abnormal pain, and decreased tendon reflexes [22]. The main side effect of UTD1 is PN. The incidence of PN caused by UTD1 combined with capecitabine was 85.4%, and the incidence of grade 3 PN was as high as 25.1% in heavily pretreated, anthracycline- and taxanes-rafractory metastatic breast cancer [4]. The mechanisms of PN include the damage of sensory neurons in the dorsal root ganglion, oxidative stress, mitochondrial dysfunction, microtubule destruction, axon degeneration, upregulation of proinflammatory cytokines, and others factors [23-25]. In the present study, the overall incidence of PN caused by UTD1 combined with other anticancer drugs was 62%, and the incidence of grade 3 PN was 2%. When UTD1 was administrated as monotherapy, the incidence of PN of any grade was as low as 37.5%, and the incidence of grade 3 PN was 12.5%. In breast cancer patients, the overall incidence of PN was 65%, and the incidence of grade 3 PN was 10%, respectively, significantly lower than those in NCT02253459 [4,5]. Moreover, none of patients reported drug reduction or withdrawal during the study. These results indicated that the incidence and severity of PN has significantly decreased due to changes in administration mode of UTD1.
The mode of administration or dose schedule of chemotherapeutic drugs appear to have a differential impact on its mechanism of action. The influence of the mode of administration on the mechanism of action of 5-fluorouracil may offer an explanation for the difference in toxicity and efficacy between bolus infusion and continuous infusion 5-fluorouracil [9,26]. In this study, we changed the administration mode of UTD1 from 5 days intermittent infusion to 120 h continuous intravenous infusion with total dose unchanged, despite all tumors were refractory to standard therapies and more than 50% patients had received at least three lines of previous therapies, the UTD1-based therapy demonstrated convincing anticancer activity. Notably, continuous intravenous infusion UTD1 exhibited an improvement of safety and tolerability over traditional administration mode.
Conclusion
In summary, our study demonstrated a promising anti-tumor activity of UTD1 in patients with advanced or metastatic solid tumors after failure of the standard therapies, especially in patients with breast and gynecological cancers. Moreover, 120 h continuous intravenous infusion is a more tolerable administration method than 5 days intermittent infusion, worthy of further study.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82274261), the Shanghai Pujiang Program (21PJD051), the Shanghai science and technology innovation action plan (23Y11905700), the medical center for cancer diagnosis and treatment in Changning District of Shanghai (20232001) and the Medical engineering cross project of Shanghai Jiao Tong University (NO. YG2022QN115).
Informed consent was obtained from the participants before sample collection and analysis.
Disclosure of conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villegas C, Gonzalez-Chavarria I, Burgos V, Iturra-Beiza H, Ulrich H, Paz C. Epothilones as natural compounds for novel anticancer drugs development. Int J Mol Sci. 2023;24:6063. doi: 10.3390/ijms24076063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Mu X, Du G. Microtubule-stabilizing agents: new drug discovery and cancer therapy. Pharmacol Ther. 2016;162:134–143. doi: 10.1016/j.pharmthera.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Xu B, Sun T, Zhang Q, Zhang P, Yuan Z, Jiang Z, Wang X, Cui S, Teng Y, Hu XC, Yang J, Pan H, Tong Z, Li H, Yao Q, Wang Y, Yin Y, Sun P, Zheng H, Cheng J, Lu J, Zhang B, Geng C, Liu J, Shen K, Yu S, Li H, Tang L, Qiu R study group of BG01-1323L. Efficacy of utidelone plus capecitabine versus capecitabine for heavily pretreated, anthracycline- and taxane-refractory metastatic breast cancer: final analysis of overall survival in a phase III randomised controlled trial. Ann Oncol. 2021;32:218–228. doi: 10.1016/j.annonc.2020.10.600. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Sun T, Zhang Q, Yuan Z, Jiang Z, Wang XJ, Cui S, Teng Y, Hu XC, Yang J, Pan H, Tong Z, Li H, Yao Q, Wang Y, Yin Y, Sun P, Zheng H, Cheng J, Lu J, Zhang B, Geng C, Liu J, Peng R, Yan M, Zhang S, Huang J, Tang L, Qiu R, Xu B BG01-1323L study group. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: a multicentre, open-label, superiority, phase 3, randomised controlled trial. Lancet Oncol. 2017;18:371–383. doi: 10.1016/S1470-2045(17)30088-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Sun M, Qiu R, Tang L, Dou G, Xu B. Phase I clinical and pharmacokinetic study of UTD1, a genetically engineered epothilone analog in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:971–978. doi: 10.1007/s00280-011-1571-6. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Huang T, Tang Y, Li Q, Wang J, Cheng X, Zhang W, Zhang B, Zhou C, Tu S. Utidelone inhibits growth of colorectal cancer cells through ROS/JNK signaling pathway. Cell Death Dis. 2021;12:338. doi: 10.1038/s41419-021-03619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C, Li G, Deng D, Li R, Li X, Feng X, Wu T, Shao X, Chen W. Efficacy of electroacupuncture in the treatment of peripheral neuropathy caused by Utidelone: study protocol for a randomized controlled trial. Front Neurol. 2023;14:1065635. doi: 10.3389/fneur.2023.1065635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Lenz HJ. Should continuous infusion 5-fluorouracil become the standard of care in the USA as it is in Europe? Cancer Invest. 2006;24:50–55. doi: 10.1080/07357900500449694. [DOI] [PubMed] [Google Scholar]
- 10.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward RA, Fawell S, Floc’h N, Flemington V, McKerrecher D, Smith PD. Challenges and opportunities in cancer drug resistance. Chem Rev. 2021;121:3297–3351. doi: 10.1021/acs.chemrev.0c00383. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang H, Yu B, Tao D, Xu X, Xu Y, Wang J, Jiao Y, Wang L. The role of m6A methylation in therapy resistance in cancer. Mol Cancer. 2023;22:91. doi: 10.1186/s12943-023-01782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao M, Jiang Q, Hu H, Han J, She L, Yao L, Ding D, Huang J. Cost-effectiveness analysis of utidelone plus capecitabine for metastatic breast cancer in China. J Med Econ. 2019;22:584–592. doi: 10.1080/13696998.2019.1588125. [DOI] [PubMed] [Google Scholar]
- 15.Meurer-Grob P, Kasparian J, Wade RH. Microtubule structure at improved resolution. Biochemistry. 2001;40:8000–8008. doi: 10.1021/bi010343p. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)) J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 17.Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med Res Rev. 2012;32:1220–1262. doi: 10.1002/med.20239. [DOI] [PubMed] [Google Scholar]
- 18.Bae EH, Greenwald MK, Schwartz AG. Chemotherapy-induced peripheral neuropathy: mechanisms and therapeutic avenues. Neurotherapeutics. 2021;18:2384–2396. doi: 10.1007/s13311-021-01142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molassiotis A, Cheng HL, Lopez V, Au JSK, Chan A, Bandla A, Leung KT, Li YC, Wong KH, Suen LKP, Chan CW, Yorke J, Farrell C, Sundar R. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019;19:132. doi: 10.1186/s12885-019-5302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess J, Ferdousi M, Gosal D, Boon C, Matsumoto K, Marshall A, Mak T, Marshall A, Frank B, Malik RA, Alam U. Chemotherapy-induced peripheral neuropathy: epidemiology, pathomechanisms and treatment. Oncol Ther. 2021;9:385–450. doi: 10.1007/s40487-021-00168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Bao T, Baser R, Chen C, Weitzman M, Zhang YL, Seluzicki C, Li QS, Piulson L, Zhi WI. Health-related quality of life in cancer survivors with chemotherapy-induced peripheral neuropathy: a randomized clinical trial. Oncologist. 2021;26:e2070–e2078. doi: 10.1002/onco.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20:1451. doi: 10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol. 2021;11:626687. doi: 10.3389/fimmu.2020.626687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsusaka S, Yamasaki H, Kitayama Y, Okada T, Maeda S. Differential effects of two fluorouracil administration regimens for colorectal cancer. Oncol Rep. 2003;10:109–113. [PubMed] [Google Scholar]