Abstract
HER2-positive breast cancer is highly aggressive, with a significant risk of recurrence and metastasis, leading to a poor prognosis. While most early-stage HER2-positive breast cancer patients benefit from combining trastuzumab monoclonal antibody with chemotherapy, the therapeutic response to various drug combinations varies across the HER2+ patient population. Therefore, predicting the prognosis and treatment response of HER2+ breast cancer patients to specific regimens is crucial for selecting appropriate precision individualized therapies. HER2DX is the first genomic tool designed to guide the treatment of HER2+ breast cancer patients. The three scores provided by HER2DX inform the entire treatment process, including predicting survival outcomes, recurrence, metastasis, and treatment responses like Pathological Complete Response Rate (pCR). It offers recommendations on follow-up intervals, treatment plans, and the duration of drug therapy. This review examines the literature and analyzes studies applying HER2DX to guide the comprehensive treatment and predict prognosis in HER2+ breast cancer patients, aiming to promote the widespread use of HER2DX in individualized treatment.
Keywords: Breast cancer, HER2, HER2DX, pCR, Outcomes
Introduction
The latest data from the International Agency for Research on Cancer (IARC)’s Global Cancer Observatory indicates that 2.3 million people were diagnosed with breast cancer in 2022, making it the second most common cancer worldwide. About 670,000 people died from breast cancer, ranking it fourth in cancer mortality rates [1]. Among women, breast cancer, lung cancer, and colorectal cancer are the most common cancer types, accounting for 51% of all new cancer cases in women, with breast cancer alone constituting 32% [2]. In the majority of countries (157 out of 185), breast cancer is the most common malignant tumor in women [3]. In China, there were approximately 2.29 million new cancer cases among women in 2022, with 357,161 new cases of breast cancer, making it the second most common cancer among Chinese women [1], underscoring the significance of breast cancer as a major health concern.
Breast cancer is categorized into four molecular subtypes based on gene expression profiles and biomarkers: Luminal A, Luminal B, HER2-positive (HER2+), and triple-negative breast cancer (TNBC), with HER2+ breast cancer accounting for 15%-20% of all cases [4]. The human epidermal growth factor receptor-2 (HER2/ERBB2) is a proto-oncogenes associated with inhibiting apoptosis and promoting cell proliferation, which enhances tumor invasiveness and promotes angiogenesis and lymphangiogenesis [5,6]. The HER2 protein, encoded by the ERBB2 gene, is a transmembrane protein with tyrosine kinase activity and is part of the EGFR family [5,7]. This family includes HER1, HER2, HER3, and HER4, all characterized by an extracellular domain, an α-helical transmembrane region, and an intracellular tyrosine kinase domain [8-10]. The HER2 receptor does not directly bind to any known ligand; its extracellular domain remains in an “open” conformation [11]. It functions in signal transduction either by forming homodimers or heterodimers with other HER family members upon ligand binding to their extracellular domains, predominantly through heterodimerization [12,13]. Upon dimerization, HER2 undergoes a conformational change, activating its intracellular tyrosine kinase activity, which initiates downstream signaling pathways, promoting cell proliferation through the RAS-ERK pathway and inhibiting cell death via the PI3K-Akt-mTOR pathway [14-17].
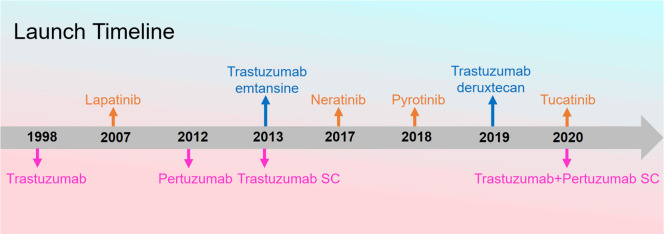
HER2+ breast cancer is highly aggressive, with patients often experiencing early recurrence and metastasis, leading to poor prognosis [18-20]. Initially, systemic chemotherapy was the primary treatment for HER2+ breast cancer patients, but it demonstrated unsatisfactory clinical efficacy [21]. The advent of anti-HER2 targeted therapy drugs, characterized by their specificity, effectiveness, and fewer adverse reactions, has significantly improved the prognosis for patients with HER2+ breast cancer and has become the main treatment approach [22,23]. Currently, anti-HER2 therapy drugs are mainly categorized into three groups: monoclonal antibodies (such as trastuzumab, pertuzumab, margetuximab, and ado-trastuzumab emtansine), small molecule tyrosine kinase inhibitors (TKIs) (such as pyrotinib, lapatinib, neratinib, and tucatinib), and antibody-drug conjugates (ADCs) (such as T-DM1, T-DXd, and RC-48). With the growing clinical demand for diverse anti-HER2 targeted drugs, the development and clinical application of these therapies continue to advance (Figure 1).
Figure 1.
Global launch timeline of anti-HER2 targeted drugs.
Large molecule monoclonal antibodies primarily target the extracellular domain of HER2, aiming to block the HER2-mediated signaling pathways. For instance, trastuzumab, a monoclonal antibody, specifically binds to the IV domain of the HER2 extracellular region, accelerating HER2 internalization and degradation, thereby inhibiting downstream signaling and exerting its antitumor activity. It also mediates antibody-dependent cellular cytotoxicity, killing cells that express HER2-related proteins [24-26]. Pertuzumab monoclonal antibody binds to the II domain of the HER2 extracellular region, preventing the formation of heterodimers between HER2 and HER1, HER3, HER4 [27,28]. Clinical studies such as CLEOPATRA, PUFFIN, NeoSphere, and PEONY have confirmed the significant efficacy of combining trastuzumab and pertuzumab in HER2+ breast cancer patients [29-32]. Small molecule TKIs diffuse through the cell membrane and competitively inhibit the binding of ATP to the ATP-binding site of the intracellular kinase domain of the HER family proteins, thereby blocking tyrosine phosphorylation and the activation of downstream signaling cascades, which suppresses the growth and proliferation of cancer cells [25,33-36]. Numerous clinical studies have demonstrated the significant therapeutic effects of TKIs in patients with HER2+ breast cancer [37-40]. ADCs consist of an antibody that selectively recognizes the HER2 receptor on the surface of cancer cells, a cytotoxic drug payload capable of killing cancer cells, and a linker connecting the antibody to the payload. Some ADCs also possess cytotoxic effects through their antibody component. These ADCs bind to the HER2 receptors on the tumor cell surface, mediate endocytosis, and are subsequently degraded within the lysosomes of tumor cells, releasing the active cytotoxic drug that damages DNA or inhibits tumor cell division, leading to tumor cell death [41-43]. Furthermore, certain ADCs like T-DXd and RC-48 exhibit a bystander effect, where cytotoxic drugs released from dying tumor cells exert lethal effects on surrounding non-HER2+ tumor cells [44-47]. Clinical studies such as EMILIA, KATHERINE, DESTINY-Breast01, 02, and 03 have confirmed that ADCs like T-DM1 and T-DXd significantly improve the prognosis of HER2+ breast cancer patients who did not achieve expected results from previous treatment with monoclonal antibodies or TKIs [48-52].
As anti-HER2 targeted therapies have advanced, the prognosis of patients with HER2+ breast cancer has improved. However, clinically, some patients exhibit low response or even resistance to targeted therapies. Therefore, selecting the most suitable targeted therapy regimen for each patient, and deciding between or combining different targeted drugs, is crucial. Breast cancer is a highly heterogeneous disease; each molecular subtype has its unique molecular characteristics and signaling pathways [53]. Previously, decisions to escalate or de-escalate breast cancer systemic therapy were primarily based on traditional parameters such as tumor size, regional lymph node status, hormone receptor (HR) status, tumor-infiltrating lymphocytes (TILs), Ki-67 proliferation index, and pathological type. Among these, TILs can predict chemotherapy efficacy and prognosis for most breast cancers, including TNBC and HER2+ breast cancer subtypes [54,55]. To more accurately predict the efficacy and prognosis of breast cancer treatment regimens, various prognostic auxiliary models that combine pathology, molecular biology, and biomarkers are currently used, such as Oncotype DX, ProsignaTM, Mammaprint, and EndoPredict (Table 1). These models assist physicians in making adjuvant treatment decisions and predicting prognoses for early-stage HR+/HER2- breast cancer patients who are premenopausal or postmenopausal, with either negative regional lymph nodes or 1-3 positive regional lymph nodes [56-58]. However, the treatment plans for HER2+ breast cancer patients still primarily rely on traditional parameters and the clinical experience of physicians, lacking genomic tools for aiding decision-making in HER2+ breast cancer. To achieve more precise personalized treatment for HER2+ breast cancer patients, Reveal Genomics has developed the first genomic tool targeted for these patients-HER2DX [59,60].
Table 1.
Gene testing tools used for HR+ breast cancer
| Gene Testing Tool | Number of Genes | Clinical Application | Involved Clinical Trials | References |
|---|---|---|---|---|
| Oncotype DX | 21 | Predicts 10-year recurrence risk in ER+ and LN- patients | In the NSABP B-14 trial, patients receiving endocrine therapy showed 10-year distant recurrence rates of 6.8%, 14.3%, and 30.5% in the low, intermediate, and high-risk groups, respectively (P < 0.001). | [61] |
| Prosigna | 55 | Predicts prognosis in postmenopausal women with Stage I or II ER+ and LN+/- breast cancer | In the ABCSG-8 trial, the ROR score significantly enhanced prognostic information beyond clinical predictors (P < 0.0001). Luminal A subtype showed a significantly lower 10-year ROR compared to Luminal B (P < 0.0001). | [62] |
| MammaPrint | 70 | Predicts distant recurrence risk in Stage I or II ER+/- and LN- patients | MammaPrint demonstrated the strongest prognostic ability for distant metastasis-free survival (dMFS) within the first 5 years after cancer diagnosis (HR=9.6; 95% CI: 4.2-22.1). | [63] |
| EndoPredict | 11 | Predicts 10-year recurrence risk in women with ER+ and LN+/- disease receiving endocrine therapy only | In the ABCSG-6 cohort, the 10-year distant recurrence rates were 8% (3%-13%) in the EP low-risk group and 22% (15%-29%) in the high-risk group (P < 0.001); in ABCSG-8, these rates were 6% (2%-9%) and 15% (11%-20%) in the low- and high-risk groups, respectively (P < 0.001). | [64] |
HER2DX is a more comprehensive genomic tool compared to earlier ones, which have limitations when used alone in clinical settings. Sestak et al. compared six clinical features (CTS, IHC 4, Oncotype DX, Prosigna, BCI, and EPclin) in the TransATAC cohort to assess their prognostic abilities for distant recurrence over 0-10 years. CTS and EPclin provided the most accurate prognostic information in the 0-10 and 5-10 year periods. In node-negative patients, ROR of Prosigna had the highest prognostic value, while RS of Oncotype DX had the lowest. In node-positive patients, CTS and EPclin were the most prognostic, while the other four features had less predictive value [65,66]. These findings suggest that genetic testing alone cannot provide comprehensive prognostic information. Effective clinical application of genetic testing requires integration with clinical features. Tools like Oncotype DX, Prosigna, MammaPrint, and EndoPredict were among the first to evaluate prognosis in HR+ breast cancer patients, but multiple studies [65,67-69] indicate that using these tools alone may introduce bias. Combining them with clinical features is essential for accurate prognosis and appropriate treatment selection.
Unlike these tools, HER2DX is the first designed specifically for HER2+ breast cancer. It combines genetic analysis with clinical features like tumor size and lymph node status. HER2DX not only predicts clinical prognosis but also treatment response to various therapies, making it well-suited for precise personalized treatment in clinical practice. HER2DX integrates genomic data with clinical features to generate three scores: the HER2DX Risk Score, the HER2DX pCR Score, and the HER2DX ERBB2 Expression Score. These scores help predict survival outcomes and the risks of recurrence and metastasis in HER2+ breast cancer patients, guiding treatment strategies. This approach reduces the risks of disease progression due to under-treatment and mitigates economic burdens and adverse drug reactions associated with over-treatment [70]. Furthermore, Marín-Aguilera et al. demonstrated in the lab that HER2DX shows high reproducibility and stability in quantifying the risk of early HER2-positive breast cancer recurrence, the likelihood of pCR, and ERBB2 mRNA expression using Formalin-Fixed, Paraffin-Embedded (FFPE) tumor tissues and purified RNA analysis [71]. The introduction of HER2DX marks a significant advancement towards precision medicine in treating HER2+ breast cancer.
The development and refinement of HER2DX
The emergence of HER2DX (2020)
In 2020, Prat et al. developed HER2DX (2020), a tool designed to assess whether escalating or de-escalating systemic treatment regimens for early-stage HER2+ breast cancer patients could improve outcomes [59]. These strategies included reducing chemotherapy dosage, shortening the duration of targeted therapy, replacing traditional single-target treatments with dual-target therapies, and substituting monoclonal antibodies with ADC drugs in patients who did not achieve a pCR after neoadjuvant therapy with monoclonal antibodies. The study revealed that most early-stage HER2+ breast cancer patients could achieve desired outcomes with chemotherapy combined with trastuzumab alone, avoiding overtreatment. Further analysis highlighted that traditional parameters, such as tumor size, lymph node status, HR status, and stromal TILs, along with biomarkers like PAM50 subtypes and PIK3CA mutations, were associated with prognosis. Using data from the Short-HER3 clinical trial [72], Pret integrated these factors to form the HER2DX (2020) prognostic model. This model includes tumor size, lymph node status, TILs, subtypes, and 13 genomic markers, and was found to correlate with distant metastasis-free survival (DMFS) in HER2+ patients (P < 0.0001). HER2DX (2020) stratified patients into low, medium, and high-risk groups, with distinct 5-year DMFS rates: 98.1%, 88.9%, and 73.9%, respectively, showing significant differences between low and high-risk groups (HR = 0.04, 95% CI: 0.0-0.1, P < 0.0001) [59].
The refinement of HER2DX (2022)
As HER2DX (2020) was applied and researched further, Pret et al. identified several limitations: (1) The subjective nature of TILs evaluation, causing inconsistencies across pathologists; (2) HER2DX (2020) assessed only 55 genes, insufficient for a comprehensive genetic tool; (3) With neoadjuvant therapy becoming more common, HER2DX (2020) could not evaluate the pCR rate post-neoadjuvant therapy, limiting its use in guiding comprehensive treatment for early-stage HER2+ patients. Therefore, based on research from various study cohorts and databases, Pret refined HER2DX, resulting in HER2DX (2022) [60].
HER2DX (2022) introduced several improvements compared to its predecessor (Table 2). (1) Genetic Markers Expansion: The number of genes analyzed expanded from 55 to 185, enhancing the comprehensiveness of genomic analysis. (2) Adjustment in Scoring for TILs: To address the subjectivity of TILs evaluation [73], HER2DX (2022) adjusted its approach, possibly incorporating quantitative gene expression related to immune infiltration [54,70,74]. (3) Optimization of Cut-off Values: HER2DX (2022) optimized cut-off values for risk and pCR scores, offering refined predictions tailored to different patient groups. (4) Increased Focus on Treatment Response Prediction: HER2DX (2022) introduced or refined scores to better predict responses to neoadjuvant and adjuvant therapies. (5) Enhanced Predictive Capabilities for ERBB2 Expression: HER2DX (2022) improved its prediction of ERBB2 expression, aiding in selecting the most suitable patients for anti-HER2 therapies. (6) Application to a Broader Range of Clinical Scenarios: HER2DX (2022) extended its applicability to include patients with advanced disease. (7) Validation Across Diverse Populations: HER2DX (2022) was validated across a more diverse population, enhancing its global utility.
Table 2.
Differences between HER2DX (2022) and HER2DX (2020) content
| HER2DX (2020) | HER2DX (2022) | |||
|---|---|---|---|---|
| Clinical feature | Tumour size, Nodal status, Number of tumour-infiltrating lymphocytes (TILS), PAM50 subtype | Tumour size, Nodal status | ||
| Genomic feature | Genes associated with better survival outcome (6 genes): | Genes related to luminal differentiation (BAG1) | Immunoglobulin (IGG) module (14 genes): | CD27, CD79A, HLA-C, IGJ, IGKC, IGL, IGLV3-25, IL2RG, CXCL8, LAX1, NTN3, PIM2, POU2AF1 and TNFRSF17 |
| Genes related to the normal cell phenotype (KRT5, KRT14, MLPH, MYC) | The tumour cell proliferation signature (4 genes): | EXO1, ASPM, NEK2 and KIF23 | ||
| Basal-like-related genes (PHGDH) | The luminal differentiation signature (5 genes): | BCL2, DNAJC12, AGR3, AFF3 and ESR1 | ||
| Genes associated with poor survival outcomes (7 genes): | Genes related to proliferation (CDC6, EXO1, RRM2) | The HER2 amplicon signature (4 genes): | ERBB2, GRB7, STARD3 and TCAP | |
| Genes related to HER2 amplicion (TMEM45B, FGFR4) | ||||
| Basal-like-related biology (CDH3) | ||||
| Genes related to cell invasion (MMP11) | ||||
| Application | Predicting survival outcomes | Predicting survival outcomes, the likelihood of pathological remission from treatment, and assessing ERBB2 expression | ||
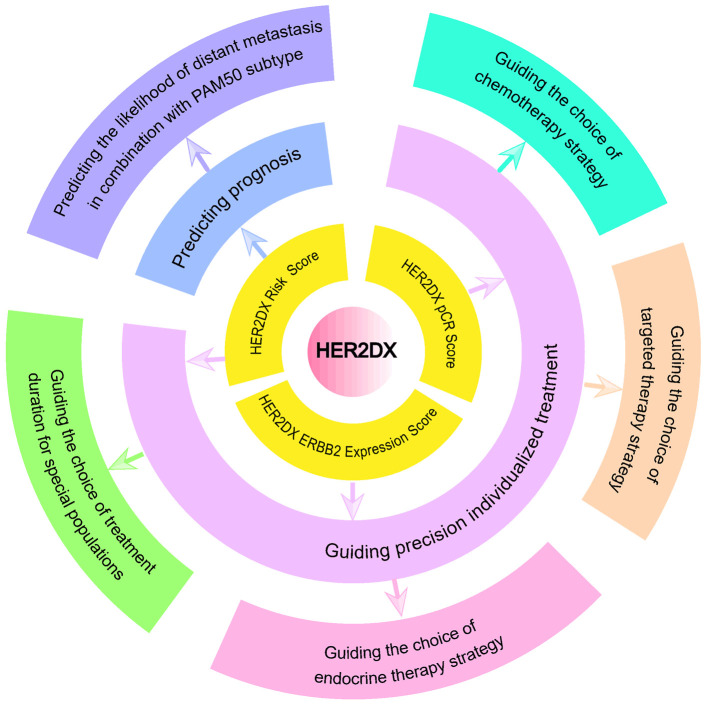
HER2DX (2022) is developed based on two clinical-pathological features and 27 genes, divided into four genetic marker groups, aiming to predict survival outcomes, distant relapse risk, and treatment response in HER2+ breast cancer patients using three scoring systems: the HER2DX Risk Score, the HER2DX pCR Score, and the HER2DX ERBB2 Expression Score, thus aiding treatment decisions (Figure 2). The two clinical features include tumor size and lymph node staging. The four genetic groups in HER2DX include 14 immune infiltration-related genes (CD27, CD79A, HLA-C, IGJ, IGKC, IGL, IGLV3-25, IL2RG, CXCL8, LAX1, NTN3, PIM2, POU2AF1, and TNFRSF17), 4 tumor cell proliferation-related genes (EXO1, ASPM, NEK2, and KIF23), 5 luminal differentiation-related genes (BCL2, DNAJC12, AGR3, AFF3, and ESR1), and 4 HER2 amplicon-related genes (ERBB2, GRB7, STARD3, and TCAP). Pret et al. [60] developed the three scoring systems by integrating data from retrospective studies, prospective studies, and international database data. HER2DX pCR Likelihood Score: developed within the H.Clinic HER2+ cohort, this score is based on HER2, IGG, luminal and proliferation signatures, tumor stage, and nodal stage. It was validated and explored in multiple cohorts (PAMELA/H.Clinic/Padova HER2+, CALGB-40601, and ISPY-2 cohorts), showing a significant correlation with pCR as both a continuous and categorical variable. Group cutoff values were established as low-pCR groups (0, 33.3), medium-pCR groups (33.3-66.7), and high-pCR groups (66.7-100). HER2DX Risk Score: Developed in the Short-HER HER2+ cohort, this score is primarily based on IGG, luminal and proliferation signatures, tumor stage, and nodal stage. It was validated across various cohorts (H.Clinic/Padova/PAMELA HER2+ cohorts and TCGA, METABRIC, SCAN-B, CALGB-40601 databases), showing a significant correlation with disease-free survival (DFS), overall survival (OS), and other prognostic factors. Group cutoff values were established as low-risk groups (0, 50) and high-risk groups (50, 100). HER2DX ERBB2 Expression Score: Developed in the Short-HER and H.Clinic cohorts and validated in multiple HER2+ cohorts (H.Clinic/Padova/PAMELA HER2+ and SOLTI HER2- cohorts), this score is correlated with the clinical HER2 status in HER2+ breast cancer patients. An optimal cutoff value of -0.98 was determined using Youden’s analysis to predict HER2 receptor status.
Figure 2.
The application of HER2DX in clinical practice.
These scoring systems reveal the long-term risk of recurrence and metastasis, the probability of pCR, and the tumor HER2 expression level, enabling more accurate treatment decisions and survival predictions. HER2DX is currently being applied and evaluated in multiple clinical studies (Table 3).
Table 3.
Summary of studies related to HER2DX
| Clinicaltrials Number | Study | Molecular typing | Number of analyzed samples | References |
|---|---|---|---|---|
| NCT02411344 | PerELISA | HR+ HER2+ | 55 | [75] |
| NCT01853748 | ATEMPT | HER2+ | 187 | 2022 SABCS Abstract PD18-01 |
| NCT00542451 | APT | HER2+ | 284 | [76] |
| NCT03716180 | DAPHNe | HER2+ | 80 | [77] |
| NCT00770809 | CALGB-40601 | HER2+ | 263 | [78,79] |
| NCT01973660 | PAMELA | HER2+ | 91 | [79,80] |
| NCT01042379 | ISPY-2 | HER2+ | 127 | [79,81] |
| NCT05912062 | BiOnHER | HER2+ | 46 | [79] |
| NEOHER | HER2+ | 67 | [79] | |
| GOM | HER2+ | 155 | [79,82] |
The prognostic value of HER2DX
The HER2DX Risk Score, whether analyzed as a continuous or categorical variable, is statistically associated with survival outcomes and distant relapse in HER2+ breast cancer patients. This score predicts survival and the likelihood of relapse and distant metastasis across different patient populations following systemic therapy [59,60]. Villacampa et al. evaluated survival in patients treated with trastuzumab, followed up long-term within the combined NEOHER and PAMELA cohorts [79]. They categorized patients using HER2DX Risk Score cutoff values. Among those achieving pCR, the 6-year event-free survival (EFS) was 98.1% in the low-risk group compared to 89.4% in the high-risk group. For patients not achieving pCR, the 6-year EFS was 93.5% in the low-risk group versus 78.8% in the high-risk group. Overall, the HER2DX Risk Score, as a categorical variable, correlated with EFS (P < 0.001) and OS (P = 0.006), helping to identify patients at low recurrence risk. Similarly, in the SCAN-B dataset [83], Villacampa et al. found a statistically significant correlation between HER2DX Risk Score, as a continuous variable, and OS (HR = 1.31 per 10-unit increment, 95% CI: 1.13-1.51, P < 0.001). As a categorical variable, 7-year OS was 94.5% in the low-risk group and 78.6% in the high-risk group (HR = 3.87, 95% CI: 2.26-6.65, P < 0.001). In the APT phase II clinical trial [76], Tolaney et al. used HER2DX to assess the association between HER2DX Risk Score and invasive disease-free survival (iDFS) and relapse-free interval (RFI). They found that HER2DX Risk Score, as a continuous variable, significantly correlated with iDFS (P = 0.047) and RFI (P = 0.011). Grouping patients by a predefined HER2DX Risk Score cutoff of 50 revealed a significant increase in recurrence risk in the high-risk group. However, using the Contal and O’Quigley method [84], the optimal cutoff to differentiate low and high-risk patients in the APT trial was determined to be a HER2DX Risk Score of 32. Patients with a score below 32 had a 1.4% probability of recurrence after 10 years, compared to 13.3% for those with a score of 32 or higher. The HER2DX genomic tool accurately identifies patients at increased recurrence risk. For these higher-risk patients, it is advisable to shorten follow-up intervals, monitor them closely, and conduct regular exams to prevent and detect recurrence early for timely intervention.
Although advances in anti-HER2 therapy have significantly improved the prognosis of patients with HER2+ breast cancer, more than 10% of these patients may still develop distant metastases during follow-up [49,85]. Further research has shown that the HER2DX Risk Score, in combination with the PAM50 subtype, can predict the likelihood of distant metastasis. Dieci et al. demonstrated that the HER2DX Risk Score is associated with the risk of any distant metastasis: the 10-year cumulative incidence of relapse and metastasis was 19.7% for patients with high risk scores and 5.3% for those with low risk scores (P < 0.001) [86]. The combination of the HER2DX Risk Score and the PAM50 subtype can predict the likelihood of specific metastatic sites. Tumors of the HER2-enriched intrinsic molecular subtype are more likely to metastasize to the brain, basal-like tumors are associated with an increased risk of lung metastasis, and luminal tumors are more prone to bone metastasis. When combining the HER2DX Risk Score with PAM50 subtypes, Luminal A subtype showed a lower incidence of any distant metastasis in both low and high HER2DX risk groups, although this difference was not statistically significant. In terms of brain metastasis, the cumulative incidence was very low in the low HER2DX risk group, regardless of the intrinsic subtype. In contrast, in the high HER2DX risk score group, patients with HER2-enriched subtype had a significantly higher incidence of brain metastasis compared to other subtypes. For lung metastasis, the incidence was significantly higher in basal-like subtypes than in other subtypes, both in low and high HER2DX risk groups [86]. These results indicate that patients with a low HER2DX Risk Score and Luminal A tumors have a very low probability of developing metastasis within 10 years, suggesting that these patients may not require intensified follow-up. For other patient groups classified by combining HER2DX and PAM50 subtypes, intensified follow-up targeted at specific potential metastatic sites and corresponding intensified treatment measures may be necessary.
HER2DX-guided precision individualized comprehensive treatment
HER2DX pCR score predicts the likelihood of pCR in anti-HER2 neoadjuvant therapy
The HER2DX pCR Score, both as a continuous and categorical variable, is statistically correlated with the pCR rate in HER2+ breast cancer patients [60]. Research by Villacampa et al. demonstrated that across different patient subgroups from clinical trials such as ISPY-2, CALGB-40601, DAPHNe, GOM, BiOnHER, NEOHER, and PAMELA, the HER2DX pCR Score was significantly associated with pCR. Specifically, the pCR rates for low, medium, and high HER2DX pCR groups were 20.2%, 55.3%, and 74%, respectively, indicating that patients with higher HER2DX pCR Scores are more likely to achieve pathological complete remission, regardless of the treatment modality used [79]. Therefore, grouping HER2+ breast cancer patients based on the HER2DX pCR Score can guide the selection of different treatment plans, enhancing the personalization and effectiveness of therapy.
Guiding the personalized selection of ADCs
Research by Brasó-Maristany et al. indicated that in second-line treatment of advanced HER2+ breast cancer, the HER2DX ERBB2 expression score correlates with response to T-DM1 therapy. The overall response rates to T-DM1 in the low, medium, and high HER2DX ERBB2 expression groups were 0%, 29%, and 56%, respectively (P < 0.001) [87]. Similarly, Villacampa et al. found in the SCAN-B dataset that patients with low HER2DX ERBB2 scores showed no significant benefit from T-DM1 (HR = 1.00, 95% CI: 0.21-4.77). However, significant benefits were observed in patients with medium (HR = 0.10, 95% CI: 0.01-0.92) and high (HR = 0.15, 95% CI: 0.10-0.23) HER2DX ERBB2 scores [83]. Patients in the medium/high HER2DX ERBB2 score groups may be ideal candidates for T-DM1, which, compared to T-DXd, offers higher efficacy, lower cost, and reduced toxicity. Considering efficacy, economic costs, and safety, T-DM1 is a strong treatment option for patients with high HER2DX ERBB2 scores.
Personalized choice of anti-HER2 therapy combined with chemotherapy
Chemotherapy drugs, such as anthracyclines, have significant side effects, potentially causing cardiotoxicity, neutropenia, diarrhea, and other adverse events [88-90]. Although the combination of trastuzumab monoclonal antibody with chemotherapy remains the first-line choice for many patients with HER2+ breast cancer [23], for some patients, the use of various combination treatments or chemotherapy drugs may constitute overtreatment. The HER2DX pCR Score plays an essential role in this regard, as it can effectively select those patient groups who can still achieve the expected treatment outcomes with reduced or even no chemotherapy drug use. This precise treatment selection helps to reduce adverse events and improve the quality of life for patients. Through the HER2DX pCR Score, physicians can more accurately assess patients’ responses to chemotherapy, thereby developing more suitable and personalized treatment plans for patients with HER2+ breast cancer. This approach not only improves treatment efficacy but also reduces the physical burden on patients, enhancing their overall treatment experience.
Villacampa et al. reported that, within cohorts receiving trastuzumab monoclonal antibody combined with multi-drug chemotherapy, single-agent taxane, or no chemotherapy, grouping by the HER2DX pCR Score showed differences in pCR rates between multi-drug chemotherapy and single-agent taxane as -4.5%, 25.5%, and -3.2% across different pCR score groups. The increase in pCR rate due to multi-drug chemotherapy was statistically significant only in tumors of the HER2DX medium pCR group (OR = 3.11, 95% CI: 1.54-6.49, P = 0.002) [79]. This suggests that for patients in the HER2DX medium pCR group, multi-drug chemotherapy could lead to a higher pCR rate, while for patients in the high and low pCR groups, the effect of multi-drug chemotherapy was not significantly superior to single-agent taxane. This finding is significant for the treatment strategy of patients with HER2+ breast cancer. The HER2DX pCR Score can help identify patients who may benefit more from a dual anti-HER2 combination with single-agent taxane, especially those with a medium HER2DX pCR score. For patients with high or low HER2DX pCR scores, using multi-drug chemotherapy does not significantly improve the pCR rate and may increase unnecessary toxic side effects. Furthermore, the PerELISA trial results showed that without using chemotherapy, the HER2DX ERBB2 Expression Score was significantly associated with pCR (P = 0.003), and this relationship was independent of the clinical HER2 immunohistochemistry level (2+ vs 3+) [75]. This suggests that patients with a high HER2DX ERBB2 Expression Score have a higher likelihood of achieving pCR after anti-HER2 therapy and can opt for dual-targeted therapy to avoid chemotherapy.
Personalized choice between dual anti-HER2 therapy and single Anti-HER2 therapy
Previous studies have shown that single anti-HER2 therapy with trastuzumab monoclonal antibody combined with chemotherapy achieved a pCR rate of 29-46% [78,91,92]. The addition of a second anti-HER2 drug, such as pertuzumab monoclonal antibody or lapatinib, increased the pCR rate by 10-20% [85,93], with a slight improvement in long-term survival rates. However, this raises a critical question: can all HER2+ breast cancer patients benefit from dual anti-HER2 therapy? This is where the HER2DX pCR Score demonstrates its value. This score effectively identifies patient groups most likely to benefit from dual anti-HER2 therapy.
Villacampa et al. categorized patients into high, medium, and low pCR groups based on the HER2DX pCR Score. The differences in pCR rates between patients receiving trastuzumab monoclonal antibody combined with chemotherapy and those receiving dual anti-HER2 therapy combined with chemotherapy were 17.6%, 5.4%, and 4.6% across these groups, respectively, with a statistically significant difference only in the high HER2DX pCR group (OR = 2.36, 95% CI: 1.09-5.42, P = 0.03) [79]. The HER2DX pCR Score can identify those in the high HER2DX pCR group who would benefit from dual anti-HER2 therapy. The study’s results suggest that the HER2DX pCR Score can help physicians determine which HER2+ breast cancer patients might benefit from dual anti-HER2 therapy. For patients in the high pCR group, dual therapy might be more suitable, while for those in the medium and low pCR groups, considering the economic cost and potential toxic side effects of dual therapy, single anti-HER2 therapy might be a more reasonable choice.
Personalized endocrine therapy choices for HER2+/HR+ patient
The HER2+/HR+ subtype of breast cancer constitutes about 70% of HER2+ cases [94]. This subtype is generally more aggressive and associated with a poorer prognosis compared to HR-negative (HR-) breast cancer [95]. The complexity of treating HER2+/HR+ breast cancer lies in the potential insensitivity to anti-HER2 therapy, possibly due to interactions between the estrogen receptor (ER) pathway and the HER2 pathway [96]. In treating HER2+/HR+ breast cancer, it is often necessary to consider both anti-HER2 therapy and endocrine therapy. However, resistance to endocrine therapy can be a significant challenge. The HER2DX pCR Score is crucial here, as it can help predict which HER2+/HR+ patients may respond well to endocrine therapy, guiding treatment decisions effectively.
In the perELISA phase III clinical trial [75], pre-treatment biopsies were conducted on HER2+/HR+ patients to assess the Ki-67 index, followed by another biopsy after 2 weeks of letrozole treatment. This was to evaluate changes in Ki-67 and distinguish between estrogen-sensitive disease (ESD) and estrogen-resistant disease (ERD). The HER2DX pCR Score showed a significant correlation with the Ki-67 response after letrozole treatment (P = 0.002). The response rates (a reduction of more than 20% in baseline Ki-67 levels) were 89.7%, 65.0%, and 16.7% in the low, medium, and high HER2DX pCR score groups, respectively. This suggests that a lower HER2DX pCR Score predicts a better response to letrozole, while a higher score indicates reduced tumor sensitivity to endocrine therapy. Additionally, the study revealed that the pCR rate in ESD patients was 22.5%, significantly lower than the 80% observed in ERD patients. The HER2DX pCR Score was significantly correlated with the pCR rate in ESD patients (P = 0.008), with rates of 7.7%, 46.2%, and 100.0% in the low, medium, and high HER2DX pCR score groups, respectively (P < 0.004). However, the score was not significantly associated with pCR in ERD patients (P = 1). These findings suggest that the HER2DX pCR Score can not only predict early response to letrozole monotherapy but also help identify patients who may benefit from combined endocrine and anti-HER2 therapy. For ESD patients with a lower HER2DX pCR Score, escalated treatment may be necessary to improve outcomes.
HER2DX for predicting prognosis in special populations and personalizing de-escalation treatment choices
Research on trastuzumab therapy duration has shown that long-term treatment (12 months) offers a lower risk of recurrence compared to short-term treatment (e.g., 9 weeks or 6 months), but with an increased risk of side effects like cardiotoxicity [72,97-99]. Exploratory research within the Short-Her trial suggested that a low HER2DX Risk Score might guide the choice of treatment regimen for specific patient groups [60,72]. Patients with a low HER2DX Risk Score, particularly those with significant comorbidities or a history of cardiotoxicity, might be suitable candidates for short-term trastuzumab therapy. This finding helps physicians create more personalized treatment plans for HER2+ breast cancer patients, aiming for optimal outcomes while minimizing side effects.
Conclusion and future perspective
The HER2DX pCR Score and Risk Score, despite their weak correlation (correlation coefficient about -0.19) [60], provide complementary information critical for guiding treatment decisions and selecting appropriate escalation or de-escalation strategies. The core value of HER2DX lies in its pCR and Risk Scores, while the HER2DX ERBB2 Expression Score serves mainly as supplementary information. Although most early-stage HER2+ breast cancer patients can achieve treatment goals with trastuzumab and chemotherapy, the effectiveness of different drug combinations varies. HER2DX can help predict pCR rates and survival outcomes after various treatments, offering personalized treatment options: for some patients, de-escalation (e.g., reducing chemotherapy types or cycles) may achieve similar therapeutic effects as standard treatment, minimizing toxicity and adverse events, thus avoiding overtreatment. Other patients may require escalated treatment (e.g., adding more effective drugs or extending treatment duration) to achieve expected results, especially when trastuzumab and chemotherapy alone do not meet therapeutic needs. HER2DX provides a tool for making more precise treatment choices based on individual patient conditions, predicting survival outcomes, recurrence, and the risk of distant metastasis, guiding follow-up intervals, and enabling more effective treatment and monitoring.
As the field of HER2+ breast cancer treatment advances, challenges remain for specific patients. Long-term use of the same anti-HER2 therapy may lead to disease progression or ineffective outcomes when switching therapies. This could be due to tumor biology, individual patient differences, or resistance development. Resistance is a crucial issue in HER2+ breast cancer treatment, potentially caused by genetic mutations, expression changes, or signaling pathway alterations, rendering previously effective treatments ineffective. The HER2DX model, while effective in predicting pCR, may also help predict drug resistance. Although research in this area is limited, the correlation between drug resistance and reduced pCR rates suggests HER2DX’s potential in predicting resistance. Further research into the genes within the HER2DX model and their roles in resistance mechanisms could unveil new therapeutic targets and strategies to overcome resistance.
Despite the promising results, HER2DX has limitations that future research must address. (1) Grouping Threshold Values: Different trials may identify different optimal cutoff values. For example, the APT trial found “32” as the optimal HER2DX Risk Score cutoff, differing from the standard threshold, suggesting the need for score adjustments based on patient populations and treatment contexts. (2) Geographical Applicability and Case Numbers: HER2DX has been evaluated in over 2,000 patients, with relatively few Chinese participants. Genetic and environmental differences suggest that effectiveness may vary between Asian and Western populations, necessitating broader evaluations. (3) Applicability to Advanced Breast Cancer: HER2DX was developed for early-stage HER2+ breast cancer. Its accuracy and applicability in advanced cases, particularly for predicting systemic treatment outcomes, may require further validation. (4) Validation Scope for Dual Anti-HER2 Therapy: HER2DX primarily validated trastuzumab and pertuzumab combination therapy, possibly overlooking the roles of TKIs and ADCs in dual-targeted therapy. Evaluating HER2DX in broader treatment regimens is necessary. (5) Lack of Prospective Studies: Most HER2DX research is retrospective, with few prospective studies that are crucial for validating its clinical applicability and effectiveness. (6) Cost Issues: HER2DX, based on genetic testing, may involve higher costs, limiting its widespread clinical use. Cost-effectiveness and financial burden must be considered.
Future research should address these limitations to enhance HER2DX’s clinical value. With more studies, HER2DX can be optimized for broader drug combinations, patient groups, and treatment stages. As new drugs and strategies emerge, HER2DX may evolve to meet new clinical challenges.
Acknowledgements
We extend our gratitude for the support from the Health Industry Research Project (grant number 201302016), the Southwest Hospital Clinical Research Incubation Major Project (grant number 2023IITZD03), the Chongqing Talents Project (grant number 414Z393), and the Chongqing Key Project of Technology Innovation and Application Development (grant number CSTB2022TIAD-KPX0168). Special thanks to the corresponding authors for their invaluable guidance and to the co-authors for their collaboration. We also acknowledge Junyi Xing (Medical Science Manager at Shanghai Roche Pharmaceuticals Ltd.) for assisting in collecting data for Figure 1 and Table 3.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; 2024. https://gco.iarc.who.int/today. (Accessed 8 February 2024) [Google Scholar]
- 2.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 3.(IARC) TIAfRoC, Global cancer burden growing, amidst mounting need for services. 2024. https://www.iarc.who.int/wp-content/uploads/2024/02/pr345_E.pdf. (Accessed 1 February 2024) [PMC free article] [PubMed]
- 4.Al-Thoubaity FK. Molecular classification of breast cancer: a retrospective cohort study. Ann Med Surg (Lond) 2019;49:44–48. doi: 10.1016/j.amsu.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Read LD, Keith D Jr, Slamon DJ, Katzenellenbogen BS. Hormonal modulation of HER-2/neu protooncogene messenger ribonucleic acid and p185 protein expression in human breast cancer cell lines. Cancer Res. 1990;50:3947–3951. [PubMed] [Google Scholar]
- 6.Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 7.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, Carpenter G, Gazdar AF, Muthuswamy SK, Arteaga CL. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sliwkowski MX. Ready to partner. Nat Struct Biol. 2003;10:158–159. doi: 10.1038/nsb0303-158. [DOI] [PubMed] [Google Scholar]
- 10.Niazi S, Purohit M, Sonawani A, Niazi JH. Revealing the molecular interactions of aptamers that specifically bind to the extracellular domain of HER2 cancer biomarker protein: an in silico assessment. J Mol Graph Model. 2018;83:112–121. doi: 10.1016/j.jmgm.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 12.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 15.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britten CD. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother Pharmacol. 2013;71:1395–1409. doi: 10.1007/s00280-013-2121-1. [DOI] [PubMed] [Google Scholar]
- 17.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40:862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, Debled M, Jacot W, Mouret-Reynier MA, Goncalves A, Dalenc F, Patsouris A, Ferrero JM, Levy C, Lorgis V, Vanlemmens L, Lefeuvre-Plesse C, Mathoulin-Pelissier S, Petit T, Uwer L, Jouannaud C, Leheurteur M, Lacroix-Triki M, Courtinard C, Perol D, Robain M, Delaloge S. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 20.Ganz PA, Goodwin PJ. Breast cancer survivorship: where are we today? Adv Exp Med Biol. 2015;862:1–8. doi: 10.1007/978-3-319-16366-6_1. [DOI] [PubMed] [Google Scholar]
- 21.Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25(Suppl 1):S142–S150. doi: 10.3747/co.25.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialists’ Collaborative group (EBCTCG) Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13864 women in seven randomised trials. Lancet Oncol. 2021;22:1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Ménard S, Costa A, Fagnoni FF. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 25.Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, Vahidian F, Gholamiyan Moghadam A, Aghadoukht A, Hajiasgharzadeh K, Baradaran B. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235:3142–3156. doi: 10.1002/jcp.29216. [DOI] [PubMed] [Google Scholar]
- 26.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26(Suppl 12):60–70. [PubMed] [Google Scholar]
- 27.Badache A, Hynes NE. A new therapeutic antibody masks ErbB2 to its partners. Cancer Cell. 2004;5:299–301. doi: 10.1016/s1535-6108(04)00088-1. [DOI] [PubMed] [Google Scholar]
- 28.Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, Clark E, Knott A, Restuccia E, Benyunes MC, Cortés J CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 30.Xu B, Li W, Zhang Q, Li Q, Wang X, Li H, Sun T, Yin Y, Zheng H, Feng J, Zhu H, Siddiqui A, Macharia H, Knott A. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): final analysis of a phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. 2023;197:503–513. doi: 10.1007/s10549-022-06775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, Starosławska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi GV, Magazzù D, McNally V, Douthwaite H, Ross G, Valagussa P. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 32.Shao Z, Pang D, Yang H, Li W, Wang S, Cui S, Liao N, Wang Y, Wang C, Chang YC, Wang H, Kang SY, Seo JH, Shen K, Laohawiriyakamol S, Jiang Z, Li J, Zhou J, Althaus B, Mao Y, Eng-Wong J. Efficacy, safety, and tolerability of Pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. 2020;6:e193692. doi: 10.1001/jamaoncol.2019.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abunada A, Sirhan Z, Thyagarajan A, Sahu RP. Tyrosine kinase inhibitors and human epidermal growth factor receptor-2 positive breast cancer. World J Clin Oncol. 2023;14:198–202. doi: 10.5306/wjco.v14.i5.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, Wang J, You F, Li X, Xiao C. The role of irreversible pan-HER tyrosine kinase inhibitors in the treatment of HER2-Positive metastatic breast cancer. Front Pharmacol. 2023;14:1142087. doi: 10.3389/fphar.2023.1142087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Deng S, Chen J, Xu X, Hu X, Kong D, Liang G, Yuan X, Li Y, Wang X. The synergistic effects of pyrotinib combined with adriamycin on HER2-positive breast cancer. Front Oncol. 2021;11:616443. doi: 10.3389/fonc.2021.616443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Du F, Diéras V, Curigliano G. The role of tyrosine kinase inhibitors in the treatment of HER2+ metastatic breast cancer. Eur J Cancer. 2021;154:175–189. doi: 10.1016/j.ejca.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Müller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP. Tucatinib, trastuzumab, and capecitabine for hER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 38.Chan A, Moy B, Mansi J, Ejlertsen B, Holmes FA, Chia S, Iwata H, Gnant M, Loibl S, Barrios CH, Somali I, Smichkoska S, Martinez N, Alonso MG, Link JS, Mayer IA, Cold S, Murillo SM, Senecal F, Inoue K, Ruiz-Borrego M, Hui R, Denduluri N, Patt D, Rugo HS, Johnston SRD, Bryce R, Zhang B, Xu F, Wong A, Martin M ExteNET Study Group. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21:80–91. e87. doi: 10.1016/j.clbc.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M NeoALTTO Study Team. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirhan Z, Thyagarajan A, Sahu RP. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil Med Res. 2022;9:39. doi: 10.1186/s40779-022-00401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9:33–46. doi: 10.1007/s13238-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-drug conjugates for cancer therapy. Molecules. 2020;25:4764. doi: 10.3390/molecules25204764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammood M, Craig AW, Leyton JV. Impact of endocytosis mechanisms for the receptors targeted by the currently approved antibody-drug conjugates (ADCs)-A necessity for future ADC research and development. Pharmaceuticals (Basel) 2021;14:674. doi: 10.3390/ph14070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giugliano F, Corti C, Tarantino P, Michelini F, Curigliano G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep. 2022;24:809–817. doi: 10.1007/s11912-022-01266-4. [DOI] [PubMed] [Google Scholar]
- 45.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117:1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu B, Wang J, Fang J, Chen X, Han Y, Li Q, Zhang P, Yuan P, Ma F, Luo Y, Fan Y, Cai R, Chen S, Li Q. Abstract PD4-06: early clinical development of RC48-ADC in patients with HER2 positive metastatic breast cancer. Cancer Res. 2020;80:PD4-06. [Google Scholar]
- 48.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A, Fischer HH, Jacot W, Conlin AK, Arce-Salinas C, Wapnir IL, Jackisch C, DiGiovanna MP, Fasching PA, Crown JP, Wülfing P, Shao Z, Rota Caremoli E, Wu H, Lam LH, Tesarowski D, Smitt M, Douthwaite H, Singel SM, Geyer CE Jr KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 50.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.André F, Hee Park Y, Kim SB, Takano T, Im SA, Borges G, Lima JP, Aksoy S, Gavila Gregori J, De Laurentiis M, Bianchini G, Roylance R, Miyoshi Y, Armstrong A, Sinha R, Ruiz Borrego M, Lim E, Ettl J, Yerushalmi R, Zagouri F, Duhoux FP, Fehm T, Gambhire D, Cathcart J, Wu C, Chu C, Egorov A, Krop I. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401:1773–1785. doi: 10.1016/S0140-6736(23)00725-0. [DOI] [PubMed] [Google Scholar]
- 52.Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, Chiu JWY, Xu B, Hamilton E, Madhusudan S, Iwata H, Altintas S, Henning JW, Curigliano G, Perez-Garcia JM, Kim SB, Petry V, Huang CS, Li W, Frenel JS, Antolin S, Yeo W, Bianchini G, Loi S, Tsurutani J, Egorov A, Liu Y, Cathcart J, Ashfaque S, Cortés J. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 53.Januškevičienė I, Petrikaitė V. Heterogeneity of breast cancer: the importance of interaction between different tumor cell populations. Life Sci. 2019;239:117009. doi: 10.1016/j.lfs.2019.117009. [DOI] [PubMed] [Google Scholar]
- 54.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 55.Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017;7:156. doi: 10.3389/fonc.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein LJ, Chia SKL, Dhesy-Thind S, Rastogi P, Alba E, Delaloge S, Martin M, Kelly CM, Ruiz-Borrego M, Gil-Gil M, Arce-Salinas CH, Brain EGC, Lee ES, Pierga JY, Bermejo B, Ramos-Vazquez M, Jung KH, Ferrero JM, Schott AF, Shak S, Sharma P, Lew DL, Miao J, Tripathy D, Pusztai L, Hortobagyi GN. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385:2336–2347. doi: 10.1056/NEJMoa2108873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piccart M, van ’t Veer LJ, Poncet C, Lopes Cardozo JMN, Delaloge S, Pierga JY, Vuylsteke P, Brain E, Vrijaldenhoven S, Neijenhuis PA, Causeret S, Smilde TJ, Viale G, Glas AM, Delorenzi M, Sotiriou C, Rubio IT, Kümmel S, Zoppoli G, Thompson AM, Matos E, Zaman K, Hilbers F, Fumagalli D, Ravdin P, Knox S, Tryfonidis K, Peric A, Meulemans B, Bogaerts J, Cardoso F, Rutgers EJT. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–488. doi: 10.1016/S1470-2045(21)00007-3. [DOI] [PubMed] [Google Scholar]
- 59.Prat A, Guarneri V, Paré L, Griguolo G, Pascual T, Dieci MV, Chic N, González-Farré B, Frassoldati A, Sanfeliu E, Cejalvo JM, Muñoz M, Bisagni G, Brasó-Maristany F, Urso L, Vidal M, Brandes AA, Adamo B, Musolino A, Miglietta F, Conte B, Oliveira M, Saura C, Pernas S, Alarcón J, Llombart-Cussac A, Cortés J, Manso L, López R, Ciruelos E, Schettini F, Villagrasa P, Carey LA, Perou CM, Piacentini F, D’Amico R, Tagliafico E, Parker JS, Conte P. A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: a retrospective study with an external evaluation. Lancet Oncol. 2020;21:1455–1464. doi: 10.1016/S1470-2045(20)30450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prat A, Guarneri V, Pascual T, Brasó-Maristany F, Sanfeliu E, Paré L, Schettini F, Martínez D, Jares P, Griguolo G, Dieci MV, Cortés J, Llombart-Cussac A, Conte B, Marín-Aguilera M, Chic N, Puig-Butillé JA, Martínez A, Galván P, Tsai YH, González-Farré B, Mira A, Vivancos A, Villagrasa P, Parker JS, Conte P, Perou CM. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine. 2022;75:103801. doi: 10.1016/j.ebiom.2021.103801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 62.Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, Mlineritsch B, Kwasny W, Knauer M, Singer C, Jakesz R, Dubsky P, Fitzal F, Bartsch R, Steger G, Balic M, Ressler S, Cowens JW, Storhoff J, Ferree S, Schaper C, Liu S, Fesl C, Nielsen TO Austrian Breast and Colorectal Cancer Study Group. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 63.Drukker CA, van Tinteren H, Schmidt MK, Rutgers EJ, Bernards R, van de Vijver MJ, Van’t Veer LJ. Long-term impact of the 70-gene signature on breast cancer outcome. Breast Cancer Res Treat. 2014;143:587–592. doi: 10.1007/s10549-013-2831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, Dietze O, Greil R, Jelen A, Sevelda P, Freibauer C, Müller V, Jänicke F, Schmidt M, Kölbl H, Rody A, Kaufmann M, Schroth W, Brauch H, Schwab M, Fritz P, Weber KE, Feder IS, Hennig G, Kronenwett R, Gehrmann M, Gnant M EP Investigators. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17:6012–6020. doi: 10.1158/1078-0432.CCR-11-0926. [DOI] [PubMed] [Google Scholar]
- 65.Sestak I, Buus R, Cuzick J, Dubsky P, Kronenwett R, Ferree S, Sgroi D, Schnabel C, Baehner R, Mallon E, Dowsett M. Abstract S6-05: comprehensive comparison of prognostic signatures for breast cancer in TransATAC. Cancer Res. 2017;77:S6-05. [Google Scholar]
- 66.Kwa M, Makris A, Esteva FJ. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol. 2017;14:595–610. doi: 10.1038/nrclinonc.2017.74. [DOI] [PubMed] [Google Scholar]
- 67.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J. Clin. Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 68.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M MINDACT Investigators. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 70.Hurvitz SA. HER2DX: a tool that might inform treatment choices for HER2-positive breast cancer. Lancet Oncol. 2020;21:1392–1393. doi: 10.1016/S1470-2045(20)30552-0. [DOI] [PubMed] [Google Scholar]
- 71.Marín-Aguilera M, Jares P, Sanfeliu E, Villacampa G, Hernández-Lllán E, Martínez-Puchol AI, Shankar S, González-Farré B, Waks AG, Brasó-Maristany F, Pardo F, Manning DK, Abery JA, Curaba J, Moon L, Gordon O, Galván P, Wachirakantapong P, Castillo O, Nee CM, Blasco P, Senevirathne TH, Sirenko V, Martínez-Sáez O, Aguirre A, Krop IE, Li Z, Spellman P, Metzger Filho O, Polyak K, Michaels P, Puig-Butillé JA, Vivancos A, Matito J, Buckingham W, Perou CM, Villagrasa-González P, Prat A, Parker JS, Paré L. Analytical validation of HER2DX genomic test for early-stage HER2-positive breast cancer. ESMO Open. 2024;9:102903. doi: 10.1016/j.esmoop.2024.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conte P, Bisagni G, Frassoldati A, Brandes A, Cavanna L, Giotta F, Aieta M, Gebbia V, Musolino A, Garrone O, Donadio M, Cavazzini G, Turletti A, Zamagni C, Danese S, Ferro A, Piacentini F, Balduzzi S, D’Amico R, Guarneri V. 3*Final analysis of the phase III multicentric Italian study Short-HER: 9 weeks vs 1 year adjuvant trastuzumab for HER2+ early breast cancer. Ann Oncol. 2017:28. doi: 10.1093/annonc/mdy414. [DOI] [PubMed] [Google Scholar]
- 73.Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, Bago-Horvath Z, Bane A, Bedri S, Brock J, Chmielik E, Christgen M, Colpaert C, Demaria S, Van den Eynden G, Floris G, Fox SB, Gao D, Ingold Heppner B, Kim SR, Kos Z, Kreipe HH, Lakhani SR, Penault-Llorca F, Pruneri G, Radosevic-Robin N, Rimm DL, Schnitt SJ, Sinn BV, Sinn P, Sirtaine N, O’Toole SA, Viale G, Van de Vijver K, de Wind R, von Minckwitz G, Klauschen F, Untch M, Fasching PA, Reimer T, Willard-Gallo K, Michiels S, Loi S, Salgado R. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 74.Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, Fernandez-Martinez A, Parker JS, Hoadley KA, Hu Z, Li Y, Soloway MG, Spears PA, Singh B, Tolaney SM, Somlo G, Port ER, Ma C, Kuzma C, Mamounas E, Golshan M, Bellon JR, Collyar D, Hahn OM, Hudis CA, Winer EP, Partridge A, Hyslop T, Carey LA, Perou CM, Sikov WM. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. J. Clin. Oncol. 2022;40:1323–1334. doi: 10.1200/JCO.21.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guarneri V, Bras-Maristany F, Dieci MV, Griguolo G, Par L, Mar Ín-Aguilera M, Miglietta F, Bottosso M, Giorgi CA, Blasco P, Castillo O, Galv N P, Vivancos A, Villagrasa P, Parker JS, Perou CM, Conte P, Prat A. HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: a correlative analysis from the PerELISA trial. EBioMedicine. 2022;85:104320. doi: 10.1016/j.ebiom.2022.104320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tolaney SM, Tarantino P, Graham N, Tayob N, Parè L, Villacampa G, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis MJ, Shapira I, Wolff AC, Carey LA, Barroso-Sousa R, Villagrasa P, DeMeo M, DiLullo M, Zanudo JGT, Weiss J, Wagle N, Partridge AH, Waks AG, Hudis CA, Krop IE, Burstein HJ, Prat A, Winer EP. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273–285. doi: 10.1016/S1470-2045(23)00051-7. [DOI] [PubMed] [Google Scholar]
- 77.Waks AG, Ogayo ER, Paré L, Marín-Aguilera M, Brasó-Maristany F, Galván P, Castillo O, Martínez-Sáez O, Vivancos A, Villagrasa P, Villacampa G, Tarantino P, Desai N, Guerriero J, Metzger O, Tung NM, Krop IE, Parker JS, Perou CM, Prat A, Winer EP, Tolaney SM, Mittendorf EA. Assessment of the HER2DX assay in patients with ERBB2-positive breast cancer treated with neoadjuvant paclitaxel, trastuzumab, and pertuzumab. JAMA Oncol. 2023;9:835–840. doi: 10.1001/jamaoncol.2023.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, Ollila DW, Krop IE, Henry NL, Weckstein DJ, Anders CK, Singh B, Hoadley KA, Iglesia M, Cheang MC, Perou CM, Winer EP, Hudis CA. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J. Clin. Oncol. 2016;34:542–549. doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villacampa G, Tung NM, Pernas S, Paré L, Bueno-Muiño C, Echavarría I, López-Tarruella S, Roche-Molina M, Del Monte-Millán M, Marín-Aguilera M, Brasó-Maristany F, Waks AG, Pascual T, Martínez-Sáez O, Vivancos A, Conte PF, Guarneri V, Vittoria Dieci M, Griguolo G, Cortés J, Llombart-Cussac A, Muñoz M, Vidal M, Adamo B, Wolff AC, DeMichele A, Villagrasa P, Parker JS, Perou CM, Fernandez-Martinez A, Carey LA, Mittendorf EA, Martín M, Prat A, Tolaney SM. Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer. Ann Oncol. 2023;34:783–795. doi: 10.1016/j.annonc.2023.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Llombart-Cussac A, Cortés J, Paré L, Galván P, Bermejo B, Martínez N, Vidal M, Pernas S, López R, Muñoz M, Nuciforo P, Morales S, Oliveira M, de la Peña L, Peláez A, Prat A. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18:545–554. doi: 10.1016/S1470-2045(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 81.Clark AS, Yau C, Wolf DM, Petricoin EF, van ’t Veer LJ, Yee D, Moulder SL, Wallace AM, Chien AJ, Isaacs C, Boughey JC, Albain KS, Kemmer K, Haley BB, Han HS, Forero-Torres A, Elias A, Lang JE, Ellis ED, Yung R, Tripathy D, Nanda R, Wulfkuhle JD, Brown-Swigart L, Gallagher RI, Helsten T, Roesch E, Ewing CA, Alvarado M, Crane EP, Buxton M, Clennell JL, Paoloni M, Asare SM, Wilson A, Hirst GL, Singhrao R, Steeg K, Asare A, Matthews JB, Berry S, Sanil A, Melisko M, Perlmutter J, Rugo HS, Schwab RB, Symmans WF, Hylton NM, Berry DA, Esserman LJ, DeMichele AM. Neoadjuvant T-DM1/pertuzumab and paclitaxel/trastuzumab/pertuzumab for HER2(+) breast cancer in the adaptively randomized I-SPY2 trial. Nat Commun. 2021;12:6428. doi: 10.1038/s41467-021-26019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bueno-Muiño C, Echavarría I, López-Tarruella S, Roche-Molina M, Del Monte-Millán M, Massarrah T, Jerez Y, Ayala de la Peña F, García-Sáenz JÁ, Moreno F, Rodríguez-Lescure Á, Malón-Giménez D, Ballesteros García AI, Marín-Aguilera M, Galván P, Brasó-Maristany F, Waks AG, Tolaney SM, Mittendorf EA, Vivancos A, Villagrasa P, Parker JS, Perou CM, Paré L, Villacampa G, Prat A, Martín M. Assessment of a genomic assay in patients with ERBB2-positive breast cancer following neoadjuvant trastuzumab-based chemotherapy with or without pertuzumab. JAMA Oncol. 2023;9:841–846. doi: 10.1001/jamaoncol.2023.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villacampa G, Pascual T, Brasó-Maristany F, Paré L, Martínez-Sáez O, Cortés J, Ciruelos E, Martin M, Conte P, Carey LA, Fernandez A, Harbeck N, Marín-Aguilera M, Vivancos A, Curigliano G, Villagrasa P, Parker JS, Perou CM, Prat A, Tolaney SM. Prognostic value of HER2DX in early-stage HER2-positive breast cancer: a comprehensive analysis of 757 patients in the Sweden cancerome analysis network-breast dataset (SCAN-B) ESMO Open. 2024;9:102388. doi: 10.1016/j.esmoop.2024.102388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cécile C, John OQ. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics & Data Analysis. 1999;30:253–270. [Google Scholar]
- 85.Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, Restuccia E, Jerusalem G, Dent S, Reaby L, Bonnefoi H, Krop I, Liu TW, Pieńkowski T, Toi M, Wilcken N, Andersson M, Im YH, Tseng LM, Lueck HJ, Colleoni M, Monturus E, Sicoe M, Guillaume S, Bines J, Gelber RD, Viale G, Thomssen C APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J. Clin. Oncol. 2021;39:1448–1457. doi: 10.1200/JCO.20.01204. [DOI] [PubMed] [Google Scholar]
- 86.Dieci MV, Conte P, Bisagni G, Bartolini S, Frassoldati A, Generali D, Piacentini F, Griguolo G, Tagliafico E, Brasó Maristany F, Chic N, Paré L, Miglietta F, Vicini R, D’Amico R, Balduzzi S, Prat A, Guarneri V. Metastatic site patterns by intrinsic subtype and HER2DX in early HER2-positive breast cancer. J Natl Cancer Inst. 2024;116:69–80. doi: 10.1093/jnci/djad179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brasó-Maristany F, Griguolo G, Chic N, Pascual T, Paré L, Maues J, Galván P, Dieci MV, Miglietta F, Giarratano T, Martínez-Sáez O, Marín-Aguilera M, Schettini F, Conte B, Angelats L, Vidal M, Adamo B, Muñoz M, Sanfeliu E, González B, Vivancos A, Villagrasa P, Parker JS, Perou CM, Conte P, Prat A, Guarneri V. HER2DX ERBB2 mRNA expression in advanced HER2-positive breast cancer treated with T-DM1. J Natl Cancer Inst. 2023;115:332–336. doi: 10.1093/jnci/djac227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang JN, Mu YX, Li Q, Fan Y, Wang JY, Ma F, Luo Y, Yuan P, Chen SS, Li Q, Cai RG, Zhang P, Xu BH. Feasibility and toxicity of EC-T dose-dense adjuvant chemotherapy: a real world study in Chinese early-stage breast cancer patients with high recurrence risk. Zhonghua Zhong Liu Za Zhi. 2019;41:368–372. doi: 10.3760/cma.j.issn.0253-3766.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 89.Sharma P, Kimler BF, O’Dea A, Nye L, Wang YY, Yoder R, Staley JM, Prochaska L, Wagner J, Amin AL, Larson K, Balanoff C, Elia M, Crane G, Madhusudhana S, Hoffmann M, Sheehan M, Rodriguez R, Finke K, Shah R, Satelli D, Shrestha A, Beck L, McKittrick R, Pluenneke R, Raja V, Beeki V, Corum L, Heldstab J, LaFaver S, Prager M, Phadnis M, Mudaranthakam DP, Jensen RA, Godwin AK, Salgado R, Mehta K, Khan Q. Randomized phase II trial of anthracycline-free and anthracycline-containing neoadjuvant carboplatin chemotherapy regimens in stage I-III triple-negative breast cancer (NeoSTOP) Clin Cancer Res. 2021;27:975–982. doi: 10.1158/1078-0432.CCR-20-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Electronic address: bc.overview@ctsu.ox.ac.uk; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: a patient-level meta-analysis of 100000 women from 86 randomised trials. Lancet. 2023;401:1277–1292. doi: 10.1016/S0140-6736(23)00285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 92.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 93.Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, Zujewski JA, Goldhirsch A, Armour A, Pritchard KI, McCullough AE, Dolci S, McFadden E, Holmes AP, Tonghua L, Eidtmann H, Dinh P, Di Cosimo S, Harbeck N, Tjulandin S, Im YH, Huang CS, Diéras V, Hillman DW, Wolff AC, Jackisch C, Lang I, Untch M, Smith I, Boyle F, Xu B, Gomez H, Suter T, Gelber RD, Perez EA. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2016. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 95.Agostinetto E, Debien V, Marta GN, Lambertini M, Piccart-Gebhart M, de Azambuja E. CDK4/6 and PI3K inhibitors: a new promise for patients with HER2-positive breast cancer. Eur J Clin Invest. 2021;51:e13535. doi: 10.1111/eci.13535. [DOI] [PubMed] [Google Scholar]
- 96.Debien V, de Azambuja E, Piccart-Gebhart M. Optimizing treatment for HER2-positive HR-positive breast cancer. Cancer Treat Rev. 2023;115:102529. doi: 10.1016/j.ctrv.2023.102529. [DOI] [PubMed] [Google Scholar]
- 97.Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espié M, Fumoleau P, Serin D, Jacquin JP, Jouannaud C, Rios M, Abadie-Lacourtoisie S, Venat-Bouvet L, Cany L, Catala S, Khayat D, Gambotti L, Pauporté I, Faure-Mercier C, Paget-Bailly S, Henriques J, Grouin JM PHARE trial investigators. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393:2591–2598. doi: 10.1016/S0140-6736(19)30653-1. [DOI] [PubMed] [Google Scholar]
- 98.Joensuu H, Fraser J, Wildiers H, Huovinen R, Auvinen P, Utriainen M, Nyandoto P, Villman KK, Halonen P, Granstam-Björneklett H, Lundgren L, Sailas L, Turpeenniemi-Hujanen T, Tanner M, Yachnin J, Ritchie D, Johansson O, Huttunen T, Neven P, Canney P, Harvey VJ, Kellokumpu-Lehtinen PL, Lindman H. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4:1199–1206. doi: 10.1001/jamaoncol.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, Hughes-Davies L, Harnett AN, Ah-See ML, Simcock R, Rea D, Raj S, Woodings P, Harries M, Howe D, Raynes K, Higgins HB, Wilcox M, Plummer C, Mansi J, Gounaris I, Mahler-Araujo B, Provenzano E, Chhabra A, Abraham JE, Caldas C, Hall PS, McCabe C, Hulme C, Miles D, Wardley AM, Cameron DA, Dunn JA PERSEPHONE Steering Committee and Trial Investigators. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]