Abstract
Lenvatinib (LEN) is a multi-target TKI, which plays a pivotal role in the treatment of advanced hepatocellular carcinoma (HCC). The inevitable occurrence of drug resistance still prevents curative potential and is deleterious for the prognosis, and a growing body of studies is accumulating, which have devoted themselves to unveiling its underlying resistance mechanism and made some progress. The dysregulation of crucial signaling pathways, non-coding RNA and RNA modifications were proven to be associated with LEN resistance. A range of drugs were found to influence LEN therapeutic efficacy. In addition, the superiority of LEN combination therapy has been shown to potentially overcome the limitations of LEN monotherapy in a series of research, and a range of promising indicators for predicting treatment response and prognosis have been discovered in recent years. In this review, we summarize the latest developments in LEN resistance, the efficacy and safety of LEN combination therapy as well as associated indicators, which may provide new insight into its resistance as well as ideas in the treatment of advanced HCC.
Keywords: Lenvatinib, drug resistance, combination therapy, prognostic factors
Introduction
Liver cancer is the fifth most common tumor, and also the third leading of cancer mortality worldwide. There are approximately 870,000 new cases and approximately 760,000 deaths of liver cancer in 2022, and the overall situation is not optimistic. Hepatocellular carcinoma (HCC) is the most common type of liver cancer with an insidious onset and a rapid progression, accounting for 75-85% of the total, and more than half of patients are diagnosed at the advanced stage and have lost the opportunity for surgical resection [1]. Systemic chemotherapy is traditionally considered the main curative approach contributing to the improvement of both life quality and survival, but associated severe adverse reactions and extremely high drug resistance incidences made it difficult to achieve ideal therapeutic efficacy [2].
In 2007, the arrival of sorafenib (SOR), initially a multi-targeted tyrosine kinase inhibitor (TKI), considerably transformed this circumstance, which undoubtedly opened a new door for targeted therapy and served as the solitary first-line targeted drug for HCC therapy over the past decade, albeit the overall survival (OS) was prolonged only 2.8 months (10.7 vs 7.9 months) [3]. In recent years, systemic therapy for HCC has achieved remarkable advancements, and the advent of lenvatinib (LEN) broke the embarrassing situation. The continuous approval of molecular targeted drugs including cabozantinib, ramucirumab and bevacizumab, and immune checkpoint inhibitors (ICIs) including nivolumab and pembrolizumab, as well as the emergence of the breakthrough combination therapy of atezolizumab/bevacizumab (ATEZ/BEV), which has greatly improved the prognosis of HCC [4]. However, the rise in the incidence of HCC has overshadowed this favorable scenario to a certain extent, and drug resistance, adverse reactions, individual differences, and other factors persistently impede our progress.
LEN, a multi-target TKI, directly inhibits related RTKs, including the platelet-derived growth factor receptor (PDGFR), KIT, and RET, as well as selectively suppresses the VEGFR1-3 and fibroblast growth factor receptors (FGFR1-3) [5]. Six years ago, the REFLECT study reported the superiority of LEN in OS, progression-free survival (PFS), time to progression (TTP), and objective response rate (ORR) [6,7]. Despite LEN’s considerable role in systemic therapy in the past few years, acquired resistance is clinically inevitable. A growing number of scientists have devoted themselves to deciphering the underlying mechanisms of LEN resistance and searching for new therapeutic targets, potential diagnostic markers, and prognostic indicators. Besides, combination therapy is undoubtedly identified as the future direction, with the aim of investigating the safety and clinical efficacies of various combination therapies, and a burgeoning number of studies have emerged. In our study, we concentrated our topics on these two aspects, collated and summarized the safety and therapeutic efficacy of LEN combination therapy in HCC (Supplementary Table 1), and the LEN resistance mechanism.
Advances of lenvatinib monotherapy in advanced HCC
Advances of lenvatinib monotherapy in advanced HCC
The REFLECT trial, a multicenter, phase III, randomized, noninferiority study, has brought a new dawn to the targeted molecular-targeted therapy of HCC [7]. It revealed that, compared with the SOR group, the LEN group exhibited the longer mOS (13.6 vs 12.3 months) and mPFS (7.3 and 3.6 months), the higher ORR (40.6 vs 12.4%) and DCR (73.8 vs 58.4%), according to mRECIST criteria, respectively. The tumor response in a series of studies referred to in our review was evaluated by the RECIST v.1.1 or mRECIST criteria, the evaluation method of tumor response not mentioned is mRECIST criteria by default. The relatively satisfactory efficacy and safety of LEN determined its clinical superiority, and it was approved by the Food and Drug Administration of the United States in August 2018 for the first-line treatment of patients with advanced uHCC. This section primarily concentrated on identifying potential prognostic indicators that may be capable of directing clinical medication and prediction of prognosis.
Biomarkers for guiding lenvatinib therapy and predicting prognosis
Within the context of examining the linkages between nutritional status and the efficacy of LEN therapy, the geriatric nutritional risk index (GNRI) and skeletal muscle index (SMI) were identified as key indicators of LEN treatment. GNRI is used to assess the nutritional status of aging patients, matched formula is calculated as follows: GNRI = 1.489 × serum albumin (g/L) + [41.7 × body weight (kg)/ideal body weight (kg)] [8]. Low GNRI scores have been linked to unfavorable outcomes in patients afflicted with heart failure [9], chronic hemodialysis [10], and malignancy [11], etc. Akiyoshi et al. conducted a retrospective analysis involving 61 HCC patients undergoing LEN treatment and observed that those with high GNRI levels (>98, n=35) exhibited a reduced discontinuation rate (46.2 vs 17.1%, P=0.014), higher PFS (HR: 1.83; 95% CI: 0.996-3.351, P=0.047) [12]. Concurrently, SMI signifies skeletal muscle mass, and the matched formula is calculated as follows: SMI = subcutaneous fat area (cm2)/height2 (m2), which has been detected intimately associated with poor prognosis in tumors [13-15]. Haruki et al. retrospectively enrolled 100 uHCC patients receiving LEN therapy and examined the influence of SMI on patient survival prognosis. Based on the creation of sarcopenia assessment criteria of the Japan Society of Hepatology, low SMI is defined as <42 cm2/m2 for men and <38 cm2/m2 for women [16]. Their finding indicated that the high SMI group has the lower withdrawal rate (17.1 vs 39.0%, P=0.042), the longer mOS (11.77 vs 8.80 months, P=0.021), and mTTF (7.67 vs 4.63 months, P=0.010) [17].
The frequent biomarkers of systemic inflammation such as c-reactive protein (CRP) levels, CRP to albumin ratio (CAR), Glasgow prognostic score (GPS), platelet-to-lymphocyte ratio (PLR), and neutrophil to lymphocyte ratio (NLR) have been consistently confirmed to be associated with LEN therapeutic effect across multiple studies. Okumura et al. enrolled 125 uHCC patients treated with LEN and observed that those with low CRP levels (<0.5 mg/dL) exhibited the longer mOS (22.9 vs 7.8 months, P<0.001) and median time to treatment failure (TTF) (8.5 vs 4.4 months, P=0.007) [18]. Similarly, Toshifumi et al. discovered that the lower CAR ratio group (<0.108) had the longer mOS (27.2 vs 13.3 months, P<0.001) and mPFS (8.8 vs 5.6 months, P<0.001) [19]. GPS, a predictive index derived from serum CRP and albumin levels, categorizes patients into three groups: GPS 0 denotes absence of both elevated CRP and hypoalbuminemia, GPS 1 indicates presence of either condition, and GPS 2 signifies existing of both conditions. This scoring system has previously proven useful in predicting cancer outcomes [20-22]. Toshifumi et al. examined 508 Child-Pugh A uHCC patients undergoing LEN therapy and identified that high GPS scores may be related to poor outcomes. The study revealed that the mOS for patients assessed as GPS 0, 1 and 2 was 28.5, 16.0, and 9.1 months, respectively (P<0.001), while the mPFS was 8.8, 6.8 and 3.8 months, respectively (P<0.001) [23]. Otherwise, PLR, an inflammatory indicator frequently linked to poor prognosis across a range of tumor types [24,25], was further investigated by Toshifumi et al., who recruited 283 uHCC patients with LEN therapy. They found that PLR (≥150) was significantly correlated with the shorter OS and PFS [26]. Additionally, NLR has been reported to be associated with poor prognosis in many malignancies [27]. Toshifumi et al. recruited 237 uHCC patients and found that NLR≥4 was associated with poor OS and PFS as well as shorter DCR (67.3 vs 85.5%, P=0.007) [28].
To evaluate whether EGFR/ERBB2 alterations is a predictor of LEN resistance during initial treatment, Lim et al. retrospectively enrolled 46 HCC patients with baseline ctDNA profiling. These patients were categorized into three groups: Group 1 consisted of patients with EGFR/ERBB2 alterations before LEN therapy (n=6), Group 2 consisted of patients without EGFR/ERBB2 alterations before LEN therapy (n=32), and Group 3 consisted of patients with EGFR/ERBB2 alterations before PD-1 therapy (n=17). Compared to group 1, group 2 had the higher DCR (62.5 vs 20%, P<0.05), and had longer mPFS (5.9 vs 2.2 months, P<0.05) and mOS (9.2 vs 3.9 months, P=0.08). This indicated that EGFR/ERBB2 alterations in patients may be associated with poor prognosis and LEN resistance in HCC patients [29].
Bi et al. retrospectively enrolled 9 patients with uHCC who received LEN treatment after liver biopsy. Through immunohistochemical staining and multicolor flow cytometry, they observed that patients with objective responses (n=4) had significant infiltration of T cells and PD-L1-expressing macrophages in and around the HCC tissue (P<0.05). They propose that T cell infiltration and PD-L1 expression of macrophages may act as potential predictors of LEN response in HCC treatment [30].
In their research conducted by Shigesawa et al., it was found that among HCC patients exhibiting low serum FGF19 levels (<194 pg/ml) and receiving LEN therapy, the ORR of LEN therapy was 86% (12/14), which was significantly higher than 31% (4/13) in patients with high FGF19 expression [31]. Osamun et al. reported a case of HCC with high FGFR4 expression which showed a better therapeutic effect for LEN after the failure of SOR treatment [32]. Norifumi et al. retrospectively analyzed 31 recurring uHCC patients including 16 FGFR4-positive patients and 15 FGFR4-negative patients. All of these participants underwent LEN therapy and displayed evidence suggesting FGFR4-positive individuals having a superior ORR (68.8 vs 20.0%, P=0.0113) and improved progression-free survival (PFS; P=0.0052) [33]. Similarly, the longer mPFS (5.5 vs 2.5 months, P=0.01) and higher ORR (81 vs 31%, P=0.006) were also detected in 57 uHCC patients with immunohistochemically positive expression for FGFR4 [34].
To explore the relationship between well-controlled viremia and the efficacy of treatment with LEN, a total of 129 patients were enrolled in Xiao et al.’s study, including 85 HBV-associated HCC patients and 44 HCV-associated HCC patients. They identified either patients with undetectable virus or patients receiving antiviral therapy for at least 6 months prior to LEN treatment as well-controlled viremia patients. It was confirmed that both HBV- and HCV-HCC patients with well-managed viremia exhibited longer PFS (8.8 vs 3.1 months, P<0.001) and OS (30.2 vs 12.8 months, P=0.041), which were significantly better than those in the uncontrolled viremia group. Despite this, no significant correlation was elucidated between HBV or HCV infections and the course of tumor progression among LEN-treated HCC patients [35].
Advances of lenvatinib combination therapy in advanced HCC
Advances of lenvatinib plus transcatheter arterial chemoembolization therapy in advanced HCC
A comprehensive analysis was performed to assess the therapeutic potency and safety profile of the LEN+transcatheter arterial chemoembolization (TACE) therapy for uHCC with portal vein tumor thrombus (PVTT). A single study retrospectively analyzed a cohort of 12 HCC patients with PVTT who received consecutive combined therapy. The results unveiled that the mOS and mPFS were 16.9 and 6.15 months. Moreover, and the ORR and DCR were 75% and 91.7%, respectively. Notably, no treatment-related deaths or severe adverse reactions (grade 4 events) were reported in their study period [36].
LEN plus TACE vs TACE
To compare the efficacy and safety between LEN+TACE vs TACE, a number of associated clinical trials are identified. Long et al. enrolled 46 patients treated with double therapy and 57 patients treated with TACE therapy. It was shown that the ORR in the LEN+TACE group was higher than that in the TACE group (69.57 vs 40.35%, P<0.05). No significant difference in 1-year and 2-year PFS rates and 1-year OS rates between the two groups, but the 2-year OS rate in the LEN+TACE group was significantly higher than that in the TACE group (73.91 vs 50.88%, P=0.025). Although all reported TRAEs were manageable, the frequency of adverse events including hypertension, diarrhea, and gingival bleeding was notably greater in the double therapy group (P<0.05) [37]. Liu et al. retrospectively analyzed 66 patients diagnosed with BCLC stage B2 HCC, of whom 34 patients underwent LEN+TACE therapy while the remaining 32 patients received solely TACE therapy. In double therapy group, the 6-month (97.1 vs 93.8%), 1-year (85.3 vs 81.1%), and 2-year (76.3 vs 45.4%) OS rates (P=0.023) were dramatically prolonged than that in TACE group, despite no significant disparity in PFS rates (P=0.510) [38]. Xie et al. retrospectively enrolled 104 uHCC patients and classified them into the LEN+TACE group (n=53) and TACE group (n=51). Their findings elucidated an enhanced ORR (77.36 vs 56.36%, P<0.05) in the LEN+TACE group as well as prolonged 12-month (81.1 vs 64.7%, P<0.05) and 18-month OS rates (69.8 vs 49.1%, P<0.05), yet no significant difference emerged in the incidence of TRAEs and 6-month OS rate between the two groups (P>0.05) [39]. Chen et al. retrospectively evaluated a cohort of 215 uHCC patients, to minimize the selection bias, 34 patients of the LEN+TACE group and 68 patients of the TACE group were selected in a ratio of 1:2. The mPFS (8.3 vs 4.6 months, P=0.008) and mOS (27.7 vs 18.4 months, P=0.043) were prolonged, and the ORR (67.6 vs 39.7%, P=0.008) was dramatically increased in the LEN+TACE group [40]. Fu et al. retrospectively enrolled 120 patients treated with either LEN+TACE therapy (n=60) or TACE therapy (n=60). The LEN+TACE group exhibited higher ORR (68.3 vs 31.7%, P<0.001) and superior 1-year and 2-year OS rates (88.4 vs 79.8%; 79.2 vs 49.2%, P=0.047), as well as higher 1-year and 2-year PFS rates (78.4 vs 64.7%; 45.5 vs 38.0%, P<0.001). The most common TRAEs reported in the LEN+TACE group were decreased albumin (55.0%), hypertension (48.3%), and decreased platelet count (46.7%), all of which were manageable [41].
LEN plus TACE vs LEN
To evaluate the efficacy and safety of LEN+TACE therapy vs LEN monotherapy, Kuroda et al. enrolled a total of 247 uHCC patients who had been administered either LEN or LEN+TACE therapy and identified 63 patients of each group after propensity score matching (PSM). It was found that mOS (31.2 vs 13.9 months, P=0.002) and mPFS (12.2 vs 7.1 months, P=0.037) in the LEN+TACE group were significantly superior to those in the LEN therapy group. Furthermore, the double therapy group exhibited more satisfactory ORR (61.9%) and DCR (74.6%). No statistical difference was reported in TRAEs between the two groups [42]. Peng et al. initiated a multi-center Phase III trial involving 338 uHCC patients, these subjects were randomly classified into the LEN+TACE group (n=170) and LEN group (n=168) in equal proportion. It was detected that the LEN+TACE group had the longer mOS (17.8 vs 11.5 months, P<0.001) and mPFS (10.6 vs 6.4 months, P<0.001), and the higher ORR (54.1 vs 25.0%, P<0.001) and DCR (94.1 vs 73.2%, P<0.001). In addition, multivariate analysis indicated that PVTT and treatment allocation served as independent risk factors for OS [43]. Ando et al. retrospectively enrolled 88 patients classified into LEN+TACE group (n=30) and LEN group (n=58). After PSM, 19 patients from each group were chosen to analyze the prognosis. The LEN+TACE group had the relatively longer mPFS (11.6 vs 10.1 months, P=0.019) and mOS (not reached vs 16.9 months, P=0.007), and had higher incidences of AST/ALT elevation and fever [44].
To evaluate the clinical efficacy and safety of LEN+drug eluting bead-TACE (DEB-TACE) therapy and LEN monotherapy. One study showed that uHCC patients receiving LEN+DEB-TACE therapy (n=142) had the longer mOS (15.9 vs 8.6 months, P=0.0022) and mPFS (8.6 vs 4.4 months, P<0.001), and the higher ORR (46.48 vs 13.05%, P<0.001) than those receiving LEN therapy (n=69). The most common TRAEs in the LEN+DEB-TACE and LEN groups were elevated AST levels (54.9%) and fatigue (46.4%), respectively, which were almost all manageable [45]. Another retrospective study included 118 uHCC patients with LEN+DEB-TACE therapy and 182 uHCC patients with LEN therapy, 78 pairs of patients were retained after PSM. It was found that the double therapy group had the higher ORR (57.7 vs 25.6%, P<0.001), 6-month and 12-month OS rates (88.5 vs 71.4%; 67.6 vs 43.4%), and 6-month and 12-month PFS rates (60.3 vs 42.3%; 21.1 vs 10.3%), and also acquired the longer TTP (15.7 vs 11.3 months, P<0.001) and mOS (8.0 vs 5.0 months, P=0.003). Moreover, vascular invasion and treatment mode were detected as independent predictors for OS and TTP [46].
LEN plus TACE vs SOR plus TACE
To evaluate the safety and therapeutic efficacy between LEN+TACE vs SOR+TACE therapy, an extensive meta-analysis encompasses the LEN+TACE group (n=261) and the SOR+TACE group (n=337). It was shown that LEN+TACE therapy group had the higher rate of odds ratio (OR) for ORR (OR: 3.63; 95% CI: 1.89-6.95; I squared statistic (I2) =57%, P<0.001) and DCR (OR: 3.78; 95% CI: 2.00-7.16; I2=52%, P<0.001), and had the longer OS (HR: 0.67; 95% CI: 0.52-0.85; I2=1%, P=0.001) and PFS (HR: 0.49; 95% CI: 0.88-0.62, P<0.001) and TTP (HR: 0.62; 95% CI: 0.45-0.84, P=0.002) compared to SOR+TACE group. And, the incidences of hypertension and proteinuria were dramatically increased in the LEN+TACE group, which was in contrast to the decreased incidence of hand-foot-skin reaction [47]. There were several clinical studies suggesting the superiority of LEN+TACE therapy as follows. Xu et al. enrolled a total of 84 uHCC patients and divided them into the SOR+TACE group (n=24) and LEN+TACE group (n=25). The findings indicated that the LEN+TACE group had the longer mOS (13 vs 8 months, P<0.05) and mPFS (10 vs 6.5 months, P<0.05), and the higher DCR (86.0 vs 76.7%, P=0.03) and ORR (62.8 vs 46.3%, P=0.027). With respect to TRAEs, the LEN+TACE group exhibited a higher incidence of diarrhea, hand-foot syndrome, hypertension, and rash, albeit these were all controllable [48]. Yang et al. recruited 116 HCC patients with PVTT receiving LEN+TACE therapy (n=59) or SOR+TACE therapy (n=57). It was observed that the LEN+TACE group had the longer mOS (16.4 vs 12.7 months, P=0.025), and the higher ORR (60.7 vs 38.9%, P=0.022). The TRAEs of such two therapies were all comparably safe and well tolerated [49]. Zhang et al. retrospectively evaluated a cohort of 112 patients receiving either LEN+TACE therapy (n=53) or SOR+TACE therapy (n=59). The LEN+TACE group had the longer mPFS (10.7 vs 6.0 months, P=0.002) and mOS (30.5 vs 20.5 months, P=0.018). Additionally, the higher DCR (81.1 vs 61.0%, P=0.020) and ORR (54.7 vs 44.1%, P=0.260) were also observed in the LEN+TACE group. Moreover, all TRAEs were comparable between the two groups [50].
To explore the efficacy and tolerability of LEN+DEB-TACE compared to SOR+DEB-TACE in the treatment of uHCC. Xu et al. retrospectively analyzed 150 patients and found that, in the LEN+DEB-TACE group (n=50), the ORR was substantially upraised (64.0 vs 33.3%, P=0.008), the OS and TTP were significantly prolonged than that in SOR+DEB-TACE group (n=100). Furthermore, subgroup analysis indicated that among patients with PVTT, the LEN+DEB-TACE regimen exhibited a superior OS and TTP, and patients with FGF21 amplification in the LEN+DEB-TACE group had longer OS. In addition, the incidence of hand and foot skin reactions was significantly reduced in the LEN+DEB-TACE group (32.0 vs 49.0%, P=0.048), yet the incidence of proteinuria (26.0 vs 10.0%, P=0.010) was significantly higher than that in the SOR+DEB-TACE group [51].
Advances of lenvatinib and atezolizumab/bevacizumab therapy in advanced HCC
LEN therapy after ATEZ/BEV therapy failure
Yano et al. reported a 68-year-old male uHCC patient with adrenal metastasis, who was discontinued due to adrenal metastatic tumor enlargement after 3 weeks of treatment with ATEZ/BEV, and his drug was replaced with LEN monotherapy. Intriguingly, following 1 month treatment of LEN, he retrieved partial response (PR) and received conversion surgery therapy. Else, there were no significant complications during a 4-month follow-up without adjuvant therapy [52].
To evaluate the therapeutic efficacy of LEN in uHCC patients who have previously undergone ATEZ/BEV therapy. Hisanori et al. enrolled 20 patients who received LEN after ATEZ/BEV treatment, and found that the ORR and DCR were 25.0 and 95.0%, respectively according to RECIST v.1.1 criteria, the mPFS and mOS were 6.0 and 10.5 months, respectively [53]. In addition, another investigation encompassed 14 uHCC patients receiving LEN therapy subsequent to ATEZ/BEV therapy failure. The DCR was 57.1%, mPFS and mOS were 4.2 and 8.3 months, respectively, and the TRAEs were all tolerable [54]. Alternatively, one study involved 137 HCC patients treated with ATEZ/BEV and observed the mOS and mPFS were 21.1 and 10.5 months, respectively. And, 50 patients progressed during ATEZ/BEV therapy, of whom 24 patients re-engaged in LEN therapy and harvested the encouraging mOS of 15.3 months, mPFS of 4.0 months, and ORR of 54.2% from the initiation of LEN therapy. And, the TRAEs in LEN therapy patients were all manageable [55].
LEN vs ATEZ/BEV
To elucidate the therapeutic efficacy and safety of LEN vs ATEZ/BEV. Toshifumi et al. recruited a total of 358 uHCC patients treated with ATEZ/BEV (n=177) or LEN (n=181) and found that mPFS in the ATEZ/BEV group was substantially longer than that in the LEN group (10.8 vs 7.3 months, P=0.019) [56]. The better prognosis and safety of ATEZ/BEV as the first-line therapy were also demonstrated in several studies. Takashi et al. retrospectively enrolled 304 patients divided into ATEZ/BEV group (n=152) and LEN group (n=152). It was observed that ATEZ/BEV group had the longer mPFS (8.3 vs 6.0 months, P=0.005) and mOS (not reached vs 20.2 months, P=0.039), higher surgical conversion rate (8.6 vs 1.9%, P=0.007). The ATEZ/BEV group demonstrated a lower incidence of anorexia, fatigue, and grade 3+ proteinuria, and a higher rate of grade 3+ bleeding [57]. Similarly, one prospective multi-center study included 272 HCC patients treated with ATEZ/BEV (n=90) or LEN (n=182), and indicated that ATEZ/BEV group (n=66) had the longer mPFS (8.8 vs 5.2 months, P=0.012) after PSM (1:1). Additionally, the rate of discontinuation due to AEs was dramatically reduced in ATEZ/BEV group (12.1 vs 28.8%, P=0.018) [58].
A single study aimed to evaluate the efficacy and safety of ATEZ/BEV vs LEN in the treatment of uHCC patients with advanced age (>80 years), which encompassed 170 patients receiving LEN therapy and 92 patients receiving ATEZ/BEV therapy. Additionally, no statistical discrepancy in ORR, DCR, mPFS, and mOS was discerned between the two groups, yet the incidence of post-progressive treatment in the ATEZ/BEV group was notably higher (59.0 vs 35.7%, P=0.01), and the rate of discontinuation due to AEs was lower than that in the LEN group (20.7 vs 40.6%, P=0.001) [59].
Conversely, there still existed some studies reporting LEN’s superiority, particularly in patients with special clinical characteristics. One investigation incorporated 217 uHCC patients with Child-Pugh B, demonstrating that the mOS in 152 patients receiving LEN therapy was prolonged compared to 65 patients receiving ATEZ/BEV therapy (13.8 vs 8.2 months, P=0.005), with no statistically significant disparity observed for mPFS within their research [60]. Else, another study integrated 8 retrospective cohort studies encompassing a total of 3690 uHCC patients, subgroup analysis indicated that LEN exhibited positive results in OS (HR 1.32, 95% CI 1.04-1.67, P=0.380) among the non-viral infected patients, despite superior survival outcome of PFS and reduced incidence of 3/4 AEs being observed in ATEZ/BEV group [61]. In a retrospective study involving 2205 uHCC patients: LEN group (n=1341) and ATEZ/BEV group (n=864), no difference in OS was identified between the two groups (P=0.739), but subgroup analysis suggested that ATEZ/BEV prolonged the OS in patients with viral infection (P=0.024), while LEN prolonged OS in patients with nonalcoholic steatohepatitis/nonalcoholic fatty liver disease (P=0.014) [62].
LEN plus TACE vs ATEZ/BEV plus TACE
To evaluate the efficacy and safety of LEN+TACE vs ATEZ/BEV+TACE in treatment for uHCC. Zhao et al. enrolled 34 patients in each of two groups, and discovered no substantial difference in the 6-month, 12-month OS rates, the mPFS, ORR, and DCR between the two groups according to RECIST v.1.1 or mRECIST criteria. Besides, the LEN+TACE group has a higher incidence of hand-foot skin reaction (35.3 vs 5.9%, P=0.003) and proteinuria (17.9 vs 2.9%, P=0.046) compared to those in the ATEZ/BEV+TACE group [63].
Biomarkers for guiding atezolizumab/bevacizumab or lenvatinib therapy and predicting prognosis
C-X-C Motif Chemokine Ligand 9 (CXCL9) is a ligand of the chemokine receptor CXCR3, which can induce lymphocyte infiltration into the lesion site and inhibit tumor growth [64]. To investigate the correlation between serum levels of CXCL9 and ATEZ/BEV therapeutic efficacy in uHCC, Hosoda et al. demonstrated that the serum levels of CXCL9 were considerably diminished in uHCC with early progressive disease (PD), and patients with elevated serum levels of CXCL9 (≥333 pg/mL) exhibited a reduced early PD occurrence (2.9 vs 35.3%, P=0.0012) and prolonged mPFS (7.57 vs 4.20 months, P=0.0084). Conversely, in the LEN therapy group, it was determined that the patients with low serum levels of CXCL9 (<333 pg/mL) had a reduced early PD frequency (4.9 vs 14.6%, P=0.15) and higher ORR (69.8 vs 43.9%, P=0.027), else, patients with low serum levels of CXCL9 (<308 pg/mL) had a marginally significantly longer mOS (61.67 vs 39.75 months, P=0.096) [65].
It was observed that CD8+ tumor-infiltrating lymphocytes (TILs) have undergone exhaustion in the tumor microenvironment (TME) of HCC, and reversing this process may augment HCC therapy [66,67]. To investigate whether CD8+ TILs can predict the response of HCC patients to ATEZ/BEV or LEN therapy. Akifumi et al. enrolled 39 uHCC patients treated with ATEZ/BEV (n=24) or LEN (n=15). By immunohistochemical staining of HCC tissues prior to systematic treatment, it was found that, in the ATEZ/BEV group, patients with high CD8+ TILs expression (n=12) had the higher ORR (66.6 vs 33.3%, P=0.012) and DCR (83.3 vs 50.0%, P=0.031) and longer mPFS (6.9 vs 4.7 months, P=0.047). In addition, it was found that patients with high CD8+ TILs expression (n=5) had higher ORR (40 vs 20%, P=0.417), lower DCR (40 vs 80%, P=0.121) and shorter mOS (6.3 vs 9.5 months, P=0.315), but the sample size was too small to draw rational conclusion [68].
Advances of lenvatinib plus radiotherapy therapy in advanced HCC
It was widely acknowledged that radiotherapy (RT) exhibited encouraging advantages in treating uHCC patients with PVTT. Many studies have demonstrated the superiority of LEN+RT combined with PD-1 and/or TACE therapy in uHCC. We summarized the associated studies in this section as follows. Qian et al. reported a 62-year-old female diagnosed with recurrent HCC with right atrium (RA) and inferior vena cava (IVC) tumor thrombosis, who showed PR after receiving LEN+PD-1+RT treatment and acquired more than 7 months of PFS with maintenance therapy of LEN+PD-1 [69]. Moreover, to assess the efficacy and safety of LEN+PD-1+RT therapy for uHCC patients with main trunk portal vein tumor thrombus (Vp4), Li et al. enrolled 39 uHCC patients with PVTT (Vp4) receiving such triple therapy. Encouragingly, the mOS and mPFS were 9.4 and 4.9 months, respectively, and the ORR was 61.5%. All TRAEs were manageable and no treatment-related deaths occurred [70]. Additionally, it was found that RT upregulated PD-L1 expression in HCC patients and amplified the effects of immunotherapy [71,72].
LEN plus RT vs LEN
To evaluate the efficacy and tolerability of LEN+stereotactic body radiation therapy (SBRT) compared to LEN monotherapy in uHCC with PVTT. Ji et al. retrospectively analyzed 37 patients treated with LEN+SBRT and 77 patients treated with LEN treatment. It was discerned that, in the LEN+SBRT group, the mOS (19.3 vs 11.2 months, P<0.001) and mPFS (10.3 vs 5.3 months, P<0.001) were significantly prolonged, and the ORR (56.8 vs 20.8%, P<0.001) was improved than that in the LEN group. The subgroup analysis also confirmed the superior prognosis of combination therapy in both the Vp1-2 and Vp3-4 subgroups. Otherwise, most of the TRAEs were controllable, and no statistical difference was observed in the incidences between the two groups [73].
A single study suggested that LEN or SOR augmented radio responsiveness in uHCC therapy. In vivo experiments revealed that the xenograft tumor growth and vascular volume density were inhibited in nude mice following 2 weeks of treatment with LEN or SOR. Additionally, in contrast to treatment for SOR, LEN induced vascular normalization more efficaciously and promptly, and strongly improved the intratumoral microenvironment of HCC, augmenting its radio responsiveness [74].
LEN plus RT vs RT
To evaluate the clinical efficacy and safety of LEN+SBRT vs SBRT in the treatment of uHCC, Wang et al. retrospectively enrolled a total of 144 patients, including 106 patients who received SBRT therapy and 38 patients who underwent LEN+SBRT therapy. After the PSM, 35 patients from each group proceeded to further evaluation, and it was shown that the LEN+SBRT group exhibited notably prolonged mOS (16.8 vs 11.0 months, P=0.043) and mPFS (9.1 vs 3.7 months, P<0.001), along with an elevated ORR (54.29 vs 22.86%, P=0.007). In addition, the majority of toxicities observed in the LEN+SBRT group were mild to moderate and manageable [75].
LEN plus TACE plus RT vs LEN plus TACE
To investigate the efficacy and safety between LEN+TACE+ external RT therapy vs LEN+TACE therapy for uHCC with PVTT, Dong et al. prospectively recruited a total of 102 patients receiving LEN+TACE+RT therapy (n=51) or LEN+TACE therapy (n=51). The triple therapy group demonstrated superior mOS (22.8 vs 17.1 months, P=0.031) and mPFS (12.8 vs 10.5 months, P=0.035), albeit with an increased rate of TRAEs (100 vs 64.7%, P<0.001). However, there were no significant differences noted in the occurrence of grade 3/4 TRAEs among these groups (54.9 vs 49.0%, P=0.552) [76].
Advances of lenvatinib plus anti-PD-1 plus hepatic arterial infusion chemotherapy therapy in advanced HCC
Hepatic arterial infusion chemotherapy (HAIC) is an extensively utilized strategy for treating advanced HCC, and its indications have greatly expanded over the last few decades [77]. The synergistic effect of LEN+PD-1+HAIC has demonstrated superior outcomes in uHCC treatment, we organized and summarized existing pertinent studies detailed below. Yuan et al. reported a 52-year-old female patient with massive uHCC, who underwent one cycle of combined therapy (mHAIC+TACE+LEN+PD-1), and three cycles of combined therapy (mHAIC+LEN+PD-1), and then achieved complete response (CR). Besides, they also reported a 57-year-old male uHCC patient with PVTT, who received 4 cycles of LEN+PD-1+HAIC therapy and achieved PR with a PFS of up to 7 months [78]. To evaluate this combined therapy’s efficacy and tolerability, a retrospective study included 61 uHCC patients receiving LEN+PD-1+HAIC therapy, revealing an ORR of 57.4%, DCR of 82.0%, and mPFS of 6.0 months. The most common TRAEs were neutropenia, abdominal pain, and elevated AST levels, which were all manageable [79]. Besides, Xu et al. retrospectively analyzed 97 uHCC patients with high-risk features: Vp4 and/or tumor occupation ≥50% liver volume (TO≥50%), who received LEN+HAIC+PD-1 therapy. The mPFS and mOS were 9.8 and 19.3 months, respectively, the ORR and DCR were 78.3% and 92.8%, respectively, and TRAEs were all manageable. In their study, it was found that the patients with low serum levels of procalcitonin (PCT) (≤0.13 ng/mL) had a better prognosis [80]. Moreover, one study retrospectively analyzed the clinical data of 98 uHCC patients receiving LEN+PD-1+TACE/HAIC therapy. The 37 patients were classified as potentially resectable (PRP), and the other 61 patients were defined as non-potentially resectable population (NPRP). The ORRs for such two groups were 67.9% and 22.9% based on RECIST v.1.1 criteria. In the PRP group, 15 patients underwent surgical resection (3 of them achieved pCR), which had the longer mPFS (25 vs 13 months, P=0.0025) and mOS (not reached vs 21 months, P=0.014) [81].
LEN plus PD-1 plus HAIC vs LEN plus PD-1
To explore the efficacy and safety of LEN+PD-1+HAIC group vs LEN+PD-1 group in uHCC patients. Fu et al. retrospectively enrolled a total of 142 uHCC patients with PVTT and categorized them into either LEN+PD-1+HAIC group (n=89) or LEN+PD-1 group (n=53). The results indicated that the triple therapy group had the superior mOS (26.3 vs 13.8 months, P<0.001), mPFS (11.5 vs 5.5 months, P<0.001), and the ORR (61.8 vs 20.8%, P<0.001) were 3 times higher than those in LEN+PD-1 group. Although the occurrence of TRAEs was upraised in the LEN+PD-1+HAIC group, most were tolerable and manageable [82]. Additionally, Chen et al. retrospectively evaluated 170 PD-L1 staining uHCC patients and subsequently divided them into two groups: LEN+PD-1+HAIC group (n=84) and LEN+PD-1 group (n=86). It was found that the mOS (17.7 vs 12.6 months, P=0.001) and mPFS (10.9 vs 6.8 months, P=0.001) were prolonged in LEN+PD-1+HAIC group. And, the incidence of TRAEs was also greatly higher than that in the LEN+PD-1 group (79.8 vs 62.8%, P=0.015), albeit those TRAEs were all controllable [83]. Furthermore, compared with LEN+PD-1 group (n=25), a separate study revealed that LEN+PD-1+HAIC group (n=45) had the higher ORR (40.0 vs 16.0%, P=0.038) and DCR (77.6 vs 44.0%, P<0.001), and the longer mOS (15.9 vs 8.6 months, P=0.0015) and mPFS (8.8 vs 5.4 months, P=0.032) [84]. Similarly, Diao et al. retrospectively analyzed a total of 121 TACE-refractory uHCC patients, who received either LEN+PD-1+HAIC therapy (n=58) or LEN+PD-1 therapy (n=63). The ORR (48.30 vs 23.80%, P=0.005) and DCR (87.90 vs 69.80%, P=0.02) were increased, and the mOS (24.0 vs 13.0 months, P=0.001) and mPFS (13.0 vs 7.2 months, P<0.001) were dramatically prolonged in LEN+PD-1+HAIC group. Multivariate analysis showed that cirrhosis, Child-Pugh B and LEN+HAIC+PD-1 therapy served as independent prognostic factors for OS and PFS, respectively. In addition, the TRAEs were all controllable in both groups [85].
LEN plus PD-1 plus HAIC vs LEN plus PD-1 plus TACE
To evaluate the clinical efficacy of LEN+PD-1+HAIC vs LEN+PD-1+TACE in the treatment of uHCC patients with PVTT and arterio-portal shingle (APF). Lin et al. enrolled a total of 95 patients, including 34 patients in the LEN+PD-1+HAIC group and 61 patients in the LEN+PD-1+TACE group. The results indicated that the LEN+PD-1+HAIC group had the higher ORR (52.9 vs 27.9%, P=0.03) and DCR (100 vs 88.5%, P=0.001) according to RECIST v.1.1 criteria, along with the longer mOS (25.0 vs 19.3 months, P=0.035), mPFS (21.74 vs 8.74 months, P=0.007) and median duration of response (mDOR) (20.43 vs 9.13 months, P=0.067) [86].
LEN plus PD-1 plus HAIC vs LEN plus HAIC
A single study assessed the therapeutic effectiveness and safety between the groups with LEN+PD-1+HAIC therapy (n=75) and LEN+HAIC therapy (n=74). And, the results showed that the LEN+PD-1+HAIC group exhibited a higher mOS (16.0 vs 9.0 months, P=0.002) and longer mPFS (11.0 vs 6.0 months, P<0.001), and were more susceptible to developing hypertension (28.00 vs 13.51%, P=0.029) [87]. Furthermore, another study retrospectively collected clinical information from 145 HAIC refractory uHCC patients who received LEN+PD-1+HAIC therapy (n=51) or LEN+HAIC therapy (n=51) and found that mOS (43.6 vs 18.9 months, P=0.009) and mPFS (35.6 vs 9.4 months, P=0.009) in the LEN+PD-1+HAIC group were dramatically prolonged [88].
LEN plus PD-1 plus HAIC vs LEN plus PD-1 plus HAIC plus TAE
To evaluate the effectiveness and safety of the combination of LEN+PD-1+HAIC, supplemented by/without TAE, a retrospective study including 100 patients (50 patients of each group) revealed the longer mOS (14.1 vs 11.3 months, P=0.041) and mPFS (5.6 vs 4.4 months, P=0.037), and higher ORR (72.0 vs 52.0%, P=0.039) and better DCR (88.0 vs 76.0%, P=0.118) in the quadruple therapy group, albeit not statistically significant. And, 59% of patients were reported to emerge with manageable TRAE of gastrointestinal discomfort [89].
LEN plus PD-1 plus HAIC vs LEN
To evaluate the effectiveness and tolerability between LEN+PD-1+HAIC therapy and LEN therapy in uHCC. One study retrospectively analyzed 157 patients, categorized as the LEN+PD-1+HAIC group (n=71) and LEN group (n=86). The results indicated that the LEN+PD-1+HAIC group had longer mPFS (11.1 vs 5.1 months, P<0.001) and mOS (not reached vs 11 months, P<0.001), and the higher ORR (67.6 vs 13.6%, P<0.001). In addition, 14.1% of patients in the LEN+PD-1+HAIC group achieved a CR for all lesions, and 21.1% attained a CR for intrahepatic target lesions. Notably, the occurrence of grade 3/4 TRAEs was higher in the LEN+PD-1+HAIC group but manageable among these participants [90].
Biomarkers for guiding LEN plus PD-1 plus HAIC therapy and predicting prognosis
There still existed several studies that had unearthed relevant biomarkers potentially advantageous in directing the LEN+PD-1+HAIC treatment regimen. We summarized these findings within this section. Lai et al. found that the low C-C motif chemokine ligand 28 (CCL28) and betacellulin (BTC) levels were positively correlated with poor prognosis, which may function as a predictive biomarker for such triple therapy [91]. Furthermore, a retrospective study enrolled 88 uHCC patients receiving LEN+PD-1+HAIC therapy and revealed that the patients belonging to the low NLR group (<3.46) exhibited superior OS (not reached vs 9.6 months, P=0.017) and mPFS (18.3 vs 5.3 months, P=0.0015) [92].
Advances of lenvatinib plus HAIC therapy in advanced HCC
LEN plus HAIC vs HAIC
To investigate the effectiveness and tolerability of LEN+HAIC vs HAIC therapy in uHCC, Long et al. enrolled 132 patients receiving HAIC therapy and 110 patients receiving LEN+HAIC therapy. The 1-year, 2-year, and 3-year OS rates of the LEN+HAIC group were all higher than those in the HAIC group (63.6 vs 47.2%, 12.1 vs 11.8% and 3.0 vs 2.7%, respectively, P<0.001). The BMI and AST levels were identified as independent prognostic factors of OS [93]. Along the same lines, another small sample research retrospectively analyzed the clinical information from 4 uHCC patients with HAIC therapy and 9 uHCC patients with LEN+HAIC therapy. The ORR was dramatically higher in the LEN+HAIC group (66.7 vs 0, P<0.05) based on RECIST v.1.1 criteria, and no significant difference in the mOS (7.0 vs 6.0 months) and mPFS (5.0 vs 3.0 months) was found between both groups [94].
LEN plus HAIC vs LEN plus HAIC plus sequential ablation
To evaluate the effectiveness and tolerability of LEN+HAIC therapy vs LEN+HAIC+ sequential ablation therapy in uHCC patients. Liu et al. retrospectively analyzed a total of 150 uHCC patients, with 97 belonging to the LEN+HAIC group and 53 belonging to the LEN+HAIC+ sequential ablation. The mOS (30 vs 13.6 months, P=0.010) and mPFS (12.8 vs 5.6 months, P<0.001) were significantly prolonged in this triple therapy group. And, there was no significant difference in the rate of TRAEs observed between these two groups [95].
Advances of lenvatinib vs HAIC therapy in advanced HCC
LEN vs HAIC
To compare the effectiveness and tolerability of LEN therapy vs HAIC therapy in uHCC patients. A comprehensive, multi-center cohort study enrolled 244 patients treated with either LEN (n=71) or HAIC (n=173). After PSM, a total of 52 patients from each group were chosen, the HAIC group had a higher DCR (73.1 vs 51.9%), and no statistical difference was found in ORR (26.0 vs 23.1%, P=0.736) based on the RECIST v1.1 criteria, mPFS (3.6 vs 4.0 months, P=0.706) and mOS (10.8 vs 7.9 months, P=0.106) between the HAIC and LEN groups. Subgroup analysis of patients with high tumor burden showed the highly longer OS in the HAIC group (10.0 vs 5.4 months, P=0.004). Furthermore, no disparity was identified in the incidence of grade 3/4 TRAEs between these two groups [96].
LEN vs HAIC plus PD-1
To evaluate the effectiveness and tolerability of LEN vs PD-1+HAIC therapy in uHCC patients. A retrospective study enrolled 118 uHCC patients with vascular invasion and/or extrahepatic spread, categorized as the LEN group (n=65) and PD-1+HAIC group (n=53). Compared with the LEN group, it was found that the PD-1+HAIC group had longer mOS (17.1 vs 10.1 months, P=0.005) and mPFS (9.3 vs 4.8 months, P=0.006), along with higher ORR (47.2 vs 9.2%, P<0.001) and DCR (86.8 vs 69.2%, P=0.002) according to RECIST v.1.1 criteria. Both groups have an acceptable safety profile [97].
Advances of lenvatinib plus anti-PD-1 therapy in advanced HCC
It has been widely demonstrated that LEN+PD-1 therapy has a synergistic effect to increase therapeutic efficacy in uHCC, in this segment, we primarily discuss the impact and outcome of uHCC patients receiving such combination therapy. Yang et al. retrospectively analyzed 378 uHCC patients receiving the LEN+PD-1 therapy, who exhibited mOS of 17.8 months, mPFS of 6.9 months, ORR of 19.6%, and DCR of 73.5% [98]. Consistently, Xu et al. retrospectively analyzed 210 patients undergoing LEN+PD-1 therapy, revealing ORR and DCR of 28.1% and 75.2% according to RECIST v.1.1 criteria, respectively, and the mOS of 17.2 and mPFS of 8.4 months, respectively. Subgroup analysis indicated that Child-Pugh A patients had significantly longer mOS (18.8 vs 5.9 months) and mPFS (9.1 vs 4.4 months) than Child-Pugh B patients, patients with albumin-bilirubin (ALBI) grade 1 also had a significantly higher mOS compared to grade 2/3 patients (23.5 vs 13.4 months), and patients with ALBI grade 2/3 had a higher incidence of grade 3/4 TRAE (57.5 vs 38.5%) [99]. Sun et al. retrospectively evaluated the clinical data of 84 uHCC patients receiving LEN+PD-1 therapy, of whom 31 patients with TO≥50% and 30 patients with Vp4 invasion. The mPFS and mOS among these patients were 6.6 and 11.4 months, respectively. Subgroup analysis showed that patients with TO≥50% had a lower ORR (P=0.015) according to RECIST v.1.1 criteria and shorter mPFS (P<0.001). On the contrary, no substantial distinction was found in ORR, mPFS and mOS between HCC patients with and without tumor thrombosis [100].
Furthermore, a retrospective study affirmed the favorable efficacy and safety of LEN+PD-1 therapy in uHCC patients after the progression of original LEN treatment (n=46). This study illustrated satisfactory therapeutic outcomes, reporting the ORR and DCR of 23.9 and 71.7% according to RECIST v.1.1 criteria, and the mPFS and mOS of 6.9 and 14.5 months. The most common TRAEs were anorexia (43.5%), hypothyroidism (43.5%), and hypertension (36.9%), all of which were all manageable [101].
Moreover, to elucidate the distinct outcomes between the synchronous and asynchronous treatment of LEN+PD-1 in uHCC, a study enrolled 213 oligometastatic advanced HCC patients and divided them into two groups: the simultaneous treatment group (121 patients received simultaneous LEN+PD-1 therapy) and the asynchronous treatment group (92 patients received PD-1 therapy 3 months after receiving LEN prior to tumor progression). Compared with the asynchronous treatment group, the synchronous treatment group exhibited greater OS rates at 12 (93.4 vs 71.5%) and 24 months (58.1 vs 25.3%) and significantly higher PFS rates at 6 (82.6 vs 63.4%), 12 (42.6 vs 14.2%) and 18 months (10.8 vs 0%) [102].
Association between LEN plus PD-1 therapy and surgical resection
For those with advanced uHCC, preoperative systemic therapy (PST) is deemed pivotal in the treatment of advanced uHCC patients, improving the surgical conversion rates, although its efficacy and safety are unclear. Zhang et al. enrolled 56 uHCC patients with BCLC stage B/C receiving the LEN+PD-1 therapy. The surgical conversion rate was 55.4%, ORR was 53.6%, and mPFS and the mOS were 8.9 and 23.9 months, respectively. Of the 31 successfully converted patients, 21 underwent surgery with the R0 resection rate of 85.7%, the pathological complete response (pCR) rate of 38.1%, and the 12-month RFS rate of 47.6% [103]. Another investigation comprised 107 uHCC patients, after LEN+PD-1 therapy, 30 patients experienced tumor regression (15 of them achieved OR) and underwent conversion surgery, and 10 patients were confirmed to reach pCR. After a median follow-up of 16.5 months, 28 patients were alive and 11 of them had tumor recurrence [104]. Overall, it was confirmed that such double therapy may improve conversion resection rates in initial uHCC patients, thereby improving prognosis [104].
Furthermore, to evaluate the efficacy and safety of LEN+PD-1 therapy before surgical resection, one study involved 147 patients, 49 of whom underwent PST prior to HCC surgery and 98 underwent upfront hepatectomy. In comparison with the UH group, patients in the PST group had more intraoperative blood loss and blood transfusion, longer postoperative hospital stay, lower ALBI score after surgery, and liver failure occurrence were more common after postoperative hepatectomy, nevertheless, the 30-day morbidity and 90-day mortality were similar in both groups [105].
LEN plus PD-1 vs LEN
To evaluate the efficacy and safety of LEN+PD-1 therapy vs LEN therapy, many researches emerged continuously in recent years. Josep et al. executed a global randomized, double-blind, Phase III study (LEAP-002), including 1309 patients, 794 of whom were randomly allocated to the LEN+PD-1 group (n=395) or the LEN+ placebo group (n=399). It was demonstrated that the LEN+PD-1 group had longer mOS (21.2 vs 19.0 months, P=0.023) and mPFS (8.2 vs 8.0 months, P=0.047). Moreover, there was no statistical disparity in TRAEs and treatment-related deaths between the two groups [106]. Concurrently, a retrospective study collated clinical data from 139 uHCC patients categorized into the LEN+PD-1 group (n=54) and the LEN group (n=85). Compared with the LEN therapy group, the double therapy group had longer mOS (21.7 vs 12.8 months, P=0.0051) and mPFS (11.3 vs 6.6 months, P=0.0128), and acquired higher DCR (92.6 vs 74.1%, P=0.006) and ORR (38.9 vs 24.7%, P=0.076). Hypertension was the most common AE in both groups, and some immune-related AEs, such as hypothyroidism (n=5) and elevated serum creatinine (n=3), etc., occurred only in the LEN+PD-1 group, all TRAEs were controllable [107]. Additionally, another retrospective study also supported that in the treatment of uHCC, the ORR was higher in the LEN+PD-1 group (n=40) than in the LEN group (n=47) (45.0 vs 23.4%, P=0.03) according to RECIST v.1.1 criteria, and the mPFS (7.5 vs 4.8 months, P=0.05), and mOS (22.9 vs 10.3 months, P=0.01) were also prolonged in the LEN+PD-1 group. The most TRAEs were dermatitis (35.0%), pruritus (27.5%), and hypothyroidism (27.5%). Only a minor proportion of patients experienced grade 3/4 toxicity reactions [108]. Similarly, to investigate the efficacy of LEN+PD-1 therapy in uHCC patients infected with HBV, another retrospective study discerned that the LEN+PD-1 therapy group exhibited superior longer mOS (21.4 vs 14 months, P=0.041) and mPFS (8.0 vs 6.3 months, P=0.015) compared to those in the LEN therapy group, and there was no significant difference in TRAEs between the two groups. The subgroup analysis was conducted to find that the Child-Pugh B HCC patients with PVTT or extrahepatic diffusion (EHS) possessed high sensitivity to LEN+PD-1 therapy which increased 12-month survival by 38% (higher than 18% in the rest of the population) [109]. In addition, many studies have compared the therapeutic efficacy of LEN+PD-1 therapy and LEN monotherapy, which consistently shed light on the superiority of such double therapy [110-112].
LEN plus PD-1 vs regorafenib+PD-1
Aiming to assess the therapeutic efficacy and safety of uHCC patients treated with LEN+PD-1 vs regorafenib (REG) +PD-1 after SOR treatment failure. Xu et al. enrolled 61 uHCC patients and divided them into the LEN+PD-1 group (n=32) and REG+PD-1 group (n=29). Based on RECIST v.1.1 criteria, the ORR (12.5 vs 10.3%, P=0.557) and DCR (71.9 vs 58.6%, P=0.207) were improved in the LEN+PD-1 group, and the mOS (5.3 vs 4.0 months, P=0.512) and mPFS (14.1 vs 13.7 months, P=0.764) were better in LEN+PD-1 group, although no statistical difference was observed between the two groups. All TRAEs that happened in these two groups were controllable. The findings of this study suggested that the PD-1+LEN/REG therapy exhibited promising therapeutic effects post-SOR therapeutic failure, and the LEN+PD-1 therapy seemed to exhibit better results [113].
LEN plus PD-1 vs SOR plus PD-1
To compare the clinical efficacy of LEN+PD-1 vs SOR+PD-1 in the treatment of uHCC patients. Hsueh et al. recruited 208 uHCC patients, 49 of whom were treated with SOR+PD-1, 39 patients were treated with LEN+PD-1, and the result showed that the LEN+PD-1 group had higher ORR (23.08 vs 18.37%, P=0.944) and DCR (41.03 vs 28.57%, P=0.561), and longer mOS (13.1 vs 7.8 months, P=0.017), along with the similar incidence of TRAEs [114].
LEN plus PD-1 vs PD-1
To evaluate the LEN+PD-1 therapy vs PD-1 therapy in uHCC treatment, Liu et al. retrospectively collected 94 patients with advanced HCC, among them, 39 patients treated with PD-1 and 30 patients treated LEN+PD-1, and found that the LEN+PD-1 group had significantly the higher ORR (32.7 vs 10.3%, P=0.013) and DCR (80.0 vs 53.8%, P=0.012) according to RECIST v.1.1 criteria, and the longer mPFS (10.6 vs 4.4 months, P<0.001) as well as mOS (18.4 vs 8.5 months, P=0.013) [115].
LEN plus PD-1 vs surgery
To assess the prognosis of HCC patients at high recurrence risk with/without postoperative adjuvant treatment of LEN+PD-1 treatment. One study enrolled 137 HCC patients, 85 of whom underwent hepatectomy alone, and 52 patients underwent hepatectomy combined with postoperative adjuvant LEN+PD-1 therapy. Compared to the hepatectomy group, the adjuvant therapy group displayed a greater mRFS (not reached vs 5.5 months, P<0.001), a higher 2-year RFS rate (56.5 vs 24.2%, P<0.001), whereas no disparity was observed in mOS of both groups (26.4 vs 26.6 months, P=0.098). Multivariate analysis indicated that adjuvant therapy loss, high Child-Pugh grade, high AFP levels, MVI, and satellite disease were independent risk factors for recurrence within 6 months postoperatively [116].
The mechanisms of action concerned with anti-PD-1 plus lenvatinib therapy
In this section, we summarized several studies dedicated to uncovering the associated mechanisms of LEN+PD-1 therapy efficacy. Yang et al. confirmed that LEN contributed to the formation of the NRP-1-PDGFRβ complex and activated the CRKL-G3G-Rap1 signaling cascade in endothelial cells, induced vascular normalization, and synergistically augmented the efficacy of PD-1 therapy [117]. Zhou et al. demonstrated that LEN notably enhanced immunogenic cell death (ICD), a crucial cell death type that occurred in anti-cancer therapy process [118], and upregulated ICD receptors, TLR3 (upregulating PD-L1 expression) and TLR4 (promoting HCC cell apoptosis). Histopathology and survival prognosis analysis also suggested that the TLR3 and TLR4 positive rates were markedly elevated in patients treated with LEN, and untreated patients with TLR3-positive expression exhibited superior OS and RFS (P<0.05) [119]. The Phosphatidylinositol-glycan biosynthesis class L (PIGL) was identified as an inhibitory metabolic enzyme involved in the regulation of TME, rendering HCC sensitive to LEN+PD-1 therapy. Mechanistically, Hua et al. discovered that elevated nuclear PIGL inhibited the cMYC/BRD2 axis to diminish the CCL2 and CCL8 expression through recruitment of macrophages and regulatory T cells involved in the formation of immunosuppressor TME, thereby inhibiting tumor immune escape and promoting the combination therapeutic effects. It was also confirmed that elevated nuclear PIGL predicted better prognosis of HCC patients and equipped potential application value to guide such double therapy through survival analysis [120].
A study revealed that physical activity synergistically improved prognosis in uHCC patients treated with LEN+PD-1. They found that the exercise group (n=28) had the better OS (HR=0.220, 95% CI: 0.060-0.799) and PFS (HR=0.158, 95% CI: 0.044-0.562), and higher ORR (95% CI: 1.482-14.102) than those in the sedentary group (n=31). Mechanistically, it was found that physical activity suppressed the Treg cell infiltration and immune checkpoint expression (CTLA4, TIGIT and TIM3), augmenting the LEN+PD-1 therapy efficacy [121]. Yi et al. also found that LEN could inhibit FGFR4 to reduce PD-L1 expression levels and Treg differentiation, improving anti-PD-1 efficacy in HCC, and proposed that high FGFR4 expression may be used as a biomarker for predicting better efficacy in HCC patients using LEN+PD-1 therapy [122]. Mex-3 RNA binding family member C (MEX3C), as a RNA-binding protein, has been shown to facilitate tumor progression [123,124]. Guo et al. found that MEX3C was upregulated in HCC tissues and was associated with poor prognosis, and deduced that targeting MEX3C may influence tumor microenvironment via regulating the abundance and proportion of immune cells including Tregs, MDSCs, and NK cells, which may potentially intensify immunotherapy efficacy [125]. Through single gene sequencing of HCC samples treated with/without LEN+PD-1 treatment, Chen et al. detected that the LEN+PD-1 therapy increased the TNF/NF-κB signaling in all immune cell types. Further studies showed that mucosal-associated invariant T (MAIT) cells secreted TNF and activated TNF receptor superfamily member 1B (TNFRSF1B) on regulatory T cells, thereby promoting immunosuppression, which contributed to HCC therapeutic resistance to the LEN+PD-1 therapy [126].
Biomarkers for guiding LEN plus PD-1 therapy and predicting prognosis
Lots of studies have shown the association between obesity and adverse prognosis in tumor patients [127], it has been reported that subcutaneous adipose tissue (SAT) parameters are linked to tumor progression [128]. Zhang et al. retrospectively enrolled 56 uHCC patients receiving the LEN+PD-1 therapy. Based on SAT volume index (SAT area divided by height squared: cm2/m2) and density, patients were divided into two groups: high-risk group (low SAT volume index and high density, n=21) and low-risk group (high SAT volume index and low density, n=35). They discovered that the ORR of high-risk patients was considerably decreased (19.0 vs 54.3%, P=0.021), the mPFS was significantly shortened (6.0 vs 12.0 months, P=0.035), and a significant reduction in OS rates was noticed in high-risk patients with BCLC stage C as well [129].
To investigate the utility of peripheral blood lymphocyte subsets in predicting the responsiveness of LEN+PD-1 therapy in uHCC patients. Zou et al. contrasted the peripheral blood lymphocyte subpopulation counts of 15 patients with OR and 16 patients without OR post-LEN+PD-1 therapy, revealing that T helper (Th) cells and natural killer (NK) cells exhibited a propensity to be more abundant in the OR group. They deduced that the patients with elevated counts of Th cells or NK cells may have a higher ORR [130]. In addition, another prospective cohort study scrutinized peripheral blood samples from 61 advanced or uHCC patients within 3 days prior to initiation of LEN+PD-1 therapy. Peripheral naive CD8 T cell subsets served as predictive biomarkers for LEN+PD-1 therapy in these patients, and were poorly expressed at cellular levels in responders. The optimal cutoff for peripheral naive CD8 T cell subsets was determined to be 6.24%, and the sensitivity, specificity, positive predictive value, and negative predictive value of double therapy response were 81.0%, 61.5%, 63.0%, and 80.0%, respectively [131]. Cao et al. retrospectively analyzed 194 uHCC patients receiving LEN+PD-1 therapy, and confirmed the ratio of white blood cell counts (×109/L)/lymphocyte proportion (%) as a novel circulating immune index (CII), which was an independent prognostic indicator for OS. The mOS were prolonged in patients with CII≤43.1 compared to patients with CII>43.1 (24.7 vs 15.1 months, P=0.019), and the low CII levels group also had better DCR (89 vs 73%, P=0.031) according to RECIST v.1.1 criteria [132].
Advances of lenvatinib plus anti-PD-1 plus TACe therapy in advanced HCC
The superiority of the LEN+PD-1+TACE therapy has been demonstrated in a series of studies. Within this segment, we mainly elucidated the efficacy and safety of such triple therapy, the specific content was as follows. Wu et al. evaluated the clinical data of 62 uHCC patients and discovered that patients treated with the LEN+PD-1+TACE therapy attained the highest ORR (80.6%) and hepatectomy conversion rates (53.2%) compared with any double therapy or single therapy [133], the incidence of TRAEs was 74.2%, which were all controllable. Similarly, Ying et al. retrospectively analyzed 53 uHCC patients receiving the LEN+PD-1+TACE therapy, the ORR was 54.9%, mPFS was 8.5 months, and all TRAEs were manageable [134]. Wu et al. recruited and analyzed 35 patients receiving the LEN+PD-1+DEB-TACE therapy. The ORR was 82.9%, the DCR was 91.4%, the mTTR was 7 weeks, the mPFS was 9 months, and 40% of patients underwent surgical intervention, all TRAEs were controllable [135]. Notably, the findings of several studies [136-141] were consistent with the outcome as mentioned above.
Recently, the LEN+PD-1+TACE therapy has come to the fore as a promising therapy in treatment of uHCC. There are also several studies focused on the efficacy of this combination therapy in uHCC patients with PVTT as follows. It was reported that a 51-year-old-aged uHCC patient with PVTT obtained a chance of surgical resection following treatment with LEN+PD-1+TACE, and achieved a cure with tumor-free status for over 34 months [142]. Li et al. retrospectively enrolled 68 uHCC patients with PVTT who received LEN+PD-1+TACE therapy, among them, the ORR and DCR were 26.1% and 78.3%, respectively, the mPFS and mOS were 9.3 and 18.2 months, respectively. The tumor number >3 was identified as an adverse risk factor for PFS and OS. And, no treatment-related deaths occurred, all TRAEs were controllable [143]. Another study further analyzed 41 uHCC patients with PVTT (Vp4), the ORR was 68.3%, the mOS and mPFS were 21.7 and 14.5 months, respectively, 12 of whom (29.3%) achieved the criteria for conversion rate to liver resection. A total of 35 patients (85.3%) experienced TRAEs that did not result in mortality during therapy [144]. Furthermore, compared with a total of 58 uHCC patients receiving triple therapy, the uHCC patients with IVC and/or RA tumor thrombosis had greater ORR (62.1%) and DCR (94.9%), and longer mPFS of 14.3 months [145].
LEN plus PD-1 plus TACE vs LEN plus TACE
In recent times, a plethora of studies have been undertaken to evaluate the efficacy and safety of LEN+PD-1+TACE therapy vs LEN+TACE therapy in uHCC treatment. A prospective study by Cai et al. encompassed 81 uHCC patients and classified them into the LEN+PD-1+TACE group (n=41) and the LEN+TACE group (n=40). It was observed that the triple therapy group had dramatically longer mOS (16.9 vs 12.1 months, P=0.009) and mPFS (7.3 vs 4.0 months, P=0.002), and higher ORR (56.1 vs 32.5%, P=0.033) and DCR (85.4 vs 62.5%, P=0.019), and there was no statistical difference in the incidence of TRAEs and its severity. Nevertheless, it was noted that the LEN+PD-1+TACE therapy failed to achieve superior clinical outcomes in patients with Vp4 [146]. Furthermore, another study mainly focused on unresectable multiple nodular and large HCC and revealed that, compared to the LEN+TACE group (n=49), the LEN+PD-1+TACE (n=33) group had longer PFS (9.4 vs 5.9 months, P<0.01) and OS (16.4 vs 11.0 months, P<0.01), higher local response rate (LRR) (51.5 vs 46.9%, P=0.233) and DCR (81.8 vs 77.6%, P=0.429), and had no massive bleeding or treatment-related deaths [147]. In agreement with the above results, Sun et al.’s research also indicated that, compared to the LEN+TACE therapy (n=52), the LEN+PD-1+TACE therapy (n=31) had longer mPFS (12.5 vs 6.6 months, P<0.001) and mOS (18.9 vs 13.9 months, P<0.001), improved the ORR (71 vs 42.3%, P=0.023), and no statistical disparity was observed in DCR between two groups (93.5 vs 80.8%, P=0.195), all TRAEs were tolerable in these two groups. After multivariate analysis, tumor number and treatment modality were identified as two independent prognosis factors for PFS and OS, else, BCLC stage was also recognized as another prognosis factor for OS [148]. Besides, the superior therapeutic efficacy in the LEN+PD-1+TACE group has been reported in several studies [149-151].
LEN plus PD-1 plus TACE vs LEN plus PD-1
To evaluate the effectiveness and safety of LEN+PD-1+TACE therapy vs LEN+PD-1 therapy, many related studies have been conducted persistently over the past few years. One study analyzed 118 uHCC patients who received either LEN+PD-1+TACE therapy (n=60) or LEN+PD-1 therapy (n=58). The results shown that the triple therapy group had longer mOS (29.0 vs 17.8 months, P<0.01) and mPFS (16.2 vs 10.2 months, P<0.01), and higher ORR (76.7 vs 44.9%, P<0.01) and DCR (96.7 vs 75.9%, P<0.01) [152]. Similarly, Wang et al. found that, after 1:1 PSM to minimize bias (n=86), the LEN+PD-1+TACE group had a higher ORR (55.8 vs 30.2%, P=0.017) and DCR (86.0 vs 65.1%, P=0.024), longer mOS (20.5 vs 12.8 months, P=0.013) and mPFS (12.1 vs 7.8 months, P=0.030), while there were no notable differences in TRAEs between the two groups, most of TRAEs were transient, manageable, and swiftly reversible [153]. Moreover, Lang et al. retrospectively analyzed 152 uHCC patients, including 39 patients receiving LEN+PD-1 and 75 patients receiving LEN+PD-1+TACE after 1:2 PSM. It was found that the LEN+PD-1+TACE group had longer mPFS (11.1 vs 5.1 months, P=0.033), mOS (not reached vs 14.0 months, P=0.0039), and higher ORR (44.0 vs 23.1%, P=0.028). No statistical disparity was detected in the incidence of grade ≥3 TRAEs between the two groups [154]. Furthermore, there was another study revealing that the LEN/SOR+PD-1+TACE group also had higher ORR (63.0 vs 29.6%, P<0.001) and DCR (85.2 vs 53.7%, P<0.001), longer mPFS (9.9 vs 5.8 months, P=0.026) and OS (not reached vs 18.5 months, P=0.003) [155].
LEN plus PD-1 plus TACE vs SOR plus PD-1 plus TACE
In addition, there exists one study showing the better efficacy of LEN+PD-1+TACE therapy than SOR+PD-1+TACE therapy in uHCC patients. Zou et al. enrolled 165 uHCC patients with PVTT who were treated with LEN+PD-1+TACE (n=80) or SOR+PD-1+TACE (n=85) and observed that the LEN+PD-1+TACE group had longer mOS (21.7 vs 15.6 months, P=0.0027) and mPFS (6.3 vs 3.2 months, P<0.001), along with higher ORR (41.25 vs 30.59%, P=0.008) and DCR (86.25 vs 62.35%, P=0.008). And, there was no significant difference noted in the incidence and severity of TRAEs between these two groups [156].
LEN plus PD-1 plus TACE vs PD-1 plus TACE vs TACE
To evaluate the efficacy and safety of LEN+PD-1+TACE therapy vs PD-1+TACE/TACE therapy in uHCC therapy. Wu et al. retrospectively analyzed a total of 141 BCLC stage C HCC patients who were divided into LEN+PD-1+TACE group (n=57), PD-1+TACE (n=41) and TACE group (n=43). The mOS of the LEN+PD-1+TACE group was significantly prolonged compared to that in both the PD-1+TACE group (19.8 vs 15.7 months, P<0.001) and the TACE group (19.8 vs 9.4 months, P<0.001). The mPFS in the LEN+PD-1+TACE group (11.4 months, 95% CI 7.6-15.3) was better than that in the PD-1+TACE groups (11.4 vs 8.4 months, P<0.001) and the TACE group (11.4 vs 4.8 months, P<0.001) as well. And, the superior outcomes of ORR (57.9%) and DCR (75.4%) were also detected in the LEN+PD-1+TACE group than those in the other two groups. The LEN+PD-1+TACE group had a higher incidence rate of grade 3+ TRAEs (28.1%) which were all acceptable [157]. In addition, Xiang et al. observed that 56 uHCC patients treated with LEN+PD-1+TACE displayed longer mPFS (22.5 vs 14.0 months, P=0.0013), and OS (26.0 vs not reached, P=0.0045), higher ORR (64.3 vs 38.3%, P=0.010) and DCR (85.7 vs 57.4%, P=0.002) compared to those in patients treated with PD-1+TACE (n=47) [158]. Compared with the TACE group (n=54), Qu et al. found the higher ORR (67.9 vs 29.6%, P<0.001), and the longer mPFS (11.9 vs 6.9 months, P=0.003) as well as mOS (23.9 vs 15.3 months, P<0.001) in the LEN+PD-1+TACE group (n=56), and the TRAEs encountered were all manageable [159].
LEN plus PD-1 plus TACE vs SOR plus TACE/LEN plus TACE
In addition, one study elucidated the therapeutic advantage of LEN+PD-1+TACE therapy relative to SOR+TACE or LEN+TACE therapy. Compared to the LEN+TACE group (n=32), it was shown that the mOS (26.7 vs 17.9 months, P=0.031) and mPFS (8.2 vs 6.6 months, P=0.047) were significantly prolonged, and ORR (86.96 vs 46.88%, P<0.001) and DCR (100 vs 75%, P<0.001) were greatly improved in the LEN+PD-1+TACE group (n=23). And, the mOS (26.7 vs 14.4 months, P=0.007) and mPFS (8.2 vs 6.0 months, P=0.005) were also prolonged, and the ORR (86.96 vs 34.48%, P<0.001) and DCR (100 vs 48.28%, P<0.001) were improved in the LEN+PD-1+TACE group compared to the SOR+TACE group (n=29). Furthermore, no obvious difference in the incidence of TRAEs and their severity was seen amongst these three groups [160].
Biomarkers for guiding LEN plus PD-1 plus TACE therapy and predicting prognosis
To date, several prognostic factors have been discovered in HCC patients with the treatment of LEN+PD-1+TACE, demonstrating considerable promise to be effective markers in guiding treatment programs and evaluating prognosis. Alpha-fetoprotein (AFP) and de-γ-carboxyprothrombin (DCP) are common tumor markers in the diagnosis of HCC [161], and alterations in these protein levels may serve as predictors of recurrence and survival outcomes in HCC patients treated with LEN+PD-1+TACE therapy [162-164]. Besides, Luo et al. observed that >50% reduction in the AFP or DCP levels after 6 weeks of the LEN+PD-1+TACE therapy may predict better ORR, longer PFS and OS [165].
Qu et al. demonstrated that NLR acted as an independent factor associated with PFS and OS in the LEN+PD-1+TACE group, and proved that mPFS (20.1 vs 6.2 months, P<0.001) were significantly prolonged in the low NLR group (≤3.11) [159]. Simultaneously, NLR level was elucidated as the only independent prognostic factor for both OS and PFS in a cohort of 63 uHCC patients receiving LEN+PD-1+TACE therapy, the low NLR group (<3.2) showed longer mPFS (19.3 vs 7.3 months, P<0.001) and mOS (28.9 vs 16.9 months, P<0.001), higher ORR (86.7 vs 39.4%, P<0.001), and were more accessible to reach early tumor shrinkage (ETS) ≥10% (73.3 vs 21.1%, P<0.001) [137]. Moreover, Li et al. also confirmed that uHCC patients with NLR≤2.165 had longer mOS (not reached vs 17.7 months, P=0.003) and mPFS (15.2 vs 7.5 months, P=0.047) [138]. Besides, Ning et al. analyzed and indicated that the serum procalcitonin (PCT) level also served as an independent prognostic factor of PFS and OS in HCC patients receiving LEN+PD-1+TACE therapy, the mPFS (15.5 vs 7.5 months, P=0.001) and mOS (25.3 and 15.3 months, P=0.016) were highly upraised in patients with low serum levels of PCT (≤0.13 ng/mL) [145].
Early tumor response has been detected to be a prognostic factor of surgical resection rates in Li et al.’s experiment. In their study including 94 uHCC patients receiving LEN+PD-1+TACE therapy, 68 (72.3%) of whom acquired early tumor response, and had significantly higher conversion surgery rates (44.1 vs 7.7%, P=0.001), longer mPFS (15.4 vs 7.8 months, P=0.005) as well as mOS (23.1 vs 12.5 months, P=0.004) [166]. Furthermore, the tumor number was also determined as an independent prognostic factor for uHCC patients receiving LEN+PD-1+TACE therapy. In patients with tumor number ≥3, the mOS (25.1 vs 14.1 months, P=0.012) and mPFS (16.4 vs 6.6 months, P=0.007) were longer than that in patients with tumor number<3 [138]. Moreover, multifactorial analysis of the LEN+PD-1+TACE group also showed that PVTT, Child-Pugh grade, interleukin-17 (IL-17), VEGF, PCT, and CRP were all independent factors of OS (P<0.05) [156].
The underlying mechanism of lenvatinib resistance
MAPK/ERK signaling
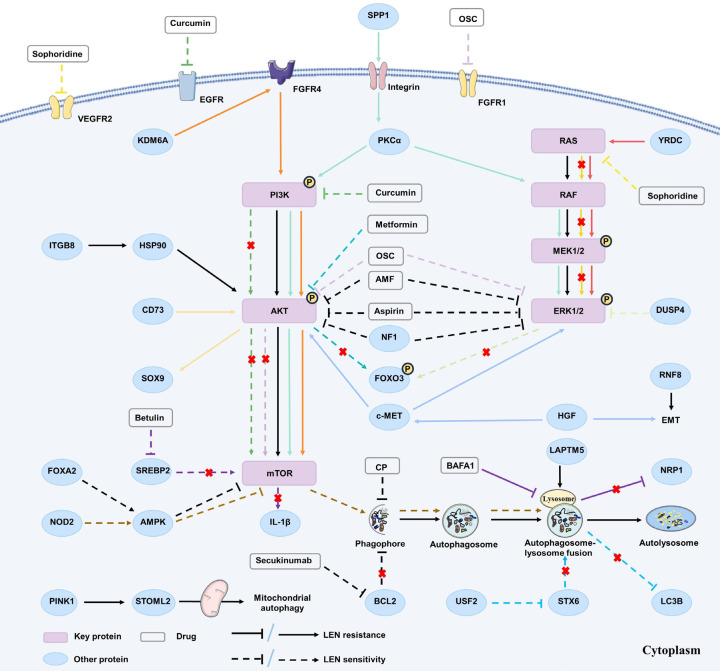
As widely known, MAPK/ERK signaling is a classic oncogenic signaling pathway, and it is discerned to be predominantly activated in HCC [167], which is highly associated with low survival and early recurrence in HCC patients [168,169]. The exploration of molecular targeted therapy to inactivate carcinogenic signaling pathways, including MAPK/ERK signaling has progressed rapidly [170]. LEN suppressed the receptor tyrosine kinase (RTK) to suppress downstream signaling pathways including MAPK/ERK signaling, inhibiting HCC occurrence and development [171]. Recent studies have indicated that the activation of MAPK/ERK signaling might be linked to LEN resistance (Figure 1).
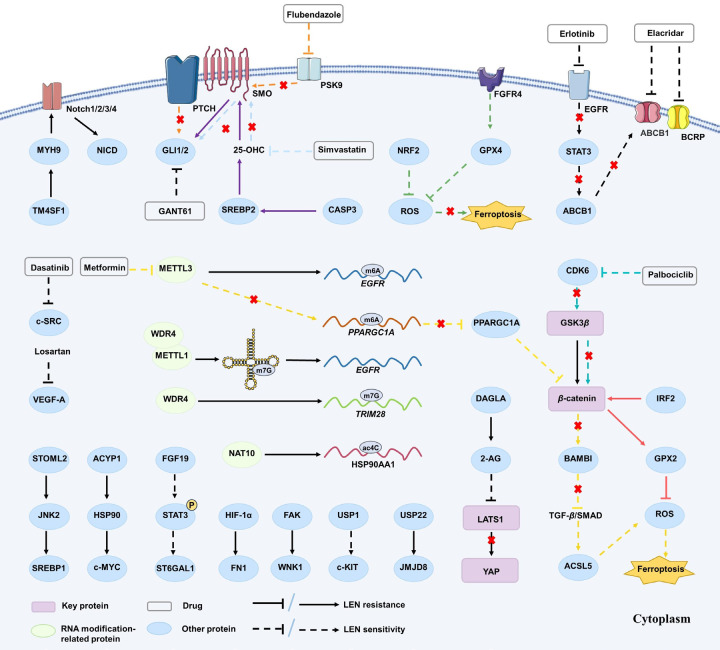
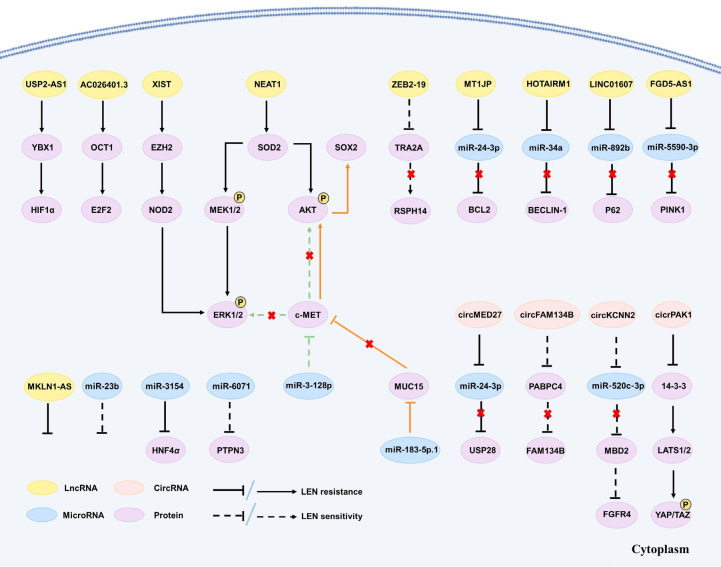
Figure 1.
The correlation between the dysregulation of three crucial signaling pathways and Lenvatinib resistance. The dysregulation of PI3K/AKT/mTOR, MAPK/ERK, and autophagy signaling pathways was proven to be associated with LEN resistance in HCC treatment. A series of proteins and a range of drugs, including Chinese herbal extracts, anti-tumor targeted drugs, NSAIDs and hypoglycemic drug etc., were observed to directly and indirectly act on corresponding targets associated with these signaling pathways, thus modulating LEN resistance.
Junjie et al. established LEN resistant (LR) -HCC cell lines and performed the sandwich enzyme immunoassay, revealing the activation of MAPK/ERK signaling pathways and upregulation of epithelial-mesenchymal transition (EMT) markers in LR-HCC cells [172]. Moreover, there are still many studies supporting the correlation between the MAPK/ERK signaling overactivation and LEN resistance. Wang et al. identified Frizzled-10 (FZD10) as a potential target for HCC prevention and treatment, which was highly expressed in hCSCs, where it may activate the β-catenin/c-Jun/MEK/ERK signaling axis to induce LEN resistance in HCC cells [173]. MAPKs are inactivated by dual-specificity phosphatases (DUSPs), such as DUSP4. Huang et al. demonstrated DUSP4 as a pivotal gene associated with LEN resistance via CRISPR/Cas9 library screening. They identified that DUSP4 was downregulated at both mRNA and protein levels in LR-HCC cells, and DUSP4 knockout improved LEN therapeutic effectiveness in vitro and in vivo, indicating that DUSP4 deficiency triggered LEN resistance by activating MAPK/ERK signaling [174]. Concurrently, by using CRISPR/Cas9 library screening, Lu et al. identified two key resistance genes, neurofibrin 1 (NF1) and DUSP9, as key drivers of LEN resistance in HCC. They further elucidated that NF1 loss activated MAPK/ERK and PI3K/AKT signaling pathways, whereas DUSP9 loss contributed to MAPK/ERK signaling pathway, thereby phosphorylating and activating FOXO3 and triggering its degradation, finally resulting in LEN resistance [175]. Cancer-associated fibroblasts (CAFs) was convinced to exert key roles in chemoresistance [176,177], the secreted phosphoprotein 1 (SPP1) produced by CAFs was identified to activate MAPK/ERK and PI3K/AKT/mTOR signaling through the integrin-protein kinase C-α (PKCα) signaling and promote EMT process, leading to LEN resistance in HCC [178]. YRDC, an ATPase integral to the biosynthesis of ubiquitous tRNA modification [179], can activate the MAPK/ERK signaling pathway and is proven positively correlated with HCC cell proliferation and metastasis [180]. Guo et al. found that YRDC knockdown inhibited the sensitivity of HCC cells to LEN and demonstrated its anti-tumor effect in vivo [181]. Further experiments confirmed that YRDC promoted the KRAS translation by regulating tRNA modification, and then activated the RAS/RAF/MEK/ERK signaling to participate in inducing LEN resistance.
In recent years, natural extracts used in tumor treatment have attracted more and more attention, and a large number of studies have focused on its potential anti-tumor and reversing-drug resistance mechanism. Amentoflavone (AMF), a biflavonoid extracted in plants, was proven to inhibit HCC progression [182]. Yang et al. found that AMF not only enhanced the LEN-induced inhibition of AKT/ERK signaling, but also promoted cell apoptosis, thus enhancing HCC sensitivity to LEN [183]. Sophoridine is a naturally bioactive alkaloid extracted in the roots of Sophora flavescens Ait, which has been proven to inhibit HCC development [184]. Zhong et al. found that sophoridine not only inhibited LR-HCC cell proliferation, migration, but also reversed LEN resistance in vitro and in vivo. Mechanistically, sophoridine decreased the expression of transcription factor E26 transformation specific sequence 1 (ETS-1) in LR-HCC cells to downregulate VEGFR2 expression and downstream RAS/RAF/MEK/ERK signaling, thereby augmenting the HCC sensitivity to LEN [185]. Oxysophocarpine (OSC) is one of the active alkaloid components extracted from the Chinese herb sophora flavescens Ait, which has been found to exert anti-tumor effects in oral squamous cell carcinoma [186]. Zhao et al. revealed that OSC downregulated FGFR1 expression and downstream AKT/mTOR and ERK signaling, entitling HCC cells sensitive to LEN [187].
PI3K/AKT signaling
The dysregulation of PI3K/AKT signaling is the most frequent aberrancy in human cancer [188]. Studies have shown that its activation plays pivotal roles in the occurrence and development of HCC by promoting angiogenesis [189,190], accelerating cell cycle [191], regulating the autophagy process [192,193], and inhibiting apoptosis [194], finally inducing drug resistance [195-197]. A large number of studies indicated that the SOR resistance of HCC is strongly correlated with the abnormal PI3K/AKT signaling activation. For example, some upstream effectors like IGF-1R [198], YB-1 [199], and FNDC5 [200] have been demonstrated to upregulate PI3K/AKT signaling, thus inducing SOR resistance in HCC. Recent studies have successively elucidated that its overactivation partially contributes to LEN resistance in HCC. In this section, we focus on the correlation between LEN resistance and aberrant PI3K/AKT pathway activation (Figure 1).
Metformin was found to not only reduce the risk of hepatocarcinogenesis [201], but also improve the sensitivity of HCC cells to SOR, and inhibit HCC recurrence and metastasis after surgical resection [202]. Furthermore, it was discerned that the combination of LEN and metformin synergistically inhibited HCC growth in vivo and in vitro. Mechanistically, Cheng et al. substantiated that metformin inhibited the activation of AKT signaling, subsequently reduced the downstream effector FOXO3 phosphorylation levels and stimulated its nuclear aggregation, thereby reversing LEN resistance [203]. Similar to the pharmacological activities of Metformin, Curcumin, a naturally derived plant extract, has been found to suppress RTKs such as EGFR, IGF, VEGFR, etc. [204], participate in inhibiting HCC progression [205] as well as functioning as a chemical sensitizer for many drugs [206]. Katsuki et al. discovered that curcumin reversed acquired LEN resistance by inhibiting EGFR and its downstream PI3K/AKT signaling [207]. Integrin subunit beta 8 (ITGB8), an important member of the integrin family [208], was detected overexpressed in various tumor cells [209]. Hou et al. observed that ITGB8 was considerably overexpressed in LR-HCC cells and found that ITGB8 knockout reversed LEN resistance in vitro and in vivo. They further revealed that ITGB8 could enhance AKT stabilization and activate AKT signaling by increasing HSP90 expression levels, thus inducing LEN resistance [210]. In addition, CD73 was found to activate AKT signaling and further activated SOX9 transcription through c-MYC and simultaneously inhibited GSK3β to prevent SOX9 ubiquitination and degradation, which promoted the HCC CSC stemness, facilitated HCC cell growth as well as induced LEN resistance [211]. Its elevated expression was correlated with unfavorable prognoses of HCC patients [212].
Autophagy signaling
Autophagy is a process stimulated in response to various environmental stresses in human body, exerts critical roles in maintaining cellular homeostasis [213]. Recently, studies have cumulatively concentrated on the correlation between autophagy regulation and tumorigenesis as well as development, however, whether autophagy progress acts tumor inhibition or promotion effect remains controversial [214]. In relation to occurrence and development of HCC, existing studies demonstrated that autophagy progress inhibited tumor growth in the early stage of HCC [215], but autophagy reversely changed its anti-tumor role into an oncogenic role when tumor developed into its advanced stage [216]. Studies have shown that the abnormal regulation of autophagy is intricately related to drug resistance [217-219], but the correlations between autophagy and LEN resistance remain poorly studied, which is shown in Figure 1.
Neuropirin 1 (NRP1), a non-tyrosine kinase transmembrane glycoprotein that exert crucial roles in angiogenesis, cell proliferation, migration, and invasion [220], has been observed abnormally highly expressed in HCC [221]. Paula et al. found a sharp increase of NRP1 expression in HCC tissues, which was strongly associated with unfavorable prognoses. They found that LEN upregulated the autophagy pathway to reduce NRP1 expression in HCC cells, thereby inhibiting cell proliferation and migration. Bafilomycin A1 (BAFA1), an autophagy inhibitor, was demonstrated to inhibit the autophagic degradation of NRP1 and upregulate its expression on HCC cell membrane, thereby inducing LEN resistance [222]. Similarly, Stomatin-like protein 2 (STOML2) is a mitochondrial intima protein contributing to tumor development in a variety of cancer types [223,224], and is notably overexpressed in HCC tissues and intimately related to the poor prognosis of HCC patients [225]. It was proven that STOML2 overexpression promoted HCC cell proliferation, migration, and invasion, and inhibited apoptosis in vitro. Mechanistically, it stabilized PINK1 to promote HCC progression, and enhance mitochondrial autophagy to induce LEN resistance [225]. Additionally, synthetic protein 6 (STX6) is one of the soluble N-ethyl maleimide-sensitive factor attachment protein receptor (SNARE) family members, which plays important roles in regulating almost all cell intimal transport events [226] and has been detected upregulated in the vast majority of tumors [227]. Zhou et al. found that STX6 was overexpressed in HCC tissues at both protein and mRNA levels, which was closely associated with poor prognosis of HCC patients. It was found that STX6 promoted the formation of autolysosomes and accelerated LC3B (one classical autophagy marker) degradation, promoting cell proliferation, migration, and invasion, and enhancing LEN resistance. Further, they found that upstream stimulus factor 2 (USF2), as an upstream transcriptional repressor, bound to the STX6 promoter to suppress its expression, inhibiting HCC cell growth and reversing LEN resistance [228]. Through genome-wide CRISPR-Cas9 screening, Pan et al. identified lysosomal protein transmembrane 5 (LAPTM5) as a key protein correlated with LEN resistance in HCC. Functionally, LAPTM5 knockdown significantly augmented the sensitivity of HCC cells to LEN, and its overexpression significantly suppressed xenogenic tumor growth with LEN treatment. Mechanistically, they found that LAPTM5 contributed to LEN resistance by promoting autolysosome formation. Importantly, it was detected that LAPTM5 expression was inversely associated with LEN sensitivity in clinical hepatocellular carcinoma samples [229]. Additionally, Ma et al. found that nucleotide-binding oligomerization domain containing 2 (NOD2) was significantly downregulated in HCC tissues, and low NOD2 expression was highly associated with a poor prognosis. NOD2 overexpression significantly inhibited the HCC cell proliferation and invasion in vitro, suppressed xenograft tumor growth in vivo, and increased the sensitivity of HCC cells to LEN. Mechanistically, NOD2 exerted its anti-tumor effect via activating AMPK signaling pathway, which activated autophagy-mediated cell apoptosis to inhibit LEN resistance [230]. Moreover, Tang et al. found that the combination of Secukinumab (a specific biological agent targeting IL-17A) and LEN significantly inhibited the growth of xenogenic tumors. Mechanistically, the combination of Secukinumab and LEN significantly increased the LC3 conversion rate in tumor tissues (the ratio of LC3II to LC3I) to decrease the BCL2 protein expression, thus promoting the autophagy pathway to inhibit HCC growth in vivo [231]. Else, Compound Phyllanthus urinaria (CP) is a kind of traditional Chinese herbal medicine, which has been proven to exhibit anti-tumor effects in experiments as well as clinics [232]. In vitro and in vivo, Liao et al. demonstrated that LEN combined with CP treatment resulted in superior therapeutic outcomes in HCC therapy than either agent used independently. Mechanistically, CP enhanced the efficacy of LEN by promoting exosome-mediated autophagy inhibition, and thus effectively inhibited HCC progression [233].
mTOR signaling
The mammalian target of rapamycin (mTOR) is an atypical serine/threonine protein kinase belonging to the PI3K-related protein kinase family, which integrates a variety of extracellular signals such as amino acids, energy states, and growth factors to participate in biological processes such as gene transcription, protein translation and ribosome synthesis, thus playing pivotal roles in cell growth, apoptosis, autophagy and metabolism [234,235]. The aberrant activation of mTOR signaling has been frequently detected in HCC, which is critical for HCC tumorigenesis and development and responsible for doxorubicin [236] and SOR [237] resistance during HCC therapy. Nowadays, mTOR has been recognized as a validated therapeutic target for the treatment of many tumor types including HCC [238,239]. Herein, we discuss the potential relationship between LEN resistance and mTOR signaling regulation (Figure 1).
Consistent with Etienne et al.’s research mentioned above, Fan et al. observed that SREBP2 was highly expressed in HCC tissue at both mRNA and protein levels, and its high expression was still closely associated with poor prognosis [240]. They confirmed that Betulin, a SREBP2 inhibitor, decreased intracellular cholesterol levels and enhanced HCC sensitivity to LEN by inhibiting the mTOR/IL-1β pathway [240]. Moreover, forkhead box protein A2 (FOXA2), a key transcription factor for liver development and metabolic homeostasis [241], was discovered to be lowly expressed in HCC tissues, and its low expression was associated with poor prognosis. Mechanistically, Wang et al. demonstrated that FOXA2 overexpression upregulated liver kinase B1 (LKB1) phosphorylation and Ste20-related adaptor α (STRADα)/LKB1 axis to activate the AMPK/mTOR signaling, thereby enhancing the HCC cell sensitivity to LEN [242].
WNT/β-catenin signaling
Wnt/β-catenin signaling is one of the most important regulatory pathways in living organisms, and β-catenin, as a key regulatory protein, is a vital biomarker for detecting the status of signaling activation [243]. The extracellular wnt protein interacts with and activates membrane receptors to transmit signals into cells, and β-catenin degradation activity is inhibited through regulating downstream protein kinase phosphorylation. Consequently, β-catenin accumulates steadily in cytoplasm, enters the nucleus, and activates downstream gene expression by activating specific transcription factors [243], which is involved in the regulation of cell proliferation and differentiation, cell cycle, and tumorigenesis, etc. [244,245]. Aberrant activation of the wnt/β-catenin signaling pathway has been observed in up to half of HCC patients, and its abnormal activation promotes HCC growth [246-248] and drug resistance [249,250]. Herein, we mainly focus on the correlation between LEN resistance and wnt/β-catenin signaling regulation in this section (Figure 2).
Figure 2.
The correlation between the dysregulation of other signaling pathways and lenvatinib resistance. The dysregulation of lipid metabolism, wnt/β-catenin signaling, hippo signaling, notch signaling, hedgehog signaling, RNA modifications and ferroptosis was found to participate the regulation of LEN resistance in uHCC treatment. And, a series of proteins and a range of drugs acted on corresponding targets to play roles in promoting or inhibiting LEN resistance in HCC treatment.
Tan et al. found that glutathione Peroxidase 2 (GPX2) was significantly overexpressed in HCC tissue and associated with poor therapeutic effectiveness of LEN. They demonstrated that LEN inhibited the nuclear translocation of β-catenin to repress GPX2 transcription, increasing reactive oxygen species (ROS) generation, and triggering HCC cell apoptosis [251]. Interferon regulatory factor 2 (IRF2), a pivotal nuclear transcription factor regulating related gene transcription [252,253], was revealed to exhibit high expression in HCC tissues and associated with poor prognosis [254]. Guo et al. found the IRF2 was upregulated in HCC at both mRNA and protein levels, which was also positively correlated with β-catenin expression. Through a series of experiments, it was confirmed that IRF2 increased β-catenin expression and augmented downstream signaling, thereby promoting HCC cell proliferation, inhibiting cell apoptosis, and inducing LEN resistance [255]. In addition, Zhang et al. identified that peroxisome proliferator-activated receptor gamma coactivator-1α (PPARGC1A), a critical receptor involved in regulating energy homeostasis, was lowly expressed in HCC and showed a poor prognosis. In hypoxic conditions, PPARGC1A inhibited bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI) expression via downregulating wnt/β-catenin signaling, thereby reversing its inhibition on the downstream TGF-β/SMAD signaling, subsequently upregulating acyl-CoA synthetase long-chain family member 5 (ACSL5) expression to facilitate ROS production, resulting in inhibition of HCC progression and LEN resistance [256]. Moreover, through functional analysis, Liang et al. showed that CDK6 played a key role in regulating LEN resistance by enhancing the stemness of HCC. Mechanistically, CDK6 bound to and phosphorylated GSK3β, thus leading to the activation of wnt/β-catenin signaling. In vitro experiments confirmed that Palbociclib, a CDK4/6 inhibitor, had a synergistic effect with LEN to exert the greatest inhibitory effect and remodel the tumor immune microenvironment [257].
Hedgehog signaling
Hedgehog signaling is an intracellular pathway with three protein ligands: Sonic hedgehog (SHH), Indian hedgehog (IHH), and Desert hedgehog (DHH). Upon ligands binding to the Patched (PTCH), the repression of Smoothened (SMO) by PTCH is reversed, which activates glioma-associated oncogene homolog 1-3 (GLI1-3) to enhance the downstream genes transcription. Hedgehog signaling serves a pivotal function in physiological embryonic development, adult tissue maintenance, renewal, and regeneration by controlling cell fate, proliferation, and differentiation [258,259]. A growing body of studies has indicated that dysregulation of this signaling is intimately linked to tumor occurrence and development, including glioblastoma [260], medulloblastoma [261], melanoma [262], pancreatic carcinoma [263] as well as HCC [264,265], etc. Recently, accumulated studies have confirmed that activation of hedgehog signaling enhanced drug resistance during tumor treatment, such as cisplatin in bladder cancer [266], azithromycin in multiple myeloma [267], as well as TKIs: erlotinib in lung cancer [268], and SOR in HCC [269]. The section presents an overview of several recently studies regarding the correlation between LEN resistance and Hedgehog signaling regulation (Figure 2).
The CD133 expression was closely related to drug resistance [270], tumor invasion, and metastasis in HCC [271,272], and it was intimately related to LEN resistance in HCC cells [273]. GANT61, as a hedgehog signaling inhibitor, inhibits the GLI1/2 and downregulates its-induced gene transcription [274], suppressing undifferentiated HCC cell growth [275]. It was discovered that GANT61 inhibited hedgehog signaling in HCC cells with high CD133 expression levels, suppressed HCC growth and reversed LEN resistance in vivo and in vitro [273]. In addition, Jing et al. first determined the anti-tumor effect of Flubendazole in HCC in vivo and in vitro, and further identified that proprotein convertase subtilisin/kexin type 9 (PCSK9), a vital serine protease regulating lipid metabolism, as the target for flubendazole, was highly expressed in HCC tissues and closely associated with the poor prognosis. The study revealed that flubendazole directly reduced PCSK9 expression, consequently inhibiting SMO and downregulating hedgehog signaling, which prevented HCC progression as well as increased LEN sensitivity [276]. Sterol regulatory element-binding protein 2 (SREBP 2), a main regulator of de novo cholesterol biosynthesis and uptake [277], has been observed overexpressed in CRC [278] and PRAD [279], and correlated to drug resistance [280]. Etienne et al. found that the SREBP2-mediated cholesterol production pathway was significantly upregulated in LR-HCC cells, confirmed that SREBP2 was highly expressed in HCC tissues and closely related to unfavorable prognosis. Mechanistically, the caspase 3 (CASP3) upregulated SREBP2 to promote cholesterol biosynthesis, and then 25-hydroxycholesterol (25-OHC), an oxysterol derivative of cholesterol, activated the SMO, GLI1/2 and downstream target genes of the hedgehog signaling [281], contributing to LEN resistance. And, they also found that Simvastatin, an inhibitor of HMG CoA reductase treating hypercholesterolemia, dramatically blocked cholesterol synthesis to inhibit the hedgehog signaling in HCC, which improved the therapeutic effectiveness of LEN in tumor organoids and xenografts experiments [282].
Epithelial-mesenchymal transition
EMT is a pathological process characterized by loss of epithelial features and high expression of mesenchymal cell-related genes [283]. It has been clearly indicated that EMT was closely related to HCC invasion and migration [284]. In recent years, an increasing number of studies have confirmed that EMT was involved in drug resistance [285,286]. We summarized several recent studies on the relationship between LEN resistance and EMT regulation in this section (Figure 2).
In HCC cells, Fang et al. elucidated that LEN increased the expression of epithelial-related proteins, such as E-cadherin and β-catenin, and concurrently downregulated the expression of intermediate mesenchymal markers, such as Fibronectin, Vimentin, and EMT-regulated transcription factors ZEB1, Snail, and Twist7 in a dose-dependent manner. Mechanistically, LEN not only accelerated the DNMT1 and UHRF1 degradation via enhancing ubiquitination modification, but also inhibited ERK signaling activation to reduce the expression of DNMT1 and UHRF1 at the mRNA level, which jointly upregulated the expression of E-cadherin and inhibited EMT process to prevent tumor metastasis in HCC [287]. Additionally, the activation of the hepatocyte growth factor (HGF)/c-MET axis was found closely associated with tumor invasion and metastasis [288]. Further investigations from Fu et al. team revealed that HGF inhibited the LEN-induced anti-proliferation, pro-apoptotic and anti-invasive effects, increased the expression of N-cadherin and Vimentin, and decreased the expression of E-cadherin in HCC cells treated with LEN. It was demonstrated that the activation of the HGF/c-MET axis promoted LEN resistance in high c-MET expression HCC cells through activating PI3K/AKT and MAPK/ERK signaling as well as intensifying EMT process [289]. Ring finger protein 8 (RNF8) is one of the E3 ubiquitin ligases linked to DNA damage repair mechanisms [290], which was also detected to suppress [291] or promote [292] tumor growth. Kuang et al. discovered that RNF8 was upregulated in HCC tissues and correlated with a poor prognosis. In vitro experiments further found that RNF8 attenuated the EMT process and HCC cell migration, enhancing LEN resistance [293].
FGF19/FGFR4 signaling
It is well known that fibroblast growth factor (FGF) signaling is essential to many intracellular biological processes [294]. FGF19 is one of the FGFs subtypes and is observed highly expressed in HCC tissues and closely associated with a poor prognosis [295]. FGFR4, the major isoform of FGFRs in human hepatocytes, is now recognized as the only receptor specific for FGF19, and is also overexpressed in HCC [296]. Nowadays, a large number of studies have demonstrated that FGF19/FGFR4 signaling significantly promotes HCC cell survival, proliferation, invasion, and metastasis [295,297,298]. In this section, we provide an overview of recent research concerning the correlation between LEN resistance and FGF19/FGFR4 axis.
Yuta et al. demonstrated that the FGF19 overexpression enhanced LEN sensitivity in HCC cells. As a tumor-derived protein, ST6 β-galactoside α-2,6-sialyltransferase 1 (ST6GAL1) was elucidated to be upregulated in several tumor types and promote tumor invasion and metastasis [299-301]. They revealed that FGF19 positively regulated ST6GAL1 expression by promoting the phosphorylation of signal transduction and transcriptional activator 3 (STAT3), one important transcription factor essential to HCC cell growth, enhancing the sensitivity of HCC cells to LEN [302]. Besides, they deduced that circulating ST6GAL1 levels had the potential to serve as a non-invasive indicator of LEN sensitivity [303]. Lysine demethylase 6A (KDM6A) plays crucial roles in various cancer types [304-306], and its aberrant elevation in HCC tissues has been correlated with poor prognosis, recently [307]. Guo et al. found that KDM6A knockdown inhibited HCC growth both in vitro and in vivo, and significantly reversed LEN resistance. Mechanistically, they revealed that KDM6A upregulated FGFR4 expression to activate the PI3K/AKT/mTOR signaling, inducing LEN resistance [307]. Furthermore, Norifumi et al. first demonstrated that LEN inhibited FGFR4 to inactivate glutathione peroxidase 4 (GPX4), accelerating lipid ROS accumulation to induce iron-dependent death in HCC cells. They further found that NRF2 overexpression inhibited iron death and induced LEN resistance [33].
Lipid metabolism regulation
Lipids are an integral component of cell biological membranes, involved in energy storage and metabolism, playing an important role in signaling transduction and maintaining cell homeostasis [308,309]. Dysregulation of lipid metabolism is a significant hallmark of carcinogenesis and is correlated with the HCC occurrence and progression [310-312]. Recently, extensive research concentrated on elucidating the correlation between dysregulation of lipid metabolism and anti-tumor drug resistance [313]. ATP citrate lyase (ACLY), a pivotal enzyme in lipid metabolism, was upregulated or activated in various tumor types [314,315]. In addition, it has been shown that targeted downregulation of ACLY could impede lipid metabolism and also reverse the SOR resistance of HCC cells [316]. Here, we mainly summarize several studies concerning the correlation between lipid regulation and LEN in HCC treatment (Figure 2).
The 2-arachidonic glycerol (2-AG) was highly overexpressed in various tumor types and positively correlated with tumor growth in most tumor types [317-319]. Diacylglycerol lipase α (DAGLA), a critical hydrolase regulating 2-AG, was found to be highly expressed in HCC tissues at both protein and mRNA levels and associated with poor prognosis. Yan et al. proved that the DAGLA/2-AG axis was activated in HCC tissues, and revealed that DAGLA activated 2-AG to inhibit LATS1 and YAP phosphorylation, and then promoted YAP nuclear translocation, which activated the hippo pathway to promote HCC progression and induce LEN resistance [320]. Additionally, the activation of the PI3K/AKT axis was also verified to inhibit downstream hippo signaling, therefore promoting the HCC progression and inducing LEN resistance. Liu et al. also observed that STOML2 was an abnormally upregulated protein in HCC, its high expression was strongly associated with poor clinicopathological features and prognosis. Mechanistically, STOML2 stabilizes c-Jun N-terminal kinase (JNK2) via inhibiting its ubiquitination and degradation, which activates the sterol regulatory element binding protein 1 (SREBP1), one transcription factor regulating the expression of several key enzymes in lipid synthesis, and accelerated its nuclear translocation to promote lipogenesis, thus promoting the HCC progression and triggering LEN resistance [321].
Glycolysis
Abnormal activation of glucose metabolism was ubiquitously discovered in HCC patients and was closely associated with high malignant phenotypes [322,323]. Acylphosphatase 1 (ACYP1), a small cytoplasmic enzyme catalyzing the hydrolysis of the carboxyl-phosphate bond of acylphosphates, was found highly expressed in HCC tissues and correlated with poor prognosis [324]. Wang et al. confirmed that ACYP1 overexpression promoted HCC cell proliferation, invasion, and migration in vitro and in vivo. Through differentially expressed genes screen and functional enrichment analysis, they showed that ACYP1 participated in regulating aerobic glycolysis, especially the production of lactic acid, and thus promoted HCC progression. Subsequent data analysis unveiled that lactate dehydrogenase (LDHA) acted as an indispensable downstream factor of ACYP1 in regulating glycolysis and promoting tumor progression. Furthermore, they observed that ACYP1 interacted with HSP90 and regulated the stability of c-MYC and revealed that ACYP1 enhanced LEN resistance through the ACYP1/HSP90/MYC/LDHA axis [325] (Figure 2).
Notch signaling
Notch signaling is an evolutionarily highly conserved signaling pathway integral to cell fate, including Notch receptors (Notch 1-4), Notch ligands (Delta-like ligands, DLL1/3/4 or Jagged ligands, JAG1/2), CBF1, Suppressor of Hairless, Lag-1 (CSL) and Notch effector. The Notch1-4 binds to the notch ligands on the neighboring cell membrane, and then releases the notch protein fragment (Notch intracellular domain, NICD), which binds to the CSL, regulating transcription of downstream target genes [326]. Nowadays, Notch signaling is proven to be crucial in normal liver development [327], and participates in regulating HCC tumorigenesis, angiogenesis, invasion, and metastasis [328] as well as promoting SOR resistance [329]. Transmembrane 4 L six family 1 (TM4SF1) was identified as an oncoprotein, which was highly expressed in HCC and closely associated with adverse prognosis [330]. Yang et al. revealed that TM4SF1 activated the Notch pathway by upregulating myosin heavy chain 9 (MYH9), thereby promoting cancer HCC cell stemness and enhancing LEN resistance [331] (Figure 2).
RNA modification
N7-methylguanosine (m7G) and N6-methyladenosine (m6A), two of the most common RNA modification types, exert pivotal roles in tumor progression [332,333]. In mammals, the methyltransferase-like 1 (METTL1)/WD repeat domain 4 (WDR4) complex is essential for normal mRNA translation, neural self-renewal, and differentiation. METTL1 binds to its cofactor WDR4 to install m7G modification in tRNA, miRNA and mRNA [334]. There existed several studies identifying that METTL1/WDR4 was upregulated in HCC tissues and played oncogenic roles [332,335,336] as well as induced SOR resistance [337].
Recently several studies explored the effects of METTL1/WDR4 on LEN therapy efficacy. Huang et al. found that METTL1/WDR4-mediated m7G tRNA modification facilitated downstream EGFR translation [338] and activated MAPK/ERK signaling in HCC cells, inducing LEN resistance. Else, Han et al.’s study demonstrated that the METTL1/WDR4, CSC markers, and m7G modification were highly upregulated in LR-HCC tissues/cells, and elevated WDR4 expression promoted HCC progression and resulted in LEN resistance via directly regulating TRIM28 to upregulate associated downstream genes [339]. In addition to METTL1, another family member-METTL3 was detected at high expression levels in LR-HCC cell lines and tissues as well, and METTL3 upregulated EGFR expression in m6A-dependent manner and enhanced MAPK/ERK signaling, thereby triggering LEN resistance [340]. Else, Metformin was proven to restore PPARGC1A expression through counteracting its m6A modification via inhibition of METTL3, subsequently suppressing downstream wnt/β-catenin signaling and enhancing the therapeutic efficacy of LEN [256].
N4-acetylcytidine (ac4C) is a highly conserved RNA modification, the only acetylation event described in eukaryotic RNA, and is catalyzed by n-acetyltransferase 10 (NAT10) [341]. Previous studies illustrated that NAT10 was upregulated in HCC tissues and associated with adverse prognosis [342], thereby accelerating EMT to enhance HCC growth and induce doxorubicin resistance [343]. Endoplasmic reticulum stress (ERS) is a pathological state and has been proven to exert oncogenic and immunosuppressive effects [344]. One recent study similarly showed that NAT10 was upregulated in HCC tissues at mRNA levels and associated with a poor prognosis. In vivo and in vitro experiments revealed that NAT10 upregulated the ac4C modification of HSP90AA1, and maintained its stabilization, which enhanced ERS to promote the HCC metastasis and induce LEN resistance [345] (Figure 2).
Hypoxia
Intratumoral hypoxia is a typical characteristic of HCC and has been proven associated with drug resistance [346]. Takahashi et al. found that HCC cells were more resistant to LEN under hypoxic conditions. Microarray analysis was performed to identify the fibronectin 1 (FN1) as the most related gene that encoded FN1 protein. They confirmed that hypoxia-induced upregulation of HIF-1α and other transcription factors, thereby enhancing FN1 expression and leading to LEN resistance [347] (Figure 2).
Drug combination
Intriguingly, some studies have found that several existing drugs and LEN may have synergistic effects potentiating anti-tumor activity in the treatment of HCC. Hirotetsu et al. found that the combination of low-dose LEN and Losartan, one angiotensin-II (AT-II) receptor blocker, achieved cellular and vascular inhibition by inhibiting VEGF-A expression, and demonstrated in xenograft trials that oral losartan combined with LEN diminished subcutaneous tumor load and intratumoral angiogenesis in nude mice [348]. Another study determined that Vitamin C increased LEN sensitivity and synergistically inhibited HCC cell proliferation, migration, and invasion in vitro experiments [349]. Graziana et al. showed that Abemaciclib, one CDK4/6 inhibitor, was involved in enhancing the LEN sensitivity in HCC cells [350]. In Takahashi et al.’s study, they identified a key differentially expressed protein c-SRC in LR-HCC cells and conducted experiments to reveal that Dasatinib, the c-SRC inhibitor, increased the sensitivity of LR-HCC cells to LEN in a dose-dependent pathway [351]. ATP binding cassette subfamily B member 1 (ABCB1, MDR1) and breast cancer resistance protein (BCRP), two important members of ATP binding cassette (ABC) family, are involved in drug efflux from tumor cells, thereby reducing the efficacious concentration of anti-tumor drugs and leading to chemotherapy failure [352,353]. Sun et al. elucidated that ABCB1 and BCRP transporters were dramatically upregulated in LR-HCC cells and found that Elacridar, an ABCB1 and BCRP inhibitor, could reverse LEN resistance in uHCC [354]. Moreover, in vivo and in vitro experiments, Hu et al. identified that the EGFR-STAT3-ABCB1 signaling was abnormally activated in the LR-HCC models, which was also suggested in the pathological tissues of LR-HCC patients. Further, they revealed that EGFR upregulated ABCB1 by activating STAT3, which induced LEN resistance by enhancing its exocytosis. In addition, Erlotinib, a type of TKI, was found to not only inhibit EGFR phosphorylation to downregulate ABCB1 expression, but also directly repress the LEN exocytosis mediated by ABCB1, which together enhanced LEN therapeutic efficacy [355].
Non-steroidal anti-inflammatory drugs (NSAIDs) have been determined to exert anti-tumor activity by blocking cyclooxygenase 2 (COX2) [356]. Yan et al. revealed that LEN+ Aspirin therapy significantly enhanced the inhibitive effects in cell proliferation, tumor growth, and angiogenesis in vivo and in vitro. Mechanistically, they found that the combination therapy downregulated the phosphorylation levels of CDK2, RB, AKT, ERK, MEK, and 4EBP1, enhanced the expression of P21 and P27, and decreased the expression of c-MYC and LDHA, synergistically participating in tumor inhibition to reverse LEN resistance [357]. Deng and his colleagues uncovered that LEN increased the recruitment of neutrophils, stimulated the polarization of neutrophils towards the N2 phenotype, and increased the PD-L1 expression in HCC cells, leading to LEN resistance. Additionally, they found that Celecoxib can decrease the survival rate of neutrophils stimulated by lactate, thereby boosting LEN’s anti-tumor effects [358].
LncRNA
Long non-coding RNA (lncRNA) is a transcript with a length of more than 200 nucleotides that regulates gene expression at different levels, thereby involving in different biological and pathological processes [359,360]. Dysregulation of lncRNA in HCC has been widely confirmed to have tumor-promoting and suppressive effects, and intensive studies have found that lncRNA plays important roles in tumor growth and drug resistance [361-365]. The correlation between dysregulation of lncRNA and drug resistance of HCC attracted increasing attention in recent years. For instance, lncRNA MALAT1 inhibits HCC cell apoptosis induced by 5-fluorouracil [366]. LncRNA SNHG1 triggers SOR resistance in HCC by activating the AKT signaling [367], etc. To date, there has been little research concerning LEN resistance caused by lncRNA dysregulation, which is shown in Figure 3.
Figure 3.
The correlation between the dysregulation of non-coding RNAs (ncRNAs) and lenvatinib resistance. The dysregulation of non-coding RNA, including lncRNA, circRNA and microRNA, was demonstrated to be closely related to LEN resistance in uHCC. A series of lincRNA and circRNA, as ceRNAs, regulated downstream mRNA expression by competitively binding with miRNA, thus involving in regulation of LEN resistance. In addition, ncRNAs participated in regulating associated signaling pathways, including MAPK/ERK, AKT, hippo, autophagy, NF-κB and FGFR4 signaling, which promoted or inhibited LEN resistance in HCC treatment. Solid lines represent the role in promoting LEN resistance, and dashed lines represent the role in suppressing LEN resistance in uHCC treatment. Pointed arrows represent activating effects, and blunt arrows represent inhibitory effect. The same is true for arrows and lines described below.
LncRNA HOTAIRM1 was upregulated in LR-HCC cells, and knockdown of HOTAIRM1 was confirmed to dramatically increase the sensitivity of LEN in HCC cells. Mechanistically, Gu et al. revealed that HOTAIRM1 downregulated miR-34a and upregulated BECLIN-1 to result in autophagy activation, inducing LEN resistance [368]. LncRNA XIST was upregulated in LR-HCC cells and its high expression was associated with LEN resistance in vitro. Duan et al. revealed that XIST induced LEN resistance via activating the EZH2/NOD2/ERK axis [369]. In addition, lncRNA AC026401.3 was found highly expressed in HCC tissues and was positively correlated with poor prognosis, while AC026401.3 knockdown enhanced LEN sensitivity in HCC cells. Notably, Wang et al. uncovered that AC026401.3 interacted with octamer-binding transcription factor 1 (OCT1) to enhance the E2F2 transcription, thereby triggering LEN resistance in HCC [370]. Besides, Ting et al. found that lncRNA MT1JP was significantly upregulated under LEN stimulation in HCC cells, and in vitro experiments demonstrated that MT1JP overexpression promoted LEN resistance in HCC cells. Mechanistically, they found that MT1JP, as a competitive endogenous RNA (ceRNA) of miR-24-3p, inhibited miR-24-3p expression, thereby reversing its inhibitory effect on B-cell lymphoma 2 (BCL2), an anti-apoptotic protein, suppressing cell apoptosis and inducing LEN resistance [371]. Furthermore, Chen et al. detected that lncRNA USP2-AS1 expression was significantly upregulated in response to hypoxic conditions and confirmed that USP2-AS1 was highly expressed in HCC tissues and positively correlated with tumor size. In vitro experiments showed that USP2-AS1 overexpression significantly increased HCC cell proliferation, migration, and invasion, and USP2-AS1 knockdown enhanced the LEN treatment efficacy. Mechanistically, USP2-AS1 increased hypoxia-inducible factor 1α (HIF1α) expression and its downstream target genes expression by enhancing the binding of Y-box binding protein 1 (YBX1) to HIF1α under hypoxia conditions, thereby impeding HCC progression and inducing LEN resistance [372]. Furthermore, Chen et al. observed that lncRNA MKLN1-AS was upregulated in HCC tissues and patients with higher MKLN1-AS expression had remarkably shorter DFS and OS. Functionally, MKLN1-AS knockdown significantly inhibited the HCC cell metastasis and growth in vitro and in vivo, and enhanced the effect of LEN-induced apoptosis [373]. Moreover, Hiroyuki et al. showed that lncRNA NEAT1 induced LEN resistance by upregulating SOD2 expression to change the growth mode of HCC cell lines from MEK/ERK to AKT-dependent mode [374]. Inhibition of ROS levels and reduction of redox state status were observed in LR-HCC cells. Zhang et al. first show that lncRNA LINC01607, as a ceRNA, competed with miroRNA-892b and triggered protective mitochondrial autophagy by upregulating P62, thereby promoting LEN resistance in HCC cells [375]. Song et al. found that PINK1 was elevated in LR-HCC cells and tissues, and through a series of experiments, they identified that lncRNA FGD5-AS1 competitively regulated PINK1 with miR-5590-3p, promoted PINK1-mediated mitochondrial homeostasis, and led to LEN resistance [376].
Different from the several lncRNAs above which were involved in facilitating HCC development and inducing LEN resistance, the downregulation of lncRNA ZEB2-19 was identified in HCC tissues and significantly linked to poor prognosis. A series of experiments was conducted by Cao et al. and demonstrated that ZEB2-19 significantly inhibited cell proliferation, metastasis, dryness maintenance as well as LEN resistance in HCC cells. Mechanistically, ZEB2-19 interacted with transformer 2α (TRA2A), promoted its degradation and inhibited the radial spoke head 14 homolog (RSPH14) expression at mRNA levels, consequently attenuating the NF-κB signaling to suppress LEN resistance [377].
CircRNA
CircRNA is a special type of lncRNA characterized by a single-stranded covalent closed-loop structure [378]. In recent years, a series of studies have demonstrated that the dysregulation of circRNA is intimately linked to HCC occurrence and development, including cell proliferation [379,380], invasion, migration [381,382], and apoptosis [383,384]. Although there exist several studies focused on the correlations between circRNAs and drug resistance, including SOR resistance [385-387], few investigations have been carried out on its underlying mechanisms associated with LEN resistance, which is shown in Figure 3.
Zhang et al. discovered that circMED27 was significantly increased in the serum and tissues of HCC patients, and associated with a poor prognosis. They revealed that circMED27, as a ceRNA of miR-655-3p, competed with miR-655-3p and enhanced ubiquitin-specific peptidase 28 (USP28) expression, thus facilitating HCC progression and triggering LEN resistance [388]. Hao et al. found that circPAK1 was highly expressed in HCC tissues and was associated with poor prognosis in HCC patients. In vitro experiments showed that circPAK1 knockdown inhibited HCC cell proliferation, migration, invasion, and angiogenesis. Animal experiments also confirmed that circPAK1 promoted HCC development. Mechanistically, through competitively binding 14-3-3 with YAP, circPAK1 weakened the recruitment and cytoplasmic immobilization of YAP, thereby promoting YAP nuclear localization and inactivating hippo signaling to enhance HCC progression. In addition, they also observed that circPAK1 can transport from LR-HCC cells to sensitive cells via exosomes, thereby inducing LEN resistance [389].
Instead, several studies reveal that circRNAs participate in inhibiting HCC development and reversing LEN resistance. Bi et al. found that circFAM134B, as a ceRNA, competed with poly (A) binding protein cytoplasmic 4 (PABPC4) to upregulating FAM134B, which promoted endoplasmic reticulum autophagy and enhanced LEN-induced iron death in HCC cells [390]. CircKCNN2 was found lowly expressed in HCC tissues and patients with high circKCNN2 expression exhibited superior OS and RFS. Through a series of experiments, Liu et al. found that circKCNN2 overexpression inhibited HCC cell proliferation and migration in vitro, promoted tumor growth in vivo, and simultaneously inhibited LEN resistance. Mechanistically, they confirmed that circKCNN2 targeted miR-520c-3p and downregulated its expression, then upregulating methyl-DNA-binding domain protein 2 (MBD2) expression to downregulate FGFR4 expression, which inhibited HCC progression and increased the sensitivity of HCC cells to LEN [391].
MicroRNA
MicroRNA (miRNA, miRs) are a special class of small non-coding RNA molecules targeting specific mRNAs to prevent or degrade their translation, essential in biological processes, including HCC development [392,393]. Based on the specificity of the target genes, miRNAs can act as tumor suppressors or oncogenes, and its dysregulation is closely related to HCC cell proliferation, invasion, metastasis, autophagy, etc. [394,395]. In recent years, research on drug resistance and miRNA has been continuously carried out, including LEN resistance (Figure 3).
The c-MET was overexpressed in more than 80% of HCC tissues and associated with poor prognosis [396]. Mucin 15 (MUC15) is a membrane-associated protein and its downregulation is closely linked to the adverse prognosis of HCC [397]. Han et al. found that MUC15 was downregulated in relapsed HCC tissues and LR-HCC tissues, and revealed that miR-183-5p.1 directly bound to MUC15 3’-UTR to inhibit its expression and reverse downstream c-MET/PI3K/AKT/SOX2 signaling inactivation, promoting HCC progression and inducing LEN resistance [398]. In addition, Yuan et al. found that miR-3154 was upregulated in HCC tissues and correlated with poor OS and DFS of uHCC patients. In vitro and in vivo experiments showed that miR-3154 knockdown inhibited HCC cell self-renewal, proliferation, metastasis, and tumorigenesis. Mechanistically, miR-3154 directly targeted HNF4α to upregulate its expression, thus promoting HCC progression. Notably, through cohort analysis and patient-derived xenografts (PDX) model analysis, patients with high miR-3154 expression might exhibit strong LEN resistance [399].
Furthermore, several studies have elaborated on the oncogenic role of miRNAs in HCC. For instance, miR-3-128p was significantly downregulated in LR-HCC cells, and its overexpression inhibited LR-HCC cell proliferation, induced apoptosis, and enhanced the anti-cancer efficacy of LEN in cellular culture and animal models. Mechanistically, miR-3-128p downregulated c-MET expression and then inhibited AKT signaling and ERK signaling, which inhibited HCC progression and enhanced LEN sensitivity to HCC [400]. In vitro experiments, Yan et al. elucidated that miR-23b overexpression synergized with LEN to inhibit HCC cell proliferation, migration, invasion, and angiogenesis, thus enhancing its sensitivity in HCC [401]. Moreover, the clinical cohort analysis demonstrated that HCC patients with high miR-6071 expression had more survival benefits from LEN treatment, it was detected that miR-6071 was downregulated in hepatic cancer stem cells (hCSCs) and its overexpression inhibited hCSCs self-renewal and tumorigenesis, along with HCC cell proliferation and migration. Mechanistically, Chen et al. identified that miR-6071 targeted the 11’UTR of PTPN3 to inhibit its translation, thereby inhibiting HCC development and rendering HCC cells sensitive to LEN [402].
Others
Zinc transporter 1 (ZNT1) is the unique transporter regulating Zn2+ from the intracellular to extracellular compartment [403] and has been detected highly expressed in HCC tissues from patients with poor prognoses [404]. Dan et al. found that ZNT1 was significantly downregulated in macrophages of HCC in humans, and its low expression was associated with shorter survival time. Mechanistically, ZNT1 regulated Zn2+ transport to control the endocytosis of toll-like receptor 2 (TLR2) and PD-L1, and then reducing macrophage-induced inflammation and immunosuppression, which augmented the therapeutic efficacy of LEN [405]. Focal adhesion kinase (FAK) plays important roles in a wide range of tumor progression, including HCC [406-408], which has been observed to be elevated at protein and mRNA expression as well as phosphorylation levels in LR-HCC cells. In vitro and in vivo experiments demonstrated that FAK inhibition reversed resistance to LEN in HCC. Mechanistically, FAK promoted LEN resistance through activation of WNK lysine-deficient kinase 1 (WNK1) [409].
Ubiquitin-specific proteases (USPs) are one of the largest deubiquitinate families regulating the stability of downstream effectors [410], its dysregulation has been demonstrated to play important roles in tumor progression, including HCC [411]. Chen et al. found elevated expression of USP1 in HCC tissues at mRNA and histological levels, which was associated with poor prognoses in both OS and DFS. It was found that USP1 enhanced the stability of c-KIT to promote HCC cell growth. In addition, they demonstrated that USP1 overexpression promoted LEN-induced apoptosis in HCC cells [412]. USP 22, a deubiquitinating enzymes closely associated with tumor progress, has been shown to induce tumor drug resistance [413,414]. Guo et al. discovered overexpression of USP22 in LR-HCC cells, and its knockout suppressed cell invasion, migration, and dryness. Additionally, cytological and animal experiments revealed that USP22 knockdown promoted the LEN sensitivity of HCC cells. Moreover, they found that USP22 positively regulated jumonji domain-containing protein 8 (JMJD8), an oncoprotein instrumental in promoting EMT and tumor immune escape [415], leading to LEN resistance in HCC [416].
In addition, there were still many studies elucidating that several key proteins played significant roles in regulating LEN resistance in HCC, but the specific resistance mechanism remained unclear. For instance, MEX3C, as an RNA-binding protein essential to regulating cellular energy balance [417], was detected to be upregulated in HCC tissues and positively associated with a poor prognosis. And, the combination of MEX3C knockdown and LEN showed a stronger inhibitory effect on HCC cells in vitro [125]. Aldo-keto reductase family 1 member C1 (AKR1C1) was highly expressed in HCC tissues and was speculated as a predictive biomarker signifying poor prognosis and LEN resistance [418]. Brain-expressed X-linked 4 (BEX4) was positively correlated with resistance to LEN in HCC cells [419]. 1-acyl-sn-glycerol-3-phosphate acyltransferaseδ (APGAT4) was significantly highly expressed in HCC tissues and was associated with poor prognosis, which was suggested to promote HCC progression and induce LEN resistance [420].
Adverse effects of lenvatinib in HCC treatment
Although LEN exerted better curative efficacy, there existed adverse reactions difficult to avoid. The REFLECT study has reported that the adverse reactions of LEN in the treatment of HCC mainly include hypertension, fatigue, diarrhea, decreased appetite, weight loss, and arthralgia/myalgia, etc. [7]. In addition to some common side effects, LEN may occasionally induce a multitude of relatively unexpected and adverse events. Herein, we primarily summarized the researches associated with rare side effects.
Hemorrhage and bleeding
Sawako et al. conducted a retrospective study with a total of 68 uHCC patients treated with consecutive LEN therapy and observed that 5 of them had an intraperitoneal or intratumoral hemorrhage, the tumor volume of the patients with hemorrhage was relatively large, and their prognosis was worse than that of patients without hemorrhage. They perceived that while LEN treatment could rapidly inhibit tumor blood supply, it still increases the risk of tumor-related bleeding [421,422]. Similarly, Aya et al. reported two cases of HCC rupture hemorrhage shortly after treatment with LEN and suggested that LEN acted on VEGFR to inhibit the regeneration of damaged vascular endothelial cells, which may lead to bleeding [423]. Masayuki et al. reported a case of a 77-year-old patient who appeared with spontaneous rupture of lung metastases from HCC after LEN treatment [424]. Yoshiaki et al. report a case of a 72-year-old male with HCC who developed tumor lysis syndrome (TLS) and HCC rupture on the second day after LEN treatment [425]. Katsutoshi reported a case of an 80-year-old man with multiple HCC who developed multiple internal tumor bleeding eight days after receiving LEN therapy and perceived that TACE may deserve careful consideration before LEN treatment in large HCC, which may substantially decrease the risk of tumor rupture and bleeding [426].
Digestive system adverse effects
Digestive tract ulcers, bleeding and perforation
Maito et al. reported an 82-year-old male HCC patient who exhibited epigastric pain, vomiting, and jaundice after three months of LEN treatment [427]. Endoscopic evaluations revealed multiple duodenal ulcers, one of which was located at the Vater’s ampulla, causing cholestasis. They deduced that LEN could potentially trigger duodenal ulcers, leading to obstructive jaundice. Saori et al. reported a 78-year-old male patient with uHCC who was diagnosed with colitis after one month of LEN treatment and regained with discontinuation of LEN [428]. Naomi et al. reported a rare case of a 75-year-old advanced HCC patient with small bowel metastases who suddenly developed abdominal pain and was diagnosed with perforation of the small intestine by imaging after one month of LEN treatment [429]. They speculated that the perforation of the small intestine might correlate with the strong antiangiogenic effects of LEN. Additionally, it was reported that advanced HCC patients associated with alcoholic liver disease and non-alcoholic fatty liver disease had a higher incidence of adverse events such as intestinal leakage following LEN treatment [430]. Mizokami et al. reported a case of intestinal fistula after treatment with LEN and the postoperative pathology revealed notable mitotic arrest of the colon epithelium, suggesting a potential link between intestinal perforation and LEN [431]. Moreover, Keiichiro et al. first reported a case of tracheoesophageal fistula during LEN therapy [432].
Cholecystitis and gallbladder perforation
Kazunaga et al. reported a 67-year-old man with advanced HCC, who developed acute right upper quadrant abdominal pain accompanied by fever after six days of LEN treatment and was diagnosed with acute non-calculous cholecystitis. Although the patient improved after antibiotics and endoscopic nasobiliary drainage treatment (ENBD), acute non-calculous cholecystitis recurred three days after taking a low dose of LEN again. They perceived that recurrent acute non-calculous cholecystitis in this patient was closely related to LEN treatment [433]. Shuya et al. reported on a 59-year-old male HCC patient with multiple bone metastases, who developed general fatigue and was subsequently diagnosed with gallbladder perforation four months after treatment with LEN. This patient reinitiated with LEN treatment after receiving conservative treatment and an unexpected occurred gallbladder rupture one month later, thus indicating that LEN was the causative agent for gallbladder perforation [434].
Diarrhea
Furthermore, diarrhea was also noted to be common during TKI therapy. Ecombe et al. suggested that TKI-induced diarrhea might be caused by disorders in intestinal function induced by modifications within the intestinal microbiome [435]. Yosuke et al. explored the relationship between the intestinal microbiome and LEN-associated diarrhea through the examination of stool samples between diarrheal and non-diarrheal groups and found the butyrate metabolic pathway was notably enriched via KEGG enrichment analysis, which induced the regulatory T cells development and was essential for the regulation of intestinal inflammation [436].
Hepatic encephalopathy
Liu et al. reported a 42-year-old uHCC patient with cirrhosis, classified as Child-Pugh C, who developed hepatic encephalopathy following three consecutive days of LEN+PD-1 therapy. After the discontinuation and positive treatment, the symptoms of this patient were continuously improved, and his blood ammonia gradually decreased to normal levels [437].
Tiredness, fatigue and decreased appetite
Cancer patients occasionally experience fatigue during the treatment period, known as cancer-related fatigue. It has been reported that abnormal adenosine triphosphate synthesis is closely related to the occurrence of fatigue [438]. Carnitine serves a crucial role in mitochondrial β-oxidation of fatty acids to generate ATP. Studies have shown that the reduction of serum carnitine levels in patients receiving cisplatin chemotherapy is related to fatigue occurrence [439,440]. Hironao et al. assessed carnitine levels in blood and urine samples from 20 HCC patients treated with LEN within 1 month and confirmed that carnitine deficiency may be a common cause of fatigue during LEN treatment [441]. Kohya et al. identified FGF2, a key metabolic regulator and appetite modulator [442,443], as a potential predictor of decreased appetite in HCC patients treated with LEN [444].
Respiratory adverse effects
Takeshi et al. reported a relatively slow progression of LEN-induced interstitial pneumonia case. For this patient, the focal ground glass opacity with infiltrating shadows in both lung fields was found two months after LEN treatment. Four months later, the patient presented respiratory symptoms, had to withdraw the LEN treatment, and died shortly thereafter [445]. In addition, Yasuhiro et al. first reported a 60-year-old HCC patient with multiple lung metastases who developed pneumothorax after six months of LEN treatment [446].
Cutaneous adverse reactions
Kanzaki et al. reported a HCC patient who acquired pyoderma gangrenosum during the LEN therapy [447]. Yukari et al. first reported a case of generalized erythema rash (GER) during LEN treatment [448]. It has been reported that extragenital condyloma acuminatum occurred in HCC patients treated with LEN in combination with PD-1 inhibitors [449]. Iwasa et al. took the lead in reporting a case of an HCC patient with skin ulcers after receiving LEN combined with proton beam therapy [450]. Cha et al. reported a 60-year-old uHCC male who developed stage IV skin ulcers with full skin shedding and muscle tissue necrosis after receiving LEN treatment for two weeks and had complete skin recovery after LEN withdrawal [451]. Rachel et al. report a 66-year-old male uHCC patient with end-stage renal disease who developed symptoms of psoriasiform eruption after two weeks of LEN treatment, and such symptoms improved after drug reduction and active treatment. In addition, they proposed that the increase in the proportion of T lymphocytes induced by LEN-mediated immunomodulatory may be responsible for the initiation of psoriasiform eruption [452].
Endocrine adverse effects
Katherine et al. reported a case of a 24-year-old HCC patient with multiple episodes of severe hypoglycemia over several months of SOR treatment, whose hypoglycemia is under control after the change of medication with LEN [453]. Yoichi et al. first reported a case of ovarian insufficiency in a 25-year-old HCC woman post a forty-eight-day treatment of LEN. In addition, the patient underwent hepatectomy, and within two months after the end of LEN treatment, the levels of related hormones returned to normal and the normal menstrual cycle resumed [454]. Maito et al. reported a case of a 74-year-old HCC male who ensured fatigue and palpitation after forty-two-day treatment of LEN and was diagnosed with destructive thyroiditis. In addition, he was further diagnosed with hypothyroidism three months later [455]. To investigate the incidence of the LEN-induced hypothyroidism in HCC patients. Shusuke et al. executed a single-center retrospective study including a total of 61 HCC patients. The findings revealed a striking high rate of high-grade hypothyroidism at 36.1% (22/61). Multivariate analysis identified non-smoking and eosinophil count ≤150/µL as risk factors for LEN-induced high-grade hypothyroidism [456]. Additionally, Takenori et al. found that in comparison with LEN or ATEZ/BEV therapy group, there may be a higher incidence of thyroid dysfunction in HCC patients treated with LEN+ATEZ/BEV [457].
Renal adverse effects
Nakashima et al. reported a 77-year-old Japanese woman with HCC who developed severe renal insufficiency and refractory hypertension within one month of the lowest dose of LEN therapy. The drug-induced thrombotic microangiopathy, podocytopathies, as well as polar vascular disease were further verified through renal biopsy [458]. Thaninee et al. reported a 67-year-old uHCC patient with BCLC B stage, who was diagnosed with nephrotic syndrome (NS) after two weeks of LEN treatment, and the symptoms gradually improved within a week after LEN discontinuation [459].
Blood system adverse effects
Laurence et al. retrospectively analyzed 23 uHCC patients who had been treated with LEN for at least one month. The 20 patients (87%) occurred a significant increase in hemoglobin (Hb) levels (P<0.001), and the Hb was elevated above 16.5 g/dL in 10 patients (all male), 7 of whom received low-dose aspirin to prevent thrombosis, 2 patients received phlebotomy, and no thromboembolic complications occurred. The Hb decreased significantly after the withdrawal of LEN (P<0.05). In addition, they detected elevated levels of erythropoietin (EPO) in histopathological liver biopsies, and deduced that LEN may increase EPO secretion through its anti-angiogenic activity, thereby leading to elevated Hb levels [460].
Discussion
HCC is an aggressive malignancy with a poor prognosis, which seriously endangers human health. Given its insidious onset, HCC is frequently diagnosed at advanced and unresectable stages [461]. LEN is widely used in clinical treatment due to its excellent therapeutic effect, albeit the occurrence of drug resistance remains unavoidable. Nowadays, combination therapy is widely and firmly considered the futural trend of HCC treatment, combined targeted drugs, and immune checkpoint inhibitors with or without local treatment shine brilliantly in the treatment of advanced HCC [4]. Besides, a lot of prospective and retrospective studies have also focused on the efficacy and tolerability of LEN combination therapy and found several associated prognostic indicatorss (Table 1), which may aid in the treatment section in clinical practice.
Table 1.
The indicators for predicting prognosis and therapeutic efficacy in uHCC
| Therapeutic approaches | Indicators reflecting good therapeutic efficacy | References |
|---|---|---|
| LEN | Low serum CRP levels (<0.5 mg/dL) | [18] |
| Low CAR ratio (<0.108) | [19] | |
| Low GPS scores | [23] | |
| High GNRI scores (>98) | [12] | |
| Low PLR (<150) | [26] | |
| Low NLR (<4) | [28] | |
| High SMI (≥42 cm2/m2 for men and ≥38 cm2/m2 for women) | [17] | |
| Significant infiltration of T cells and PD-L1-expressing macrophages | [30] | |
| Low serum levels of FGF19 (<194 pg/mL) | [31] | |
| High histological expression of FGFR4 | [32,33] | |
| With well-controlled HBV or HCV viremia | [35] | |
| Without EGFR/ERBB2 alterations | [29] | |
| Low serum levels of CXCL9 (<308/333 pg/mL) | [65] | |
| LEN+HAIC+PD-1 | Low serum levels of PCT (≤0.13 ng/mL) | [80] |
| LEN+HAIC+PD-1 | High serum levels of CCL28 (>5.9 ng/ml) and BTC (>387.8 pg/mL) | [91] |
| LEN+PD-1 | Low NLR (<3.46) | [92] |
| High expression of nuclear PIGL | [120] | |
| LEN+PD-1 | High FGFR4 expression | [122] |
| LEN+PD-1+TACE | High counts of peripheral Th (>153 cells/uL) and NK cells (>214 cells/uL) | [130] |
| High SAT volume index and low density | [129] | |
| Low peripheral naive CD8 T cell subsets (<6.24%) | [131] | |
| Low white blood cell counts (×109/L)/lymphocyte proportion (%) (≤43.1) | [132] | |
| >50% decrease in the serum levels of AFP or DCP | [165] | |
| LEN+PD-1+TACE | Low NLR (≤3.11) | [159] |
| Low NLR (<3.2) | [137] | |
| Low NLR (≤2.165) | [138] | |
| Low serum levels of PCT (≤0.13 ng/mL) | [145] |
Abbreviations: BTC: Betacellulin; CAR: CRP to albumin ratio; CCL28: C-C motif chemokine ligand 28; CRP: c-reactive protein; CXCL9: C-X-C motif chemokine ligand 9; GNRI: Geriatric nutritional risk index; GSP: Glasgow prognostic score; HAIC: Hepatic arterial infusion chemotherapy; LEN: Lenvatinib; NK cell: Natural killer cell; NLR: Neutrophil to lymphocyte ratio; PCT: Procalcitonin; PIGL: Phosphatidylinositol-glycan biosynthesis class L; PLR: Platelet-to-lymphocyte ratio; SAT: Subcutaneous adipose tissue; SMI: Skeletal muscle index; TACE: Transcatheter arterial chemoembolization.
Aberrant regulations of signaling pathways including PI3K/AKT [207], wnt [255], autophagy process [222] and MAPK/ERK signaling [173], etc., are strongly related to LEN resistance. Studies revealed that several targeted drugs synergistically amplified the LEN therapeutic efficacy and delayed or even reversed drug resistance process by regulating key proteins in such signaling (Table 2). Concretely, Tan et al. first found that the EGFR inhibitor gefitinib reversed LEN resistance by inhibiting the negative feedback activation of ERK and its downstream signaling during LEN treatment. Besides, Secukinumab has also been reported to promote autophagy by inhibiting IL-17A-induced BCL2 degradation, thereby antagonizing drug resistance [231]. It provides us with new reflections on whether the combination of other clinically targeted drugs with LEN similarly could potentially augment LEN sensitivity and yield superior survival. For example, Tepotinib was observed to inhibit SOR resistance by inhibiting c-MET in HCC, and it remains unknown whether it antagonizes LEN resistance by inhibiting c-MET and its downstream factors [462]. Alternatively, the activation of RAS and its downstream MAPK/ERK pathway has been extensively identified in HCC [463], and whether the existing MEK inhibitors and most recent proposed pan-RAS inhibitors (BI-2865) [464] can cooperate with LEN in the treatment of advanced HCC is still waiting to be explored in future.
Table 2.
The correlation between associated drugs and lenvatinib resistance in uHCC treatment
| Drug name | Drug types | Roles | LEN resistance | Reference |
|---|---|---|---|---|
| Elacridar | ABCB1 and BCRP inhibitor | Inhibition of ABCB1 and BCRP | Inhibition | [354] |
| Flubendazole | Anthelmintic | Inhibition of PCSK9 and hedgehog signaling | Inhibition | [276] |
| Losartan | AT-II receptor blocker | Suppressing VEGF-A expression | Inhibition | [348] |
| Secukinumab | Biologic agent targeting IL-17A | Inhibition of BCL2 expression and promotion of autophagy pathway | Inhibition | [231] |
| Palbociclib | CDK4/6 inhibitor | Inhibition of wnt/β-catenin signaling | Inhibition | [257] |
| Abemaciclib | Chinese herbal extracts | Inhibition of c-MYC | Inhibition | [350] |
| Amentoflavone | Inhibition of AKT/ERK signaling | Inhibition | [183] | |
| Sophoridine | Inhibition of VEGFR2 and MAPK/ERK signaling | Inhibition | [185] | |
| OSC | Inhibition of FGFR1, AKT/mTOR and MAPK/ERK signaling | Inhibition | [187] | |
| Betulin | Inhibition of the mTOR/IL-1β signaling | Inhibition | [240] | |
| CP | Inhibition of exosome-mediated autophagy | Inhibition | [233] | |
| Curcumin | Inhibition of EGFR and PI3K/AKT signaling | Inhibition | [207] | |
| Dasatinib | c-SRC inhibitor | Inhibition of c-SRC | Inhibition | [351] |
| Erlotinib | EGFR inhibitor | Inhibit of STAT/ABCB1 axis and inhibition of LEN exocytosis | Inhibition | [355] |
| GANT61 | GLI1/2 inhibitor | Inhibition of hedgehog signaling | Inhibition | [273] |
| Simvastatin | HMG CoA Reductase inhibitor | Inhibition of cholesterol synthesis and hedgehog signaling | Inhibition | [282] |
| Metformin | Hypoglycemic drug | Inhibition of AKT signaling | Inhibition | [203] |
| Metformin | Inhibition of wnt/β-catenin signaling | Inhibition | [256] | |
| BAFA1 | Macrolide antibiotics | Inhibit autophagic degradation of NRP1 | Promotion | [222] |
| Aspirin | NSAIDs | Downregulation of AKT and MAPK/ERK signaling | Inhibition | [357] |
| Celecoxib | Decrease the survival rate of PD-L1 neutrophils | Inhibition | [358] | |
| Vitamin C | Vitamins | / | Inhibition | [349] |
Abbreviations: ABCB1: ATP binding cassette subfamily B member 1; BCRP: Breast cancer resistance protein; PCSK9: Proprotein convertase subtilisin/kexin type 9; BCL2: B-cell lymphoma 2; OSC: Oxysophocarpine; CP: Compound phyllanthus urinaria; BAFA1: Bafilomycin A1; NSAIDs: Non-steroidal anti-inflammatory drugs.
Interestingly, several studies have determined that chronic disease-related drugs could synergistically potentiate the clinical efficacy of LEN (Table 2). Metformin, as the first-line hypoglycemic agent for type 2 diabetes treatment, was found to enhance LEN sensitivity by inhibiting AKT pathway and downstream signaling activation [203]. Furthermore, aspirin is widely used in the treatment of rheumatism or arthritis, and anti-thrombotic prevention of atrial fibrillation and myocardial infarction, which has been also detected to improve the efficacy of LEN by inhibiting AKT/ERK signaling [357]. It is viable to collect and analyze the clinical information of HCC patients with chronic illness mentioned above and evaluate their treatment response to LEN.
In conclusion, while LEN combination therapy has indeed improved prognoses compared with LEN monotherapy, the specific mechanism remains elusive. In recent years, although many studies have revealed partial LEN resistance mechanisms, there are still many unknown fields waiting to be explored, the precision therapy for HCC is still a long way to go. We sincerely hope that this could offer some inspiration to researchers, which may help them to choose individual treatment precisely, as well as acquire new research ideas to further reveal the mechanism of LEN resistance in HCC.
Acknowledgements
This research was funded by The Medical and health science and Technology project of Zhejiang Province (Grant No. 2019337934), Natural Science Foundation of Ningbo (Grant No. 2021J065), The Fundamental Research Funds for the Provincial Universities of Zhejiang (Grant No. SJLZ2022004), and The K.C. Wong Magna Fund in Ningbo University.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15:1008–1014. doi: 10.1245/s10434-007-9705-0. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203–222. doi: 10.1038/s41575-022-00704-9. [DOI] [PubMed] [Google Scholar]
- 5.Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79:665–674. doi: 10.1007/s40265-019-01116-x. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, Vogel A, Tovoli F, Ueshima K, Aikata H, López CL, Pracht M, Meng Z, Daniele B, Park JW, Palmer D, Tamai T, Saito K, Dutcus CE, Lencioni R. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: a subanalysis of the REFLECT study. J Hepatol. 2023;78:133–141. doi: 10.1016/j.jhep.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Cen K, Sun W, Feng B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. 2021;33:1477–1486. doi: 10.1007/s40520-020-01656-3. [DOI] [PubMed] [Google Scholar]
- 10.Xiong J, Wang M, Zhang Y, Nie L, He T, Wang Y, Huang Y, Feng B, Zhang J, Zhao J. Association of geriatric nutritional risk index with mortality in hemodialysis patients: a meta-analysis of cohort studies. Kidney Blood Press Res. 2018;43:1878–1889. doi: 10.1159/000495999. [DOI] [PubMed] [Google Scholar]
- 11.Lv GY, An L, Sun DW. Geriatric nutritional risk index predicts adverse outcomes in human malignancy: a meta-analysis. Dis Markers. 2019;2019:4796598. doi: 10.1155/2019/4796598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita A, Hagiwara N, Osawa A, Akasu T, Matsumoto Y, Ueda K, Saeki C, Oikawa T, Koike K, Saruta M. The geriatric nutritional risk index predicts tolerability of lenvatinib in patients with hepatocellular carcinoma. In Vivo. 2022;36:865–873. doi: 10.21873/invivo.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Z, Cheng L, Li K, Shuai Y, Xue K, Zhong Y, Chen L. Correlation between L3 skeletal muscle index and prognosis of patients with stage IV gastric cancer. J Gastrointest Oncol. 2021;12:2073–2081. doi: 10.21037/jgo-21-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jogiat UM, Bédard ELR, Sasewich H, Turner SR, Eurich DT, Filafilo H, Baracos V. Sarcopenia reduces overall survival in unresectable oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13:2630–2636. doi: 10.1002/jcsm.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascarella MA, Gardiner L, Patel T, Vendra V, Khan N, Kergoat MJ, Kubik MW, Solari MG, Snyderman CH, Traylor KS, Sridharan SS. Cervical paraspinal skeletal muscle index outperforms frailty indices to predict postoperative adverse events in operable head and neck cancer with microvascular reconstruction. Microsurgery. 2022;42:209–216. doi: 10.1002/micr.30848. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 17.Uojima H, Chuma M, Tanaka Y, Hidaka H, Nakazawa T, Iwabuchi S, Kobayashi S, Hattori N, Ogushi K, Morimoto M, Kagawa T, Tanaka K, Kako M, Koizumi W. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer. 2020;9:193–206. doi: 10.1159/000504604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okumura T, Kimura T, Iwadare T, Wakabayashi SI, Kobayashi H, Yamashita Y, Sugiura A, Joshita S, Fujimori N, Kunimoto H, Komatsu M, Fukushima H, Mori H, Umemura T. Prognostic significance of c-reactive protein in lenvatinib-treated unresectable hepatocellular carcinoma: a multi-institutional study. Cancers (Basel) 2023;15:5343. doi: 10.3390/cancers15225343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Naganuma A, Aoki T, Koizumi Y, Nakamura S, Joko K, Hiasa Y, Kudo M. C-reactive protein to albumin ratio predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Sci Rep. 2022;12:8421. doi: 10.1038/s41598-022-12058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Li H, Cai J, Chen L, Yao J, Zhang Y, Xu W, Geng L, Yang M, Chen P, Zheng J, Yang Y, Gong S. Prognostic value of the glasgow prognostic score or modified glasgow prognostic score for patients with colorectal cancer receiving various treatments: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;51:1237–1249. doi: 10.1159/000495500. [DOI] [PubMed] [Google Scholar]
- 21.Kudou K, Kusumoto T, Nambara S, Tsuda Y, Kusumoto E, Yoshida R, Sakaguchi Y, Ikejiri K. New index combining multiple inflammation-based prognostic scores for predicting the prognosis of gastric cancer patients. JGH Open. 2022;6:171–178. doi: 10.1002/jgh3.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834–1836. doi: 10.1038/sj.bjc.6602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Naganuma A, Aoki T, Koizumi Y, Nakamura S, Joko K, Hiasa Y, Kudo M Real-life Practice Experts for HCC (RELPEC) Study Group and the Hepatocellular Carcinoma Experts from 48 Clinics in Japan (HCC 48) Group. Glasgow prognostic score predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter analysis. Eur J Gastroenterol Hepatol. 2022;34:857–864. doi: 10.1097/MEG.0000000000002398. [DOI] [PubMed] [Google Scholar]
- 24.Dalpiaz O, Krieger D, Ehrlich GC, Pohlmann K, Stojakovic T, Pummer K, Zigeuner R, Pichler M, Hutterer GC. Validation of the preoperative platelet-to-lymphocyte ratio as a prognostic factor in a european cohort of patients with upper tract urothelial carcinoma. Urol Int. 2017;98:320–327. doi: 10.1159/000452109. [DOI] [PubMed] [Google Scholar]
- 25.You J, Zhu GQ, Xie L, Liu WY, Shi L, Wang OC, Huang ZH, Braddock M, Guo GL, Zheng MH. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2016;7:25516–25527. doi: 10.18632/oncotarget.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Yasuda S, Toyoda H, Fukunishi S, Ohama H, Kawata K, Nakamura S, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Arai T, Imai M, Joko K, Koizumi Y, Hiasa Y Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Platelet-lymphocyte ratio predicts survival in patients with hepatocellular carcinoma who receive lenvatinib: an inverse probability weighting analysis. Eur J Gastroenterol Hepatol. 2021;32:261–268. doi: 10.1097/MEG.0000000000001734. [DOI] [PubMed] [Google Scholar]
- 27.Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol. 2015;22(Suppl 3):S603–613. doi: 10.1245/s10434-015-4571-7. [DOI] [PubMed] [Google Scholar]
- 28.Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Yasuda S, Toyoda H, Fukunishi S, Ohama H, Kawata K, Nakamura S, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Arai T, Imai M, Joko K, Koizumi Y, Hiasa Y. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;40:968–976. doi: 10.1111/liv.14405. [DOI] [PubMed] [Google Scholar]
- 29.Lim M, Franses JW, Imperial R, Majeed U, Tsai J, Hsiehchen D. EGFR/ERBB2 amplifications and alterations associated with resistance to lenvatinib in hepatocellular carcinoma. Gastroenterology. 2023;164:1006–1008. e1003. doi: 10.1053/j.gastro.2023.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Sung PS, Cho SW, Lee J, Yang H, Jang JW, Bae SH, Choi JY, Yoon SK. Infiltration of T cells and programmed cell death ligand 1-expressing macrophages as a potential predictor of lenvatinib response in hepatocellular carcinoma. J Liver Cancer. 2020;20:128–134. doi: 10.17998/jlc.20.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigesawa T, Suda G, Kimura M, Shimazaki T, Maehara O, Yamada R, Kitagataya T, Suzuki K, Nakamura A, Ohara M, Umemura M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N. Baseline angiopoietin-2 and FGF19 levels predict treatment response in patients receiving multikinase inhibitors for hepatocellular carcinoma. JGH Open. 2020;4:880–888. doi: 10.1002/jgh3.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata O, Kamimura K, Ko M, Sakai N, Abe H, Morita S, Mizusawa T, Sato H, Sakamaki A, Terai S. Effect of lenvatinib on a hepatocellular carcinoma with fibroblast growth factor receptor 4 expression: a case report and review of the literature. Intern Med. 2021;60:1709–1715. doi: 10.2169/internalmedicine.6580-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iseda N, Itoh S, Toshida K, Tomiyama T, Morinaga A, Shimokawa M, Shimagaki T, Wang H, Kurihara T, Toshima T, Nagao Y, Harada N, Yoshizumi T, Mori M. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci. 2022;113:2272–2287. doi: 10.1111/cas.15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi M, Ono A, Ishikawa A, Kodama K, Uchikawa S, Hatooka H, Zhang P, Teraoka Y, Morio K, Fujino H, Nakahara T, Murakami E, Miki D, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Hayes CN, Fujita M, Nakagawa H, Yasui W, Aikata H, Chayama K. Tumor fibroblast growth factor receptor 4 level predicts the efficacy of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol. 2020;11:e00179. doi: 10.14309/ctg.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao YW, Sou FM, Wang JH, Chen YH, Tsai MC, Hu TH, Hung CH, Chen CH, Kuo YH. Well-controlled viremia reduces the progression of hepatocellular carcinoma in chronic viral hepatitis patients treated with lenvatinib. Kaohsiung J Med Sci. 2023;39:1233–1242. doi: 10.1002/kjm2.12757. [DOI] [PubMed] [Google Scholar]
- 36.Chen R, Li Y, Song K, Li L, Shen C, Ma P, Wang Z. Efficacy and safety of transarterial chemoembolization-lenvatinib sequential therapy for the treatment of hepatocellular carcinoma with portal vein tumor thrombus: a retrospective study. J Gastrointest Oncol. 2022;13:780–786. doi: 10.21037/jgo-22-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long J, Liu L, Yang X, Lu X, Qin L. Impact of combining lenvatinib with transarterial chemoembolization for unresectable hepatocellular carcinoma. Pak J Med Sci. 2023;39:1847–1852. doi: 10.12669/pjms.39.6.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Yan S, Zhang G, Yang L, Wei S, Yi P. A retrospective study of transarterial chemoembolization (TACE) combined with lenvatinib compared with TACE monotherapy for BCLC B2 stage hepatocellular carcinoma. Oncol Lett. 2023;26:507. doi: 10.3892/ol.2023.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie QY, Huang LP, Gao FW, Liu DQ, Wang X, Jiang KY, Gong J, Zhao X, Gao BJ, Lei ZH. Efficacy of lenvatinib combined with sequential transarterial chemoembolization for primary hepatocellular carcinoma and the effects on serum basic fibroblast growth factor and vascular endothelial growth factor. Front Pharmacol. 2022;13:965770. doi: 10.3389/fphar.2022.965770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YX, Zhang JX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ. Comparison of the efficacy and safety of transarterial chemoembolization with or without lenvatinib for unresectable hepatocellular carcinoma: a retrospective propensity score-matched analysis. J Hepatocell Carcinoma. 2022;9:685–694. doi: 10.2147/JHC.S373250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, Liu L, Zhang X, Zhai J, Qu Z. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–675. doi: 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuroda H, Oikawa T, Ninomiya M, Fujita M, Abe K, Okumoto K, Katsumi T, Sato W, Igarashi G, Iino C, Endo T, Tanabe N, Numao H, Fukuda S, Iijima K, Masamune A, Ohira H, Ueno Y, Takikawa Y. Objective response by mRECIST to initial lenvatinib therapy is an independent factor contributing to deep response in hepatocellular carcinoma treated with lenvatinib-transcatheter arterial chemoembolization sequential therapy. Liver Cancer. 2022;11:383–396. doi: 10.1159/000522424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase iii, randomized clinical trial (LAUNCH) J. Clin. Oncol. 2023;41:117–127. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 44.Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, Kikukawa C, Kosaka Y, Uchikawa S, Morio K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Tsuge M, Hiramatsu A, Fukuhara T, Mori N, Takaki S, Tsuji K, Nonaka M, Hyogo H, Aisaka Y, Masaki K, Honda Y, Moriya T, Naeshiro N, Takahashi S, Imamura M, Chayama K, Aikata H. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99:507–517. doi: 10.1159/000515865. [DOI] [PubMed] [Google Scholar]
- 45.Xia D, Bai W, Wang E, Li J, Chen X, Wang Z, Huang M, Huang M, Sun J, Yang W, Lin Z, Wu J, Li Z, Yang S, Zhu X, Chen Z, Zhang Y, Fan W, Mai Q, Ding R, Nie C, Feng L, Li X, Huang W, Sun J, Wang Q, Lv Y, Li X, Luo B, Wang Z, Yuan J, Guo W, Li K, Li B, Li R, Yin Z, Xia J, Han G. Lenvatinib with or without concurrent drug-eluting beads transarterial chemoembolization in patients with unresectable, advanced hepatocellular carcinoma: a real-world, multicenter, retrospective study. Liver Cancer. 2022;11:368–382. doi: 10.1159/000523849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan W, Zhu B, Yue S, Zheng X, Zou X, Li F, Qiao L, Wu Y, Xue M, Wang H, Tang Y, Li J. Idarubicin-Loaded DEB-TACE plus lenvatinib versus lenvatinib for patients with advanced hepatocellular carcinoma: a propensity score-matching analysis. Cancer Med. 2023;12:61–72. doi: 10.1002/cam4.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu JN, Li JJ, Yan S, Zhang GN, Yi PS. Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2023;13:1074793. doi: 10.3389/fonc.2023.1074793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu R, Ji X, Pei X, Yu Y. Comparison of efficacy and safety between transarterial chemoembolization (TACE) combined with lenvatinib versus TACE combined with sorafenib in the treatment of intermediate and advanced hepatocellular carcinoma. Am J Transl Res. 2023;15:1117–1128. [PMC free article] [PubMed] [Google Scholar]
- 49.Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, Zhang Y, Wu H, Liao Z. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol. 2021;11:821599. doi: 10.3389/fonc.2021.821599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang JX, Chen YX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ. Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a comparative retrospective study. Hepatol Res. 2022;52:794–803. doi: 10.1111/hepr.13801. [DOI] [PubMed] [Google Scholar]
- 51.Xue M, Wu Y, Zhu B, Zou X, Fan W, Li J. Advanced hepatocellular carcinoma treated by transcatheter arterial chemoembolization with drug-eluting beads plus lenvatinib versus sorafenib, a propensity score matching retrospective study. Am J Cancer Res. 2021;11:6107–6118. [PMC free article] [PubMed] [Google Scholar]
- 52.Yano S, Kawaoka T, Johira Y, Miura R, Kosaka M, Shirane Y, Murakami S, Amioka K, Naruto K, Ando Y, Kosaka Y, Yamaoka K, Kodama K, Uchikawa S, Fujino H, Ohno A, Nakahara T, Murakami E, Okamoto W, Yamauchi M, Imamura M, Mori K, Arihiro K, Kuroda S, Kobayashi T, Ohdan H, Aikata H. Advanced hepatocellular carcinoma with response to lenvatinib after atezolizumab plus bevacizumab. Medicine (Baltimore) 2021;100:e27576. doi: 10.1097/MD.0000000000027576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muto H, Kuzuya T, Kawabe N, Ohno E, Funasaka K, Nagasaka M, Nakagawa Y, Miyahara R, Shibata T, Hashimoto S, Katano Y, Hirooka Y. Clinical outcomes with lenvatinib in patients previously treated with atezolizumab/bevacizumab for advanced hepatocellular carcinoma. Anticancer Res. 2023;43:4673–4682. doi: 10.21873/anticanres.16663. [DOI] [PubMed] [Google Scholar]
- 54.Chen YH, Chen YY, Wang JH, Hung CH. Efficacy and safety of lenvatinib after progression on first-line atezolizumab plus bevacizumab treatment in advanced hepatocellular carcinoma patients. Anticancer Res. 2023;43:1377–1384. doi: 10.21873/anticanres.16286. [DOI] [PubMed] [Google Scholar]
- 55.Yano S, Kawaoka T, Yamasaki S, Johira Y, Kosaka M, Shirane Y, Miura R, Amioka K, Naruto K, Yamaoka K, Fujii Y, Uchikawa S, Fujino H, Ono A, Nakahara T, Murakami E, Miki D, Tsuge M, Teraoka Y, Kouno H, Takaki S, Mori N, Tsuji K, Oka S. Therapeutic efficacy and safety of lenvatinib after atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Cancers (Basel) 2023;15:5406. doi: 10.3390/cancers15225406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tada F, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Kosaka H, Naganuma A, Matono T, Aoki T, Kuroda H, Yata Y, Koizumi Y, Nakamura S, Kaibori M, Iijima H, Hiasa Y, Kudo M Real-life Practice Experts for HCC (RELPEC) Study Group and the Hepatocellular Carcinoma Experts from 48 clinics in Japan (HCC 48) Group. Comparison of prognostic impact of atezolizumab plus bevacizumab versus lenvatinib in patients with intermediate-stage hepatocellular carcinoma. Liver Int. 2024;44:113–124. doi: 10.1111/liv.15753. [DOI] [PubMed] [Google Scholar]
- 57.Niizeki T, Tokunaga T, Takami Y, Wada Y, Harada M, Shibata M, Nakao K, Sasaki R, Hirai F, Shakado S, Yoshizumi T, Itoh S, Yatsuhashi H, Bekki S, Ido A, Mawatari S, Honda K, Sugimoto R, Senju T, Takahashi H, Kuwashiro T, Maeshiro T, Nakamuta M, Aratake Y, Yamashita T, Otsuka Y, Matsumoto S, Sohda T, Shimose S, Murotani K, Tanaka Y. Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a propensity score matching analysis. Target Oncol. 2022;17:643–653. doi: 10.1007/s11523-022-00921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maesaka K, Sakamori R, Yamada R, Doi A, Tahata Y, Miyazaki M, Ohkawa K, Mita E, Iio S, Nozaki Y, Yakushijin T, Imai Y, Kodama T, Hikita H, Tatsumi T, Takehara T. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res. 2022;52:630–640. doi: 10.1111/hepr.13771. [DOI] [PubMed] [Google Scholar]
- 59.Hatanaka T, Kakizaki S, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Yokohama K, Nishikawa H, Nishimura T, Shimada N, Kawata K, Kosaka H, Naganuma A, Yata Y, Ohama H, Kuroda H, Aoki T, Tanaka K, Tanaka T, Tada F, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Koizumi Y, Nakamura S, Kaibori M, Iijima H, Hiasa Y, Kudo M, Kumada T Real-life Practice Experts for HCC (RELPEC) Study Group, and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Comparative analysis of the therapeutic outcomes of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma patients aged 80 years and older: multicenter study. Hepatol Res. 2024;54:382–391. doi: 10.1111/hepr.13991. [DOI] [PubMed] [Google Scholar]
- 60.Rimini M, Persano M, Tada T, Suda G, Shimose S, Kudo M, Cheon J, Finkelmeier F, Lim HY, Presa J, Salani F, Lonardi S, Piscaglia F, Kumada T, Sakamoto N, Iwamoto H, Aoki T, Chon HJ, Himmelsbach V, Schirripa M, Montes M, Vivaldi C, Soldà C, Hiraoka A, Sho T, Niizeki T, Nishida N, Steup C, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tada F, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Kosaka H, Naganuma A, Koizumi Y, Nakamura S, Kaibori M, Iijima H, Hiasa Y, Burgio V, Scartozzi M, Cascinu S, Casadei-Gardini A. Survival outcomes from atezolizumab plus bevacizumab versus Lenvatinib in Child Pugh B unresectable hepatocellular carcinoma patients. J Cancer Res Clin Oncol. 2023;149:7565–7577. doi: 10.1007/s00432-023-04678-2. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Yang L, Wei S, Li J, Yi P. Efficacy and safety of atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2023;149:16191–16201. doi: 10.1007/s00432-023-05342-5. [DOI] [PubMed] [Google Scholar]
- 62.Casadei-Gardini A, Rimini M, Tada T, Suda G, Shimose S, Kudo M, Cheon J, Finkelmeier F, Lim HY, Rimassa L, Presa J, Masi G, Yoo C, Lonardi S, Tovoli F, Kumada T, Sakamoto N, Iwamoto H, Aoki T, Chon HJ, Himmelsbach V, Pressiani T, Montes M, Vivaldi C, Soldà C, Piscaglia F, Hiraoka A, Sho T, Niizeki T, Nishida N, Steup C, Iavarone M, Di Costanzo G, Marra F, Scartozzi M, Tamburini E, Cabibbo G, Foschi FG, Silletta M, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tada F, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Kosaka H, Naganuma A, Koizumi Y, Nakamura S, Kaibori M, Iijima H, Hiasa Y, Burgio V, Persano M, Della Corte A, Ratti F, De Cobelli F, Aldrighetti L, Cascinu S, Cucchetti A. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a large real-life worldwide population. Eur J Cancer. 2023;180:9–20. doi: 10.1016/j.ejca.2022.11.017. [DOI] [PubMed] [Google Scholar]
- 63.Zhao C, Xiang Z, Li M, Wang H, Liu H, Yan H, Huang M. Transarterial chemoembolization combined with atezolizumab plus bevacizumab or lenvatinib for unresectable hepatocellular carcinoma: a propensity score matched study. J Hepatocell Carcinoma. 2023;10:1195–1206. doi: 10.2147/JHC.S418256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Q, Lu P, Xia Y, Ding S, Fan Y, Li X, Han P, Liu J, Tian D, Liu M. CXCL9: evidence and contradictions for its role in tumor progression. Cancer Med. 2016;5:3246–3259. doi: 10.1002/cam4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hosoda S, Suda G, Sho T, Ogawa K, Kimura M, Yang Z, Yoshida S, Kubo A, Tokuchi Y, Kitagataya T, Maehara O, Ohnishi S, Nakamura A, Yamada R, Ohara M, Kawagishi N, Natsuizaka M, Nakai M, Morikawa K, Furuya K, Baba M, Yamamoto Y, Suzuki K, Izumi T, Meguro T, Terashita K, Ito J, Miyagishima T, Sakamoto N. Low baseline CXCL9 predicts early progressive disease in unresectable HCC with atezolizumab plus bevacizumab treatment. Liver Cancer. 2022;12:156–170. doi: 10.1159/000527759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li KK, Adams DH. Antitumor CD8+ T cells in hepatocellular carcinoma: present but exhausted. Hepatology. 2014;59:1232–1234. doi: 10.1002/hep.26779. [DOI] [PubMed] [Google Scholar]
- 67.Ji J, Yin Y, Ju H, Xu X, Liu W, Fu Q, Hu J, Zhang X, Sun B. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis. 2018;9:478. doi: 10.1038/s41419-018-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuwano A, Yada M, Miyazaki Y, Tanaka K, Kurosaka K, Ohishi Y, Masumoto A, Motomura K. Tumor-infiltrating CD8(+) T cells as a biomarker for chemotherapy efficacy in unresectable hepatocellular carcinoma. Oncol Lett. 2023;25:259. doi: 10.3892/ol.2023.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian Y, Gong L, Li S, Mao K, Li X, Liao G. Case report: radiotherapy plus immunotherapy and lenvatinib for the treatment of recurrent hepatocellular carcinoma with a right atrium and inferior vena cava tumor thrombus. Front Oncol. 2022;12:879454. doi: 10.3389/fonc.2022.879454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li G, Zhao Y, Li K, Yang S, Xiang C, Song J, Yang Y, Li G, Dong J. Effectiveness and safety of the PD-1 inhibitor lenvatinib plus radiotherapy in patients with HCC with main PVTT: real-world data from a tertiary centre. J Hepatocell Carcinoma. 2023;10:2037–2048. doi: 10.2147/JHC.S432542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du SS, Chen GW, Yang P, Chen YX, Hu Y, Zhao QQ, Zhang Y, Liu R, Zheng DX, Zhou J, Fan J, Zeng ZC. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys. 2022;112:1243–1255. doi: 10.1016/j.ijrobp.2021.12.162. [DOI] [PubMed] [Google Scholar]
- 72.Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J. Clin. Oncol. 2019;37:2141–2151. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji X, Xu Z, Sun J, Li W, Duan X, Wang Q. Lenvatinib with or without stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis: a retrospective study. Radiat Oncol. 2023;18:101. doi: 10.1186/s13014-023-02270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Une N, Takano-Kasuya M, Kitamura N, Ohta M, Inose T, Kato C, Nishimura R, Tada H, Miyagi S, Ishida T, Unno M, Kamei T, Gonda K. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med Oncol. 2021;38:60. doi: 10.1007/s12032-021-01503-z. [DOI] [PubMed] [Google Scholar]
- 75.Wang Q, Ji X, Sun J, Li W, Duan X, Zhang A. Comparison of stereotactic body radiotherapy with and without lenvatinib for advanced hepatocellular carcinoma: a propensity score analysis. J Cancer Res Clin Oncol. 2023;149:7441–7452. doi: 10.1007/s00432-023-04652-y. [DOI] [PubMed] [Google Scholar]
- 76.Dong A, Zhu M, Zhang Z, Fan W, Wu Z, Chen Y, Tu J, Zhang Y, Zhuang W, He X, Peng Z. Efficacy of radiation plus transarterial chemoembolization and lenvatinib in hepatocellular carcinoma with portal vein tumor thrombus. Front Oncol. 2023;13:1320818. doi: 10.3389/fonc.2023.1320818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao ZW, Cheng BQ, Zhou T, Gao YJ. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: a narrative review. Hepatobiliary Pancreat Dis Int. 2022;21:134–144. doi: 10.1016/j.hbpd.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Tian Y, Jin W, Sun H, Jin D, Kang D, Li Z, Piao L. Modified hepatic arterial infusion chemotherapy combined with lenvatinib and camrelizumab for advanced HCC: two case reports. J Hepatocell Carcinoma. 2023;10:1587–1593. doi: 10.2147/JHC.S426174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y, Fu S, Mao Y, Huang S, Li D, Wu J. Efficacy and safety of hepatic arterial infusion chemotherapy combined with programmed cell death protein-1 antibody and lenvatinib for advanced hepatocellular carcinoma. Front Med (Lausanne) 2022;9:919069. doi: 10.3389/fmed.2022.919069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang X, Wu H, Ning S, Li X, Xie Y, Shao W, Yu J. Hepatic arterial infusion chemotherapy combined with lenvatinib plus humanized programmed death receptor-1 in patients with high-risk advanced hepatocellular carcinoma: a real-world study. J Hepatocell Carcinoma. 2023;10:1497–1509. doi: 10.2147/JHC.S418387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gan L, Lang M, Tian X, Ren S, Li G, Liu Y, Han R, Zhu K, Li H, Wu Q, Cui Y, Zhang W, Fang F, Li Q, Song T. A retrospective analysis of conversion therapy with lenvatinib, sintilimab, and arterially-directed therapy in patients with initially unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:673–686. doi: 10.2147/JHC.S404675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu Y, Peng W, Zhang W, Yang Z, Hu Z, Pang Y, Hu D, Chen J, Wang J, Zhou Z, Xu L, Chen M, Zhang Y. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. 2023;58:413–424. doi: 10.1007/s00535-023-01976-x. [DOI] [PubMed] [Google Scholar]
- 83.Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z, Huang X, Wu Y, Li T, Guo W. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: a multicenter retrospective study. BMC Cancer. 2021;21:1126. doi: 10.1186/s12885-021-08858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, Guo RP. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi: 10.3389/fonc.2021.618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diao L, Wang C, You R, Leng B, Yu Z, Xu Q, Cheng Y, Yin G. Hepatic arterial infusion chemotherapy combined with lenvatinib and PD-1 inhibitors versus lenvatinib and PD-1 inhibitors for HCC refractory to TACE. J Gastroenterol Hepatol. 2024;39:746–753. doi: 10.1111/jgh.16463. [DOI] [PubMed] [Google Scholar]
- 86.Lin Z, Chen D, Hu X, Huang D, Chen Y, Zhang J, Li X, Zou X. Clinical efficacy of HAIC (FOLFOX) combined with lenvatinib plus PD-1 inhibitors vs. TACE combined with lenvatinib plus PD-1 inhibitors in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombus and arterioportal fistulas. Am J Cancer Res. 2023;13:5455–5465. [PMC free article] [PubMed] [Google Scholar]
- 87.Lin LW, Ke K, Yan LY, Chen R, Huang JY. Efficacy and safety of hepatic artery infusion chemotherapy combined with tyrosine kinase inhibitors plus programmed death-1 inhibitors for hepatocellular carcinoma refractory to transarterial chemoembolization. Front Oncol. 2023;13:1178428. doi: 10.3389/fonc.2023.1178428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.An C, Fu Y, Li W, Zuo M, Wu P. Postprogression treatment of lenvatinib plus PD-1 inhibitor in advanced hepatocellular carcinoma refractory to hepatic arterial infusion chemotherapy. Cancer. 2023;129:2235–2244. doi: 10.1002/cncr.34764. [DOI] [PubMed] [Google Scholar]
- 89.Chen S, Shi F, Wu Z, Wang L, Cai H, Ma P, Zhou Y, Mai Q, Wang F, Tang S, Zhuang W, Lai J, Chen X, Chen H, Guo W. Hepatic arterial infusion chemotherapy plus lenvatinib and tislelizumab with or without transhepatic arterial embolization for unresectable hepatocellular carcinoma with portal vein tumor thrombus and high tumor burden: a multicenter retrospective study. J Hepatocell Carcinoma. 2023;10:1209–1222. doi: 10.2147/JHC.S417550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, Chen MS, Guo RP, Li QJ, Shi M. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi: 10.1177/17588359211002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, Li Q, Xu L, Zhang Y, Wei W, Chen M, Kan A, Shi M. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi: 10.1016/j.ejca.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Xiao Y, Zhu G, Xie J, Luo L, Deng W, Lin L, Tao J, Hu Z, Shan R. Pretreatment neutrophil-to-lymphocyte ratio as prognostic biomarkers in patients with unresectable hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy combined with lenvatinib and camrelizumab. J Hepatocell Carcinoma. 2023;10:2049–2058. doi: 10.2147/JHC.S432134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Long F, Chen S, Li R, Lin Y, Han J, Guo J, Chen Y, Li C, Song P. Efficacy and safety of HAIC alone vs. HAIC combined with lenvatinib for treatment of advanced hepatocellular carcinoma. Med Oncol. 2023;40:147. doi: 10.1007/s12032-023-02012-x. [DOI] [PubMed] [Google Scholar]
- 94.Yuan W, Yue W, Wen H, Wang X, Wang Q. Analysis on efficacy of hepatic artery infusion chemotherapy with or without lenvatinib for unresectable hepatocellular carcinoma. Eur Surg Res. 2023;64:268–277. doi: 10.1159/000529475. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Qiao Y, Zhou M, Guo J, Lin Y, Li W, An C, Li C. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and sequential ablation in the treatment of advanced hepatocellular carcinoma. Cancer Med. 2023;12:5436–5449. doi: 10.1002/cam4.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J, Han JW, Sung PS, Lee SK, Yang H, Nam HC, Yoo SH, Lee HL, Kim HY, Lee SW, Kwon JH, Jang JW, Kim CW, Nam SW, Oh JS, Chun HJ, Bae SH, Choi JY, Yoon SK. Comparative analysis of lenvatinib and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma: a multi-center, propensity score study. J Clin Med. 2021;10:4045. doi: 10.3390/jcm10184045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu YJ, Lai ZC, He MK, Bu XY, Chen HW, Zhou YM, Xu L, Wei W, Zhang YJ, Chen MS, Guo RP, Shi M, Li QJ. Toripalimab combined with hepatic arterial infusion chemotherapy versus lenvatinib for advanced hepatocellular carcinoma. Technol Cancer Res Treat. 2021;20:15330338211063848. doi: 10.1177/15330338211063848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang X, Chen B, Wang Y, Wang Y, Long J, Zhang N, Xue J, Xun Z, Zhang L, Cheng J, Lei J, Sun H, Li Y, Lin J, Xie F, Wang D, Pan J, Hu K, Guan M, Huo L, Shi J, Yu L, Zhou L, Zhou J, Lu Z, Yang X, Mao Y, Sang X, Lu Y, Zhao H. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023;17:709–719. doi: 10.1007/s12072-022-10480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu MH, Huang C, Li ML, Zhu XD, Tan CJ, Zhou J, Fan J, Sun HC, Shen YH. Effectiveness and safety of lenvatinib plus anti-programmed death-1 antibodies in patients with hepatocellular carcinoma: a real-world cohort study. Cancer Med. 2023;12:9202–9212. doi: 10.1002/cam4.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun X, Zhang Q, Mei J, Yang Z, Chen M, Liang T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer. 2022;22:293. doi: 10.1186/s12885-022-09405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zou J, Huang P, Ge N, Xu X, Wang Y, Zhang L, Chen Y. Anti-PD-1 antibodies plus lenvatinib in patients with unresectable hepatocellular carcinoma who progressed on lenvatinib: a retrospective cohort study of real-world patients. J Gastrointest Oncol. 2022;13:1898–1906. doi: 10.21037/jgo-22-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang XH, Liu CJ, Wen HQ, Duan XH, Jiao YQ, Liu YJ, Chen MS, Zhu KS, Mao XH, Zhou QF. Effectiveness of lenvatinib plus immune checkpoint inhibitors in primary advanced hepatocellular carcinoma beyond oligometastasis. Clin Transl Med. 2023;13:e1214. doi: 10.1002/ctm2.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W, Tong S, Hu B, Wan T, Tang H, Zhao F, Jiao T, Li J, Zhang Z, Cai J, Ye H, Wang Z, Chen S, Wang Y, Li X, Wang F, Cao J, Tian L, Zhao X, Chen M, Wang H, Cai S, Hu M, Bai Y, Lu S. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer. 2023;11:e007366. doi: 10.1136/jitc-2023-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yi Y, Sun BY, Weng JL, Zhou C, Zhou CH, Cai MH, Zhang JY, Gao H, Sun J, Zhou J, Fan J, Ren N, Qiu SJ. Lenvatinib plus anti-PD-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:1046584. doi: 10.3389/fonc.2022.1046584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen YH, Huang C, Zhu XD, Xu MH, Chen ZS, Tan CJ, Zhou J, Fan J, Sun HC. The safety profile of hepatectomy following preoperative systemic therapy with lenvatinib plus anti-PD-1 antibodies versus hepatectomy alone in patients with hepatocellular carcinoma. Ann Surg Open. 2022;3:e163. doi: 10.1097/AS9.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Masi G, Kwiatkowski M, Lim HY, Kim JH, Breder V, Kumada H, Cheng AL, Galle PR, Kaneko S, Wang A, Mody K, Dutcus C, Dubrovsky L, Siegel AB, Finn RS LEAP-002 Investigators. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1399–1410. doi: 10.1016/S1470-2045(23)00469-2. [DOI] [PubMed] [Google Scholar]
- 107.Zhao L, Chang N, Shi L, Li F, Meng F, Xie X, Xu Z, Wang F. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: a retrospective, real-world study. Heliyon. 2022;8:e09538. doi: 10.1016/j.heliyon.2022.e09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu WC, Lin TY, Chen MH, Hung YP, Liu CA, Lee RC, Huang YH, Chao Y, Chen SC. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;40:789–797. doi: 10.1007/s10637-022-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang X, Yu S, Pang J, Zhang W, Kong H, Huang J, Zhang G, Zhang H, Gu Y, Chen Y, Yang B, Liu J, Zeng Z. Population sensitive to lenvatinib plus anti-PD-1 for unresectable hepatocellular carcinoma infected with hepatitis B virus. J Hepatocell Carcinoma. 2023;10:847–861. doi: 10.2147/JHC.S411748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Q, Cao M, Yuan G, Cheng X, Zang M, Chen M, Hu X, Huang J, Li R, Guo Y, Ruan J, Chen J. Lenvatinib plus camrelizumab vs. lenvatinib monotherapy as first-line treatment for unresectable hepatocellular carcinoma: a multicenter retrospective cohort study. Front Oncol. 2022;12:809709. doi: 10.3389/fonc.2022.809709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu Y, Sun P, Wang K, Xiao S, Cheng Y, Li X, Wang B, Li J, Yu W, Cheng Y. Efficacy and safety of lenvatinib monotreatment and lenvatinib-based combination therapy for patients with unresectable hepatocellular carcinoma: a retrospective, real-world study in China. Cancer Cell Int. 2021;21:503. doi: 10.1186/s12935-021-02200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei F, Huang Q, He J, Luo L, Zeng Y. Lenvatinib plus camrelizumab versus lenvatinib monotherapy as post-progression treatment for advanced hepatocellular carcinoma: a short-term prognostic study. Cancer Manag Res. 2021;13:4233–4240. doi: 10.2147/CMAR.S304820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu Y, Fu S, Shang K, Zeng J, Mao Y. PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus regorafenib in patients with advanced hepatocellular carcinoma after failure of sorafenib. Front Oncol. 2022;12:958869. doi: 10.3389/fonc.2022.958869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiang HC, Lee YC, Chang TT, Lin YJ, Wu HT, Wang CT, Chen CY, Chen PJ, Hsieh MT, Lin SH, Chen SH, Chuang CH, Wu IC, Hong TC, Wu JS, Han MZ, Chen WT, Chiang CM, Hung KK, Kuo HY. Real-world effectiveness of sorafenib versus lenvatinib combined with PD-1 inhibitors in unresectable hepatocellular carcinoma. Cancers (Basel) 2023;15:854. doi: 10.3390/cancers15030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Q, Li R, Li L, Wang G, Ji S, Zheng X, Jia X, Tao H, Hu Y. Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience. Ther Adv Med Oncol. 2023;15:17588359231206274. doi: 10.1177/17588359231206274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ouyang J, Wang Z, Yuan K, Yang Y, Zhou Y, Li Q, Yang N, Zhao H, Zhao H, Zhou J. Adjuvant lenvatinib plus PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy: a retrospective landmark analysis. J Hepatocell Carcinoma. 2023;10:1465–1477. doi: 10.2147/JHC.S424616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang J, Guo Z, Song M, Pan Q, Zhao J, Huang Y, Han Y, Ouyang D, Yang C, Chen H, Di M, Tang Y, Zhu Q, Wang Q, Li Y, He J, Weng D, Xiang T, Xia J. Lenvatinib improves anti-PD-1 therapeutic efficacy by promoting vascular normalization via the NRP-1-PDGFRβ complex in hepatocellular carcinoma. Front Immunol. 2023;14:1212577. doi: 10.3389/fimmu.2023.1212577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 119.Zhou C, Yang ZF, Sun BY, Yi Y, Wang Z, Zhou J, Fan J, Gan W, Ren N, Qiu SJ. Lenvatinib induces immunogenic cell death and triggers toll-like receptor-3/4 ligands in hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:697–712. doi: 10.2147/JHC.S401639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu H, Shi T, Yao L, Xu D, Ding Y, Xia Q, Liu W, Wang X. Elevated nuclear PIGL disrupts the cMyc/BRD4 axis and improves PD-1 blockade therapy by dampening tumor immune evasion. Cell Mol Immunol. 2023;20:867–880. doi: 10.1038/s41423-023-01048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu XF, Zhu XD, Feng LH, Li XL, Xu B, Li KS, Xiao N, Lei M, Sun HC, Tang ZY. Physical activity improves outcomes of combined lenvatinib plus anti-PD-1 therapy in unresectable hepatocellular carcinoma: a retrospective study and mouse model. Exp Hematol Oncol. 2022;11:20. doi: 10.1186/s40164-022-00275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74:2544–2560. doi: 10.1002/hep.31921. [DOI] [PubMed] [Google Scholar]
- 123.Chao H, Deng L, Xu F, Yu Z, Xu X, Huang J, Zeng T. MEX3C regulates lipid metabolism to promote bladder tumorigenesis through JNK pathway. Onco Targets Ther. 2019;12:3285–3294. doi: 10.2147/OTT.S199667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao Y, Jiang F, Zhang S, Yang L, Sun Y. MEX3C promotes osteosarcoma malignant progression through negatively regulating FGF14. J BUON. 2020;25:1554–1561. [PubMed] [Google Scholar]
- 125.Guo J, Zhao J, Xu Q, Huang D. MEX3C as a potential target for hepatocellular carcinoma drug and immunity: combined therapy with Lenvatinib. BMC Cancer. 2023;23:967. doi: 10.1186/s12885-023-11320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou C, Sun BY, Zhou PY, Yang ZF, Wang ZT, Liu G, Gan W, Wang Z, Zhou J, Fan J, Yi Y, Ren N, Qiu SJ. MAIT cells confer resistance to Lenvatinib plus anti-PD1 antibodies in hepatocellular carcinoma through TNF-TNFRSF1B pathway. Clin Immunol. 2023;256:109770. doi: 10.1016/j.clim.2023.109770. [DOI] [PubMed] [Google Scholar]
- 127.Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357–373. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–384. doi: 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang J, Yang X, Yang X, Xu J, Wang Y, Wang Y, Xun Z, Zhang N, Sang X, Xu Y, Wang X, Zhao H, Lu X. Prognostic value of SAT volume and density for predicting the outcome of patients with unresectable HCC treated with lenvatinib plus anti-PD-1 antibodies. Am J Cancer Res. 2023;13:912–921. [PMC free article] [PubMed] [Google Scholar]
- 130.Zou Y, Chen Z, Chen Z, Zhang Y, Zheng Z, Wu F, Xu L, Li Q, Lin Y, Shi N, Jin H. Peripheral Th and NK cells are associated with response to lenvatinib plus PD-1 inhibitor in unresectable hepatocellular carcinoma. Clin Immunol. 2023;249:109290. doi: 10.1016/j.clim.2023.109290. [DOI] [PubMed] [Google Scholar]
- 131.Huang C, Xu B, Zhu XD, Shen YH, Li ML, Zhu JJ, Zhou J, Fan J, Sun HC. Peripheral naïve CD8(+) T cells as a predictive biomarker of response to lenvatinib plus an anti-PD-1 antibody in advanced hepatocellular carcinoma: a biomarker study. Cancer Commun (Lond) 2022;42:1226–1230. doi: 10.1002/cac2.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo DZ, Zhang SY, Dong SY, Yan JY, Wang YP, Cao Y, Rao SX, Fan J, Yang XR, Huang A, Zhou J. Circulating immune index predicting the prognosis of patients with hepatocellular carcinoma treated with lenvatinib and immunotherapy. Front Oncol. 2023;13:1109742. doi: 10.3389/fonc.2023.1109742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, Zhou JY, Li YN, Qiu FN, Li B, Yan ML. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi: 10.2147/JHC.S332420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teng Y, Ding X, Li W, Sun W, Chen J. A retrospective study on therapeutic efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Technol Cancer Res Treat. 2022;21:15330338221075174. doi: 10.1177/15330338221075174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu SJ, Ruan DD, Wu QY, Tang Y, Zhang JH, Cai SL, Zhou YF, Luo JW, Fang ZT. Safety and efficacy of drug-eluting bead transarterial chemoembolization combined with lenvatinib and anti-pd-1 antibodies for unresectable hepatocellular carcinoma: a retrospective analysis. J Hepatocell Carcinoma. 2023;10:807–820. doi: 10.2147/JHC.S408819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu JY, Zhang ZB, Zhou JY, Ke JP, Bai YN, Chen YF, Wu JY, Zhou SQ, Wang SJ, Zeng ZX, Li YN, Qiu FN, Li B, Yan ML. Outcomes of salvage surgery for initially unresectable hepatocellular carcinoma converted by transcatheter arterial chemoembolization combined with lenvatinib plus anti-PD-1 antibodies: a multicenter retrospective study. Liver Cancer. 2022;12:229–237. doi: 10.1159/000528356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qu S, Wu D, Hu Z. Neutrophil-to-lymphocyte ratio and early tumor shrinkage as predictive biomarkers in unresectable hepatocellular carcinoma patients treated with lenvatinib, PD-1 inhibitors, in combination with TACE. Technol Cancer Res Treat. 2023;22:15330338231206704. doi: 10.1177/15330338231206704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X, Fu Z, Chen X, Cao K, Zhong J, Liu L, Ding N, Zhang X, Zhai J, Qu Z. Efficacy and safety of lenvatinib combined with PD-1 inhibitors plus TACE for unresectable hepatocellular carcinoma patients in China real-world. Front Oncol. 2022;12:950266. doi: 10.3389/fonc.2022.950266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, Han X. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. doi: 10.3389/fphar.2021.709060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mu C, Shen J, Zhu X, Peng W, Zhang X, Wen T. The efficacy and safety of lenvatinib plus transarterial chemoembolization in combination with PD-1 antibody in treatment of unresectable recurrent hepatocellular carcinoma: a case series report. Front Oncol. 2023;13:1096955. doi: 10.3389/fonc.2023.1096955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, Feng D, Chen Y, Zheng J. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. doi: 10.3389/fonc.2021.783480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu S, Xiong R, Duan C, Tang J, Yin T, Dai S. PD-1 combined with lenvatinib and TACE for the transformational treatment of hepatocellular carcinoma combined with portal vein tumor thrombus: a case report and literature review. Front Oncol. 2023;13:1199143. doi: 10.3389/fonc.2023.1199143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li X, Ding X, Liu M, Wang J, Sun W, Teng Y, Xu Y, Wu H, Li W, Zhou L, Chen J. A multicenter prospective study of TACE combined with lenvatinib and camrelizumab for hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med. 2023;12:16805–16814. doi: 10.1002/cam4.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li SQ, Wu JY, Wu JY, Xie H, Li JH, Zeng ZX, Fu YK, Liu DY, Li H, Chen WZ, Huang JY, Yan ML. Transarterial chemoembolization plus lenvatinib and PD-1 inhibitors for hepatocellular carcinoma with main trunk portal vein tumor thrombus: a multicenter retrospective study. J Hepatocell Carcinoma. 2023;10:1799–1811. doi: 10.2147/JHC.S428980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ning S, Li X, Ma X, Liu J, Chang X. Efficacy of TACE combined with lenvatinib plus sintilimab for hepatocellular carcinoma with tumor thrombus in the inferior vena cava and/or right atrium. J Hepatocell Carcinoma. 2023;10:1511–1525. doi: 10.2147/JHC.S410967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhou J, Zhu K. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H, Huang M. TACE combined with lenvatinib and camrelizumab for unresectable multiple nodular and large hepatocellular carcinoma (>5 cm) Technol Cancer Res Treat. 2023;22:15330338231200320. doi: 10.1177/15330338231200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang W, Zhu L, Guo Y, Gui Y, Liu F, Chen L, Xiong F, Zheng C. Safety and efficacy of lenvatinib combined with camrelizumab plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a two-center retrospective study. Front Oncol. 2022;12:982948. doi: 10.3389/fonc.2022.982948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang H, Yang T, Qiu G, Liu J. Efficacy and safety of TACE combined with lenvatinib and PD-(L)1 inhibitor in the treatment of unresectable hepatocellular carcinoma: a retrospective study. J Hepatocell Carcinoma. 2023;10:1435–1443. doi: 10.2147/JHC.S423684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guo P, Pi X, Gao F, Li Q, Li D, Feng W, Cao W. Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: a propensity score matching study. Front Oncol. 2022;12:945915. doi: 10.3389/fonc.2022.945915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu J, Wei S, Yang L, Yu J, Yan D, Yi P. Efficacy and safety of transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors in the treatment of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2023;149:14451–14461. doi: 10.1007/s00432-023-05231-x. [DOI] [PubMed] [Google Scholar]
- 152.Xin Y, Zhang X, Liu N, Peng G, Huang X, Cao X, Zhou X, Li X. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int. 2023;17:753–764. doi: 10.1007/s12072-023-10502-3. [DOI] [PubMed] [Google Scholar]
- 153.Wang J, Zhao M, Han G, Han X, Shi J, Mi L, Li N, Yin X, Duan X, Hou J, Yin F. Transarterial chemoembolization combined with pd-1 inhibitors plus lenvatinib showed improved efficacy for treatment of unresectable hepatocellular carcinoma compared with PD-1 inhibitors plus lenvatinib. Technol Cancer Res Treat. 2023;22:15330338231166765. doi: 10.1177/15330338231166765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lang M, Gan L, Ren S, Han R, Ma X, Li G, Li H, Zhang T, Wu Q, Cui Y, Zhang W, Fang F, Li Q, Lu W, Song T. Lenvatinib plus sintilimab with or without transarterial chemoembolization for intermediate or advanced stage hepatocellular carcinoma: a propensity score-matching cohort study. Am J Cancer Res. 2023;13:2540–2553. [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang JX, Hua HJ, Cheng Y, Liu S, Shi HB, Zu QQ. Role of transarterial chemoembolization in the era of tyrosine kinase inhibitor and immune checkpoint inhibitor combination therapy for unresectable hepatocellular carcinoma: a retrospective propensity score matched analysis. Acad Radiol. 2024;31:1304–1311. doi: 10.1016/j.acra.2023.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Zou X, Xu Q, You R, Yin G. Evaluating the benefits of TACE combined with lenvatinib plus PD-1 inhibitor for hepatocellular carcinoma with portal vein tumor thrombus. Adv Ther. 2023;40:1686–1704. doi: 10.1007/s12325-023-02449-6. [DOI] [PubMed] [Google Scholar]
- 157.Wu J, Zeng J, Wang H, Huo Z, Hou X, He D. Efficacy and safety of transarterial chemoembolization combined with lenvatinib and camrelizumab in patients with BCLC-defined stage C hepatocellular carcinoma. Front Oncol. 2023;13:1244341. doi: 10.3389/fonc.2023.1244341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiang YJ, Wang K, Yu HM, Li XW, Cheng YQ, Wang WJ, Feng JK, Bo MH, Qin YY, Zheng YT, Shan YF, Zhou LP, Zhai J, Cheng SQ. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. 2022;52:721–729. doi: 10.1111/hepr.13773. [DOI] [PubMed] [Google Scholar]
- 159.Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, Hu L, Zhang J, Wang R, Wei L, Wu D. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: a prospective cohort study. Front Oncol. 2022;12:874473. doi: 10.3389/fonc.2022.874473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao S, Zhou M, Wang P, Yang J, Zhang D, Yin F, Song P. Sorafenib, lenvatinib, or lenvatinib combining PD-1 inhibitors plus TACE in unresectable hepatocellular carcinoma: a retrospective analysis. Technol Cancer Res Treat. 2022;21:15330338221133640. doi: 10.1177/15330338221133640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69:1983–1994. doi: 10.1002/hep.30233. [DOI] [PubMed] [Google Scholar]
- 162.Saeki I, Yamasaki T, Yamashita S, Hanazono T, Urata Y, Furutani T, Yokoyama Y, Oishi T, Maeda M, Kimura T, Kotoh Y, Sasaki R, Miyaji T, Oono T, Aibe Y, Hisanaga T, Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Takami T, Sakaida I. Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers (Basel) 2020;12:779. doi: 10.3390/cancers12040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun X, Mei J, Lin W, Yang Z, Peng W, Chen J, Zhang Y, Xu L, Chen M. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer. 2021;21:775. doi: 10.1186/s12885-021-08428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ichikawa T, Machida N, Sasaki H, Tenmoku A, Kaneko H, Negishi R, Oi I, Fujino MA. Early prediction of the outcome using tumor markers and mRECIST in unresectable hepatocellular carcinoma patients who underwent transarterial chemoembolization. Oncology. 2016;91:317–330. doi: 10.1159/000448999. [DOI] [PubMed] [Google Scholar]
- 165.Luo MC, Wu JY, Wu JY, Lin ZT, Li YN, Zeng ZX, Wei SM, Yan ML. Early tumor marker response predicts treatment outcomes in patients with unresectable hepatocellular carcinoma receiving combined lenvatinib, immune checkpoint inhibitors, and transcatheter arterial chemoembolization therapy. J Hepatocell Carcinoma. 2023;10:1827–1837. doi: 10.2147/JHC.S425674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li X, Chen J, Wang X, Bai T, Lu S, Wei T, Tang Z, Huang C, Zhang B, Liu B, Li L, Wu F. Outcomes and prognostic factors in initially unresectable hepatocellular carcinoma treated using conversion therapy with lenvatinib and TACE plus PD-1 inhibitors. Front Oncol. 2023;13:1110689. doi: 10.3389/fonc.2023.1110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141:160–171. doi: 10.1016/j.pharmthera.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 168.Chen L, Shi Y, Jiang CY, Wei LX, Wang YL, Dai GH. Expression and prognostic role of pan-Ras, Raf-1, pMEK1 and pERK1/2 in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2011;37:513–520. doi: 10.1016/j.ejso.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 169.Delire B, Stärkel P. The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications. Eur J Clin Invest. 2015;45:609–623. doi: 10.1111/eci.12441. [DOI] [PubMed] [Google Scholar]
- 170.Gnoni A, Licchetta A, Memeo R, Argentiero A, Solimando AG, Longo V, Delcuratolo S, Brunetti O. Role of BRAF in hepatocellular carcinoma: a rationale for future targeted cancer therapies. Medicina (Kaunas) 2019;55:754. doi: 10.3390/medicina55120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Han KH. Treatment of hepatocellular carcinoma with lenvatinib. Gastroenterol Hepatol (N Y) 2018;14:662–664. [PMC free article] [PubMed] [Google Scholar]
- 172.Ao J, Chiba T, Shibata S, Kurosugi A, Qiang N, Ma Y, Kan M, Iwanaga T, Sakuma T, Kanzaki H, Kanayama K, Kojima R, Kusakabe Y, Nakamura M, Saito T, Nakagawa R, Kondo T, Ogasawara S, Suzuki E, Muroyama R, Kato J, Mimura N, Kanda T, Maruyama H, Kato N. Acquisition of mesenchymal-like phenotypes and overproduction of angiogenic factors in lenvatinib-resistant hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2021;549:171–178. doi: 10.1016/j.bbrc.2021.02.097. [DOI] [PubMed] [Google Scholar]
- 173.Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W, Jiang X, Li H, Yang P, Xiang D. N6-methyladenosine-mediated up-regulation of FZD10 regulates liver cancer stem cells’ properties and lenvatinib resistance through WNT/β-catenin and hippo signaling pathways. Gastroenterology. 2023;164:990–1005. doi: 10.1053/j.gastro.2023.01.041. [DOI] [PubMed] [Google Scholar]
- 174.Huang S, Ma Z, Zhou Q, Wang A, Gong Y, Li Z, Wang S, Yan Q, Wang D, Hou B, Zhang C. Genome-wide CRISPR/Cas9 library screening identified that DUSP4 deficiency induces lenvatinib resistance in hepatocellular carcinoma. Int J Biol Sci. 2022;18:4357–4371. doi: 10.7150/ijbs.69969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lu Y, Shen H, Huang W, He S, Chen J, Zhang D, Shen Y, Sun Y. Genome-scale CRISPR-Cas9 knockout screening in hepatocellular carcinoma with lenvatinib resistance. Cell Death Discov. 2021;7:359. doi: 10.1038/s41420-021-00747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Peng H, Xue R, Ju Z, Qiu J, Wang J, Yan W, Gan X, Tian Y, Shen H, Wang X, Wang X, Ni X, Yu Y, Lu L. Cancer-associated fibroblasts enhance the chemoresistance of CD73(+) hepatocellular carcinoma cancer cells via HGF-Met-ERK1/2 pathway. Ann Transl Med. 2020;8:856. doi: 10.21037/atm-20-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Eun JW, Yoon JH, Ahn HR, Kim S, Kim YB, Lim SB, Park W, Kang TW, Baek GO, Yoon MG, Son JA, Weon JH, Kim SS, Cho HJ, Cheong JY. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun (Lond) 2023;43:455–479. doi: 10.1002/cac2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Harris KA, Shekhtman A, Agris PF. Specific RNA-protein interactions detected with saturation transfer difference NMR. RNA Biol. 2013;10:1307–1311. doi: 10.4161/rna.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang S, Zhu P, Sun B, Guo J, Zhou H, Shu Y, Li Q. Modulation of YrdC promotes hepatocellular carcinoma progression via MEK/ERK signaling pathway. Biomed Pharmacother. 2019;114:108859. doi: 10.1016/j.biopha.2019.108859. [DOI] [PubMed] [Google Scholar]
- 181.Guo J, Zhu P, Ye Z, Wang M, Yang H, Huang S, Shu Y, Zhang W, Zhou H, Li Q. YRDC mediates the resistance of lenvatinib in hepatocarcinoma cells via modulating the translation of KRAS. Front Pharmacol. 2021;12:744578. doi: 10.3389/fphar.2021.744578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen WL, Hsieh CL, Chen JH, Huang CS, Chen WT, Kuo YC, Chen CY, Hsu FT. Amentoflavone enhances sorafenib-induced apoptosis through extrinsic and intrinsic pathways in sorafenib-resistant hepatocellular carcinoma SK-Hep1 cells in vitro. Oncol Lett. 2017;14:3229–3234. doi: 10.3892/ol.2017.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang CJ, Wu MH, Tsai JJ, Hsu FT, Hsia TC, Liu KC, Kuo YC. Inactivation of AKT/ERK signaling and induction of apoptosis are associated with amentoflavone sensitization of hepatocellular carcinoma to lenvatinib. Anticancer Res. 2022;42:2495–2505. doi: 10.21873/anticanres.15728. [DOI] [PubMed] [Google Scholar]
- 184.Wang B, Xu J, Wang H, Chang S, Liu N. Effect and mechanism of sophoridine to suppress hepatocellular carcinoma in vitro and vivo. Biomed Pharmacother. 2017;95:324–330. doi: 10.1016/j.biopha.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 185.Zhao Z, Zhang D, Wu F, Tu J, Song J, Xu M, Ji J. Sophoridine suppresses lenvatinib-resistant hepatocellular carcinoma growth by inhibiting RAS/MEK/ERK axis via decreasing VEGFR2 expression. J Cell Mol Med. 2021;25:549–560. doi: 10.1111/jcmm.16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu R, Peng J, Wang H, Li L, Wen X, Tan Y, Zhang L, Wan H, Chen F, Nie X. Oxysophocarpine retards the growth and metastasis of oral squamous cell carcinoma by targeting the Nrf2/HO-1 axis. Cell Physiol Biochem. 2018;49:1717–1733. doi: 10.1159/000493615. [DOI] [PubMed] [Google Scholar]
- 187.Zhao Z, Song J, Zhang D, Wu F, Tu J, Ji J. Oxysophocarpine suppresses FGFR1-overexpressed hepatocellular carcinoma growth and sensitizes the therapeutic effect of lenvatinib. Life Sci. 2021;264:118642. doi: 10.1016/j.lfs.2020.118642. [DOI] [PubMed] [Google Scholar]
- 188.Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2016;2:1565–1573. doi: 10.1001/jamaoncol.2016.0891. [DOI] [PubMed] [Google Scholar]
- 189.Ma YS, Wu TM, Qian B, Liu YS, Ding H, Fan MM, Liu JB, Yu F, Wang HM, Shi Y, Gu LP, Li L, Tian LL, Wang PY, Wang GR, Wu ZJ, Zou QF, Ling CC, Fu D. KDM5A silencing transcriptionally suppresses the FXYD3-PI3K/AKT axis to inhibit angiogenesis in hepatocellular cancer via miR-433 up-regulation. J Cell Mol Med. 2021;25:4040–4052. doi: 10.1111/jcmm.16371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liao ZH, Zhu HQ, Chen YY, Chen RL, Fu LX, Li L, Zhou H, Zhou JL, Liang G. The epigallocatechin gallate derivative Y(6) inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/ HIF-1α/VEGF dependent pathways. J Ethnopharmacol. 2020;259:112852. doi: 10.1016/j.jep.2020.112852. [DOI] [PubMed] [Google Scholar]
- 191.Gao X, Jiang Y, Xu Q, Liu F, Pang X, Wang M, Li Q, Li Z. 4-hydroxyderricin promotes apoptosis and cell cycle arrest through regulating PI3K/AKT/mTOR pathway in hepatocellular cells. Foods. 2021;10:2036. doi: 10.3390/foods10092036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen Y, Yin S, Liu R, Yang Y, Wu Q, Lin W, Li W. β-Sitosterol activates autophagy to inhibit the development of hepatocellular carcinoma by regulating the complement C5a receptor 1/alpha fetoprotein axis. Eur J Pharmacol. 2023;957:175983. doi: 10.1016/j.ejphar.2023.175983. [DOI] [PubMed] [Google Scholar]
- 193.Kouroumalis E, Tsomidis I, Voumvouraki A. Pathogenesis of hepatocellular carcinoma: the interplay of apoptosis and autophagy. Biomedicines. 2023;11:1166. doi: 10.3390/biomedicines11041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang R, Lian Y, Xie K, Cai Y, Pan Y, Zhu Y. Ropivacaine suppresses tumor biological characteristics of human hepatocellular carcinoma via inhibiting IGF-1R/PI3K/AKT/mTOR signaling axis. Bioengineered. 2021;12:9162–9173. doi: 10.1080/21655979.2021.1995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR, Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, Gao DM, Fan J, Ke AW, Shi GM. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016;7:e2201. doi: 10.1038/cddis.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shen Q, Jiang S, Wu M, Zhang L, Su X, Zhao D. LncRNA HEIH confers cell sorafenib resistance in hepatocellular carcinoma by regulating miR-98-5p/PI3K/AKT pathway. Cancer Manag Res. 2020;12:6585–6595. doi: 10.2147/CMAR.S241383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li Y, Ye Y, Feng B, Qi Y. Long noncoding RNA lncARSR promotes doxorubicin resistance in hepatocellular carcinoma via modulating PTEN-PI3K/Akt pathway. J Cell Biochem. 2017;118:4498–4507. doi: 10.1002/jcb.26107. [DOI] [PubMed] [Google Scholar]
- 198.Cai W, Ma Y, Song L, Cao N, Gao J, Zhou S, Tang X. IGF-1R down regulates the sensitivity of hepatocellular carcinoma to sorafenib through the PI3K/akt and RAS/raf/ERK signaling pathways. BMC Cancer. 2023;23:87. doi: 10.1186/s12885-023-10561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu T, Xie XL, Zhou X, Chen SX, Wang YJ, Shi LP, Chen SJ, Wang YJ, Wang SL, Zhang JN, Dou SY, Jiang XY, Cui RL, Jiang HQ. Y-box binding protein 1 augments sorafenib resistance via the PI3K/Akt signaling pathway in hepatocellular carcinoma. World J Gastroenterol. 2021;27:4667–4686. doi: 10.3748/wjg.v27.i28.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu H, Zhao L, Wang M, Yang K, Jin Z, Zhao C, Shi G. FNDC5 causes resistance to sorafenib by activating the PI3K/Akt/Nrf2 pathway in hepatocellular carcinoma cells. Front Oncol. 2022;12:852095. doi: 10.3389/fonc.2022.852095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 202.You A, Cao M, Guo Z, Zuo B, Gao J, Zhou H, Li H, Cui Y, Fang F, Zhang W, Song T, Li Q, Zhu X, Yin H, Sun H, Zhang T. Metformin sensitizes sorafenib to inhibit postoperative recurrence and metastasis of hepatocellular carcinoma in orthotopic mouse models. J Hematol Oncol. 2016;9:20. doi: 10.1186/s13045-016-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cheng Y, Zhan P, Lu J, Lu Y, Luo C, Cen X, Wang F, Xie C, Yin Z. Metformin synergistically enhances the antitumour activity of Lenvatinib in hepatocellular carcinoma by altering AKT-FOXO3 signalling pathway. Liver Int. 2023;43:1577–1592. doi: 10.1111/liv.15611. [DOI] [PubMed] [Google Scholar]
- 204.Golonko A, Lewandowska H, Świsłocka R, Jasińska UT, Priebe W, Lewandowski W. Curcumin as tyrosine kinase inhibitor in cancer treatment. Eur J Med Chem. 2019;181:111512. doi: 10.1016/j.ejmech.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 205.Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D, Breuhahn K, Conner EA, Galle PR, Andersen JB, Factor VM, Thorgeirsson SS. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661–669. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 207.Miyazaki K, Morine Y, Xu C, Nakasu C, Wada Y, Teraoku H, Yamada S, Saito Y, Ikemoto T, Shimada M, Goel A. Curcumin-mediated resistance to lenvatinib via EGFR signaling pathway in hepatocellular carcinoma. Cells. 2023;12:612. doi: 10.3390/cells12040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang Y, Li X, Wu Z, Yang D, Zhou Y, Wang H, Liao Q, Wang W. Circular RNAs in cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol Cancer. 2019;18:90. doi: 10.1186/s12943-019-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu S, Chen L, Zhao H, Li Q, Hu R, Wang H. Integrin β8 facilitates tumor growth and drug resistance through a Y-box binding protein 1-dependent signaling pathway in bladder cancer. Cancer Sci. 2020;111:2423–2430. doi: 10.1111/cas.14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hou W, Bridgeman B, Malnassy G, Ding X, Cotler SJ, Dhanarajan A, Qiu W. Integrin subunit beta 8 contributes to lenvatinib resistance in HCC. Hepatol Commun. 2022;6:1786–1802. doi: 10.1002/hep4.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ma XL, Hu B, Tang WG, Xie SH, Ren N, Guo L, Lu RQ. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13:11. doi: 10.1186/s13045-020-0845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv LH, Wang H, Zhou Y, Jin AL, Sun YF, Zhang CY, Qiu SJ, Pan BS, Zhou J, Fan J, Yang XR, Guo W. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12:37. doi: 10.1186/s13045-019-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huang F, Wang BR, Wang YG. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4643–4651. doi: 10.3748/wjg.v24.i41.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cui J, Gong Z, Shen HM. The role of autophagy in liver cancer: molecular mechanisms and potential therapeutic targets. Biochim Biophys Acta. 2013;1836:15–26. doi: 10.1016/j.bbcan.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 215.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee CW, Chan FK, Yu J, Sung JJ. The autophagic paradox in cancer therapy. Oncogene. 2012;31:939–953. doi: 10.1038/onc.2011.295. [DOI] [PubMed] [Google Scholar]
- 217.Li D, Yao Y, Rao Y, Huang X, Wei L, You Z, Zheng G, Hou X, Su Y, Varghese Z, Moorhead JF, Chen Y, Ruan XZ. Cholesterol sensor SCAP contributes to sorafenib resistance by regulating autophagy in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41:116. doi: 10.1186/s13046-022-02306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Feng Y, Zhang D, He G, Liu Y, Zhao Y, Ren X, Sun H, Lu G, Zhang Z, Ren L, Yin Y, Li H, He S. AZD4547 and the alleviation of hepatoma cell sorafenib resistance via the promotion of autophagy. Anticancer Agents Med Chem. 2022;22:3107–3113. doi: 10.2174/1871520622666220425124419. [DOI] [PubMed] [Google Scholar]
- 219.Zheng R, Weng S, Xu J, Li Z, Wang Y, Aizimuaji Z, Ma S, Zheng L, Li H, Ying W, Rong W, Xiao T. Autophagy and biotransformation affect sorafenib resistance in hepatocellular carcinoma. Comput Struct Biotechnol J. 2023;21:3564–3574. doi: 10.1016/j.csbj.2023.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lyu Z, Jin H, Yan Z, Hu K, Jiang H, Peng H, Zhuo H. Effects of NRP1 on angiogenesis and vascular maturity in endothelial cells are dependent on the expression of SEMA4D. Int J Mol Med. 2020;46:1321–1334. doi: 10.3892/ijmm.2020.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lin J, Zhang Y, Wu J, Li L, Chen N, Ni P, Song L, Liu X. Neuropilin 1 (NRP1) is a novel tumor marker in hepatocellular carcinoma. Clin Chim Acta. 2018;485:158–165. doi: 10.1016/j.cca.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 222.Fernández-Palanca P, Payo-Serafín T, San-Miguel B, Méndez-Blanco C, Tuñón MJ, González-Gallego J, Mauriz JL. Hepatocellular carcinoma cells loss lenvatinib efficacy in vitro through autophagy and hypoxia response-derived neuropilin-1 degradation. Acta Pharmacol Sin. 2023;44:1066–1082. doi: 10.1038/s41401-022-01021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hu G, Zhang J, Xu F, Deng H, Zhang W, Kang S, Liang W. Stomatin-like protein 2 inhibits cisplatin-induced apoptosis through MEK/ERK signaling and the mitochondrial apoptosis pathway in cervical cancer cells. Cancer Sci. 2018;109:1357–1368. doi: 10.1111/cas.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma W, Chen Y, Xiong W, Li W, Xu Z, Wang Y, Wei Z, Mou T, Wu Z, Cheng M, Zou Y, Zhu Y, Zhou W, Liu F, Geng Y. STOML2 interacts with PHB through activating MAPK signaling pathway to promote colorectal Cancer proliferation. J Exp Clin Cancer Res. 2021;40:359. doi: 10.1186/s13046-021-02116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zheng Y, Huang C, Lu L, Yu K, Zhao J, Chen M, Liu L, Sun Q, Lin Z, Zheng J, Chen J, Zhang J. STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J Hematol Oncol. 2021;14:16. doi: 10.1186/s13045-020-01029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dingjan I, Linders PTA, Verboogen DRJ, Revelo NH, Ter Beest M, van den Bogaart G. Endosomal and phagosomal SNAREs. Physiol Rev. 2018;98:1465–1492. doi: 10.1152/physrev.00037.2017. [DOI] [PubMed] [Google Scholar]
- 227.Riggs KA, Hasan N, Humphrey D, Raleigh C, Nevitt C, Corbin D, Hu C. Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J Cell Sci. 2012;125:3827–3839. doi: 10.1242/jcs.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhou L, Wang Z, Chen X, Li X, Ge C, Min X, Zhao F, Chen T, Li J. Syntaxin-6 promotes the progression of hepatocellular carcinoma and alters its sensitivity to chemotherapies by activating the USF2/LC3B axis. Int J Biol Sci. 2023;19:3892–3907. doi: 10.7150/ijbs.86636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pan J, Zhang M, Dong L, Ji S, Zhang J, Zhang S, Lin Y, Wang X, Ding Z, Qiu S, Gao D, Zhou J, Fan J, Gao Q. Genome-Scale CRISPR screen identifies LAPTM5 driving lenvatinib resistance in hepatocellular carcinoma. Autophagy. 2023;19:1184–1198. doi: 10.1080/15548627.2022.2117893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ma X, Qiu Y, Sun Y, Zhu L, Zhao Y, Li T, Lin Y, Ma D, Qin Z, Sun C, Han L. NOD2 inhibits tumorigenesis and increases chemosensitivity of hepatocellular carcinoma by targeting AMPK pathway. Cell Death Dis. 2020;11:174. doi: 10.1038/s41419-020-2368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tang R, Zheng L, Zheng J, Wu J, Chen P, Chen J, Xu D, Zeng Y, Li Q, Zhang Z. Secukinumab plays a synergistic role with starvation therapy in promoting autophagic cell death of hepatocellular carcinoma via inhibiting IL-17A-increased BCL2 level. In Vitro Cell Dev Biol Anim. 2023;59:381–393. doi: 10.1007/s11626-023-00770-6. [DOI] [PubMed] [Google Scholar]
- 232.Tong GD, Zhang X, Zhou DQ, Wei CS, He JS, Xiao CL, Liu XL, Zheng YJ, Chen SN, Tang HH. Efficacy of early treatment on 52 patients with preneoplastic hepatitis B virus-associated hepatocellular carcinoma by compound Phyllanthus Urinaria L. Chin J Integr Med. 2014;20:263–271. doi: 10.1007/s11655-013-1320-7. [DOI] [PubMed] [Google Scholar]
- 233.Liao M, Qin M, Liu L, Huang H, Chen N, Du H, Huang D, Wang P, Zhou H, Tong G. Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound Phyllanthus urinaria and lenvatinib in hepatocellular carcinoma. Phytomedicine. 2024;122:155091. doi: 10.1016/j.phymed.2023.155091. [DOI] [PubMed] [Google Scholar]
- 234.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 235.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li J, Zhou W, Mao Q, Gao D, Xiong L, Hu X, Zheng Y, Xu X. HMGB1 promotes resistance to doxorubicin in human hepatocellular carcinoma cells by inducing autophagy via the AMPK/mTOR signaling pathway. Front Oncol. 2021;11:739145. doi: 10.3389/fonc.2021.739145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tan XP, Xiong BH, Zhang YX, Wang SL, Zuo Q, Li J. FXYD5 promotes sorafenib resistance through the Akt/mTOR signaling pathway in hepatocellular carcinoma. Eur J Pharmacol. 2022;931:175186. doi: 10.1016/j.ejphar.2022.175186. [DOI] [PubMed] [Google Scholar]
- 238.Ashworth RE, Wu J. Mammalian target of rapamycin inhibition in hepatocellular carcinoma. World J Hepatol. 2014;6:776–782. doi: 10.4254/wjh.v6.i11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 240.Fan M, Chen Z, Shao W, Chen Y, Lin Z, Yi C, Li Y, Lu L, Zhou Y, Lin J. SREBP2 inhibitor betulin sensitizes hepatocellular carcinoma to lenvatinib by inhibiting the mTOR/IL-1beta pathway. Acta Biochim Biophys Sin (Shanghai) 2023;55:1479–1486. doi: 10.3724/abbs.2023122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 242.Wang Z, Shen J, Chen C, Wen T, Li C. FOXA2 plays a critical role in hepatocellular carcinoma progression and lenvatinib-associated drug resistance. Biosci Trends. 2023;17:136–147. doi: 10.5582/bst.2022.01535. [DOI] [PubMed] [Google Scholar]
- 243.Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4:a011163. doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ng LF, Kaur P, Bunnag N, Suresh J, Sung ICH, Tan QH, Gruber J, Tolwinski NS. WNT signaling in disease. Cells. 2019;8:826. doi: 10.3390/cells8080826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yuan J, Han B, Hu H, Qian Y, Liu Z, Wei Z, Liang X, Jiang B, Shao C, Gong Y. CUL4B activates Wnt/β-catenin signalling in hepatocellular carcinoma by repressing Wnt antagonists. J Pathol. 2015;235:784–795. doi: 10.1002/path.4492. [DOI] [PubMed] [Google Scholar]
- 248.Chang YS, Chou YP, Chung CC, Lee YT, Yen JC, Jeng LB, Chang JG. Molecular classification of hepatocellular carcinoma using wnt-hippo signaling pathway-related genes. Cancers (Basel) 2022;14:4580. doi: 10.3390/cancers14194580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wei Y, Shen N, Wang Z, Yang G, Yi B, Yang N, Qiu Y, Lu J. Sorafenib sensitizes hepatocellular carcinoma cell to cisplatin via suppression of Wnt/β-catenin signaling. Mol Cell Biochem. 2013;381:139–144. doi: 10.1007/s11010-013-1695-6. [DOI] [PubMed] [Google Scholar]
- 250.Liu LJ, Lv Z, Xue X, Xing ZY, Zhu F. Canonical WNT signaling activated by WNT7B contributes to l-hbs-mediated sorafenib resistance in hepatocellular carcinoma by inhibiting mitophagy. Cancers (Basel) 2022;14:5781. doi: 10.3390/cancers14235781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tan W, Zhang K, Chen X, Yang L, Zhu S, Wei Y, Xie Z, Chen Y, Shang C. GPX2 is a potential therapeutic target to induce cell apoptosis in lenvatinib against hepatocellular carcinoma. J Adv Res. 2023;44:173–183. doi: 10.1016/j.jare.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, Tang M, Jiang S, Ma X, Chen P, Katkhuda R, Korphaisarn K, Chakravarti D, Chang A, Spring DJ, Chang Q, Zhang J, Maru DM, Maeda DY, Zebala JA, Kopetz S, Wang YA, DePinho RA. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell. 2019;35:559–572. e557. doi: 10.1016/j.ccell.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang F, Li J, Zhu J, Liu L, Zhu K, Cheng S, Lv R, Zhang P. IRF2-INPP4B-mediated autophagy suppresses apoptosis in acute myeloid leukemia cells. Biol Res. 2019;52:11. doi: 10.1186/s40659-019-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yi Y, Wu H, Gao Q, He HW, Li YW, Cai XY, Wang JX, Zhou J, Cheng YF, Jin JJ, Fan J, Qiu SJ. Interferon regulatory factor (IRF)-1 and IRF-2 are associated with prognosis and tumor invasion in HCC. Ann Surg Oncol. 2013;20:267–276. doi: 10.1245/s10434-012-2487-z. [DOI] [PubMed] [Google Scholar]
- 255.Guo Y, Xu J, Du Q, Yan Y, Geller DA. IRF2 regulates cellular survival and Lenvatinib-sensitivity of hepatocellular carcinoma (HCC) through regulating β-catenin. Transl Oncol. 2021;14:101059. doi: 10.1016/j.tranon.2021.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang Q, Xiong L, Wei T, Liu Q, Yan L, Chen J, Dai L, Shi L, Zhang W, Yang J, Roessler S, Liu L. Hypoxia-responsive PPARGC1A/BAMBI/ACSL5 axis promotes progression and resistance to lenvatinib in hepatocellular carcinoma. Oncogene. 2023;42:1509–1523. doi: 10.1038/s41388-023-02665-y. [DOI] [PubMed] [Google Scholar]
- 257.Leung CON, Yang Y, Leung RWH, So KKH, Guo HJ, Lei MML, Muliawan GK, Gao Y, Yu QQ, Yun JP, Ma S, Zhao Q, Lee TKW. Broad-spectrum kinome profiling identifies CDK6 upregulation as a driver of lenvatinib resistance in hepatocellular carcinoma. Nat Commun. 2023;14:6699. doi: 10.1038/s41467-023-42360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nguyen NT, Lin DP, Yen SY, Tseng JK, Chuang JF, Chen BY, Lin TA, Chang HH, Ju JC. Sonic hedgehog promotes porcine oocyte maturation and early embryo development. Reprod Fertil Dev. 2009;21:805–815. doi: 10.1071/RD08277. [DOI] [PubMed] [Google Scholar]
- 259.Dunaeva M, Waltenberger J. Hh signaling in regeneration of the ischemic heart. Cell Mol Life Sci. 2017;74:3481–3490. doi: 10.1007/s00018-017-2534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kundu S, Nandhu MS, Longo SL, Longo JA, Rai S, Chin LS, Richardson TE, Viapiano MS. The scaffolding protein DLG5 promotes glioblastoma growth by controlling Sonic Hedgehog signaling in tumor stem cells. Neuro Oncol. 2022;24:1230–1242. doi: 10.1093/neuonc/noac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang ZW, Teng X, Zhao F, Ma C, Zhang J, Xiao LF, Wang Y, Chang M, Tian Y, Li C, Zhang Z, Song S, Tong WM, Liu P, Niu Y. METTL3 regulates m(6)A methylation of PTCH1 and GLI2 in Sonic hedgehog signaling to promote tumor progression in SHH-medulloblastoma. Cell Rep. 2022;41:111530. doi: 10.1016/j.celrep.2022.111530. [DOI] [PubMed] [Google Scholar]
- 262.Shamsoon K, Hiraki D, Yoshida K, Takabatake K, Takebe H, Yokozeki K, Horie N, Fujita N, Nasrun NE, Okui T, Nagatsuka H, Abiko Y, Hosoya A, Saito T, Shimo T. The role of hedgehog signaling in the melanoma tumor bone microenvironment. Int J Mol Sci. 2023;24:8862. doi: 10.3390/ijms24108862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dosch JS, Pasca di Magliano M, Simeone DM. Pancreatic cancer and hedgehog pathway signaling: new insights. Pancreatology. 2010;10:151–157. doi: 10.1159/000225923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hu A, Hu Z, Ye J, Liu Y, Lai Z, Zhang M, Ji W, Huang L, Zou H, Chen B, Zhong J. Metformin exerts anti-tumor effects via Sonic hedgehog signaling pathway by targeting AMPK in HepG2 cells. Biochem Cell Biol. 2022;100:142–151. doi: 10.1139/bcb-2021-0409. [DOI] [PubMed] [Google Scholar]
- 265.Lin J, Tan H, Nie Y, Wu D, Zheng W, Lin W, Zhu Z, Yang B, Chen X, Chen T. Krüppel-like factor 2 inhibits hepatocarcinogenesis through negative regulation of the Hedgehog pathway. Cancer Sci. 2019;110:1220–1231. doi: 10.1111/cas.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu T, Zhang Z, Wang C, Huang H, Li Y. BRD4 promotes the migration and invasion of bladder cancer cells through the Sonic hedgehog signaling pathway and enhances cisplatin resistance. Biochem Cell Biol. 2022;100:179–187. doi: 10.1139/bcb-2021-0552. [DOI] [PubMed] [Google Scholar]
- 267.Zhang Y, Yao G, Yang X, Qiu T, Wang S. Mechanism of targeting the hedgehog signaling pathway against chemotherapeutic resistance in multiple myeloma. J Oncol. 2022;2022:1399697. doi: 10.1155/2022/1399697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Haque I, Kawsar HI, Motes H, Sharma M, Banerjee S, Banerjee SK, Godwin AK, Huang CH. Downregulation of miR-506-3p facilitates EGFR-TKI resistance through induction of sonic hedgehog signaling in non-small-cell lung cancer cell lines. Int J Mol Sci. 2020;21:9307. doi: 10.3390/ijms21239307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wang S, Wang Y, Xun X, Zhang C, Xiang X, Cheng Q, Hu S, Li Z, Zhu J. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient-derived organoids. J Exp Clin Cancer Res. 2020;39:22. doi: 10.1186/s13046-020-1523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bodzin AS, Wei Z, Hurtt R, Gu T, Doria C. Gefitinib resistance in HCC mahlavu cells: upregulation of CD133 expression, activation of IGF-1R signaling pathway, and enhancement of IGF-1R nuclear translocation. J Cell Physiol. 2012;227:2947–2952. doi: 10.1002/jcp.23041. [DOI] [PubMed] [Google Scholar]
- 271.Hou Y, Zou Q, Ge R, Shen F, Wang Y. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res. 2012;22:259–272. doi: 10.1038/cr.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB, Lo CM, Guan XY, Chan KW. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807–820. doi: 10.1002/hep.24739. [DOI] [PubMed] [Google Scholar]
- 273.Hu Q, Hu X, Zhang L, Zhao Y, Li L. Targeting Hedgehog signalling in CD133-positive hepatocellular carcinoma: improving Lenvatinib therapeutic efficiency. Med Oncol. 2021;38:41. doi: 10.1007/s12032-021-01487-w. [DOI] [PubMed] [Google Scholar]
- 274.Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: beyond smoothened. Oncotarget. 2015;6:13899–13913. doi: 10.18632/oncotarget.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Harada K, Ohashi R, Naito K, Kanki K. Hedgehog signal inhibitor GANT61 inhibits the malignant behavior of undifferentiated hepatocellular carcinoma cells by targeting non-canonical GLI signaling. Int J Mol Sci. 2020;21:3126. doi: 10.3390/ijms21093126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jin W, Yu J, Su Y, Lin H, Liu T, Chen J, Ge C, Zhao F, Geng Q, Mao L, Jiang S, Cui Y, Chen T, Jiang G, Li J, Miao C, Xiao X, Li H. Drug repurposing flubendazole to suppress tumorigenicity via PCSK9-dependent Inhibition and potentiate lenvatinib therapy for hepatocellular carcinoma. Int J Biol Sci. 2023;19:2270–2288. doi: 10.7150/ijbs.81415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 278.Jacobs RJ, Voorneveld PW, Kodach LL, Hardwick JC. Cholesterol metabolism and colorectal cancers. Curr Opin Pharmacol. 2012;12:690–695. doi: 10.1016/j.coph.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 279.Alfaqih MA, Nelson ER, Liu W, Safi R, Jasper JS, Macias E, Geradts J, Thompson JW, Dubois LG, Freeman MR, Chang CY, Chi JT, McDonnell DP, Freedland SJ. CYP27A1 loss dysregulates cholesterol homeostasis in prostate cancer. Cancer Res. 2017;77:1662–1673. doi: 10.1158/0008-5472.CAN-16-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kondo A, Yamamoto S, Nakaki R, Shimamura T, Hamakubo T, Sakai J, Kodama T, Yoshida T, Aburatani H, Osawa T. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 2017;18:2228–2242. doi: 10.1016/j.celrep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 281.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mok EHK, Leung CON, Zhou L, Lei MML, Leung HW, Tong M, Wong TL, Lau EYT, Ng IOL, Ding J, Yun JP, Yu J, Zhu HL, Lin CH, Lindholm D, Leung KS, Cybulski JD, Baker DM, Ma S, Lee TKW. Caspase-3-induced activation of SREBP2 drives drug resistance via promotion of cholesterol biosynthesis in hepatocellular carcinoma. Cancer Res. 2022;82:3102–3115. doi: 10.1158/0008-5472.CAN-21-2934. [DOI] [PubMed] [Google Scholar]
- 283.Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808. doi: 10.1016/j.jhep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 284.Yang Y, Liu Q, Zhang H, Zhao H, Mao R, Li Z, Ya S, Jia C, Bao Y. Silencing of GP73 inhibits invasion and metastasis via suppression of epithelial-mesenchymal transition in hepatocellular carcinoma. Oncol Rep. 2017;37:1182–1188. doi: 10.3892/or.2017.5351. [DOI] [PubMed] [Google Scholar]
- 285.Dong J, Zhai B, Sun W, Hu F, Cheng H, Xu J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS One. 2017;12:e0185088. doi: 10.1371/journal.pone.0185088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, van Pelt J. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329:74–83. doi: 10.1016/j.canlet.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 287.Fang T, Jiao Z, You Y, Cao J, Wang C, Liu J, Zhao W. Lenvatinib inhibited HCC cell migration and invasion through regulating the transcription and ubiquitination of UHRF1 and DNMT1. Biochem Pharmacol. 2023;210:115489. doi: 10.1016/j.bcp.2023.115489. [DOI] [PubMed] [Google Scholar]
- 288.You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008;41:833–839. doi: 10.5483/bmbrep.2008.41.12.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fu R, Jiang S, Li J, Chen H, Zhang X. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med Oncol. 2020;37:24. doi: 10.1007/s12032-020-01350-4. [DOI] [PubMed] [Google Scholar]
- 290.Tan Q, Niu K, Zhu Y, Chen Z, Li Y, Li M, Wei D, Balajee AS, Fang H, Zhao Y. RNF8 ubiquitinates RecQL4 and promotes its dissociation from DNA double strand breaks. Oncogenesis. 2021;10:24. doi: 10.1038/s41389-021-00315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li L, Guturi KKN, Gautreau B, Patel PS, Saad A, Morii M, Mateo F, Palomero L, Barbour H, Gomez A, Ng D, Kotlyar M, Pastrello C, Jackson HW, Khokha R, Jurisica I, Affar EB, Raught B, Sanchez O, Alaoui-Jamali M, Pujana MA, Hakem A, Hakem R. Ubiquitin ligase RNF8 suppresses Notch signaling to regulate mammary development and tumorigenesis. J Clin Invest. 2018;128:4525–4542. doi: 10.1172/JCI120401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lee HJ, Li CF, Ruan D, Powers S, Thompson PA, Frohman MA, Chan CH. The DNA damage transducer RNF8 facilitates cancer chemoresistance and progression through twist activation. Mol Cell. 2016;63:1021–1033. doi: 10.1016/j.molcel.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kuang J, Duan T, Gao C, Liu C, Chen S, Zhu LY, Min L, Lu C, Wang W, Zhu L. RNF8 depletion attenuates hepatocellular carcinoma progression by inhibiting epithelial-mesenchymal transition and enhancing drug sensitivity. Acta Biochim Biophys Sin (Shanghai) 2023;55:661–671. doi: 10.3724/abbs.2023076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, Ni Z, Zhang B, Zhang D, Luo F, Chen H, Sun X, Feng JQ, Qi H, Chen L. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5:181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D, Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, Loo HL, Aung MO, Lim SG, Ullrich A. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118–127. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 297.Sandhu DS, Baichoo E, Roberts LR. Fibroblast growth factor signaling in liver carcinogenesis. Hepatology. 2014;59:1166–1173. doi: 10.1002/hep.26679. [DOI] [PubMed] [Google Scholar]
- 298.Hyeon J, Ahn S, Lee JJ, Song DH, Park CK. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci. 2013;58:1916–1922. doi: 10.1007/s10620-013-2609-x. [DOI] [PubMed] [Google Scholar]
- 299.Hsieh CC, Shyr YM, Liao WY, Chen TH, Wang SE, Lu PC, Lin PY, Chen YB, Mao WY, Han HY, Hsiao M, Yang WB, Li WS, Sher YP, Shen CN. Elevation of β-galactoside α2,6-sialyltransferase 1 in a fructoseresponsive manner promotes pancreatic cancer metastasis. Oncotarget. 2017;8:7691–7709. doi: 10.18632/oncotarget.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wei A, Fan B, Zhao Y, Zhang H, Wang L, Yu X, Yuan Q, Yang D, Wang S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin signaling pathway. Oncotarget. 2016;7:65374–65388. doi: 10.18632/oncotarget.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wichert B, Milde-Langosch K, Galatenko V, Schmalfeldt B, Oliveira-Ferrer L. Prognostic role of the sialyltransferase ST6GAL1 in ovarian cancer. Glycobiology. 2018;28:898–903. doi: 10.1093/glycob/cwy065. [DOI] [PubMed] [Google Scholar]
- 302.Zhang X, Zhang H, Liao Z, Zhang J, Liang H, Wang W, Yu J, Dong K. SHC4 promotes tumor proliferation and metastasis by activating STAT3 signaling in hepatocellular carcinoma. Cancer Cell Int. 2022;22:24. doi: 10.1186/s12935-022-02446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Myojin Y, Kodama T, Maesaka K, Motooka D, Sato Y, Tanaka S, Abe Y, Ohkawa K, Mita E, Hayashi Y, Hikita H, Sakamori R, Tatsumi T, Taguchi A, Eguchi H, Takehara T. ST6GAL1 is a novel serum biomarker for lenvatinib-susceptible FGF19-driven hepatocellular carcinoma. Clin Cancer Res. 2021;27:1150–1161. doi: 10.1158/1078-0432.CCR-20-3382. [DOI] [PubMed] [Google Scholar]
- 304.Liu L, Cui J, Zhao Y, Liu X, Chen L, Xia Y, Wang Y, Chen S, Sun S, Shi B, Zou Y. KDM6A-ARHGDIB axis blocks metastasis of bladder cancer by inhibiting Rac1. Mol Cancer. 2021;20:77. doi: 10.1186/s12943-021-01369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Leng X, Wang J, An N, Wang X, Sun Y, Chen Z. Histone 3 lysine-27 demethylase KDM6A coordinates with KMT2B to play an oncogenic role in NSCLC by regulating H3K4me3. Oncogene. 2020;39:6468–6479. doi: 10.1038/s41388-020-01449-y. [DOI] [PubMed] [Google Scholar]
- 306.Chen X, Yang Z, Feng J, Duan T, Pan T, Yan L, Jin T, Xiang Y, Zhang M, Chen P, Wang W, Zhang R, Chen B, Zhao L, Xie T, Sui X. Combination of lysine-specific demethylase 6A (KDM6A) and mismatch repair (MMR) status is a potential prognostic factor in colorectal cancer. Cancer Med. 2021;10:317–324. doi: 10.1002/cam4.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Guo W, Li S, Qian Y, Li L, Wang F, Tong Y, Li Q, Zhu Z, Gao WQ, Liu Y. KDM6A promotes hepatocellular carcinoma progression and dictates lenvatinib efficacy by upregulating FGFR4 expression. Clin Transl Med. 2023;13:e1452. doi: 10.1002/ctm2.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218:e20201606. doi: 10.1084/jem.20201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 310.Sangineto M, Villani R, Cavallone F, Romano A, Loizzi D, Serviddio G. Lipid metabolism in development and progression of hepatocellular carcinoma. Cancers (Basel) 2020;12:1419. doi: 10.3390/cancers12061419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Pope ED 3rd, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA 3rd, Mody K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets. 2019;23:473–483. doi: 10.1080/14728222.2019.1615883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hu B, Lin JZ, Yang XB, Sang XT. Aberrant lipid metabolism in hepatocellular carcinoma cells as well as immune microenvironment: a review. Cell Prolif. 2020;53:e12772. doi: 10.1111/cpr.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Germain N, Dhayer M, Boileau M, Fovez Q, Kluza J, Marchetti P. Lipid metabolism and resistance to anticancer treatment. Biology (Basel) 2020;9:474. doi: 10.3390/biology9120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Khwairakpam AD, Shyamananda MS, Sailo BL, Rathnakaram SR, Padmavathi G, Kotoky J, Kunnumakkara AB. ATP citrate lyase (ACLY): a promising target for cancer prevention and treatment. Curr Drug Targets. 2015;16:156–163. doi: 10.2174/1389450115666141224125117. [DOI] [PubMed] [Google Scholar]
- 315.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 316.Sun H, Wang F, Huang Y, Wang J, Zhang L, Shen Y, Lin C, Guo P. Targeted inhibition of ACLY expression to reverse the resistance of sorafenib in hepatocellular carcinoma. J Cancer. 2022;13:951–964. doi: 10.7150/jca.52778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Guida M, Ligresti A, De Filippis D, D’Amico A, Petrosino S, Cipriano M, Bifulco G, Simonetti S, Orlando P, Insabato L, Nappi C, Di Spiezio Sardo A, Di Marzo V, Iuvone T. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology. 2010;151:921–928. doi: 10.1210/en.2009-0883. [DOI] [PubMed] [Google Scholar]
- 318.Ramer R, Wittig F, Hinz B. The endocannabinoid system as a pharmacological target for new cancer therapies. Cancers (Basel) 2021;13:5701. doi: 10.3390/cancers13225701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yang J, Tian Y, Zheng R, Li L, Qiu F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol Lett. 2019;18:1530–1538. doi: 10.3892/ol.2019.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yan YC, Meng GX, Yang CC, Yang YF, Tan SY, Yan LJ, Ding ZN, Ma YL, Dong ZR, Li T. Diacylglycerol lipase alpha promotes hepatocellular carcinoma progression and induces lenvatinib resistance by enhancing YAP activity. Cell Death Dis. 2023;14:404. doi: 10.1038/s41419-023-05919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Liu Y, Sun L, Guo H, Zhou S, Wang C, Ji C, Meng F, Liang S, Zhang B, Yuan Y, Ma K, Li X, Guo X, Cui T, Zhang N, Wang J, Liu Y, Liu L. Targeting SLP2-mediated lipid metabolism reprograming restricts proliferation and metastasis of hepatocellular carcinoma and promotes sensitivity to Lenvatinib. Oncogene. 2023;42:374–388. doi: 10.1038/s41388-022-02551-z. [DOI] [PubMed] [Google Scholar]
- 322.Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen T, Chen Z, Huang S, Gu J, Li J, Yao M, Zhao Y, He X. MiR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology. 2015;62:1132–1144. doi: 10.1002/hep.27929. [DOI] [PubMed] [Google Scholar]
- 323.Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu X, Zhu G, Zhao Y, Chen Y, Yu Y, Pan Q, Wang J, Sun F. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun. 2017;8:15280. doi: 10.1038/ncomms15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhou L, Fu Z, Wang S, Jia J, Cheng Y, Zheng Y, Zhang N, Lu W, Yao Z. ACYP1 is a pancancer prognostic indicator and affects the immune microenvironment in LIHC. Front Oncol. 2022;12:875097. doi: 10.3389/fonc.2022.875097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wang S, Zhou L, Ji N, Sun C, Sun L, Sun J, Du Y, Zhang N, Li Y, Liu W, Lu W. Targeting ACYP1-mediated glycolysis reverses lenvatinib resistance and restricts hepatocellular carcinoma progression. Drug Resist Updat. 2023;69:100976. doi: 10.1016/j.drup.2023.100976. [DOI] [PubMed] [Google Scholar]
- 326.Henrique D, Schweisguth F. Mechanisms of Notch signaling: a simple logic deployed in time and space. Development. 2019;146:dev172148. doi: 10.1242/dev.172148. [DOI] [PubMed] [Google Scholar]
- 327.Ahn S, Hyeon J, Park CK. Notch1 and Notch4 are markers for poor prognosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:286–294. doi: 10.1016/s1499-3872(13)60046-6. [DOI] [PubMed] [Google Scholar]
- 328.Huang Q, Li J, Zheng J, Wei A. The carcinogenic role of the notch signaling pathway in the development of hepatocellular carcinoma. J Cancer. 2019;10:1570–1579. doi: 10.7150/jca.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Yang X, Liu J, Liang Q, Sun G. Valproic acid reverses sorafenib resistance through inhibiting activated Notch/Akt signaling pathway in hepatocellular carcinoma. Fundam Clin Pharmacol. 2021;35:690–699. doi: 10.1111/fcp.12608. [DOI] [PubMed] [Google Scholar]
- 330.Wei Y, Shen X, Li L, Cao G, Cai X, Wang Y, Shen H. TM4SF1 inhibits apoptosis and promotes proliferation, migration and invasion in human gastric cancer cells. Oncol Lett. 2018;16:6081–6088. doi: 10.3892/ol.2018.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yang SB, Zhou ZH, Lei J, Li XW, Chen Q, Li B, Zhang YW, Ge YZ, Zuo S. TM4SF1 upregulates MYH9 to activate the NOTCH pathway to promote cancer stemness and lenvatinib resistance in HCC. Biol Direct. 2023;18:18. doi: 10.1186/s13062-023-00376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Luo Y, Yao Y, Wu P, Zi X, Sun N, He J. The potential role of N(7)-methylguanosine (m7G) in cancer. J Hematol Oncol. 2022;15:63. doi: 10.1186/s13045-022-01285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, Huang JT, Chen SM, Xu ZG, Leng XH, Yu XC, Cao J, Zhang Z, Liu J, Lengyel E, He C. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–1083. doi: 10.1038/s41556-018-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Alexandrov A, Martzen MR, Phizicky EM. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA. 2002;8:1253–1266. doi: 10.1017/s1355838202024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Tian QH, Zhang MF, Zeng JS, Luo RG, Wen Y, Chen J, Gan LG, Xiong JP. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J Mol Med (Berl) 2019;97:1535–1545. doi: 10.1007/s00109-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 336.Chen Z, Zhu W, Zhu S, Sun K, Liao J, Liu H, Dai Z, Han H, Ren X, Yang Q, Zheng S, Peng B, Peng S, Kuang M, Lin S. METTL1 promotes hepatocarcinogenesis via m(7) G tRNA modification-dependent translation control. Clin Transl Med. 2021;11:e661. doi: 10.1002/ctm2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Xia P, Zhang H, Xu K, Jiang X, Gao M, Wang G, Liu Y, Yao Y, Chen X, Ma W, Zhang Z, Yuan Y. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma. Cell Death Dis. 2021;12:691. doi: 10.1038/s41419-021-03973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Huang M, Long J, Yao Z, Zhao Y, Zhao Y, Liao J, Lei K, Xiao H, Dai Z, Peng S, Lin S, Xu L, Kuang M. METTL1-mediated m7G tRNA modification promotes lenvatinib resistance in hepatocellular carcinoma. Cancer Res. 2023;83:89–102. doi: 10.1158/0008-5472.CAN-22-0963. [DOI] [PubMed] [Google Scholar]
- 339.Han WY, Wang J, Zhao J, Zheng YM, Chai XQ, Gao C, Cai JB, Ke AW, Fan J, Gao PT, Sun HX. WDR4/TRIM28 is a novel molecular target linked to lenvatinib resistance that helps retain the stem characteristics in hepatocellular carcinomas. Cancer Lett. 2023;568:216259. doi: 10.1016/j.canlet.2023.216259. [DOI] [PubMed] [Google Scholar]
- 340.Wang L, Yang Q, Zhou Q, Fang F, Lei K, Liu Z, Zheng G, Zhu L, Huo J, Li X, Peng S, Kuang M, Lin S, Huang M, Xu L. METTL3-m(6)A-EGFR-axis drives lenvatinib resistance in hepatocellular carcinoma. Cancer Lett. 2023;559:216122. doi: 10.1016/j.canlet.2023.216122. [DOI] [PubMed] [Google Scholar]
- 341.Ito S, Horikawa S, Suzuki T, Kawauchi H, Tanaka Y, Suzuki T, Suzuki T. Human NAT10 is an ATP-dependent RNA acetyltransferase responsible for N4-acetylcytidine formation in 18 S ribosomal RNA (rRNA) J Biol Chem. 2014;289:35724–35730. doi: 10.1074/jbc.C114.602698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhang X, Liu J, Yan S, Huang K, Bai Y, Zheng S. High expression of N-acetyltransferase 10: a novel independent prognostic marker of worse outcome in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:14765–14771. [PMC free article] [PubMed] [Google Scholar]
- 343.Zhang X, Chen J, Jiang S, He S, Bai Y, Zhu L, Ma R, Liang X. N-acetyltransferase 10 enhances doxorubicin resistance in human hepatocellular carcinoma cell lines by promoting the epithelial-to-mesenchymal transition. Oxid Med Cell Longev. 2019;2019:7561879. doi: 10.1155/2019/7561879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pan Z, Bao Y, Hu M, Zhu Y, Tan C, Fan L, Yu H, Wang A, Cui J, Sun G. Role of NAT10-mediated ac4C-modified HSP90AA1 RNA acetylation in ER stress-mediated metastasis and lenvatinib resistance in hepatocellular carcinoma. Cell Death Discov. 2023;9:56. doi: 10.1038/s41420-023-01355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, Jiang H, Liu J, Liu L. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 347.Takahashi M, Okada K, Ouch R, Konno T, Usui K, Suzuki H, Satoh M, Kogure T, Satoh K, Watanabe Y, Nakamura H, Murai Y. Fibronectin plays a major role in hypoxia-induced lenvatinib resistance in hepatocellular carcinoma PLC/PRF/5 cells. Pharmazie. 2021;76:594–601. doi: 10.1691/ph.2021.1854. [DOI] [PubMed] [Google Scholar]
- 348.Takagi H, Kaji K, Nishimura N, Ishida K, Ogawa H, Takaya H, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. The angiotensin ii receptor blocker losartan sensitizes human liver cancer cells to lenvatinib-mediated cytostatic and angiostatic effects. Cells. 2021;10:575. doi: 10.3390/cells10030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang X, Qian S, Wang S, Jia S, Zheng N, Yao Q, Gao J. Combination of Vitamin C and Lenvatinib potentiates antitumor effects in hepatocellular carcinoma cells in vitro. PeerJ. 2023;11:e14610. doi: 10.7717/peerj.14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Digiacomo G, Fumarola C, La Monica S, Bonelli M, Cavazzoni A, Galetti M, Terenziani R, Eltayeb K, Volta F, Zoppi S, Bertolini P, Missale G, Alfieri R, Petronini PG. CDK4/6 inhibitors improve the anti-tumor efficacy of lenvatinib in hepatocarcinoma cells. Front Oncol. 2022;12:942341. doi: 10.3389/fonc.2022.942341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Takahashi M, Araki T, Yashima H, Nagamine A, Nagano D, Yamamoto K. Increased c-SRC expression is involved in acquired resistance to lenvatinib in hepatocellular carcinoma. Oncol Lett. 2023;26:529. doi: 10.3892/ol.2023.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wang JQ, Wu ZX, Yang Y, Teng QX, Li YD, Lei ZN, Jani KA, Kaushal N, Chen ZS. ATP-binding cassette (ABC) transporters in cancer: a review of recent updates. J Evid Based Med. 2021;14:232–256. doi: 10.1111/jebm.12434. [DOI] [PubMed] [Google Scholar]
- 354.Sun D, Liu J, Wang Y, Dong J. Co-administration of MDR1 and BCRP or EGFR/PI3K inhibitors overcomes lenvatinib resistance in hepatocellular carcinoma. Front Oncol. 2022;12:944537. doi: 10.3389/fonc.2022.944537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Hu B, Zou T, Qin W, Shen X, Su Y, Li J, Chen Y, Zhang Z, Sun H, Zheng Y, Wang CQ, Wang Z, Li TE, Wang S, Zhu L, Wang X, Fu Y, Ren X, Dong Q, Qin LX. Inhibition of EGFR overcomes acquired lenvatinib resistance driven by STAT3-ABCB1 signaling in hepatocellular carcinoma. Cancer Res. 2022;82:3845–3857. doi: 10.1158/0008-5472.CAN-21-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Thiruchenthooran V, Sánchez-López E, Gliszczyńska A. Perspectives of the application of non-steroidal anti-inflammatory drugs in cancer therapy: attempts to overcome their unfavorable side effects. Cancers (Basel) 2023;15:475. doi: 10.3390/cancers15020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yan X, Yu H, Liang J, Hu Z, Li X, Liu H, Yao J, Sui X, Zheng J, Li R. Synergistic antitumor efficacy of aspirin plus lenvatinib in hepatocellular carcinoma via regulating of diverse signaling pathways. Cell Death Discov. 2023;9:416. doi: 10.1038/s41420-023-01664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, Li S, Lu W, Xie Q, Chen H, Lai J, Chen Q, Jiang X, Liu S, Zhang Z, Zhao M. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9:e002305. doi: 10.1136/jitc-2020-002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sun X, Qiu JJ, Zhu S, Cao B, Sun L, Li S, Li P, Zhang S, Dong S. Oncogenic features of PHF8 histone demethylase in esophageal squamous cell carcinoma. PLoS One. 2013;8:e77353. doi: 10.1371/journal.pone.0077353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Huang JL, Ren TY, Cao SW, Zheng SH, Hu XM, Hu YW, Lin L, Chen J, Zheng L, Wang Q. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget. 2015;6:33791–33804. doi: 10.18632/oncotarget.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ma Y, Sun W, Zhang Q, Gao B, Cai W, Liu Q, Liao J, Wang X. lncRNA BSG-AS1 is hypoxia-responsive and promotes hepatocellular carcinoma by enhancing BSG mRNA stability. Biochem Biophys Res Commun. 2021;566:101–107. doi: 10.1016/j.bbrc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 363.Han Y, Chen M, Wang A, Fan X. STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2019;508:472–479. doi: 10.1016/j.bbrc.2018.11.092. [DOI] [PubMed] [Google Scholar]
- 364.Liu S, Bu X, Kan A, Luo L, Xu Y, Chen H, Lin X, Lai Z, Wen D, Huang L, Shi M. SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 2022;528:16–30. doi: 10.1016/j.canlet.2021.12.026. [DOI] [PubMed] [Google Scholar]
- 365.Li W, Fu Q, Man W, Guo H, Yang P. LncRNA OR3A4 participates in the angiogenesis of hepatocellular carcinoma through modulating AGGF1/akt/mTOR pathway. Eur J Pharmacol. 2019;849:106–114. doi: 10.1016/j.ejphar.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 366.Yuan P, Cao W, Zang Q, Li G, Guo X, Fan J. The HIF-2α-MALAT1-miR-216b axis regulates multi-drug resistance of hepatocellular carcinoma cells via modulating autophagy. Biochem Biophys Res Commun. 2016;478:1067–1073. doi: 10.1016/j.bbrc.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 367.Li W, Dong X, He C, Tan G, Li Z, Zhai B, Feng J, Jiang X, Liu C, Jiang H, Sun X. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2019;38:183. doi: 10.1186/s13046-019-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gu D, Tong M, Wang J, Zhang B, Liu J, Song G, Zhu B. Overexpression of the lncRNA HOTAIRM1 promotes lenvatinib resistance by downregulating miR-34a and activating autophagy in hepatocellular carcinoma. Discov Oncol. 2023;14:66. doi: 10.1007/s12672-023-00673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Duan A, Li H, Yu W, Zhang Y, Yin L. Long noncoding RNA XIST promotes resistance to lenvatinib in hepatocellular carcinoma cells via epigenetic inhibition of NOD2. J Oncol. 2022;2022:4537343. doi: 10.1155/2022/4537343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Wang Y, Tan K, Hu W, Hou Y, Yang G. LncRNA AC026401.3 interacts with OCT1 to intensify sorafenib and lenvatinib resistance by activating E2F2 signaling in hepatocellular carcinoma. Exp Cell Res. 2022;420:113335. doi: 10.1016/j.yexcr.2022.113335. [DOI] [PubMed] [Google Scholar]
- 371.Yu T, Yu J, Lu L, Zhang Y, Zhou Y, Zhou Y, Huang F, Sun L, Guo Z, Hou G, Dong Z, Wang B. MT1JP-mediated miR-24-3p/BCL2L2 axis promotes Lenvatinib resistance in hepatocellular carcinoma cells by inhibiting apoptosis. Cell Oncol (Dordr) 2021;44:821–834. doi: 10.1007/s13402-021-00605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Chen SP, Zhu GQ, Xing XX, Wan JL, Cai JL, Du JX, Song LN, Dai Z, Zhou J. LncRNA USP2-AS1 promotes hepatocellular carcinoma growth by enhancing YBX1-mediated HIF1α protein translation under hypoxia. Front Oncol. 2022;12:882372. doi: 10.3389/fonc.2022.882372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Chen X, Ye Q, Chen Z, Lin Q, Chen W, Xie C, Wang X. Long non-coding RNA muskelin 1 antisense RNA as a potential therapeutic target in hepatocellular carcinoma treatment. Bioengineered. 2022;13:12237–12247. doi: 10.1080/21655979.2022.2074703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Tsuchiya H, Shinonaga R, Sakaguchi H, Kitagawa Y, Yoshida K. NEAT1-SOD2 axis confers sorafenib and lenvatinib resistance by activating AKT in liver cancer cell lines. Curr Issues Mol Biol. 2023;45:1073–1085. doi: 10.3390/cimb45020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Zhang Y, Zhang Y, Tao H, Zhu J, Lu Y, Cheng F, Xiong Y, Liu J, Cai G, Zhang Z, Liang H, Chen Y, Zhang W. Targeting LINC01607 sensitizes hepatocellular carcinoma to Lenvatinib via suppressing mitophagy. Cancer Lett. 2023;576:216405. doi: 10.1016/j.canlet.2023.216405. [DOI] [PubMed] [Google Scholar]
- 376.Song M, Ma L, Shen C, Liu W, Zhang P, Bi R, Zhao C. FGD5-AS1/miR-5590-3p/PINK1 induces Lenvatinib resistance in hepatocellular carcinoma. Cell Signal. 2023;111:110828. doi: 10.1016/j.cellsig.2023.110828. [DOI] [PubMed] [Google Scholar]
- 377.Cao M, Ren Y, Li Y, Deng J, Su X, Tang Y, Yuan F, Deng H, Yang G, He Z, Liu B, Yao Z, Deng M. Lnc-ZEB2-19 inhibits the progression and lenvatinib resistance of hepatocellular carcinoma by attenuating the NF-κB signaling pathway through the TRA2A/RSPH14 axis. Int J Biol Sci. 2023;19:3678–3693. doi: 10.7150/ijbs.85270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Fu X, Zhang J, He X, Yan X, Wei J, Huang M, Liu Y, Lin J, Hu H, Liu L. Circular RNA MAN2B2 promotes cell proliferation of hepatocellular carcinoma cells via the miRNA-217/MAPK1 axis. J Cancer. 2020;11:3318–3326. doi: 10.7150/jca.36500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Xu L, Feng X, Hao X, Wang P, Zhang Y, Zheng X, Li L, Ren S, Zhang M, Xu M. CircSETD3 (Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting hepatocellular carcinoma growth. J Exp Clin Cancer Res. 2019;38:98. doi: 10.1186/s13046-019-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zhang X, Luo P, Jing W, Zhou H, Liang C, Tu J. circSMAD2 inhibits the epithelial-mesenchymal transition by targeting miR-629 in hepatocellular carcinoma. Onco Targets Ther. 2018;11:2853–2863. doi: 10.2147/OTT.S158008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Meng J, Chen S, Han JX, Qian B, Wang XR, Zhong WL, Qin Y, Zhang H, Gao WF, Lei YY, Yang W, Yang L, Zhang C, Liu HJ, Liu YR, Zhou HG, Sun T, Yang C. Twist1 regulates vimentin through Cul2 circular RNA to promote EMT in hepatocellular carcinoma. Cancer Res. 2018;78:4150–4162. doi: 10.1158/0008-5472.CAN-17-3009. [DOI] [PubMed] [Google Scholar]
- 383.Huang G, Liang M, Liu H, Huang J, Li P, Wang C, Zhang Y, Lin Y, Jiang X. CircRNA hsa_circRNA_104348 promotes hepatocellular carcinoma progression through modulating miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell Death Dis. 2020;11:1065. doi: 10.1038/s41419-020-03276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Qiu L, Huang Y, Li Z, Dong X, Chen G, Xu H, Zeng Y, Cai Z, Liu X, Liu J. Circular RNA profiling identifies circADAMTS13 as a miR-484 sponge which suppresses cell proliferation in hepatocellular carcinoma. Mol Oncol. 2019;13:441–455. doi: 10.1002/1878-0261.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Yang C, Dong Z, Hong H, Dai B, Song F, Geng L, Lu J, Yang J, Sui C, Xu M. circFN1 mediates sorafenib resistance of hepatocellular carcinoma cells by sponging miR-1205 and regulating E2F1 expression. Mol Ther Nucleic Acids. 2020;22:421–433. doi: 10.1016/j.omtn.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L, Gorshkov K, Mao Q, Xia S, Cen D, Zheng J, Liang X, Cai X. N(6)-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol Cancer. 2020;19:163. doi: 10.1186/s12943-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Weng H, Zeng L, Cao L, Chen T, Li Y, Xu Y, Peng Y, Ye Y. circFOXM1 contributes to sorafenib resistance of hepatocellular carcinoma cells by regulating MECP2 via miR-1324. Mol Ther Nucleic Acids. 2021;23:811–820. doi: 10.1016/j.omtn.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Zhang P, Sun H, Wen P, Wang Y, Cui Y, Wu J. circRNA circMED27 acts as a prognostic factor and mediator to promote lenvatinib resistance of hepatocellular carcinoma. Mol Ther Nucleic Acids. 2022;27:293–303. doi: 10.1016/j.omtn.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hao X, Zhang Y, Shi X, Liu H, Zheng Z, Han G, Rong D, Zhang C, Tang W, Wang X. CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3ζ. J Exp Clin Cancer Res. 2022;41:281. doi: 10.1186/s13046-022-02494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Bi T, Lu Q, Pan X, Dong F, Hu Y, Xu Z, Xiu P, Liu Z, Li J. circFAM134B is a key factor regulating reticulophagy-mediated ferroptosis in hepatocellular carcinoma. Cell Cycle. 2023;22:1900–1920. doi: 10.1080/15384101.2023.2249302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Liu D, Liu W, Chen X, Yin J, Ma L, Liu M, Zhou X, Xian L, Li P, Tan X, Zhao J, Liao Y, Cao G. circKCNN2 suppresses the recurrence of hepatocellular carcinoma at least partially via regulating miR-520c-3p/methyl-DNA-binding domain protein 2 axis. Clin Transl Med. 2022;12:e662. doi: 10.1002/ctm2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Matsuyama H, Suzuki HI. Systems and synthetic microRNA biology: from biogenesis to disease pathogenesis. Int J Mol Sci. 2019;21:132. doi: 10.3390/ijms21010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Toro AU, Shukla SK, Bansal P. Emerging role of MicroRNA-based theranostics in hepatocellular carcinoma. Mol Biol Rep. 2023;50:7681–7691. doi: 10.1007/s11033-023-08586-z. [DOI] [PubMed] [Google Scholar]
- 394.Song SK, Jung WY, Park SK, Chung CW, Park Y. Significantly different expression levels of microRNAs associated with vascular invasion in hepatocellular carcinoma and their prognostic significance after surgical resection. PLoS One. 2019;14:e0216847. doi: 10.1371/journal.pone.0216847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Vychytilova-Faltejskova P, Slaby O. MicroRNA-215: from biology to theranostic applications. Mol Aspects Med. 2019;70:72–89. doi: 10.1016/j.mam.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 396.Giordano S, Columbano A. Met as a therapeutic target in HCC: facts and hopes. J Hepatol. 2014;60:442–452. doi: 10.1016/j.jhep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 397.Wang RY, Chen L, Chen HY, Hu L, Li L, Sun HY, Jiang F, Zhao J, Liu GM, Tang J, Chen CY, Yang YC, Chang YX, Liu H, Zhang J, Yang Y, Huang G, Shen F, Wu MC, Zhou WP, Wang HY. MUC15 inhibits dimerization of EGFR and PI3K-AKT signaling and is associated with aggressive hepatocellular carcinomas in patients. Gastroenterology. 2013;145:1436–1448. e1–12. doi: 10.1053/j.gastro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 398.Han T, Zheng H, Zhang J, Yang P, Li H, Cheng Z, Xiang D, Wang R. Downregulation of MUC15 by miR-183-5p.1 promotes liver tumor-initiating cells properties and tumorigenesis via regulating c-MET/PI3K/AKT/SOX2 axis. Cell Death Dis. 2022;13:200. doi: 10.1038/s41419-022-04652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wei Y, Wei L, Han T, Ding S. miR-3154 promotes hepatocellular carcinoma progression via suppressing HNF4α. Carcinogenesis. 2022;43:1002–1014. doi: 10.1093/carcin/bgac067. [DOI] [PubMed] [Google Scholar]
- 400.Xu X, Jiang W, Han P, Zhang J, Tong L, Sun X. MicroRNA-128-3p mediates lenvatinib resistance of hepatocellular carcinoma cells by downregulating c-Met. J Hepatocell Carcinoma. 2022;9:113–126. doi: 10.2147/JHC.S349369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Yan WN, Li SB, Ma HJ, Chen DD, Wang J, Le T, Zhang GQ. Effect of miR-23b on the malignant phenotype and the sensitivity of lenvatinib in human hepatocellular carcinoma cells. Zhonghua Gan Zang Bing Za Zhi. 2021;29:433–438. doi: 10.3760/cma.j.cn501113-20210416-00187. [DOI] [PubMed] [Google Scholar]
- 402.Chen M, Wang H, Shi S, Zhang H, Xu S, Jiang Y. miR-6071 inhibits hepatocellular carcinoma progression via targeting PTPN11. Arch Biochem Biophys. 2022;727:109345. doi: 10.1016/j.abb.2022.109345. [DOI] [PubMed] [Google Scholar]
- 403.Lehvy AI, Horev G, Golan Y, Glaser F, Shammai Y, Assaraf YG. Alterations in ZnT1 expression and function lead to impaired intracellular zinc homeostasis in cancer. Cell Death Discov. 2019;5:144. doi: 10.1038/s41420-019-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kakita N, Katayama K, Yasui T, Satake S, Aoi K, Jo H, Kim YK, Yamazaki M, Hashimoto A, Kambe T. Zinc transporter 1 expression in hepatocellular carcinoma correlates with prognosis: a single-center retrospective study. J Trace Elem Med Biol. 2024;82:127354. doi: 10.1016/j.jtemb.2023.127354. [DOI] [PubMed] [Google Scholar]
- 405.Yang D, Tian T, Li X, Zhang B, Qi L, Zhang F, Han M, Wang S, Xiao J, Gou Y, Zhang R, Liu Q, Su S, Liu J, Huang X, Gao Q, Hui L, Tang H, Chen Y, Wang H, Wei B. ZNT1 and Zn2+ control TLR4 and PD-L1 endocytosis in macrophageS to improve chemotherapy efficacy against liver tumor. Hepatology. 2024;80:312–329. doi: 10.1097/HEP.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 406.Gnani D, Romito I, Artuso S, Chierici M, De Stefanis C, Panera N, Crudele A, Ceccarelli S, Carcarino E, D’Oria V, Porru M, Giorda E, Ferrari K, Miele L, Villa E, Balsano C, Pasini D, Furlanello C, Locatelli F, Nobili V, Rota R, Leonetti C, Alisi A. Focal adhesion kinase depletion reduces human hepatocellular carcinoma growth by repressing enhancer of zeste homolog 2. Cell Death Differ. 2017;24:889–902. doi: 10.1038/cdd.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ichihara E, Westover D, Meador CB, Yan Y, Bauer JA, Lu P, Ye F, Kulick A, de Stanchina E, McEwen R, Ladanyi M, Cross D, Pao W, Lovly CM. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res. 2017;77:2990–3000. doi: 10.1158/0008-5472.CAN-16-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Skinner HD, Giri U, Yang L, Woo SH, Story MD, Pickering CR, Byers LA, Williams MD, El-Naggar A, Wang J, Diao L, Shen L, Fan YH, Molkentine DP, Beadle BM, Meyn RE, Myers JN, Heymach JV. Proteomic profiling identifies PTK2/FAK as a driver of radioresistance in hpv-negative head and neck cancer. Clin Cancer Res. 2016;22:4643–4650. doi: 10.1158/1078-0432.CCR-15-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hou W, Gad SA, Ding X, Dhanarajan A, Qiu W. Focal adhesion kinase confers lenvatinib resistance in hepatocellular carcinoma via the regulation of lysine-deficient kinase 1. Mol Carcinog. 2024;63:173–189. doi: 10.1002/mc.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Li W, Cui K, Prochownik EV, Li Y. The deubiquitinase USP21 stabilizes MEK2 to promote tumor growth. Cell Death Dis. 2018;9:482. doi: 10.1038/s41419-018-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Chen Z, Ma Y, Guo Z, Song D, Chen Z, Sun M. Ubiquitin-specific protease 1 acts as an oncogene and promotes lenvatinib efficacy in hepatocellular carcinoma by stabilizing c-kit. Ann Hepatol. 2022;27:100669. doi: 10.1016/j.aohep.2022.100669. [DOI] [PubMed] [Google Scholar]
- 413.Huang X, Zhang Q, Lou Y, Wang J, Zhao X, Wang L, Zhang X, Li S, Zhao Y, Chen Q, Liang T, Bai X. USP22 deubiquitinates CD274 to suppress anticancer immunity. Cancer Immunol Res. 2019;7:1580–1590. doi: 10.1158/2326-6066.CIR-18-0910. [DOI] [PubMed] [Google Scholar]
- 414.Feng T, Ling S, Xu C, Ying L, Su D, Xu X. Ubiquitin-specific peptidase 22 in cancer. Cancer Lett. 2021;514:30–37. doi: 10.1016/j.canlet.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 415.Boeckel JN, Derlet A, Glaser SF, Luczak A, Lucas T, Heumüller AW, Krüger M, Zehendner CM, Kaluza D, Doddaballapur A, Ohtani K, Treguer K, Dimmeler S. JMJD8 regulates angiogenic sprouting and cellular metabolism by interacting with pyruvate kinase M2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2016;36:1425–1433. doi: 10.1161/ATVBAHA.116.307695. [DOI] [PubMed] [Google Scholar]
- 416.Guo J, Zhao J. USP22-JMJD8 axis promotes Lenvatinib resistance in hepatocellular carcinoma. Biochim Biophys Acta Mol Cell Res. 2024;1871:119617. doi: 10.1016/j.bbamcr.2023.119617. [DOI] [PubMed] [Google Scholar]
- 417.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 418.Gao C, Chang L, Xu T, Li X, Chen Z. AKR1C1 overexpression leads to lenvatinib resistance in hepatocellular carcinoma. J Gastrointest Oncol. 2023;14:1412–1433. doi: 10.21037/jgo-23-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Liu L, Yu K, Huang C, Huo M, Li X, Yin R, Liu C, Lu L, Sun H, Zhang J. Sex differences in hepatocellular carcinoma indicated BEX4 as a potential target to improve efficacy of lenvatinib plus immune checkpoint inhibitors. J Cancer. 2022;13:3221–3233. doi: 10.7150/jca.73051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Li Z, Yang NH, Li B, Sun CY. The regulation of APGAT4 on the growth and lenvatinib resistance of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2023;31:408–414. doi: 10.3760/cma.j.cn501113-20230225-00080. [DOI] [PubMed] [Google Scholar]
- 421.Kotani K, Uchida-Kobayashi S, Yoshida K, Kawamura E, Fujii H, Hagihara A, Enomoto M, Tamori A, Kawada N. Lenvatinib-induced tumor-related hemorrhage in patients with unresectable hepatocellular carcinoma. Am J Gastroenterol. 2021;116:631. doi: 10.14309/ajg.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 422.Uchida-Kobayashi S, Kageyama K, Yamamoto A, Ikenaga H, Yoshida K, Kotani K, Kimura K, Odagiri N, Hagihara A, Fujii H, Enomoto M, Tamori A, Kubo S, Miki Y, Kawada N. Lenvatinib-induced tumor-related hemorrhages in patients with large hepatocellular carcinomas. Oncology. 2021;99:186–191. doi: 10.1159/000510911. [DOI] [PubMed] [Google Scholar]
- 423.Sato A, Imai Y, Uchiya H, Uchida Y, Nakazawa M, Sugawara K, Nakayama N, Mochida S. Two cases of intraabdominal bleeding caused by hepatocellular carcinoma rupture soon after the initiation of chemotherapy with lenvatinib. Intern Med. 2022;61:2301–2305. doi: 10.2169/internalmedicine.8733-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Higashino M, Sugiura R, Yamamoto Y, Naruse H, Sakamoto N. A spontaneous rupture of lung metastasis from hepatocellular carcinoma after the introduction of lenvatinib. J Gastrointestin Liver Dis. 2021;30:169–170. doi: 10.15403/jgld-3025. [DOI] [PubMed] [Google Scholar]
- 425.Shimizu Y, Sunagozaka H, Yamagata K, Hirai H, Miura M, Yonemoto Y, Naito Y, Hasatani K, Yoshikawa J, Aoyagi H, Kaneko S. Lenvatinib-induced tumor lysis syndrome in a patient with advanced hepatocellular carcinoma: a case report. Clin J Gastroenterol. 2021;14:645–649. doi: 10.1007/s12328-020-01306-1. [DOI] [PubMed] [Google Scholar]
- 426.Ishihara K. A case of multiple intratumoral hemorrhage and rupture of hepatocellular carcinoma after starting lenvatinib. Case Reports Hepatol. 2020;2020:6659388. doi: 10.1155/2020/6659388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Suoh M, Hagihara A, Yamamura M, Maruyama H, Taira K, Enomoto M, Tamori A, Fujiwara Y, Kawada N. Obstructive jaundice due to duodenal ulcer induced by lenvatinib therapy for hepatocellular carcinoma. Intern Med. 2021;60:545–552. doi: 10.2169/internalmedicine.5097-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Miyajima S, Tsuji K, Kito Y, Yoshida N, Matsunaga K, Tsuji S, Katayanagi K, Yonezawa M, Kubo A, Ushijima K, Minato H, Doyama H. Colitis induced by Lenvatinib in a patient with advanced hepatocellular carcinoma. Clin J Gastroenterol. 2021;14:187–192. doi: 10.1007/s12328-020-01249-7. [DOI] [PubMed] [Google Scholar]
- 429.Suzuki N, Tajiri K, Futsukaichi Y, Tanaka S, Murayama A, Entani T, Kobayashi S, Takahashi K, Fujii T, Imura J, Yasuda I. Perforation of the small intestine after introduction of lenvatinib in a patient with advanced hepatocellular carcinoma. Case Rep Gastroenterol. 2020;14:63–69. doi: 10.1159/000505774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Fujimoto Y, Namisaki T, Takeda S, Murata K, Enomoto M, Takaya H, Tsuji Y, Fujinaga Y, Sawada Y, Nishimura N, Kitagawa K, Kaji K, Inoue T, Kawaratani H, Moriya K, Akahane T, Mitoro A, Yoshiji H. Leaky gut and severe adverse events in advanced hepatocellular carcinoma treated with lenvatinib. Anticancer Res. 2022;42:4895–4905. doi: 10.21873/anticanres.15995. [DOI] [PubMed] [Google Scholar]
- 431.Mizokami K, Watanabe A, Yamaguchi E, Saito A. Bowel perforation in a patient with hepatocellular carcinoma during lenvatinib treatment. Case Rep Gastroenterol. 2022;16:502–506. doi: 10.1159/000525569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yokota K, Kitagawa H, Okamoto K, Namikawa T, Maeda H, Kobayashi M, Seo S. A case of tracheoesophageal fistula in a non-metastatic site during lenvatinib treatment for hepatocellular carcinoma. Cancer Diagn Progn. 2023;3:475–478. doi: 10.21873/cdp.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ishigaki K, Hamada T, Nakai Y, Ishigaki Y, Oyama H, Kanai S, Suzuki T, Nakamura T, Sato T, Hakuta R, Saito K, Saito T, Takahara N, Mizuno S, Kogure H, Tateishi R, Tada M, Koike K. Lenvatinib-induced acute acalculous cholecystitis in a patient with hepatocellular carcinoma. Clin J Gastroenterol. 2020;13:568–571. doi: 10.1007/s12328-020-01116-5. [DOI] [PubMed] [Google Scholar]
- 434.Honda S, Saito Y, Sawada K, Hasebe T, Nakajima S, Okumura T. Repeated perforation of the gallbladder in a patient with hepatocellular carcinoma receiving lenvatinib. Intern Med. 2020;59:657–662. doi: 10.2169/internalmedicine.3806-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Secombe KR, Van Sebille YZA, Mayo BJ, Coller JK, Gibson RJ, Bowen JM. Diarrhea induced by small molecule tyrosine kinase inhibitors compared with chemotherapy: potential role of the microbiome. Integr Cancer Ther. 2020;19:1534735420928493. doi: 10.1177/1534735420928493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Inukai Y, Yamamoto K, Honda T, Ito T, Imai N, Ishizu Y, Nakamura M, Kawashima H, Ishigami M. Differences in the intestinal microbiome associated with diarrhea during lenvatinib treatment for hepatocellular carcinoma. Dig Dis. 2023;41:138–147. doi: 10.1159/000524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Liu Z, Meng Y, Xiao W, Wang H, Sun L, Fu Z, Xiong X, Zhang S. Hepatic encephalopathy induced by Lenvatinib and anti-PD-1 mAb in a patient with advanced hepatocellular carcinoma: a case report. Mol Clin Oncol. 2021;14:110. doi: 10.3892/mco.2021.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Khan HA, Alhomida AS. A review of the logistic role of L-carnitine in the management of radiation toxicity and radiotherapy side effects. J Appl Toxicol. 2011;31:707–713. doi: 10.1002/jat.1716. [DOI] [PubMed] [Google Scholar]
- 439.Endo K, Ueno T, Ishikawa K, Nakanishi Y, Kondo S, Wakisaka N, Yoshizaki T. Effects of l-carnitine administration on health-related quality of life during cisplatin-based chemoradiotherapy in patients with head and neck squamous cell carcinoma. Auris Nasus Larynx. 2019;46:431–436. doi: 10.1016/j.anl.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 440.Lancaster CS, Hu C, Franke RM, Filipski KK, Orwick SJ, Chen Z, Zuo Z, Loos WJ, Sparreboom A. Cisplatin-induced downregulation of OCTN2 affects carnitine wasting. Clin Cancer Res. 2010;16:4789–4799. doi: 10.1158/1078-0432.CCR-10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Okubo H, Ando H, Ishizuka K, Kitagawa R, Okubo S, Saito H, Kokubu S, Miyazaki A, Ikejima K, Shiina S, Nagahara A. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS One. 2020;15:e0229772. doi: 10.1371/journal.pone.0229772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Flippo KH, Potthoff MJ. Metabolic messengers: FGF21. Nat Metab. 2021;3:309–317. doi: 10.1038/s42255-021-00354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Santoso P, Nakata M, Shiizaki K, Boyang Z, Parmila K, Otgon-Uul Z, Hashimoto K, Satoh T, Mori M, Kuro OM, Yada T. Fibroblast growth factor 21, assisted by elevated glucose, activates paraventricular nucleus NUCB2/Nesfatin-1 neurons to produce satiety under fed states. Sci Rep. 2017;7:45819. doi: 10.1038/srep45819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kohya R, Suda G, Ohara M, Sasaki T, Yoda T, Sakurai N, Yoshida S, Fu Q, Yang Z, Hosoda S, Maehara O, Ohnishi S, Tokuchi Y, Kitagataya T, Suzuki K, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Ogawa K, Sakamoto N. Potential correlation between changes in serum FGF21 levels and lenvatinib-induced appetite loss in patients with unresectable hepatocellular carcinoma. Cancers (Basel) 2023;15:3257. doi: 10.3390/cancers15123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Imakura T, Sato S, Tomonari T, Murakami K, Takahashi N, Naito N, Mima M, Kagawa K, Koyama K, Nishimura H, Kawano H, Nokihara H, Azuma M, Takayama T, Nishioka Y. Lenvatinib-induced interstitial pneumonia in a patient with hepatocellular carcinoma. Intern Med. 2022;61:1211–1217. doi: 10.2169/internalmedicine.7300-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Kawanishi Y, Kuwahara M, Utsunomiya M, Ishida N, Ishikawa Y, Hiroi M, Akimori T. Pneumothorax during lenvatinib treatment for hepatocellular carcinoma with lung metastasis. Clin J Gastroenterol. 2021;14:288–292. doi: 10.1007/s12328-020-01273-7. [DOI] [PubMed] [Google Scholar]
- 447.Kanzaki M, Honda A, Sawai Y, Suda K, Suda A, Kawachi Y. Lenvatinib-induced pyoderma gangrenosum in a patient with hepatocellular carcinoma: a case report. J Dermatol. 2024;51:e31–e32. doi: 10.1111/1346-8138.16896. [DOI] [PubMed] [Google Scholar]
- 448.Matsumoto Y, Fukumoto T, Takahashi W, Nishigori C. Lenvatinib-induced severe generalized erythematous rash in a patient with hepatocellular carcinoma. Dermatol Reports. 2022;15:9617. doi: 10.4081/dr.2023.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Chi CC, Jing J. Extragenital giant condyloma acuminatum in a hepatocellular carcinoma patient treated with lenvatinib plus PD-1 inhibitor. Int J Dermatol. 2023;62:1423–1424. doi: 10.1111/ijd.16757. [DOI] [PubMed] [Google Scholar]
- 450.Iwasa T, Adachi S, Ogiso H, Takada E, Mabuchi M, Suzuki Y, Yamauchi O, Saito K, Iwashita T, Ogino H, Shimizu M. Severe skin ulcer caused by taking lenvatinib after proton beam therapy. Clin J Gastroenterol. 2023;16:588–592. doi: 10.1007/s12328-023-01802-0. [DOI] [PubMed] [Google Scholar]
- 451.Cha S, Kim DW, Choe JW, Kim TH, Kim SY, Hyun JJ, Jung SW, Koo JS, Jung YK, Yim HJ. A case report of a patient presented with skin ulcer after treatment of lenvatinib. J Liver Cancer. 2021;21:194–198. doi: 10.17998/jlc.2021.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Sally R, Ugonabo N, Nguyen A, Kim RH, Lo Sicco K. Lenvatinib-induced psoriasiform eruption and palmoplantar erythema in a patient with hepatocellular carcinoma. JAAD Case Rep. 2021;15:1–3. doi: 10.1016/j.jdcr.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Gómez Torres KM, Molina Villalba C, Estévez Escobar M. Lenvatinib in severe hypoglycemia associated with hepatocellular carcinoma. Rev Esp Enferm Dig. 2021;113:799. doi: 10.17235/reed.2021.8016/2021. [DOI] [PubMed] [Google Scholar]
- 454.Aoki Y, Inoue Y, Sasahira N, Ono M, Inamura K, Kataoka A, Takano T, Kanao H, Watanabe M. Primary ovarian insufficiency associated with lenvatinib therapy in a patient with hepatocellular carcinoma: a case report. Oncol Lett. 2023;26:450. doi: 10.3892/ol.2023.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Suoh M, Fujii H, Nagata Y, Kotani K, Hagihara A, Enomoto M, Tamori A, Inaba M, Kawada N. Destructive thyroiditis presenting as thyrotoxicosis followed by hypothyroidism during lenvatinib therapy for hepatocellular carcinoma. Clin J Gastroenterol. 2020;13:860–866. doi: 10.1007/s12328-020-01107-6. [DOI] [PubMed] [Google Scholar]
- 456.Uekusa S, Nemoto M, Hanai Y, Nakashin M, Miyagawa A, Yanagino S, Arita Y, Matsumoto T, Nishizawa K, Nagai H, Higai K, Matsuo K. Risk factors for lenvatinib-induced high-grade hypothyroidism in patients with hepatocellular carcinoma: a retrospective study. Pharmacology. 2023;108:460–468. doi: 10.1159/000531881. [DOI] [PubMed] [Google Scholar]
- 457.Ichimura T, Ichikura D, Hinata M, Hida N, Baba T. Thyroid dysfunction with atezolizumab plus bevacizumab after lenvatinib in hepatocellular carcinoma: a case series. SAGE Open Med Case Rep. 2023;11:2050313x231164488. doi: 10.1177/2050313X231164488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Nakashima S, Sekine A, Sawa N, Kawamura Y, Kono K, Kinowaki K, Kawada M, Hasegawa E, Akuta N, Suzuki Y, Ohashi K, Takaichi K, Ubara Y, Hoshino J. Thrombotic microangiopathy, podocytopathy, and damage to the renal tubules with severe proteinuria and acute renal dysfunction induced by lenvatinib. Intern Med. 2022;61:3083–3088. doi: 10.2169/internalmedicine.8365-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Prasoppokakorn T, Thanapirom K, Treeprasertsuk S. Nephrotic syndrome induced by lenvatinib treatment for hepatocellular carcinoma. Case Reports Hepatol. 2022;2022:5101856. doi: 10.1155/2022/5101856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Legros L, Pascale A, Guettier C, Eftekhari P, Merabet YB, Stang M, Bossevot R, Goldschmidt E, Ulusakarya A, Morisset S, Lewin M, Samuel D, Rosmorduc O. Progressive erythrocytosis under lenvatinib treatment in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2023;91:337–344. doi: 10.1007/s00280-023-04519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Decaens T, Barone C, Assenat E, Wermke M, Fasolo A, Merle P, Blanc JF, Grando V, Iacobellis A, Villa E, Trojan J, Straub J, Bruns R, Berghoff K, Scheele J, Raymond E, Faivre S. Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression. Br J Cancer. 2021;125:190–199. doi: 10.1038/s41416-021-01334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Li L, Zhao GD, Shi Z, Qi LL, Zhou LY, Fu ZX. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol Lett. 2016;12:3045–3050. doi: 10.3892/ol.2016.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Kim D, Herdeis L, Rudolph D, Zhao Y, Böttcher J, Vides A, Ayala-Santos CI, Pourfarjam Y, Cuevas-Navarro A, Xue JY, Mantoulidis A, Bröker J, Wunberg T, Schaaf O, Popow J, Wolkerstorfer B, Kropatsch KG, Qu R, de Stanchina E, Sang B, Li C, McConnell DB, Kraut N, Lito P. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023;619:160–166. doi: 10.1038/s41586-023-06123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.