Abstract
Cellular senescence is an irreversible state of growth arrest, and induction of senescence is considered a potential therapeutic strategy against cancer. Indoleamine 2,3-dioxygenase 1 (IDO1), an enzyme catabolizing L-tryptophan into kynurenine, plays a key role in tumor immune tolerance. However, the roles of IDO1 in cellular senescence and chemoresistance remain elusive. Herein, we observed a significant elevation of IDO1 expression in colorectal cancer (CRC) tissues compared to non-neoplastic controls, based on both the GEPIA database and mouse model. Functionally, ectopic expression of IDO1 blunted 5-fluorouracil (5-FU)-induced cell senescence and rendered CRC cells more refractory towards 5-FU treatment, whereas IDO1 silencing resulted in opposing effects. Further studies demonstrated that IDO1 overexpression decreased the levels of senescent-related proteins, including p16, p21, p53, and cyclin D1. Mechanistically, the kynurenine released from IDO1-expressing CRC cells inhibited the IGFBP5/p53 signaling pathway, accounting for IDO1-mediated suppression of cell senescence and induction of chemoresistance. Collectively, these data revealed an unrecognized role of IDO1 in senescence escape and chemoresistance via releasing its catabolite kynurenine, implicating that therapeutically targeting IDO1 or IGFBP5/p53 signaling pathway holds great promise for CRC treatment.
Keywords: IDO1, cell senescence, chemoresistance, colorectal cancer, kynurenine
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths worldwide. It has been estimated that CRC mortality is as high as 0.9 million annually around the globe [1]. Despite the recent improvements in CRC therapeutic strategies, the 5-year survival rate of CRC patients is still not optimistic. Therefore, understanding the molecular mechanisms of colorectal carcinogenesis is a prerequisite for identifying the therapeutically actionable targets that could provide more sophisticated clinically intervening strategies for CRC.
Cellular senescence has been described as a state of permanent cell cycle arrest and is regarded as a potential therapeutic tool against carcinogenesis [2,3]. Fundamentally, senescence is the adaptive response to various cellular stresses such as telomere shortening, oncogene-induced senescence, genetic mutations, radiotherapy, and chemotherapy [4]. The senescent phenotype is a common hallmark of the cell under stress, which is often characterized by flattened shape, enlarged cell size, and enhanced senescence-associated β-galactosidase (SA-β-gal) activity [5]. Senescent cells often exhibit the characteristics of the upregulation of cyclin-dependent protein kinase inhibitors including p16, p21, or p27, and the induction of p53 [6]. Intriguingly, senescent tumor cells can be eliminated by the immune system [7]. Therefore, cellular senescence has been regarded as one of the compelling therapeutic strategies for cancer treatment.
Indoleamine 2,3 dioxygenase 1 (IDO1), a well-characterized tryptophan metabolic enzyme, catalyzes tryptophan into kynurenine (Kyn). IDO1 is often highly expressed in several types of tumors and is tightly correlated with adverse clinical outcomes [8]. Accumulating evidence has demonstrated that IDO1 rewires the immunosuppressive milieu to facilitate cancer progression through a variety of mechanisms, such as inhibition of effector T and NK cells, induction of T regulatory cell differentiation, and activation of myeloid-derived suppressor cells [9]. Recently, the immune-independent function of IDO1 has attracted considerable attentions. It has been reported that IDO1 and its metabolite Kyn accentuate CRC development via the activation of PI3K/Akt and the β-catenin pathway [10]. Moreover, IDO1 silencing could inhibit tumor cell proliferation, and angiogenesis, and induce apoptosis [8,11,12]. However, the effect of IDO1 and Kyn on cellular senescence and chemoresistance in cancer cells is not yet delineated.
In this study, we demonstrated that IDO1 is overexpressed in CRC cells and plays key roles in escaping cellular senescence, and confers CRC cells more refractory to 5-FU. Essentially, IDO1-expressing CRC cells release catabolite Kyn, rather than other soluble factors, to attenuate the IGFBP5/p53 signaling pathway, thereby evoking senescence evasion, and protecting CRC cells from 5-FU toxicity. Our findings highlight that intervening with the IDO1-mediated Kyn metabolism may represent a promising approach for CRC treatment.
Materials and methods
Reagents and cell culture
Kyn was purchased from Targetmol (Shanghai, China). 5-FU was obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). The transfection reagent Lipofectamine 3000 was bought from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies against p53, p21, and p27 were obtained from Proteintech (Wuhan, China). Cyclin D1 antibody was bought from Cell Signaling Technology (Beverly, MA, USA). Antibodies for IDO1, GAPDH, and γ-H2AX were obtained from Abcam (Cambridge, MA, USA). IGFBP5 antibody was obtained from Cloud-Clone Corp (Wuhan, China).
Human colorectal cancer cells HCT-116 and HCT-8 were obtained from the ATCC (Manassas, VA, USA). HCT-116 and HCT-8 cells were cultured in RPMI-1640 with 10% FBS at 37°C with 5% CO2.
Cell viability assay and colony formation assay
Cell viability assay was performed as previously described [13]. The indicated cells were inoculated into the 96-well culture plates with 5000 cells/well and cultured for 24, 48, and 72 h. The cell survival ratio was determined using an MTT assay.
A clonogenic assay was conducted as previously described [14]. After HCT-116 and HCT-8 cells were transfected with IDO1 overexpression plasmids or specific shIDO1 plasmids for 72 h, cells were trypsinized and 1 × 104 cells were seeded in a 60 mm culture dish in the presence of 5-FU (20 μM). The cell medium was changed every alternate day with 5-FU (20 μM). After 2 weeks, the surviving colonies were fixed stained with 0.1% crystal violet and photographed.
Senescence-associated β-galactosidase assay
The senescence-associated β-galactosidase (SA-β-gal) activity was measured using the SA-β-gal Staining Kit (Beyotime Biotech, Beijing, China). Briefly, HCT-8 and HCT-116 cells were seeded into sterile six-well plates with indicated treatment for 5 days. Then, the cells were washed with PBS and fixed in 4% paraformaldehyde. Subsequently, the cells were incubated with fresh β-gal Staining Solution. The SA-β-gal positive cells were photographed and counted with a bright field microscope (Zeiss, Oberkochen, German).
Quantitative real-time PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen, MA, USA) and purified with DNase I and then cDNA was generated by PrimeScript RT Master Mix (Takara, Dalian, China). qPCR was performed as previously described [15]. The primer sequences were shown in Table S1.
Western blot analysis
Whole-cell extracts were prepared and western blot was carried out as previously described [16]. The specific protein band was visualized and scanned by the western blot imaging system (AI600 images, GE, USA).
Plasmid construction, cell transfection, and lentivirus infection
For amplification of the human full-length coding sequence of the IDO1 gene, the forward (5’-CGACGATAAGGAATTCATGGCACACGCTATGGAAAAC-3’) and reverse primer (5’-AGATGCATGCGGATCCTTAACCTTCCTTCAAAAGGGATTTC-3’) was performed. For amplification of the human full-length coding sequence of the IGFBP5 gene, the forward (5’-CGACGATAAGGAATTCATGGTGTTGCTCACCGCGGTCCT-3’) and reverse primer (5’-AGATGCATGCGGTCACTCAACGTTGCTGCTGTCGA-3’) were designed. The PCR amplification is cloned to the pSin-FLAG vector via seamless cloning master mix as per the manufacturer’s recommendations (Sangon Biotech, Shanghai, China) and the constructs were identified by sequencing.
The constructions of two sets of shRNA targeted for IDO1 was as follows, shIDO1-1 (forward 5’-CCGGCCATCTGCAAATCGTGACTAACTCGAGTTAGTCACGATTTGCAGATGGTTTTTG-3’, reverse, 5’-AATTCAAAAACCATCTGCAAATCGTGACTAACTCGAGTTAGTCACGATTTGCAGATGG-3’) and shIDO1-2 (forward 5’-CCGGGCAGACTGTGTCTTGGCAAACCTCGAGGTTTGCCAAGACACAGTCTGCTTTTTG-3’, reverse, 5’-AATTCAAAAAGCAGACTGTGTCTTGGCAAACCTCGAGGTTTGCCAAGACACAGTCTGC-3’). The sequences were synthesized, annealed, and ligated into the pLKO.1 plasmid. The constructs were validated via sequencing.
For stable cell line selection, IDO1 overexpression, or shRNA plasmid was co-transfected with both psPAX2 packaging plasmid and the pMD2.G envelope plasmid at a 4:3:1 ratio into 293 T cells using the Calcium Phosphate Cell Transfection Kit (Beyotime, China). Medium containing the virus was collected and concentrated using ultrafiltration membranes (Millipore). CRC cells were infected with the viruses and subjected to puromycin selection as previously described [6].
For transient transfection, HCT-116 and HCT-8 cells grow up to 70% confluency and are then transfected with indicated plasmids using Lipofectamine 3000 as previously described [6].
Enzyme-linked immunosorbent assay (ELISA)
The Kyn concentration in the supernatant of the culture medium was detected using a human kynurenine ELISA Kit (SenBeiJia Biological Technology Co., Ltd., Nanjing, China) as previously described [17].
Xenograft model
All animal experiments were approved by the ethics committee of Animal Experiments of Shanxi University and followed the Guidelines of ethical regulatory standards. Six-week-old female nude mice were injected s.c. with HCT-116 cells stably expressing IDO1. When the volume of the tumor reached approximately 4 mm × 4 mm, tumor-bearing mice were randomly assigned (n = 5 per group) and intraperitoneally injected with 5-FU (25 mg/kg) or vehicle control every 3 days for 3 weeks to evaluate the dynamic tumor growth. At the endpoint, the mice were euthanized and the tumors were collected, weighed, and subsequently used for IHC analysis.
Immunohistochemistry (IHC)
The paraffin sections from the AOM/DSS group and control group [18] were incubated with specific antibodies against IDO1 at 4°C. IHC analysis was performed as previously described [6].
Statistical analysis
All data are shown as mean ± SD. The differences between the two groups were analyzed using two-tailed t tests. GraphPad Prism Software (San Diego, CA, USA) was performed for statistical analysis. P < 0.05 was deemed statistically significant.
Results
IDO1 overexpression impairs CRC cell senescence and favors chemoresistance
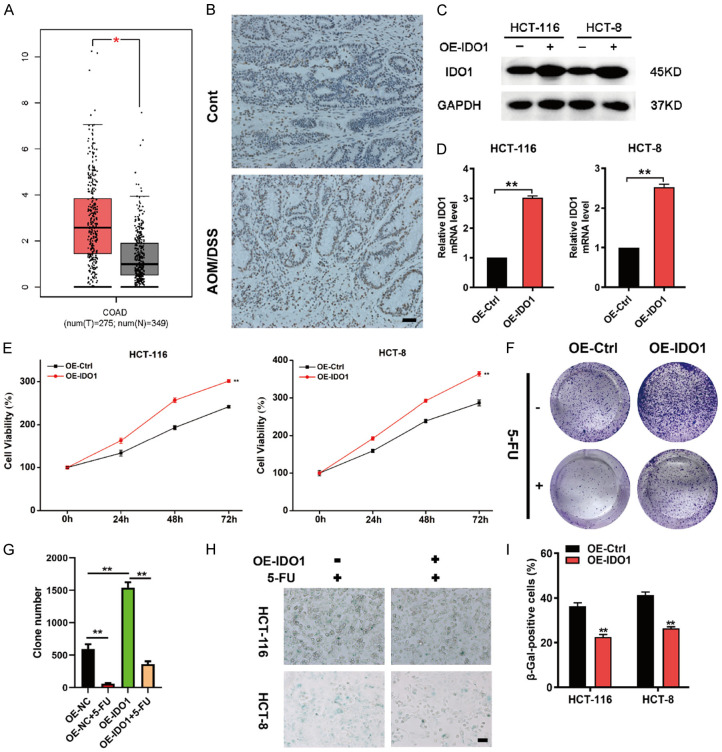
To illuminate the potential function of IDO1, the transcriptional levels of IDO1 in CRC versus benign counterparts were analyzed using the GEPIA database. The results showed that IDO1 expression was significantly higher in CRC tissues compared to normal tissues (Figure 1A). We also observed an obvious upregulation of IDO1 expression in AOM/DSS-induced colon cancer mouse model (Figure 1B). To investigate the functions of IDO1 in CRC proliferation and chemoresistance, the stable cell lines expressing human IDO1 was established by lentivirus infection and verified by western blot and qPCR analysis (Figure 1C and 1D). IDO1 overexpression significantly increased CRC cell viability in both HCT-116 and HCT-8 cells (Figure 1E). Moreover, IDO1-overexpressing cells were more resistant to 5-FU treatment (Figure 1F and 1G). Senescence is an irreversible cycle arrest state, which has been regarded as a powerful tool to prevent tumor progression [4]. In the presence of chemotherapy drugs, the impact of specific genes on tumor cell senescence can be more clearly determined [4]. Next, we interrogated whether IDO1-mediated CRC cell growth was associated with cellular senescence using SA-β-gal staining. As shown in Figure 1H and 1I, IDO1 overexpression dramatically decreased the proportion of SA-β-gal-positive cells in the conditions of 5-FU treatment. Collectively, these findings demonstrated that IDO1 overexpression impeded cellular senescence and facilitated chemoresistance.
Figure 1.
IDO1 overexpression impedes cellular senescence and confers CRC cells more tolerable to 5-FU. (A) The expression of IDO1 in colorectal adenocarcinoma (COAD) was analyzed using GEPIA. (B) Immunohistochemical staining was performed to analyze IDO1 expression in intestinal tissue sections from the AOM/DSS group and control. (C, D) HCT-116 and HCT-8 cells were infected with IDO1 overexpression or control lentivirus followed by the detection of IDO1 expression via western blot (C) and qPCR analysis (D). (E) Cell viability of control cells and IDO1-overexpression cells was analyzed via MTT assay. (F) Colony formation assays were performed using HCT-116 cells with stable IDO1 overexpression in the presence or absence of 20 μM 5-FU treatment. (G) The summarized colony numbers were derived from (F). (H) SA-β-gal staining of CRC control cells and IDO1-overexpression cells in the presence of 20 μM 5-FU treatment for 48 h. (I) The quantitative results of SA-β-gal positive cells from (H). Data are presented as means ± SD. *P < 0.05; **P < 0.01.
IDO1 loss restores CRC cell senescence and enhances chemosensitivity
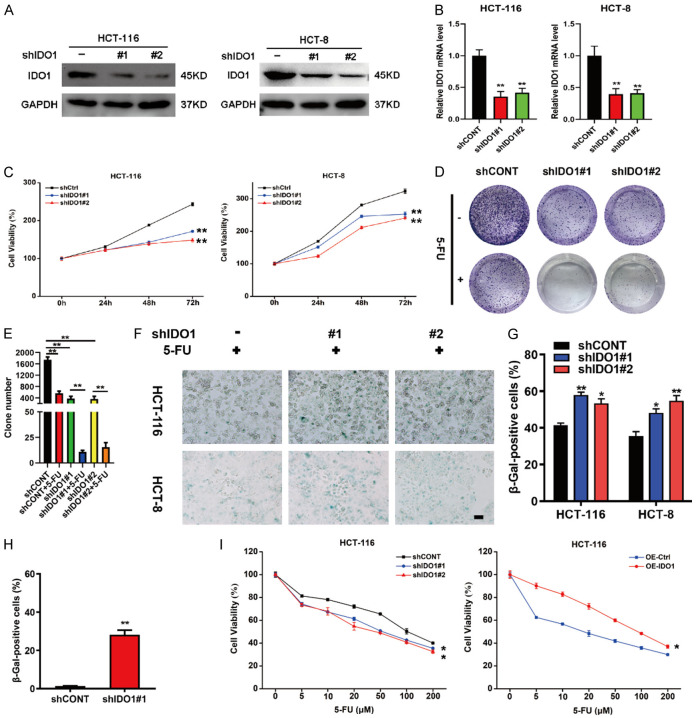
To further substantiate the functional relevance of IDO1 with cellular senescence and chemoresistance, we knocked down IDO1 expression by infecting CRC cells with the lentivirus carrying short hairpin RNAs (shRNA) targeting IDO1, and stable cell lines were obtained by puromycin selection. As shown in Figure 2A and 2B, IDO1 knockdown obviously attenuated its expression in CRC cells. MTT assays showed that CRC cells expressing IDO1 shRNAs displayed a notable decrease in cell viability compared to those containing nonspecific shRNA (Figure 2C). Next, the clonogenic analysis was conducted to simulate the clinical scenario of long-term treatment. In accordance with aforementioned results, the stable knockdown of IDO1 elicited an obvious increase in the 5-FU sensitivity of CRC cells (Figure 2D and 2E). Consistent with the idea that induction of cell senescence is advantageous for circumventing drug resistance [3], we noticed that IDO1 knockdown significantly boosted the proportion of senescent CRC cells caused by 5-FU (Figure 2F and 2G). Furthermore, cells with stable knockdown of IDO1 had higher SA-β-gal activity than the control cell lines even in the absence of 5-FU treatment (Figure 2H). In addition, stable overexpression of IDO1 blunted CRC cells to chemotherapy, while knockdown of IDO1 enhanced the chemosensitivity of CRC cells (Figure 2I). Taken together, these data indicated that IDO1 silencing promoted cellular senescence and rendered CRC cells more sensitive towards 5-FU treatment.
Figure 2.
IDO1 silencing drives CRC cell senescence and blunts the sensitivity of CRC cells to 5-FU. (A, B) HCT-116 and HCT-8 cells were stably infected with two different IDO1 shRNAs or scramble shRNA (shCtrl), and knockdown efficiencies were determined by western blot (A) and qPCR analysis (B). (C) Cell viability of CRC control cells and IDO1-knockdown cells was analyzed by MTT assay. (D) Colony formation assays were performed in CRC control cells and IDO1-knockdown cells in the presence or absence of 20 μM 5-FU treatment. (E) The colony numbers were summarized from (D). (F) SA-β-gal staining of CRC control cells and IDO1-knockdown cells in the presence of 20 μM 5-FU treatment for 48 h. (G) The SA-β-gal positive cells from (F) were summarized. (H) SA-β-gal activity staining was performed in HCT-116 cells expressing shCONT or shIDO1 without 5-FU treatment, and the SA-β-gal positive cells were calculated. (I) HCT-116 cells with stable IDO1 knockdown or overexpression were exposed to various concentrations of 5-FU for 48 h and the cell viabilities were determined by MTT assay. *P < 0.05; **P < 0.01.
IDO1 causes senescence bypass and chemoresistance via the suppression of IGFBP5/p53 signaling
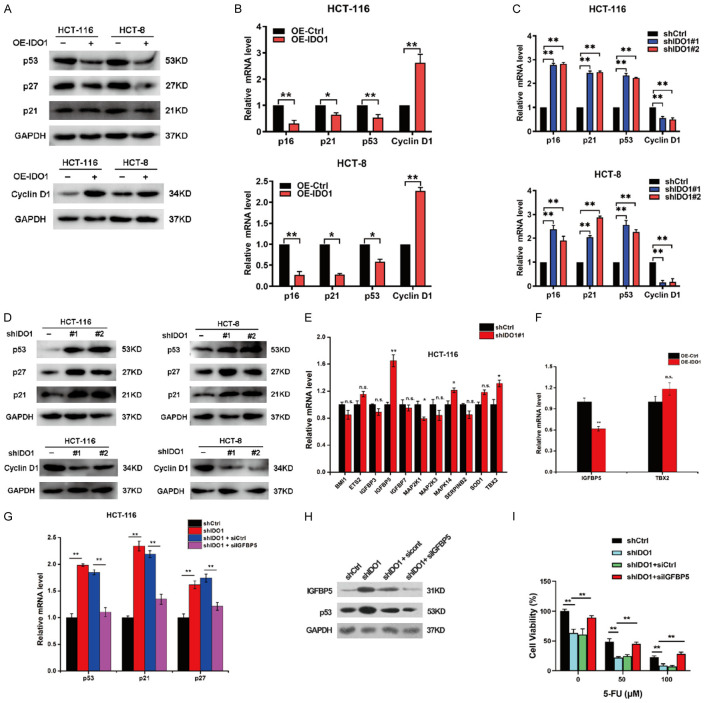
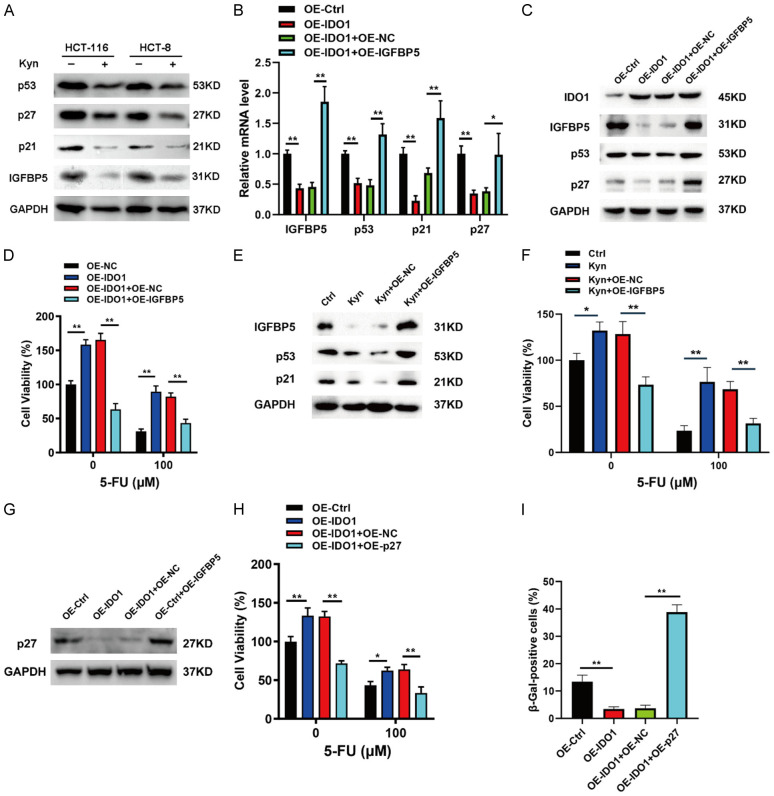
Numerous studies have demonstrated that persistent activation of tumor suppressor p53 and cyclin-dependent kinase inhibitors (CDKIs), including p16, p21, and p27, played a pivotal role in cellular senescence [5]. Therefore, these senescence-associated proteins were evaluated in IDO1-overexpressing CRC cells. The results of qPCR and western blot revealed that IDO1 overexpression caused a substantial decline of p16, p21and p53 and an increase of cyclin D1 (Figure 3A and 3B). In contrast, IDO1 silencing significantly upregulated the expressions of p16, p21, and p53, and attenuated cyclin D1 expression (Figure 3C and 3D). To address the possible molecular mechanism by which IDO1 restricted cellular senescence, we designed primers for senescence-associated genes based on Cellular Senescence RT2 Profiler PCR Array (Qiagen) [19]. It is worth noting that IDO1 depletion significantly upregulated the levels of IGFBP5, while overexpression of IDO1 significantly inhibits IGFBP5 expression (Figure 3E and 3F).
Figure 3.
IDO1 disables senescence and confers chemoresistance of CRC cells via attenuating IGFBP5/p53 signaling. A. The levels of p53, p27, p21, and cyclin D1 in CRC cells stably expressing IDO1 were determined by western blot. B. The levels of p53, p16, p21, and cyclin D1 were measured by qPCR analysis in HCT-116 and HCT-8 cells expressing IDO1. C. Relative mRNA expression of p53, p16, p21, and cyclin D1 was analyzed by qPCR analysis in the CRC cells with IDO1 knockdown. D. Western blot was performed to detect protein levels of p53, p27, p21, and cyclin D1 in the CRC cells with IDO1 knockdown. E. The expression of the indicated senescent-related genes was detected in HCT-116 cells with IDO1 silencing. F. Relative mRNA expression of IGFBP5 and TBX2 was analyzed by qPCR analysis in HCT-116 cells overexpressing IDO1. G. Relative mRNA expression of p53, p21, and p27 were analyzed by qPCR analysis in the IDO1-knockdown HCT-116 cells with or without IGFBP5 silencing. H. Western blot of IGFBP5 and p53 in IDO1-depleted HCT-116 cells transfected with or without siIGFBP5. I. Cell viability of IDO1-depleted HCT-116 cells with or without IGFBP5 silencing in the presence of indicated concentrations of 5-FU was measured by MTT assay. *P < 0.05; **P < 0.01.
Numerous studies have shown that IGFBP5 functions as a tumor suppressor to inhibit cell proliferation, invasion, metastasis, and chemoresistance [20,21]. IGFBP5 can promote the mRNA and protein levels of p53 to restrict gastric cancer growth and trigger the senescence of endothelial cells and fibroblasts [22,23]. Therefore, we speculated that the senescence escape and chemoresistance mediated by IDO1 may depend on the expression of IGFBP5. As expected, IDO1 knockdown-induced transcriptional upregulation of p53, p21, and p27 was obviously abrogated by IGFBP5 silencing (Figure 3G). Furthermore, IDO1 depletion-induced p53 protein upregulation was also compromised by knockdown of IGFBP5 (Figure 3H). Moreover, cell viability of IDO1-depleted CRC cells with IGFBP5 silencing in the presence or absence of 5-FU treatment was determined by MTT assay. IGFBP5 knockdown reversed IDO1 depletion-elicited chemosensitization (Figure 3I). Altogether, these findings indicated that IDO1 impaired the IGFBP5/p53 signaling pathway, thereby rendering senescence escape and chemoresistance of CRC cells.
Kynurenine is responsible for senescence bypass and chemoresistance caused by IDO1
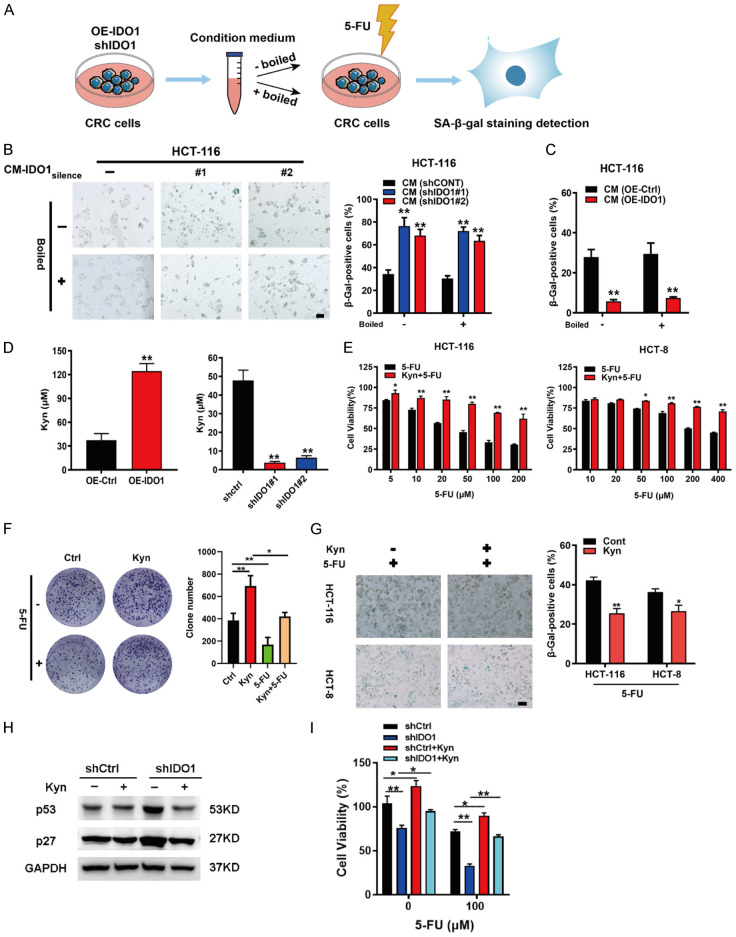
Accumulating evidence has suggested that senescence is a tumor-suppressive response that can be transmitted to the surrounding tumor cells to influence their senescence status [24,25]. Since IDO1 functions as a metabolic enzyme rather than a transcription factor, we assume that IDO1-expressing CRC cells may secrete soluble factors to modulate the cellular senescence. To this end, the conditioned medium from IDO1-overexpressing (CM-IDO1over) or depleted (CM-IDO1silence) CRC cells were collected to incubate the CRC cells in the presence of low dose of 5-FU (Figure 4A). We observed that, compared to the control medium, CM-IDO1silence enhanced 5-FU-induced HCT-116 cellular senescence (Figure 4B). By contrast, CM-IDO1over significantly blunted 5-FU-elicited HCT-116 cell senescence (Figure 4C). To clarify the effective components responsible for the aforesaid effect, the CM-IDO1over was boiled for 1 h to inactivate proteins in the CM. We found that the CM-IDO1over still could reduce 5-FU-triggered senescence even after boiling (Figure 4C). These results implied that it is likely the thermally stable metabolite that accounts for the function of IDO1. IDO1 is a tryptophan metabolizing enzyme, which converts tryptophan into Kyn [9]. It is tempting to speculate whether Kyn is a mediator for IDO1-mediated cell senescence and drug resistance. The ELISA detection indicated that the Kyn concentrations in the CM were obviously increased or decreased upon IDO1 overexpression or IDO1 silencing, respectively (Figure 4D). As shown in Figure 4E, Kyn treatment is sufficed to protect CRC cells from 5-FU toxicity. Furthermore, we validated the sufficiency of Kyn to blunt 5-FU efficacy towards CRC cells and attenuate 5-FU-triggered CRC cell senescence (Figure 4F and 4G). We further determined the effects of Kyn on cellular senescence and chemoresistance in CRC control cells and IDO1-knockdown cells. The results showed that IDO1 silencing mediated the upregulation of senescent-related proteins p53 and p27 and chemoresistance was reversed by the addition of Kyn (Figure 4H and 4I). Collectively, these observations demonstrated that Kyn secreted by IDO1-overexpressing CRC cells was responsible for IDO1-caused cellular senescence escape and chemoresistance.
Figure 4.
Kynurenine, the main catabolite, is indispensable for IDO1-mediated cellular senescence and chemoresistance. (A) Schematic diagram showing the process to study the effects of conditioned medium (CM) collected from IDO1-overexpressing CRC cells or IDO1-silencing CRC cells on cell senescence of CRC cells. (B, C) The CM derived from HCT-116 cells with IDO1 silencing (B) or IDO1 overexpression (C) was collected and boiled at 100°C for 1 h and removed precipitate by centrifugation. HCT-116 cells were supplemented with control CM or boiled CM for 5 days and analyzed by SA-β-gal staining. (D) ELISA was used to determine the contents of Kyn in the indicated CM. (E) MTT assay was performed to determine cell viability of HCT-116 and HCT-8 cells exposed to Kyn (100 μM) and various concentrations of 5-FU alone or in combination for 48 h. (F) HCT-116 cells were treated with Kyn (100 μM) in the presence or absence of 5-FU (20 μM) and analyzed by colony formation assay. (G) CRC cells were treated with or without Kyn (100 μM) in the presence of 5-FU (20 μM) and then analyzed by SA-β-gal staining. (H) Western blot was performed to detect protein levels of p53 and p27 in IDO1-silencing HCT-116 cells after exposure to 100 μM Kyn. (I) The IDO1-silencing HCT-116 cells were treated with Kyn (100 μM) in the presence of the indicated concentrations of 5-FU and the cell viability was measured by MTT assay. *P < 0.05; **P < 0.01.
IDO1 suppresses IGFBP5/p53 signaling to restrains cellular senescence and exacerbates chemoresistance via releasing kynurenine
To further investigate the molecular mechanisms by which Kyn, as a proto-oncogenic metabolite, regulates senescence and chemoresistance in CRC cells, western blot assay was performed to determine the effects of Kyn on the expression of p53, IGFBP5, p21, and p27. The results demonstrated that Kyn exposure reduced the expression of p53, p27, p21, and IGFBP5 proteins (Figure 5A). To investigate the role of IGFBP5 in IDO1-mediated CRC senescence and chemoresistance, we transiently transfected the IGFBP5-expressing plasmids into IDO1 overexpressing cells. The downregulation of IGFBP5, p53, p21, and p27 expression caused by IDO1 overexpression was significantly reversed by IGFBP5 overexpression (Figure 5B and 5C). Furthermore, IGFBP5 overexpression compromised IDO1 overexpression-mediated chemoresistance (Figure 5D). In addition, the effects of Kyn on the levels of IGFBP5, p53, p21, and chemoresistance in CRC cells were also abrogated by IGFBP5 overexpression (Figure 5E and 5F). To further clarify the role of p27 in IDO1-mediated suppression of CRC cell senescence and promotion of chemoresistance, we transfected the p27 overexpression plasmid into IDO1-overexpressing cells (Figure 5G). The results showed that p27 overexpression abrogated IDO1 overexpression-mediated chemoresistance and senescence bypass (Figure 5H and 5I). Taken together, these findings demonstrated that IDO1 inhibited cellular senescence and promoted chemoresistance via suppression of IGFBP5/p53 signaling.
Figure 5.
IDO1-secreted Kyn restrains cellular senescence and exacerbates chemoresistance via attenuating IGFBP5/p53 signaling. (A) Western blot was performed to detect protein levels of p53, p27, p21, and IGFBP5 in HCT-116 and HCT-8 cells after exposure to 100 μM Kyn. (B, C) Relative mRNA expression and protein levels of IGFBP5, p53, p21, and p27 were analyzed by qPCR analysis (B) and western blot (C) in the stable IDO1 overexpressed HCT-116 cells with or without IGFBP5 overexpression. (D) HCT-116 cells overexpressing IDO1 alone or together with IGFBP5 were treated with the indicated concentrations of 5-FU and the cell viability was measured by MTT assay. (E, F) HCT-116 cells were treated with Kyn (100 μM) with or without IGFBP5 overexpression and the indicated protein levels and cell viability were analyzed by western blot (E) and MTT assay (F). (G) Relative levels of p27 were analyzed by western blot in the stable IDO1 overexpressed HCT-116 cells with or without p27 overexpression. (H) HCT-116 cells overexpressing IDO1 alone or together with p27 were treated with the indicated concentrations of 5-FU and the cell viability was measured by MTT assay. (I) SA-β-gal staining of the stable IDO1 overexpressed HCT-116 cells with or without p27 overexpression in the presence of 20 μM 5-FU treatment for 48 h. The SA-β-gal positive cells were calculated. *P < 0.05; **P < 0.01.
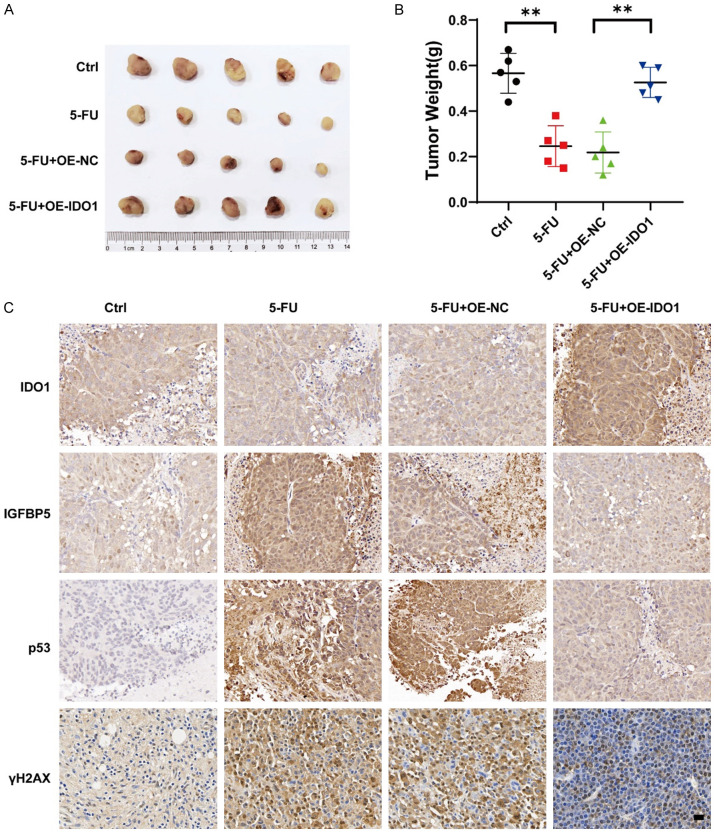
To investigate the roles of IDO1 in chemoresistance and cell senescence in vivo, xenograft mouse models were constructed. The IDO1 stably overexpressing HCT-116 cells were subcutaneously injected into the nude mice. One week after cell injection, mice with tumors were treated with either vehicle control or 5-FU via intraperitoneal injection. The results demonstrated that IDO1-overexpressing CRC cells exhibited significantly greater resistance to 5-FU compared with control cells (Figure 6A and 6B). The IHC results revealed that 5-FU treatment resulted in the upregulation of IGFBP5, p53, and γH2AX, and the effects were reversed by IDO1 overexpression (Figure 6C). These findings demonstrated that IDO1 overexpression notably facilitated chemoresistance.
Figure 6.
IDO1 facilitates CRC chemoresistance in vivo. A. HCT-116 cells with stable IDO1 overexpression were subcutaneously injected into the flanks of athymic nude mice (n = 5). The mice were intraperitoneally injected with 5-FU (25 mg/kg) or vehicle control every 3 days for 3 weeks to evaluate the tumor growth. At the end of the treatment, tumors were excised and photographed. B. The tumor weights were analyzed. C. The levels of IDO1, IGFBP5, p53, and γH2AX were detected in the tumors via IHC analysis. Representative images are shown. Scale bar: 20 μm. **P < 0.01.
Discussion
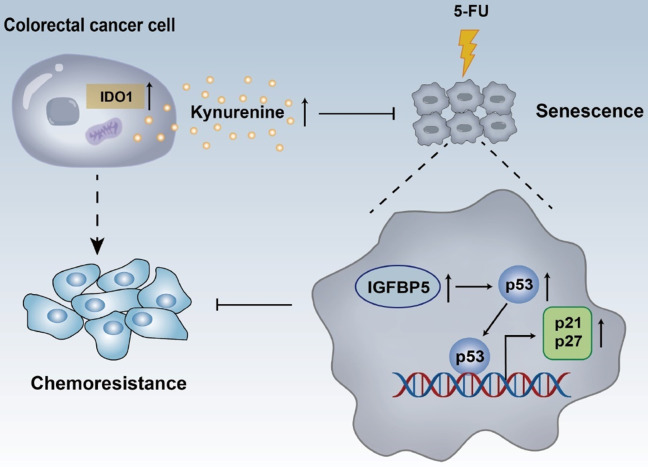
IDO1 represents a well-studied tryptophan catabolizing enzyme, which regulates the rate-limiting step of Kyn production. Numerous studies have focused on the function of IDO1 as a master regulator of immune tolerance [9,26,27]. Recently, newly identified functions of IDO1 beyond the immune regulation have attracted the attention of researchers. Mounting evidence has demonstrated that IDO1 is closely associated with tumor cell proliferation, viability, apoptosis, metastasis, and angiogenesis [8,10-12,28]. In the current study, we demonstrated that IDO1 is upregulated in CRC cells. High expression of IDO1 markedly countered 5-FU-triggered CRC cell senescence and facilitated drug resistance, irrelevantly of its canonical function of immune regulation. More importantly, IDO1-mediated Kyn production resulted in senescence escape and augmentation of chemoresistance via the inhibition of IGFBP5/p53 signaling (Figure 7). Together, these data implied that IDO1 or Kyn may present a therapeutical actionable target for CRC treatment.
Figure 7.
Schematic diagram for illustrating the roles of IDO1 on cellular senescence and chemoresistance.
Cell senescence refers to a process in which cells stop dividing permanently and resume survival. It can be evoked by various stimuli, including chemotherapy [5]. Cellular senescence has been recognized as a therapeutic arsenal for antagonizing cancer [3]. We found that the increased level of IDO1 has blocked the induction of cellular senescence caused by 5-FU, whereas IDO1 silencing accelerated 5-FU-provoked cellular senescence. Furthermore, the boiled CM from IDO1-overexpressing or IDO1-depleted CRC cells has decreased and increased the cellular senescence, respectively. As a catabolite of the IDO1 product, Kyn is highly expressed in the serum of CRC patients [10]. Studies have implicated that Kyn functions as an oncometabolite to drive tumor cell growth via activating the PI3K/Akt, β-catenin, and AHR signaling in a cell-autonomous manner [10,29,30]. Hitherto, there is no available evidence clarifying the relationship between IDO1 or Kyn and cancer cell senescence. Given the fact that intracellular concentrations of Kyn in CRC cells were higher than 70 μM [10], we found that 100 μM Kyn treatment mimicked the abrogation of cellular senescence and augmentation of chemoresistance caused by IDO1. Accumulating evidence has shown that IGFBP5 induces tumor cell apoptosis and cell cycle arrest, inhibits tumor development, and plays an important role in tumor suppression [22,31]. IGFBP5 is also a biomarker for predicting patient prognosis and chemotherapy response, and IGFBP5 silencing can promote chemoresistance in breast cancer patients [32]. It has been reported that IGFBP5 mainly exists in intracellular form and secreted proteins in cells. Various studies have shown that intracellular IGFBP5 directly promotes the mRNA and protein levels of p53 to retard tumor growth [22]. Studies have highlighted that IGFBP5 induces cellular senescence in endothelial cells and fibroblasts [23]. However, the role of IGFBP5 in CRC cell senescence and chemoresistance is still unclear. Here, we reported that IDO1 attenuated the IGFBP5/p53 signaling, thereby conferring senescence bypass and chemoresistance of CRC cells. Given that boiling CM from CRC cells with overexpression or deletion of IDO1 reduces or increases cell senescence, respectively, we speculate that IDO1 may regulate intracellular IGFBP5 to exert its effect. The mechanism by which Kyn regulates IGFBP5 transcription requires further investigations in our future work. In addition, considering that senescent tumor cells expose their Achilles’ heel [2,3], our results imply that the combination of IDO1 inhibitors and senolytics, an agent that selectively kills senescent cells, has great potential in CRC treatment.
High abundance and enzymatic activity of IDO1 were closely correlated with the unresponsiveness of breast cancer to paclitaxel treatment [33]. Previous studies have reported that the expression of IDO1 was higher in multidrug-resistant cells relative to chemosensitive cells, which reconfigured a stronger immunosuppressive state to confer chemoresistance [34]. It has been reported that IDO1 inhibition sensitized CRC cells to radiation [35]. In agreement with these findings, our data showed that IDO1 promotes chemoresistance of CRC cells via releasing Kyn and IDO1 depletion renders CRC cell senescence and more sensitivity towards 5-FU. The clinical trials of IDO1 inhibitors in treating various types of cancer have not been successful, which may be due to the neglect of the functions of Kyn metabolites themselves [36]. Our results highlight the role of Kyn as an oncometabolite in inhibiting CRC cell senescence and promoting CRC chemoresistance. How to utilize the weaknesses exposed by IDO1 depletion for novel and efficient combination therapy deserves further investigation.
Conclusions
In summary, our data revealed that IDO1 was upregulated in CRC cells, which caused cellular senescence escape and protected CRC cells from 5-FU toxicity via releasing its oncometabolite Kyn to suppress the IGFBP5/p53 signaling. These novel findings expand our understanding of IDO1 in cellular senescence and chemoresistance and imply that IDO1 could be exploited therapeutically for CRC treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82270217, 32200321, 31800657), graduate education innovation project of Shanxi Province (No. 2022Y114), Natural Science Foundation of Shanxi Province (No. 202203021211293, 20210302124252), and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2021L212).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Koga N, Suzuki H, Kato M. Promotion of cellular senescence by THG-1/TSC22D4 knockout through activation of JUNB. Biochem Biophys Res Commun. 2020;522:897–902. doi: 10.1016/j.bbrc.2019.11.145. [DOI] [PubMed] [Google Scholar]
- 3.Paez-Ribes M, González-Gualda E, Doherty GJ, Muñoz-Espín D. Targeting senescent cells in translational medicine. EMBO Mol Med. 2019;11:e10234. doi: 10.15252/emmm.201810234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou Z, Guo K, Sun X, Hu F, Chen Q, Luo X, Wang G, Hu J, Sun L. TRIB2 functions as novel oncogene in colorectal cancer by blocking cellular senescence through AP4/p21 signaling. Mol Cancer. 2018;17:172. doi: 10.1186/s12943-018-0922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Schmitt CA. The dynamic nature of senescence in cancer. Nat Cell Biol. 2019;21:94–101. doi: 10.1038/s41556-018-0249-2. [DOI] [PubMed] [Google Scholar]
- 6.Fu R, Yang P, Sajid A, Li Z. Avenanthramide a induces cellular senescence via miR-129-3p/Pirh2/p53 signaling pathway to suppress colon cancer growth. J Agric Food Chem. 2019;67:4808–4816. doi: 10.1021/acs.jafc.9b00833. [DOI] [PubMed] [Google Scholar]
- 7.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Zhou W, Zhang X, Ding Y, Du Q, Hu R. 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer by inducing CDC20 inhibition-mediated mitotic death of colon cancer cells. Int J Cancer. 2018;143:1516–1529. doi: 10.1002/ijc.31417. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, Li Y. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;11:100. doi: 10.1186/s13045-018-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, Ciorba MA. IDO1 and kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Cancer Res. 2019;79:1138–1150. doi: 10.1158/0008-5472.CAN-18-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Yuan K, Peng S, Huang Y, Zhang Y, Hu Y, Feng Y, Shi Y, Liu Y, Wang H, Zhou N, Min W. Gene silencing of indoleamine 2,3-dioxygenase hinders tumor growth through angiogenesis inhibition. Int J Oncol. 2017;50:2136–2144. doi: 10.3892/ijo.2017.3975. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Zhang Y, Zheng X, Zhang X, Wang H, Li Q, Yuan K, Zhou N, Yu Y, Song N, Fu J, Min W. Gene silencing of indoleamine 2,3-dioxygenase 2 in melanoma cells induces apoptosis through the suppression of NAD+ and inhibits in vivo tumor growth. Oncotarget. 2016;7:32329–32340. doi: 10.18632/oncotarget.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu R, Yang P, Li Z, Liu W, Amin S, Li Z. Avenanthramide A triggers potent ROS-mediated anti-tumor effects in colorectal cancer by directly targeting DDX3. Cell Death Dis. 2019;10:593. doi: 10.1038/s41419-019-1825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu R, Yang P, Amin S, Li Z. A novel miR-206/hnRNPA1/PKM2 axis reshapes the Warburg effect to suppress colon cancer growth. Biochem Biophys Res Commun. 2020;531:465–471. doi: 10.1016/j.bbrc.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Li Z, Fu R, Wu H, Li Z. Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signalling. Cell Signal. 2014;26:1853–1862. doi: 10.1016/j.cellsig.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Li Z, Li H, Lu Y, Wu H, Li Z. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell Signal. 2015;27:1525–1532. doi: 10.1016/j.cellsig.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Sun C, Li M, Zhang L, Sun F, Chen H, Xu Y, Lan Y, Zhang L, Lu S, Zhu J, Huang J, Wang J, Hu Y, Feng Y, Zhang Y. IDO1 plays a tumor-promoting role via MDM2-mediated suppression of the p53 pathway in diffuse large B-cell lymphoma. Cell Death Dis. 2022;13:572. doi: 10.1038/s41419-022-05021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang P, Li ZW, Zhang LC, Li HQ, Li ZY. Analysis of metabonomic profiling alterations in a mouse model of colitis-associated cancer and 2-deoxy-D-glucose treatment. RSC Adv. 2016;6:58862–58870. [Google Scholar]
- 19.Lazzarini R, Nicolai M, Pirani V, Mariotti C, Di Primio R. Effects of senescent secretory phenotype acquisition on human retinal pigment epithelial stem cells. Aging (Albany NY) 2018;10:3173–3184. doi: 10.18632/aging.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding M, Bruick RK, Yu Y. Secreted IGFBP5 mediates mTORC1-dependent feedback inhibition of IGF-1 signalling. Nat Cell Biol. 2016;18:319–327. doi: 10.1038/ncb3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remsing Rix LL, Sumi NJ, Hu Q, Desai B, Bryant AT, Li X, Welsh EA, Fang B, Kinose F, Kuenzi BM, Chen YA, Antonia SJ, Lovly CM, Koomen JM, Haura EB, Marusyk A, Rix U. IGF-binding proteins secreted by cancer-associated fibroblasts induce context-dependent drug sensitization of lung cancer cells. Sci Signal. 2022;15:eabj5879. doi: 10.1126/scisignal.abj5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Li W, Cao L, Xu J, Qian Y, Chen H, Zhang Y, Kang W, Gou H, Wong CC, Yu J. PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53. Oncogene. 2019;38:4590–4604. doi: 10.1038/s41388-019-0743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R. IGF binding protein-5 induces cell senescence. Front Endocrinol (Lausanne) 2018;9:53. doi: 10.3389/fendo.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teo YV, Rattanavirotkul N, Olova N, Salzano A, Quintanilla A, Tarrats N, Kiourtis C, Müller M, Green AR, Adams PD, Acosta JC, Bird TG, Kirschner K, Neretti N, Chandra T. Notch signaling mediates secondary senescence. Cell Rep. 2019;27:997–1007. e1005. doi: 10.1016/j.celrep.2019.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rattanavirotkul N, Kirschner K, Chandra T. Induction and transmission of oncogene-induced senescence. Cell Mol Life Sci. 2021;78:843–852. doi: 10.1007/s00018-020-03638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen E, Van den Eynde BJ. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res. 2017;5:695–709. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 27.Li A, Barsoumian HB, Schoenhals JE, Cushman TR, Caetano MS, Wang X, Valdecanas DR, Niknam S, Younes AI, Li G, Woodward WA, Cortez MA, Welsh JW. Indoleamine 2,3-dioxygenase 1 inhibition targets anti-PD1-resistant lung tumors by blocking myeloid-derived suppressor cells. Cancer Lett. 2018;431:54–63. doi: 10.1016/j.canlet.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thaker AI, Rao MS, Bishnupuri KS, Kerr TA, Foster L, Marinshaw JM, Newberry RD, Stenson WF, Ciorba MA. IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416–425. e1–4. doi: 10.1053/j.gastro.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkateswaran N, Conacci-Sorrell M. Kynurenine: an oncometabolite in colon cancer. Cell Stress. 2020;4:24–26. doi: 10.15698/cst2020.01.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maitituoheti M, Keung EZ, Tang M, Yan L, Alam H, Han G, Singh AK, Raman AT, Terranova C, Sarkar S, Orouji E, Amin SB, Sharma S, Williams M, Samant NS, Dhamdhere M, Zheng N, Shah T, Shah A, Axelrad JB, Anvar NE, Lin YH, Jiang S, Chang EQ, Ingram DR, Wang WL, Lazar A, Lee MG, Muller F, Wang L, Ying H, Rai K. Enhancer reprogramming confers dependence on glycolysis and IGF signaling in KMT2D mutant melanoma. Cell Rep. 2020;33:108293. doi: 10.1016/j.celrep.2020.108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn BY, Elwi AN, Lee B, Trinh DL, Klimowicz AC, Yau A, Chan JA, Magliocco A, Kim SW. Genetic screen identifies insulin-like growth factor binding protein 5 as a modulator of tamoxifen resistance in breast cancer. Cancer Res. 2010;70:3013–3019. doi: 10.1158/0008-5472.CAN-09-3108. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Wei L, Liu J, Li F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharmacol. 2020;85:77–93. doi: 10.1007/s00280-019-04009-8. [DOI] [PubMed] [Google Scholar]
- 34.Campia I, Buondonno I, Castella B, Rolando B, Kopecka J, Gazzano E, Ghigo D, Riganti C. An autocrine Cytokine/JAK/STAT-signaling induces kynurenine synthesis in multidrug resistant human cancer cells. PLoS One. 2015;10:e0126159. doi: 10.1371/journal.pone.0126159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ, Thotala D, Ciorba MA. Interferon-induced IDO1 mediates radiation resistance and is a therapeutic target in colorectal cancer. Cancer Immunol Res. 2020;8:451–464. doi: 10.1158/2326-6066.CIR-19-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiore A, Zeitler L, Russier M, Groß A, Hiller MK, Parker JL, Stier L, Köcher T, Newstead S, Murray PJ. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell. 2022;82:920–932. e7. doi: 10.1016/j.molcel.2022.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.