Abstract
Breast cancer is a multifactorial disease driven by acquired genetic and epigenetic changes that lead to aberrant regulation of cellular signaling pathways. Receptor tyrosine kinases (RTKs), a class of critical receptors, are involved in the initiation and progression of breast cancer. RTKs are cell surface receptors with unique structures and biological characteristics, which respond to environmental signals by initiating signaling cascades such as the mitogen-activated protein kinase (MAPK) pathway, Janus kinase (JAK)/signal transducer, activator of transcription (STAT) pathway, and phosphoinositide 3-kinase (PI3K)/AKT pathway. The critical role of RTKs makes them suitable targets for breast cancer treatment. Targeted therapies against RTKs have been developed in recent years, evaluated in clinical trials, and approved for several cancer types, including breast cancer. However, breast cancer displays molecular heterogeneity and exhibits different therapeutic responses to various drug types, leading to limited effectiveness of targeted therapy against RTKs. In this review, we summarize the structural and functional characteristics of selected RTKs and discuss the mechanisms and current status of drug therapy involving different protein tyrosine kinases in breast cancer progression.
Keywords: Receptor tyrosine kinases, breast cancer, tyrosine protein kinase inhibitors, anti-RTK therapy, drug resistance, targeted therapy
Introduction
Breast cancer (BC) is the most prevalent and highly fatal tumor among women worldwide [1]. It can be classified into five distinct subtypes: luminal A/B, HER2-positive (HER+), basal-like, claudin-low, and normal breast-like, based on the expression levels of estrogen receptor (ER), progesterone receptor (PR), HER2, cytokeratin 5/6 (CK5/6), and claudins 3/4/7 [2-4]. Among these, triple-negative breast cancer (TNBC) accounts for 15-20% of breast cancer and shares remarkable similarities with basal-like breast cancer. TNBC lacks the expression of ER, PR, and HER2, and is characterized by its high metastatic capacity [5,6].
Breast cancer is a consequence of dysregulation in multiple signaling pathways within the epithelial cells of the breast. The activation of growth factors and chemokines disrupts diverse signaling cascades in the tumor microenvironment, thereby contributing to cancer progression [7,8]. Playing a crucial role in this process, receptor tyrosine kinases (RTKs) are a vital family of receptors that regulate essential biological processes, including cell proliferation, differentiation, metabolism, and survival [9]. They achieve this by initiating downstream signaling pathways.
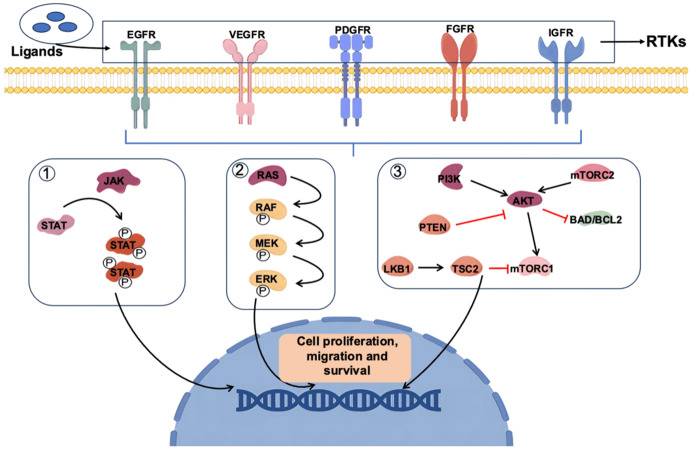
RTKs are single-pass transmembrane proteins expressed in various cell types. Multiple RTKs, such as epidermal growth factor receptors (EGFRs), vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), insulin-like growth factor receptors (IGFRs), and fibroblast growth factor receptors (FGFRs), are expressed in different types of tumors, including breast cancer [9-11]. Elevated levels of RTKs have been associated with increased invasiveness of breast cancer and decreased overall and disease-free survival rates [12]. Ligand binding induces conformational changes in RTKs, subsequently activating downstream signals. Key pathways known to be activated by RTKs include the mitogen-activated protein kinase (MAPK), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), as well as phosphoinositide 3-kinase (PI3K)/AKT pathway [13-17]. These RTK-activated pathways play pivotal roles in various aspects of breast cancer progression (Figure 1).
Figure 1.
RTK-regulated important signaling in breast cancer progression. (1) Once a ligand binds to the receptor, two STAT proteins are phosphorylated by JAK forming a dimer that enters the nucleus, causing the transcription of target genes. (2) After the TKR is activated by a ligand, Ras dimerizes and binds Raf, promoting Raf activation. Active Raf phosphorylates and activates MEK1/2 which induces ERK1/2 activation, leading to transcription activation. (3) PI3K phosphorylates phosphatidyl inositol-bisphosphate (PIP2) to PIP3, a process that can be reversed by the action of PTEN. PIP3 causes the activation of Akt in the plasma membrane, thereby activating the mTOR complex, one of the major pathways involved in tumorigenesis. The above have represented the interaction between various signaling pathways activated through RTKs and involved in tumor proliferation.
Due to the significant role of RTKs in breast cancer progression, targeting RTKs may contribute to cancer treatment. Over the years, targeted therapies against RTKs, including small molecule inhibitors and monoclonal antibodies, have demonstrated efficacy in cancer treatment [18,19]. Among them, drugs such as lapatinib, trastuzumab, and bevacizumab have gained approval from the U.S. Food and Drug Administration (FDA) for the clinical management of breast cancer. Furthermore, RTK inhibitors have shown promise in overcoming multidrug resistance and improving disease-free survival rates in patients with metastatic breast cancer [20]. Despite the clinical benefits of anti-RTK therapy for breast cancer, the primary and acquired resistance significantly limits the effectiveness of RTK-targeted treatments. Therefore, further research is essential to overcome this challenge and enhance the efficacy of RTK-targeted therapies [21]. In this review, we have delved into the intricate signaling cascades of EGFR, VEGFR, PDGFR, FGFR, and other key receptors implicated in breast cancer. Our exploration of the role of RTK inhibitors in breast cancer treatment offers valuable insights into the potential for targeted therapy. Understanding the underlying mechanisms and the potential benefits of RTK inhibitors can contribute to more effective and personalized treatment strategies for breast cancer patients, ultimately improving their clinical outcomes.
Functions and targeted drugs of RTKs
Features of RTKs
RTKs, integral membrane proteins, are activated through specific ligand interactions. These receptors consist of distinct domains, including extracellular ligand-binding regions, a transmembrane domain, and intracellular kinase domains [22]. Remarkably, each receptor class exhibits unique structural and sequence characteristics within their extracellular domains, defining their ligand specificity. Various protein motifs, such as immunoglobulin-like (Ig) domains, leucine-rich domains (L domains), cysteine-rich domains (CR domains), or fibronectin type III (Fn3) domains, are specifically present in different receptors. Conversely, the intracellular domains encompass the tyrosine kinase domain and the C-terminal region [23]. Some receptors possess insertions that separate the kinase domain when varying lengths of sequences are inserted [24]. Additionally, the C-terminal domains of RTKs differ among the family members, contributing to the specificity and diversity of downstream signaling. Their catalytic activity enables tyrosine residue phosphorylation, which is triggered by ligand binding to the extracellular domains of RTK proteins, thereby stabilizing the active state [25,26].
Mechanism of RTK activation
RTKs, a superfamily of 58 members into 20 subfamilies [27,28], share two key features: ligand-induced dimerization and auto-phosphorylation of tyrosine residues [29]. As for the first one, conformational changes occur in the monomeric or self-inhibited receptors. This enables the receptors to form dimers, which facilitates the enhancement of tyrosine kinase activity. Subsequently, the kinase domain and the C-terminal region of RTKs undergo auto-phosphorylation at specific tyrosine residues. This auto-phosphorylation plays a crucial role in the assembly of signaling molecules comprising Src homology 2 (SH2) and phosphotyrosine-binding domains [30].
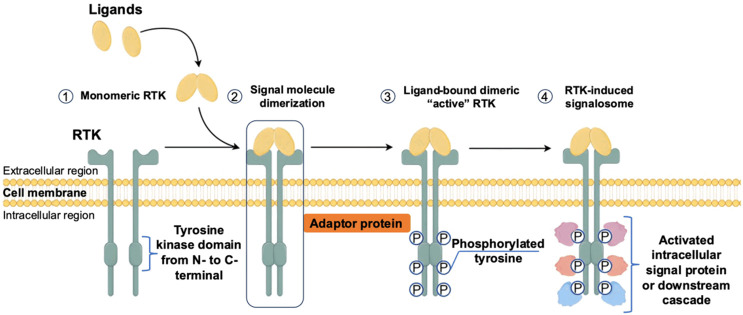
RTKs primarily interact with soluble ligands, such as growth factors, cytokines, and hormones, to initiate signal transduction pathways [25,27]. These ligands engage a repertoire of downstream signaling components, including kinases like PI3K and SRC, adaptor proteins like SHC and GRB2, transcription factors like STAT, ubiquitin ligases, and phospholipases such as phospholipase C-gamma (PLC-γ) [13-17]. These signaling cascades activate various pathways, including MAPK, PI3K/AKT/mammalian target of rapamycin (mTOR), PLC-γ/protein kinase C, and JAK/STAT pathways. It is important to note that RTK signaling can occur both intracellularly and at the cell surface, with distinct signaling pathways depending on the subcellular localization of the receptors [31]. Ultimately, the activation of RTKs leads to diverse biological responses, including cell growth, survival, inhibition of apoptosis, stimulation of angiogenesis, and promotion of cell motility (Figure 2) [32].
Figure 2.
Classical Structure of receptor tyrosine kinase and activation mechanism. (1) At first, RTKs reside at the cell membrane as a monomer. (2) Upon ligand binding, RTKs are activated through the formation of inter-molecular dimerization in the presence of ligands, resulting in kinase activation and phosphorylation of the receptor C-terminal tail. (3) Phosphorylated RTK either serves as a docking site for adaptor proteins or may directly phosphorylate signaling molecules.
Development of RTKs-targeted drugs
RTKs have emerged as central players in regulating critical cellular processes, including cell growth, survival, organ morphogenesis, angiogenesis, and tissue regeneration [33,34]. While RTK activity is intricately regulated in normal cells, dysregulated or constitutive activation of RTKs has been observed across a wide variety of cancers. Aberrant activation can stem from functional mutations, gene rearrangements, amplification, overexpression, or abnormal autocrine, endocrine, as well as paracrine signaling between receptors and ligands. Notably, these aberrations have been found to correlate with the progression of various human cancers [35-37].
Targeting RTKs has shown promising therapeutic benefits. In colorectal cancer, for instance, alterations in EGFR ligands [38], dual mutations in associated proteins, epidermal regulatory protein irregularities, and transforming growth factor-alpha (TGF-α) have been established as predictive biomarkers and prognostic indicators for the response to anti-EGFR antibodies, such as cetuximab or panitumumab [39]. Similarly, EGFR mutations, anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 (ROS1) translocations in non-small cell lung cancer (NSCLC), ret proto-oncogene (RET) mutations in medullary thyroid carcinoma (MTC), and HER2 amplification in breast cancer have been identified, paving the way for targeted therapeutic strategies [40,41]. In fact, over the last decade, RTK-targeted therapies have demonstrated significant improvements in the treatment of selected cancer patients [42-46]. Their ability to selectively inhibit constitutively activated RTKs within tumor cells holds promise as a novel approach for cancer treatment [47].
In summary, gaining insights into the mechanisms that drive the activation of RTKs, particularly within the context of specific RTKs, helps us understand crucial cellular processes with significant implications in cancer (Table 1) [48-52]. Moreover, advancements in targeting these aberrant signaling pathways have opened new possibilities for therapeutic interventions, bringing hope for improved outcomes in the treatment of cancer.
Table 1.
Classification and therapeutic applications of RTKs
| Receptor family | Receptor | Applications |
|---|---|---|
| Epidermal growth factor receptor, EGFR | HER1, HER2, HER3, HER4 | Non-small cell lung cancer, head and neck tumors, colorectal cancer, pancreatic cancer, breast cancer, ovarian cancer, cervical cancer |
| Insulin receptor, INSR | IGF-I, IGF-II, INSR, INSRR | Breast cancer, hematological malignancies, colorectal cancer, lung cancer, cervical cancer |
| Platelet-derived growth factor receptor, PDGFR | PDGFRα, PDGFRβ, CSF-1R, SCFR, FLK2, FLT3 | Hypereosinophilic syndrome, mastocytosis, gastrointestinal stromal tumor, epithelial cell tumor, leukemia |
| Fibroblast growth factor receptor, FGFR | FGFR1, FGFR2, FGFR3, FGFR4 | Angiogenesis |
| Vascular endothelial growth factor receptor, VEGFR | VEGFR1, VEGFR2, VEGFR3, VEGFR4 | Hepatocellular carcinoma, lung cancer, ovarian cancer |
| Hepatocyte growth factor receptor, HGFR | HGFR, MSPR | Breast cancer, colorectal cancer, gastric cancer, prostate cancer, renal cell carcinoma |
| Angiopoietins receptor of Tie family | Tie1, Tie2, TEK | Hemangioblastoma, epithelial cell tumor, gastric cancer, hepatocellular carcinoma |
The drugs targeting tyrosine kinases can be categorized into antibody-based therapies and small molecule inhibitors. Currently, there are 87 approved small-molecule kinase inhibitors worldwide, with the majority of the 71 small molecule kinase inhibitors approved by the FDA being tyrosine protein kinase inhibitors (TKIs), which find their primary application in oncology [53-58] (Table 2).
Table 2.
List of FDA-approved TKIs
| Targeted kinase | Name of the drugs |
|---|---|
| ALK | Alectinib, Crizotinib, Brigatinib, Lorlatinib, Ceritinib |
| Bcr-Abl | Bosutinib, Dasatinib, Nilotinib, Ponatinib, Imatinib |
| BTK | Acalabrutinib, Ibrutinib, Zarubrutinib |
| C-Met | Crizotinib, Cabozantinib |
| EGFR | Erlotinib, Afatinib, Gefitinib, Dacomitinib, Osimertinib, Neratinib |
| JAKs | Ruxolitinib, Baricitinib, Tofacitinib |
| PDGFR | Lenvatinib, Nintedanib, Ponatinib, Regorafenib, Imatinib |
| RET | Lenvatinib, Regorafenib, Sunitinib, Vandetanib |
| SRC | Dasatinib, Bosutinib, Ponatinib |
| VEGFR | Axitinib, Lenvatinib, Regorafenib, Pazopanib, Nintedanib, Sorafenib, Sunitinib |
| FGFR | Nintedanib, Erdafitinib |
| c-Kit | Pexidartinib, Avapritinib |
| FLT3 | Gelteritinib, Sunitinib |
In breast cancer, overexpression of HER2 occurs in approximately 25% of patients and is associated with lower survival rates [59,60]. Similarly, EGFR is frequently upregulated in solid tumors and plays a role in various malignant characteristics such as proliferation, apoptosis resistance, and tumor cell mobility. These discoveries have paved the way for the development of antibodies targeting HER2 and EGFR, and the success of drugs like trastuzumab and cetuximab validates the efficacy of targeting these growth factor receptors [61,62]. Moreover, successful interventions using RTK inhibitors include imatinib for the treatment of gastrointestinal stromal tumors harboring c-Kit mutations and gefitinib as well as erlotinib for NSCLC patients with EGFR mutations [49]. In addition to its inhibition of BCR-ABL and SRC, sunitinib also targets multiple RTKs such as PDGFR and VEGFR on endothelial cells, both of which play roles in tumor angiogenesis and proliferation [63].
Based on clinical trial data for small molecule kinase inhibitors (SMKIs) [64], there are currently approximately 110 novel kinases being investigated as potential targets [51,52,55,56,65-67]. The approved kinase inhibitors only cover around 30% of the human kinase, indicating that there are still numerous untapped areas within this kind of drugs waiting to be explored.
The role of RTK signaling in breast cancer progression
Under normal physiological conditions, the activity levels of RTKs are tightly balanced through the mechanisms mentioned above, as well as interactions with other molecules, including tyrosine phosphatases [68]. RTKs acquire activating capabilities through various mechanisms, such as gain-of-function mutations, genomic amplification, chromosomal rearrangements, and autocrine activation [9], ultimately resulting in the disruption of the balance between cell proliferation and cell death [22]. Moreover, when considering the intricate regulation of RTK signaling in terms of time and space, the dysregulation becomes even more intricate [69]. Constitutive activation of RTKs can endow normal cells with oncogenic properties, initiating tumorigenesis driven by these receptors [70].
The RTK pathway exhibits diverse mechanisms and clinical significance across different subtypes of breast cancer [25]. In HR+ (hormone receptor-positive) breast cancer, RTK pathways such as EGFR and HER2 contribute to tumor progression by promoting cell proliferation and survival [49]. The interplay between hormone receptor activation and RTK signaling pathways further enhances cellular dependence on hormones [71]. In HER2+ breast cancer, overexpression of HER2 leads to the activation of downstream signaling pathways, including PI3K/AKT and MAPK, thereby fostering cell proliferation and anti-apoptotic effects [72]. The RTK pathway plays a pivotal role in this subtype, and targeted therapies against HER2, like trastuzumab, have achieved remarkable success in improving patient outcomes [73].
TNBC (triple-negative breast cancer), characterized by the absence of HR and HER2 expression, may see RTK pathways like EGFR and FGFR facilitate tumorigenesis and progression through distinct signaling mechanisms [74-77]. The absence of specific targets renders TNBC treatment challenging, yet ongoing research into RTK pathways is exploring novel targeted therapeutic strategies [78-80]. Heterogeneity in mutations and expression levels of RTK pathways across different breast cancer subtypes influences tumor biology and treatment responses [81,82].
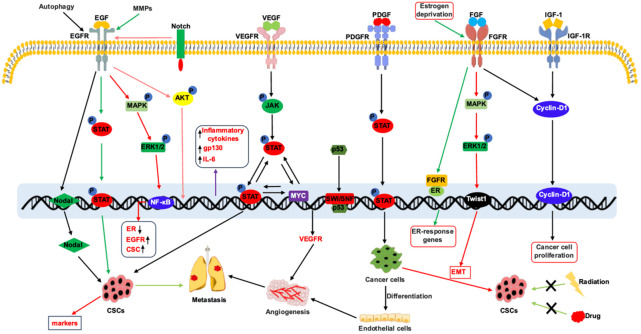
Next, we delve into the specific mechanisms of RTK pathways across different breast cancer subtypes (Figure 3), as an understanding of these mechanisms holds the potential to enhance prognosis and quality of life for breast cancer patients [25,73,75,77,83-91].
Figure 3.
RTK-regulated signaling in breast cancer progression. (1) EGFR regulates the activation of JAK/STAT and MAPK signaling pathway to induce expression of stem cell markers leading to enrichment of cancer stem cells. EGFR induces Akt phosphorylation to promote inflammation. EGFR-regulated signaling also plays pivotal role in angiogenesis and metastasis. (2) VEGFR activates JAK/STAT signaling pathway to induce cancer stem cell phenotype through MYC expression. Mutant p53 induces the expression of VEGFR through the interaction with SWI/SNF complex. (3) PDGFR is expressed on stromal cells such as fibroblasts and is a marker of fibroblast activation. PDGFR-regulated STAT activation is involved the differentiation of cancer cells to endothelial cells leading to angiogenesis. (4) FGFR-activated MAPK pathway induces EMT and CSC phenotype. (5) Cooperation between the FGFR and IGF-1R regulates nuclear translocation of Cyclin D1 leading to enhanced cancer cell proliferation.
EGFR: key regulator of cancer stem cell phenotype and metastasis
EGFR, a member of the ErbB family, is an RTK comprised of four closely related receptors: EGFR1 (EGFR, HER1, c-ErbB1), HER2 (EGFR2, c-ErbB2), EGFR3 (c-ErbB3, HER3), and EGFR4 (c-ErbB4, HER4). These receptors are situated on the cell membrane and consist of an extracellular ligand-binding domain, a transmembrane hydrophobic region, and an intracellular RTK domain [92,93]. Activation of EGFR occurs upon binding with its ligand. Ligand binding induces conformational changes in the receptor, facilitating the formation of either homodimers or heterodimers with other ErbB receptors [94]. Dimerization leads to cross-phosphorylation of conserved tyrosine residues within the kinase domain, resulting in the activation of downstream signaling pathways such as MAPK/ERK1/2 and PI3K/AKT. These pathways regulate vital cellular processes including proliferation, survival, migration, differentiation, and metastasis [95,96].
The EGFR signaling pathway is meticulously regulated in normal cells. However, alterations in EGFR expression and ligand overexpression in tumor cells can disrupt the balance, leading to abnormal autocrine or paracrine stimulation and increased activation of the tyrosine kinase domain [97]. Consequently, this aberrant signaling promotes cell proliferation, differentiation, angiogenesis, and apoptosis suppression, ultimately facilitating tumor growth and metastasis [98]. Overexpression of EGFR is commonly observed in breast cancer tissues and is associated with heightened invasiveness and poor clinical outcomes [99]. Studies have revealed that EGFR and HER-2 are overexpressed in approximately 30% of invasive breast cancers and are correlated with tumor recurrence and overall survival. Furthermore, EGFR overexpression is observed in over half of TNBC cases, accounting for 15% of all breast cancers [100]. Research has revealed a positive correlation between EGFR expression and tumor pathological grade, while a negative correlation exists between EGFR expression and ER expression in breast cancer. Additionally, ER levels negatively correlate with cancer stem cell phenotype. These findings suggest that ER-negative TNBCs exhibit higher levels of EGFR expression and harbor a population of stem cells [98].
Inflammatory breast cancer (IBC) is an aggressive, fatal type marked by a significant number of chemo- and radio-resistant CSCs. Approximately 30% of IBC cases exhibit EGFR expression, rising to 40-50% in ER- and PR-negative cases [98,100,101]. High EGFR expression predicts worse prognosis and higher recurrence risk in IBC. Studies have demonstrated that using EGFR antibodies effectively reduces SUM149 cell proliferation, indicating the potential of targeting EGFR in IBC [102-104]. EGFR-HER2 heterodimers boost breast cancer metastasis. Moreover, EGFR inversely correlates with HER2 and ER. Research has shown that EGFR-HER2 heterodimers can promote the metastasis of breast cancer cells. In TNBC patients, EGFR expression is markedly elevated. Therefore, EGFR holds great potential as a therapeutic target in TNBC, warranting further investigation and development of targeted therapies [105,106]. Recent studies conducted on various cell lines have highlighted the critical role of EGFR in promoting epithelial-mesenchymal transition (EMT). EMT involves cellular morphological changes, wherein epithelial cells transition to a mesenchymal fibroblast-like phenotype. This process is considered crucial for tumor infiltration and metastasis. While EMT is also implicated in normal mammary gland development, its significance becomes more prominent in breast cancer progression [107]. Multiple growth factors, including EGFR, hepatocyte growth factor, fibroblast growth factor, and insulin-like growth factor 1 or 2, have been shown to induce EMT in different epithelial cell lines. Among these, EGFR is particularly influential in EMT induction. For instance, EGFR activates the Ras-ERK pathway, which regulates EMT, thereby impacting tumor infiltration and metastasis. In cancer cells, the activation of RSK through ERK enhances mesenchymal activity and invasiveness [108-110]. Studies have explored the role of erlotinib, an EGFR-tyrosine kinase inhibitor, can inhibit cell viability, invasiveness, and the transition of IBC cells from a mesenchymal phenotype to an epithelial phenotype. Treatment with erlotinib resulted in increased expression of E-cadherin, an epithelial cell marker, and decreased expression of elastin, a component of the extracellular matrix. These findings suggest that erlotinib may exert its anti-metastatic effects by suppressing EMT [102-106]. Therefore, the strong correlation between EGFR and EMT indicates their potential as critical targets for inhibiting tumor metastasis. Further research in this area holds promise for providing valuable insights into the development of targeted therapies.
VEGFRs: key node in tumor angiogenesis and lymphatic genesis
VEGF, also known as vascular permeability factor or vasculotropin, plays a crucial role in regulating angiogenesis by exerting specific effects on vascular endothelial cells. The VEGF family comprises six homologues, including VEGF-A, -B, -C, -D, -E, and the placenta growth factor. Within the VEGF family, three receptors have been identified. VEGFR-1, encoded by the Flt-1 gene; VEGFR-2, encoded by the Flk-1/KDR gene; and VEGFR-3, encoded by the Flt-4 gene. These receptors are classified as transmembrane receptor tyrosine kinases [111]. VEGF expression varies among different tissues, including the heart, lymph nodes, placenta, and tumor tissues, during body development. The discovery of VEGF and its receptors in breast cancer cells indicates the presence of specific autocrine signaling pathways. These pathways can mediate the phosphorylation of VEGFR-1/2 or induce NRP1/2 signaling, promoting tumor cell proliferation, survival, and migration. Understanding these pathways is crucial in developing strategies to target VEGF signaling for therapeutic intervention in breast cancer [111-113].
Numerous studies have demonstrated the involvement of VEGF-A in promoting the survival of breast cancer cells. Blocking VEGF-A transcription, either through using VEGF siRNA or neutralizing antibodies, has been shown to induce apoptosis in tumor cells under both normal and low oxygen conditions [114]. This apoptotic effect in breast cancer cells involves downregulation of Bcl-2 expression, increased protein misfolding, and disruption of the PI3K pathway [115]. Moreover, VEGF binding to VEGF-R1 and VEGF-R2 sustains cancer cell survival. In cell models such as MCF-7 or MDA-MB-231, downregulatingVEGF-R2 or NRP-1 inhibits AKT phosphorylation, suppressing VEGF-R1 and decreasing cancer cell survival [116]. These findings shed light on the complex interplay between VEGF-A and its receptors in breast cancer cell survival and provide crucial insights for the development of therapeutic strategies aimed at targeting the VEGF signaling pathway. VEGF-A, -C, and -D are simultaneously expressed in various types of tumor cells and bind to receptors VEGFR-1, VEGFR-2, and VEGFR-3. This binding serves to protect lymphatic endothelial cells from apoptosis induced by the immune system while promoting the growth, proliferation, and migration of these cells. Consequently, lymphatic vessel formation is stimulated, leading to lymphatic metastasis in tumors [117-120]. Notably, VEGF-C and VEGF-D exhibit specific affinity for VEGFR-3, which triggers receptor phosphorylation. VEGF-C demonstrates a high affinity for lymphatic endothelial cells, thus inducing their proliferation and the formation of lymphatic sinuses. In different cancer types, VEGF-A binds to VEGFR-1 and VEGFR-2 to induce tumor angiogenesis, while VEGF-C and VEGF-D interact with VEGFR-3 to promote lymphatic vessel generation [121]. Recent studies involving 50 breast cancer patients focused on investigating the expression of VEGF-C, VEGFR-3, and angiopoietin-1 in cancerous tissue, along with their associations with different clinical and pathological characteristics, including metastasis [122]. The results revealed a robust correlation between microlymphatic vessel formation and both lymph node metastasis and VEGFR-3 expression [123]. Moreover, microvessel and microlymphatic vessel densities in breast cancer are crucial for tumor progression and lymph node metastasis. Notably, microlymphatic vessel density in adjacent tissues independently predicts lymph node metastasis. VEGF-C expression in lymphatic endothelial cells strongly correlates with lymphatic vessel formation and metastasis, highlighting its role in promoting lymphatic metastasis in breast cancer [124,125]. It is worth noting that although VEGF plays a vital role in promoting tumor angiogenesis, lymphangiogenesis, and immune modulation, the vessels and lymphatic vessels formed exhibit characteristics such as immaturity, leakiness, and inadequate support from the surrounding vasculature [126]. Targeting VEGF-C, VEGF-D, and their corresponding receptor VEGFR-3 shows promise in inhibiting tumor metastasis and improve prognosis in breast cancer [127,128]. These targeted interventions can suppress tumor vessel growth or promote apoptosis [129], holding potential for managing metastatic breast cancer and enhancing patient outcomes.
PDGFR: crucial role in tumor-stroma interaction
PDGFRs, belonging to the RTK superfamily, consist of PDGFR-α and PDGFR-β. These receptors share similar functions and play significant roles in early hematopoiesis, blood vessel formation, and organ development [130]. Both PDGFRα and PDGFRβ are crucial in both physiological and pathological conditions. The diverse binding patterns of PDGF to its receptors involve five different homodimeric or heterodimeric forms: PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC, and PDGF-DD. PDGF-AA specifically activates PDGFRα, while PDGF-BB activates PDGFRα, PDGFRα/β, and PDGFRβ [131]. PDGF-AB and PDGF-CC activate PDGFRα and PDGFRα/β, whereas PDGF-DD selectively activates its receptor, PDGFR-β. The interaction between PDGF and its receptors leads to the assembly of PDGFR subunits into dimers, which activates the intrinsic tyrosine kinase activity of the receptors [132]. Upon activation, PDGFR phosphorylates tyrosine residues on its substrates, initiating downstream signaling cascades that regulate cellular responses. These downstream signaling pathways can be divided into three categories based on their specific effects: (1) regulation of cell survival and growth, (2) regulation of cell invasion, blood vessel formation, and metastasis, and (3) regulation of EMT [133-135].
PDGF is derived from the stromal stem cells of the local tumor microenvironment. These stem cells generate various cytokines that interact with tumor cells, playing a crucial role in tumor initiation and progression [136]. PDGFs and PDGFRs are key regulators of cell growth and division, exerting significant impacts on malignant cells and the tumor microenvironment [124]. Dysregulation of PDGF signaling has been observed in various human malignancies, including prostate, lung, kidney, ovarian, brain, and pancreatic cancers. Overexpression of PDGF has been detected in the stromal cells of breast cancer, accompanied by the activation of the PI3K-AKT-mTOR signaling pathway [137]. Promising results have been demonstrated through combined treatment employing PDGFR tyrosine kinase inhibitors and mTOR inhibitors, showing potential in reducing stromal reactions and tumor proliferation, offering a novel therapeutic strategy for breast cancer [134]. PDGF plays a vital role in breast tumor invasion. Enhanced expression of PDGF promotes cell proliferation, inhibits apoptosis, and induces the expression of the CXCR4, thereby facilitating tumor growth and lymph node metastasis. Effective elimination of PDGF-induced lymph node metastasis can be achieved through blocking CXCR4 signaling pathway [138]. In breast cancer cells, there exists an interplay between PDGF, Notch, and NF-κB signaling pathways. Overexpressed PDGF leads to elevated NF-κB/Jagged-1 expression, where NF-κB activation influences Notch signaling, EMT, and tumor invasion. Downregulation of PDGFD can inhibit Notch and NF-κB pathways, partially reverse EMT, suppress cell growth, and induce apoptosis [135]. The interactions between tissue-resident stem cells and the cancer microenvironment also contribute to tumor progression. These stem cells secrete PDGF in a paracrine manner, inducing EMT in cancer cells. This PDGF-dependent mechanism promotes the expansion of cancer stem cell populations and facilitates tumor growth [139]. In summary, PDGF represents a promising therapeutic target for the treatment of breast cancer. The dysregulation of PDGF signaling in the tumor microenvironment and its interaction with tumor cells contribute to tumor initiation, progression, invasion, and metastasis [140]. Understanding the role of PDGF in breast cancer opens up opportunities for targeted therapeutic interventions aimed at disrupting PDGF signaling pathways and preventing tumor progression. Unveiling the crosstalk between PDGF, Notch, and NF-κB signaling pathways provides valuable insights into the mechanisms behind tumor invasion, EMT, and the regulation of cancer stem cells.
FGFR: aberrant expression in breast cancer
FGFRs are members of the RTK family. Encoded by the FGFR1, FGFR2, FGFR3, and FGFR4 genes, these receptors are primarily composed of single-chain glycoproteins [141]. Structurally, FGFRs consist of three main regions: an extracellular region, a transmembrane region, and an intracellular region with tyrosine kinase activity. FGFRs play a crucial role in regulating cell growth and division [142]. Upon binding with their specific ligands, FGFRs undergo receptor dimerization, leading to the activation of their tyrosine kinase activity. Activated tyrosine kinases serve as connection points between upstream signaling pathways and transmit signals to the intracellular environment, triggering various downstream signaling pathways [143]. These pathways include the MAPK, PI3K/AKT, STAT, and PLC-γ pathway. These pathways regulate gene expression and modulate cellular processes such as cell differentiation, proliferation, and the formation of tumors [144,145]. Numerous studies have demonstrated that FGFs and FGFRs play a crucial role in promoting cancer progression through diverse mechanisms. These mechanisms encompass inducing mitotic and survival signals, promoting EMT, invasion, and angiogenesis. The dysregulation of FGFR signaling has been implicated in various types of cancer, highlighting its potential as a viable therapeutic target for cancer treatment [145-148].
FGFRs and FGFs play a crucial role in breast development and tissue homeostasis regulation. An array of studies have identified a close association between ectopic expression of the FGFR family and the development of breast cancer [147]. Notably, approximately 10% of breast cancer patients exhibit amplification of the FGFR1 gene, a genetic alteration that has been linked with early recurrence and poor prognosis, particularly in ER-positive breast cancer [149]. Intriguingly, FGFR1 amplification is rarely observed in HER2-amplified breast cancer, suggesting a mutually exclusive activation of similar downstream signaling pathways between FGFR1 and HER2 [150]. FGFR1 amplification has been linked to endocrine therapy resistance. High rates of FGFR1 amplification are observed in breast cancer subtypes characterized by a high Ki-67 proliferation index and luminal B phenotype, emphasizing the significance of FGFR1 overexpression in predicting unfavorable outcomes. These findings provide a strong rationale for exploring targeted endocrine therapies against FGFR1 [151]. Recent research has increasingly associated FGFR2 with breast cancer, identifying it as one of the key non-inherited susceptibility genes, particularly in TNBC. Studies have reported FGFR2 gene amplification and overexpression in a specific subtype of TNBC, demonstrating its activation of the PI3K/AKT signaling pathway and subsequent inhibition of apoptosis. These findings underscore the potential of FGFR2 as a promising therapeutic target, especially in TNBC cases with FGFR2 amplification [152]. Besides, FGFR3 mutations have been implicated in various malignancies, such as multiple myeloma, cervical cancer, and bladder cancer, but their association with breast cancer remains limited [153]. Moreover, compelling evidence has pointed towards FGFR4 ectopic expression in human breast cancer, which is linked to chemoresistance in breast cancer. By using mouse breast cancer models has substantiated the role of FGFR4 in promoting tumor progression and metastasis [154]. Analyses of breast cancer cells that survived doxorubicin treatment have revealed an upregulation of FGFR4 expression, while interference with FGFR4 using antagonistic antibodies has demonstrated increased chemosensitivity in breast cancer cells expressing FGFR4. Collectively, these findings underscore FGFR4 as a significant factor influencing chemotherapy resistance and a promising therapeutic target for overcoming drug resistance [155]. Collectively, it is evident that FGFRs are mechanistically interlinked with the function and resistance of other RTKs, providing potential targets for breast cancer treatment.
IGFR: a high-risk factor for breast cancer
As a member of the IGFR family, IGF-1R is a ubiquitously expressed type 1 transmembrane heterotetrameric receptor consisting of two ligands, an extracellular α subunit and two β subunits, and ligand binding induces transphosphorylation of tyrosine within the TK domain by the dimeric subunit partner [156]. Phosphorylated residues act as docking sites for other signaling molecules, such as insulin receptor substrates 1 to 4 (IRS1 to IRS4) and adaptor protein SHC, which lead to the activation of the PI3K and MAPK pathways [157,158]. Under normal physiological conditions, the IGF system is tightly regulated, allowing for homeostatic growth. In tumor cells, these molecules are activated by mutations, chromosomal translocations, abnormal stimuli (autocrine, endocrine, or paracrine), or loss of genomic imprinting [159]. IGF-1R gene amplification has been reported in a variety of malignancies [160]. High concentrations of IGF-1 are present in several common cancers, including prostate cancer and premenopausal breast cancer, and higher blood concentrations of IGF-1 are associated with a higher risk of breast cancer in nonmenopausal women [161]. Therefore, IGF-IR can be a promising protein for specific and targeted therapeutics.
Current application of tyrosine kinase inhibitors in breast cancer treatment
Breast cancer is a heterogeneous disease which has been characterized molecularly into different subtypes depending on expression of ER, PR and HER2. For hormone receptor-positive breast cancer (luminal A and B), hormone therapy consists of selective estrogen receptor modulators (tamoxifen and raloxifene) is routinely used as adjuvant therapy [32,162]. Since TNBC or basal like and HER-enriched breast cancer do not express hormone receptors so that hormone therapy is not effective in these subtypes [53]. However, due to the prominent expression of RTKs in TNBC and HER2-enriched sub types, blocking the functions of RTKs is one of the promising approaches for management of TNBC and HER2-enriched breast cancer [42]. So far, various strategies have been adopted for inhibition of RTK-dependent signaling, and some are currently used in clinics (Table 3) [38,39,50,55,56,66,76,77,87,163-178].
Table 3.
Current anti-RTK therapy of breast cancer
| Molecule | Type | Target | Phase of study | Mechanism |
|---|---|---|---|---|
| Trastuzumab | Humanized mAb | HER | In clinical use | Inhibits HFR2 and HER3 dimerization, induces ADCC |
| Cetuximab | Chimeric mAb | EGFR | Phase I, II | Induces NK cell mediated ADCC |
| Panitumumab | Humanized mAb | EGFR | Phase II | Enhances sensitivity to DNA-damaging agents in TNBC |
| Nimotuzumab | Humanized mAb | EGFR | Phase I | Induces NK cell mediated ADCC |
| Necitumumab | Humanized mAb | EGFR | Phase II | Inhibits downstream targets in EGFR pathway, induces ADCC |
| Gefitinib | Reversible TKI | EGFR | Phase I, II | Reverses TAM resistance by up-regulating the ERα |
| Erlotinib | Reversible TKI | EGFR | Phase I, II | Suppresses CDK2 activity |
| Lapatinib | Reversible TKI | EGFR, HER2 | In clinical use | Used as an alternate therapy in HER2 positive breast cancer |
| Afatinib | Irreversible TKI | EGFR, HER2 | Phase II | Inhibits EGFR and HER2 signaling irreversibly |
| Varlitinib | Reversible TKI | EGFR, HER2, ErbB4 | Phase II | Inhibits HER/MAPK signaling in TNBC |
| Dacomitinib | Irreversible TKI | EGFR, HER2, ErbB4 | Phase I, Solid tumors | Inhibits HER2, EGFR, HER4, Akt and ERK phosphorylation |
| Sapitinib | Reversible TKI | EGFR, HER2, ErbB3 | Phase I, Solid tumors | Showed higher inhibitory potential in tamoxifen resistant breast cancer |
| Vandetanib | TKI | EGFR, VEGFR2-3, RET | Phase I, II | Targets angiogenesis by inhibiting VEGFR2 and 3 signaling along with EGFR pathway |
| Neratinob | Irreversible TKI | EGFR, HER2, ErbB4 | Phase I, II, III | Irreversibly blocks EGFR and HER2 pathway |
| BMS-690514 | Irreversible TKI | EGFR, HER2, ErbB4, VEGFR1-3 | Phase I, Solid tumors | Irreversibly blocks EGFR and HER2 pathway |
| AEE788 | Reversible TKI | EGFR, ErbB2, VEGFR | Phase I | Targets angiogenesis by inhibiting VEGFR2 and 3 signaling along with EGFR pathway |
| Lucitanib | TKI | FGFR1-2, PDGFRα/β, VEGFR1-3 | Phase II | Show anti-angiogenic and anti-tumoral activity by targeting FGFR and VEGFR |
Tyrosine kinase plays a pivotal role in tumor formation and progression. TKIs, which specifically target these kinases, have emerged as a focal point of cutting-edge research in molecular targeted therapy for combating tumors worldwide [47]. By effectively suppressing the biological activity of tyrosine kinases, TKIs disrupt the reparative mechanisms employed by tumor cells, leading to cell cycle arrest at the G1 phase, induction of apoptosis, inhibition of neovascularization, and the manifestation of anti-tumor effects through diverse signaling pathways [179,180]. Currently, TKIs utilized in breast cancer treatment can be classified into three main groups based on their specific targets: EGFR-targeting TKIs, VEGFR-targeting TKIs, and non-receptor TKIs.
Inhibitors of tyrosine kinase targeting EGFR
EGFR tyrosine kinase inhibitors (EGFR-TKIs) are small-molecule compounds that specifically target the EGFR [181-186]. Currently, there are two main classes of drugs used to target the EGFR pathway in cancer treatment: 1) EGFR monoclonal antibodies, represented by trastuzumab, which targets HER2. Trastuzumab interferes with ligand binding to HER2, inhibiting receptor dimerization [187-189]. Trastuzumab has achieved significant breakthroughs in breast cancer treatment and is considered the standard therapy for HER2+ breast cancer. However, some patients develop primary or acquired resistance to trastuzumab, still posing significant challenges in clinical management [190]; 2) EGFR-TKIs can penetrate cells and competitively repress the tyrosine kinase domain of EGFR. By binding to ATP, they inhibit autophosphorylation of EGFR, thereby blocking downstream signaling pathways mediated by EGFR, ultimately exerting anti-tumor effects [191]. Currently, several EGFR-TKIs are being studied for breast cancer treatment, including lapatinib, afatinib, gefitinib, erlotinib, and neratinib [192].
Inhibitors of tyrosine kinase targeting VEGFR
VEGFR Tyrosine Kinase Inhibitors (VEGFR-TKIs) are small-molecule compounds designed to specifically target the tyrosine kinase domain of VEGFR. Since angiogenesis plays a crucial role in tumor growth, invasion, and metastasis, inhibiting tumor angiogenesis has emerged as a promising approach for cancer treatment [191]. Existing anti-angiogenic therapies primarily focus on inhibiting the VEGF pathway using strategies such as VEGF monoclonal antibodies like bevacizumab, as well as VEGFR-TKIs sorafenib, sunitinib, and others [192-200]. Notably, VEGF mRNA expression has been identified in various tumors, including breast cancer. Bevacizumab has demonstrated efficacy in treating breast cancer in previous studies. Several novel VEGFR-TKIs are currently under investigation and in different stages of clinical trials, which mainly include sorafenib, sunitinib, axitinib, pazopanib, vandetanib, and others [201,202].
Non-receptor tyrosine kinase inhibitors
Among the non-receptor tyrosine kinase family, Src kinases have received significant attention. They could interact with various receptor proteins, regulating cell proliferation, differentiation, adhesion, motility, and angiogenesis. Blocking or regulating the binding of Src tyrosine kinases with these overexpressed receptors can keep tumor cell proliferation and invasion under control. Recently, TKIs such as dasatinib (BMS-354825), bosutinib (SKI-606), and saracatinib (AZD-0530) have emerged, working by competing for the ATP-binding site of Src tyrosine kinase. Numerous ongoing clinical studies hold the promise of offering a brighter future for targeted therapy in breast cancer [203].
Promising future development
Over the past two decades, a diverse range of RTKs targeted inhibitors have been developed and clinically evaluated to enhance cancer patient survival rates. In particular, the aberrant activation of RTKs has emerged as a potential therapeutic target, where molecular targeted inhibitors can hinder the activity of pathogenic tyrosine kinases. Further insights into genetics, cell biology, and structural biology have led to the development of novel treatment approaches. Disease-causing RTK mutations, deletions, translocations, and amplifications have been identified in breast cancer. Currently, therapies targeting RTKs involve both small molecule inhibitors and monoclonal antibodies, with ongoing research. The potential applications of RTKs in breast cancer treatment hold significant prospects for future advancements.
Targeting RTKs in TNBC treatment
TNBC is a highly heterogeneous subtype of breast cancer with the highest rates of recurrence and distant metastasis. Due to absence of both hormone receptors (HR) and HER2 protein, effective treatment for TNBC remain limited. Even immunotherapy has shown modest response rates of around 10-20% in patients. Particularly for patients with advanced-stage TNBC, chemotherapy remains the primary clinical treatment method. Therefore, the search for more effective breast cancer targets and treatment methods is of utmost importance.
Currently approved or clinically tested antibodies for breast cancer treatment can be classified into three main categories: 1) Monoclonal antibodies targeting tumor-surface antigens; 2) Immune checkpoint inhibitors represented by PD-1 and PD-L1 antibodies; 3) Antibody-drug conjugates (ADCs) [204]. Monoclonal antibodies targeting tumor-surface antigens primarily work by blocking the signaling pathways that promote tumor cell growth through binding to HER2 or other antigens on the tumor surface. This inhibits tumor growth or facilitates the destruction of tumor cells through antibody-dependent cellular cytotoxicity (ADCC). Various cell factor receptors such as EGFR, VEGFR, and FGFR are included in this category [205].
Research has shown that EGFR protein is frequently overexpressed in TNBC and serves as an independent prognostic indicator for disease-free and overall survival. EGFR can potentially be targeted using cetuximab and small-molecule TKIs [184]. Similarly, compared to non-TNBC patients, TNBC patients exhibit significantly higher levels of VEGF expression and have shorter disease-free survival periods [206]. Since angiogenesis is considered a key component driving tumor cell proliferation and survival, VEGF has emerged as a promising target for TNBC treatment [207]. A study evaluating bevacizumab, a monoclonal antibody targeting VEGF-A, as an adjunct therapy for TNBC showed improved immunotherapeutic effects [208]. Aberrant FGFR signaling, fueled by various genetic alterations including point mutations, activating mutations, fusions, rearrangements, and amplifications, plays a vital role in tumor progression. Therefore, FGFR is regarded as a potential target for breast cancer treatment [209]. While FGFR1 amplification is associated with poor prognosis in HR-positive breast cancer, its role in TNBC remains controversial. FGFR2 expression is correlated with poorer overall survival [210]. FGFR inhibitors have gained attention as one of the promising drugs. If TNBC patients can also benefit from FGFR inhibitors, it would significantly improve their survival rates. However, drug resistance in breast cancer patients to FGFR inhibitors is currently the major obstacle hindering clinical approval. Preclinical data have also investigated the efficacy of anti-FGFR isoform antibodies and FGFR inhibitors, showing promising results in Phase I clinical trials for solid tumors, including breast cancer [211].
Overall, targeting RTKs offers a potential therapeutic avenue for the treatment of TNBC. Further research and advancements in targeted therapies are crucial for enhancing the outcomes and survival rates of TNBC patients.
Alterations in RTKs in ER+ breast cancer with endocrine resistance
RTKs, including EGFR, HER2, IGFR, VEGFR, and FGFR, are activated upon ligand binding. These receptors are primarily involved in growth factors, cytokines, or hormones, and their activation or overexpression is associated with endocrine therapy resistance in ER+ breast cancer [212].
HER2 overexpression reduces the sensitivity to antiestrogen therapy, partly through activation of the PI3K-AKT-mTOR and MAPK pathways [213]. Additionally, HER2 expression depends on the NF-κB pathway, and in ERα-suppressed breast cancer circulating tumor cells, NF-κB signaling can increase HER2 expression. Thus, inhibiting the NF-κB pathway in combination with fulvestrant can restore the sensitivity of ER+/HER2- endocrine-resistant breast cancer cells to endocrine therapy [214]. The current treatment for ER+/HER2+ tumors involves combining estrogen targeting with HER2 inhibitors [215]. HER2 mutations are linked to acquired endocrine resistance and have been found in non-HER2 amplification metastatic breast cancer within 5% of endocrine therapy-resistant patients. ER+ breast cancer cells and xenografts that express HER2 mutations are resistant to estrogen deprivation or fulvestrant treatment and show poor response to HER2 TKI, lapatinib [216]. However, studies suggest that co-blocking HER2 and ER expression in breast cancer cells has a synergistic effect. Therefore, for patients with ER+ breast cancer and concurrent HER2 mutations, the combination of lapatinib and fulvestrant is a favorable choice [217].
EGFR amplification accounts for approximately 1.7% of endocrine-resistant metastatic breast cancer and can promote fulvestrant resistance. Co-administration of EGFR inhibitors can reverse this resistance [218-220]. In a cohort of 60 patients diagnosed with metastatic ER+ breast cancer, both before and after initiation of endocrine therapy, the comparison of whole exome sequencing data and circulating DNA analysis revealed that FGFR1 amplification accounted for 15%, FGFR2 amplification accounted for 5%, FGFR2 activating mutations accounted for 3.3%, and FGF3 amplification accounted for 28.3%. Immunohistochemical (IHC) staining and fluorescence in situ hybridization (FISH) showed that besides its typical membrane-bound intracellular signaling function, FGFR1 can also participate in endocrine resistance by regulating gene transcription in ER+ breast cancer [221]. The combination of FGFR inhibitors with fulvestrant can inhibit the growth of ER+/FGFR1-amplified cell lines and tumors. It was reported almost two decades ago that approximately 30% of breast tumors demonstrate elevated FGFR4 expression in comparison to normal tissues. Experimental studies suggest that FGFR4 may mediate acquired endocrine resistance in metastatic breast cancer [222]. Therefore, endocrine therapy in combination with novel FGFR inhibitors may offer new strategies for treating metastatic breast cancer.
Conclusion
Breast cancer is a multifactorial disease characterized by dysregulation of cellular signaling pathways due to genetic and epigenetic alterations. Numerous growth factors and their receptors, known as RTKs, are involved in the development and progression of cancer. Overexpression or dysregulation of RTKs in breast cancer cells activates downstream signaling pathways such as MAPK, PI3K/AKT, and JAK/STAT, promoting tumor growth, angiogenesis, and metastasis. The multifaceted role of RTKs makes them attractive targets for breast cancer treatment. In recent decades, significant progress has been made in understanding RTKs and targeted therapies through genomic technologies. Several drugs, including small molecule inhibitors and monoclonal antibodies, have been developed and approved for treating cancer by targeting RTK activation. While approved TKIs have led to tumor regression or prolonged survival, the lack of selectivity for individual targets and the drug resistance remain challenges. Furthermore, structural mutations, gene amplification, and alternative pathway activation pose challenges to anti-RTK therapy.
Despite research findings supporting the significance of RTK signaling as a therapeutic target in breast cancer, existing clinical trial data show modest efficacy of RTK inhibitors. The reasons for the lack of efficacy of RTK inhibitors in breast cancer patients are still inconclusive, whether it is due to drug ineffectiveness, insufficient patient selection, or a lack of oncogenic potential in RTK genomic variations. Before considering RTK signaling as a therapeutic target in breast cancer, the following issues need to be addressed: precise definition of RTK signaling abnormalities and identification of predictive biomarkers for response to RTK inhibitors; optimization of combinational strategies of RTK inhibitors with endocrine drugs or other targeted agents to enhance efficacy and reduce resistance; and development of more effective RTK inhibitors.
In conclusion, RTK signaling plays a crucial role in the pathogenesis of breast cancer, and targeted strategies against RTK signaling show promising prospects for treatment. However, further exploration is needed to appropriately block this signaling pathway in breast cancer patients to achieve optimal efficacy. Therefore, further research on acquired resistance in breast cancer is of great significance for developing novel therapeutic strategies against tumor recurrence.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81972490) and the Fundamental Research Funds for the Central Universities of Central South University (No. 2022ZZTS0877).
Disclosure of conflict of interest
None.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- ADCS
Antibody-drug conjugates
- ALK
Anaplastic lymphoma kinase
- BC
Breast cancer
- CXCR4
CXC chemokine receptor 4
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- ER
Estrogen receptor
- FDA
Food and Drug Administration
- FGFR
Fibroblast growth factor receptor
- FISH
Fluorescence in situ hybridization
- HR
Hormone receptors
- IBC
Inflammatory breast cancer
- IGFR
Insulin-like growth factor receptor
- IHC
Immunohistochemical
- JAK
Janus kinase
- MAPK
Mitogen-activated protein kinase
- MTC
Medullary thyroid carcinoma
- mTOR
Mammalian target of rapamycin
- NSCLC
Non-small cell lung cancer
- PDGFR
Platelet-derived growth factor receptor
- PI3K
Phosphoinositide 3-kinase
- PLC-γ
Phospholipase C-gamma
- PR
Progesterone receptor
- RTKs
Receptor tyrosine kinases
- SH
Src homology
- STAT
Signal transducer and activator of transcription
- TGF-α
Transforming growth factor-alpha
- TKI
Tyrosine kinase inhibitor
- TNBC
Triple-negative breast cancer
- VEGFR
Vascular endothelial growth factor receptor
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomiguchi M, Yamamoto Y, Yamamoto-Ibusuki M, Goto-Yamaguchi L, Fujiki Y, Fujiwara S, Sueta A, Hayashi M, Takeshita T, Inao T, Iwase H. Fibroblast growth factor receptor-1 protein expression is associated with prognosis in estrogen receptor-positive/human epidermal growth factor receptor-2-negative primary breast cancer. Cancer Sci. 2016;107:491–498. doi: 10.1111/cas.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D, Vortmeyer AO, Steinberg SM, Aldape K, Steeg PS. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 9.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarden Y, Shilo BZ. SnapShot: EGFR signaling pathway. Cell. 2007;131:1018. doi: 10.1016/j.cell.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Chen MK, Hung MC. Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases. FEBS J. 2015;282:3693–3721. doi: 10.1111/febs.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton AJ, Diez-Gonzalez L, Ace O, Vera-Badillo F, Seruga B, Jordán J, Amir E, Pandiella A, Ocaña A. Prognostic relevance of receptor tyrosine kinase expression in breast cancer: a meta-analysis. Cancer Treat Rev. 2014;40:1048–1055. doi: 10.1016/j.ctrv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Wise R, Zolkiewska A. Metalloprotease-dependent activation of EGFR modulates CD44(+)/CD24(-) populations in triple negative breast cancer cells through the MEK/ERK pathway. Breast Cancer Res Treat. 2017;166:421–433. doi: 10.1007/s10549-017-4440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Kim S, Joh J, Remick SC, Miller DM, Yan J, Kanaan Z, Chao JH, Krem MM, Basu SK, Hagiwara S, Kenner L, Moriggl R, Bunting KD, Tse W. MLLT11/AF1q boosts oncogenic STAT3 activity through Src-PDGFR tyrosine kinase signaling. Oncotarget. 2016;7:43960–43973. doi: 10.18632/oncotarget.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, Carragher NO, Munro A, Chang A, Bresnick AR, Lang RA, Pollard JW. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212:1433–1448. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, Greve B, El-Shinawi M, Mohamed MM, Götte M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. 2017;16:57. doi: 10.1186/s12943-017-0621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao D, Pan C, Sun J, Gilbert C, Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D, Creighton CJ, Slingerland JM. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene. 2015;34:3107–3119. doi: 10.1038/onc.2014.257. [DOI] [PubMed] [Google Scholar]
- 18.Neal JW, Sledge GW. Decade in review-targeted therapy: successes, toxicities and challenges in solid tumours. Nat Rev Clin Oncol. 2014;11:627–628. doi: 10.1038/nrclinonc.2014.171. [DOI] [PubMed] [Google Scholar]
- 19.Remon J, Morán T, Majem M, Reguart N, Dalmau E, Márquez-Medina D, Lianes P. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: a new era begins. Cancer Treat Rev. 2014;40:93–101. doi: 10.1016/j.ctrv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.He M, Wei MJ. Reversing multidrug resistance by tyrosine kinase inhibitors. Chin J Cancer. 2012;31:126–133. doi: 10.5732/cjc.011.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 23.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 24.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 25.Esteban-Villarrubia J, Soto-Castillo JJ, Pozas J, San Román-Gil M, Orejana-Martín I, Torres-Jiménez J, Carrato A, Alonso-Gordoa T, Molina-Cerrillo J. Tyrosine kinase receptors in oncology. Int J Mol Sci. 2020;21:8529. doi: 10.3390/ijms21228529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choura M, Rebaï A. Receptor tyrosine kinases: from biology to pathology. J Recept Signal Transduct Res. 2011;31:387–394. doi: 10.3109/10799893.2011.625425. [DOI] [PubMed] [Google Scholar]
- 28.Grassot J, Mouchiroud G, Perrière G. RTKdb: database of Receptor Tyrosine Kinase. Nucleic Acids Res. 2003;31:353–358. doi: 10.1093/nar/gkg036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 30.Trenker R, Jura N. Receptor tyrosine kinase activation: from the ligand perspective. Curr Opin Cell Biol. 2020;63:174–185. doi: 10.1016/j.ceb.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 32.Ebrahimi N, Fardi E, Ghaderi H, Palizdar S, Khorram R, Vafadar R, Ghanaatian M, Rezaei-Tazangi F, Baziyar P, Ahmadi A, Hamblin MR, Aref AR. Receptor tyrosine kinase inhibitors in cancer. Cell Mol Life Sci. 2023;80:104. doi: 10.1007/s00018-023-04729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 34.Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45:6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul MK, Mukhopadhyay AK. Tyrosine kinase - role and significance in cancer. Int J Med Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pytel D, Sliwinski T, Poplawski T, Ferriola D, Majsterek I. Tyrosine kinase blockers: new hope for successful cancer therapy. Anticancer Agents Med Chem. 2009;9:66–76. doi: 10.2174/187152009787047752. [DOI] [PubMed] [Google Scholar]
- 37.Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- 38.Siatis KE, Giannopoulou E, Manou D, Sarantis P, Karamouzis MV, Raftopoulou S, Fasseas K, Alzahrani FM, Kalofonos HP, Theocharis AD. Resistance to hormone therapy in breast cancer cells promotes autophagy and EGFR signaling pathway. Am J Physiol Cell Physiol. 2023;325:C708–C720. doi: 10.1152/ajpcell.00199.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda T, Tsubaki M, Matsuda T, Kimura A, Jinushi M, Obana T, Takegami M, Nishida S. EGFR inhibition reverses epithelial-mesenchymal transition, and decreases tamoxifen resistance via Snail and Twist downregulation in breast cancer cells. Oncol Rep. 2022;47:109. doi: 10.3892/or.2022.8320. [DOI] [PubMed] [Google Scholar]
- 40.Yamaoka T, Kusumoto S, Ando K, Ohba M, Ohmori T. Receptor tyrosine kinase-targeted cancer therapy. Int J Mol Sci. 2018;19:3491. doi: 10.3390/ijms19113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miraghel SA, Ebrahimi N, Khani L, Mansouri A, Jafarzadeh A, Ahmadi A, Aref AR. Crosstalk between non-coding RNAs expression profile, drug resistance and immune response in breast cancer. Pharmacol Res. 2022;176:106041. doi: 10.1016/j.phrs.2021.106041. [DOI] [PubMed] [Google Scholar]
- 42.Sudhesh Dev S, Zainal Abidin SA, Farghadani R, Othman I, Naidu R. Receptor tyrosine kinases and their signaling pathways as therapeutic targets of curcumin in cancer. Front Pharmacol. 2021;12:772510. doi: 10.3389/fphar.2021.772510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleuren EDG, Terry RL, Meyran D, Omer N, Trapani JA, Haber M, Neeson PJ, Ekert PG. Enhancing the potential of immunotherapy in paediatric sarcomas: breaking the immunosuppressive barrier with receptor tyrosine kinase inhibitors. Biomedicines. 2021;9:1798. doi: 10.3390/biomedicines9121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abella JV, Park M. Breakdown of endocytosis in the oncogenic activation of receptor tyrosine kinases. Am J Physiol Endocrinol Metab. 2009;296:E973–984. doi: 10.1152/ajpendo.90857.2008. [DOI] [PubMed] [Google Scholar]
- 45.Kam KW, Wong PPY, Young AL. Tyrosine kinase inhibitor-induced corneal ulcers. Lancet Oncol. 2019;20:e65. doi: 10.1016/S1470-2045(18)30520-5. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Zhang D, Guo Y, Lu B, Zhao ZJ, Xu X, Chen Y. Tyrosine kinase ROR1 as a target for anti-cancer therapies. Front Oncol. 2021;11:680834. doi: 10.3389/fonc.2021.680834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abbaspour Babaei M, Kamalidehghan B, Saleem M, Huri HZ, Ahmadipour F. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Devel Ther. 2016;10:2443–2459. doi: 10.2147/DDDT.S89114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia L, Zheng Z, Liu JY, Chen YJ, Ding J, Hu GS, Hu YH, Liu S, Luo WX, Xia NS, Liu W. Targeting triple-negative breast cancer with combination therapy of EGFR CAR T cells and CDK7 inhibition. Cancer Immunol Res. 2021;9:707–722. doi: 10.1158/2326-6066.CIR-20-0405. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Bhattacharya A, Peterson D, Li Y, Liu X, Marangoni E, Robila V, Zhang Y. Targeted dual degradation of HER2 and EGFR obliterates oncogenic signaling, overcomes therapy resistance, and inhibits metastatic lesions in HER2-positive breast cancer models. Drug Resist Updat. 2024;74:101078. doi: 10.1016/j.drup.2024.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boichuk S, Dunaev P, Mustafin I, Mani S, Syuzov K, Valeeva E, Bikinieva F, Galembikova A. Infigratinib (BGJ 398), a pan-FGFR inhibitor, targets P-glycoprotein and increases chemotherapeutic-induced mortality of multidrug-resistant tumor cells. Biomedicines. 2022;10:601. doi: 10.3390/biomedicines10030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernández-Nogueira P, Mancino M, Fuster G, López-Plana A, Jauregui P, Almendro V, Enreig E, Menéndez S, Rojo F, Noguera-Castells A, Bill A, Gaither LA, Serrano L, Recalde-Percaz L, Moragas N, Alonso R, Ametller E, Rovira A, Lluch A, Albanell J, Gascon P, Bragado P. Tumor-associated fibroblasts promote HER2-targeted therapy resistance through FGFR2 activation. Clin Cancer Res. 2020;26:1432–1448. doi: 10.1158/1078-0432.CCR-19-0353. [DOI] [PubMed] [Google Scholar]
- 52.Kähkönen TE, Toriseva M, Petruk N, Virta AR, Maher A, Eigéliené N, Kaivola J, Boström P, Koskivuo I, Nees M, Tuomela JM, Ivaska KK, Härkönen PL. Effects of FGFR inhibitors TKI258, BGJ398 and AZD4547 on breast cancer cells in 2D, 3D and tissue explant cultures. Cell Oncol (Dordr) 2021;44:205–218. doi: 10.1007/s13402-020-00562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rimel BJ, Crane EK, Hou J, Nakayama J, MacDonald J, Lutz K, Makker V, O’Cearbhaill RE. Tyrosine kinase inhibitor toxicities: a society of gynecologic oncology review and recommendations. Gynecol Oncol. 2023;174:148–156. doi: 10.1016/j.ygyno.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Kang J, Choi YJ, Seo BY, Jo U, Park SI, Kim YH, Park KH. A selective FGFR inhibitor AZD4547 suppresses RANKL/M-CSF/OPG-dependent ostoclastogenesis and breast cancer growth in the metastatic bone microenvironment. Sci Rep. 2019;9:8726. doi: 10.1038/s41598-019-45278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meric-Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau HT, Tran B, Kelley RK, Park JO, Javle M, He Y, Benhadji KA, Goyal L. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12:402–415. doi: 10.1158/2159-8290.CD-21-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales-Guadarrama G, Méndez-Pérez EA, García-Quiroz J, Avila E, Ibarra-Sánchez MJ, Esparza-López J, García-Becerra R, Larrea F, Díaz L. The inhibition of the FGFR/PI3K/Akt axis by AZD4547 disrupts the proangiogenic microenvironment and vasculogenic mimicry arising from the interplay between endothelial and triple-negative breast cancer cells. Int J Mol Sci. 2023;24:13770. doi: 10.3390/ijms241813770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdalla AN, Qattan A, Malki WH, Shahid I, Hossain MA, Ahmed M. Significance of targeting VEGFR-2 and cyclin D1 in luminal-a breast cancer. Molecules. 2020;25:4606. doi: 10.3390/molecules25204606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong X, Ren J, Amoozgar Z, Lee S, Datta M, Roberge S, Duquette M, Fukumura D, Jain RK. Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice. J Immunother Cancer. 2023;11:e005583. doi: 10.1136/jitc-2022-005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 60.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 61.Laird AD, Cherrington JM. Small molecule tyrosine kinase inhibitors: clinical development of anticancer agents. Expert Opin Investig Drugs. 2003;12:51–64. doi: 10.1517/13543784.12.1.51. [DOI] [PubMed] [Google Scholar]
- 62.Yook S, Cai Z, Jeong JJ, Lu Y, Winnik MA, Pignol JP, Reilly RM. Dual-receptor-targeted (DRT) radiation nanomedicine labeled with (177)Lu is more potent for killing human breast cancer cells that coexpress HER2 and EGFR than single-receptor-targeted (SRT) radiation nanomedicines. Mol Pharm. 2020;17:1226–1236. doi: 10.1021/acs.molpharmaceut.9b01259. [DOI] [PubMed] [Google Scholar]
- 63.O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 64.Huang Y, Xiong W, Ma L, Wu H. A cross-sectional study of the FDA approved indications and supporting pivotal trials of small-molecular kinase inhibitors in cancer therapies with the biomarker of cancer driver gene. Int J Cancer. 2022;151:2107–2114. doi: 10.1002/ijc.34222. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z, Tong LJ, Tang BY, Liu HY, Wang X, Zhang T, Cao XW, Chen Y, Li HL, Qian XH, Xu YF, Xie H, Ding J. C11, a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor, suppresses breast cancer metastasis and angiogenesis. Acta Pharmacol Sin. 2019;40:823–832. doi: 10.1038/s41401-018-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Qiu X, Wang X, Liu H, Geck RC, Tewari AK, Xiao T, Font-Tello A, Lim K, Jones KL, Morrow M, Vadhi R, Kao PL, Jaber A, Yerrum S, Xie Y, Chow KH, Cejas P, Nguyen QD, Long HW, Liu XS, Toker A, Brown M. FGFR-inhibitor-mediated dismissal of SWI/SNF complexes from YAP-dependent enhancers induces adaptive therapeutic resistance. Nat Cell Biol. 2021;23:1187–1198. doi: 10.1038/s41556-021-00781-z. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, Zhang S, Wang T, Shao H, Gao J, Wang Y, Ge Y. Neferine inhibits MDA-MB-231 cells growth and metastasis by regulating miR-374a/FGFR-2. Chem Biol Interact. 2019;309:108716. doi: 10.1016/j.cbi.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 68.Ostman A, Böhmer FD. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 2001;11:258–266. doi: 10.1016/s0962-8924(01)01990-0. [DOI] [PubMed] [Google Scholar]
- 69.Casaletto JB, McClatchey AI. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat Rev Cancer. 2012;12:387–400. doi: 10.1038/nrc3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDonell LM, Kernohan KD, Boycott KM, Sawyer SL. Receptor tyrosine kinase mutations in developmental syndromes and cancer: two sides of the same coin. Hum Mol Genet. 2015;24:R60–66. doi: 10.1093/hmg/ddv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wan G, Chen X, Gou R, Guan C, Chen J, Wang Q, Wu W, Chen H, Zhang Q, Wang H. Platelet membrane-based biochemotactic-targeting nanoplatform combining PDT with EGFR inhibition therapy for the treatment of breast cancer. Biomater Sci. 2024;12:691–709. doi: 10.1039/d3bm01627g. [DOI] [PubMed] [Google Scholar]
- 72.Szymczyk J, Czyrek A, Otlewski J, Zakrzewska M. FGF1 protects MCF-7 cells against taltobulin through both the MEKs/ERKs and PI3K/AKT signaling pathway. Biomedicines. 2023;11:1856. doi: 10.3390/biomedicines11071856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagan ML, Mander S, Joseph C, McGrath M, Barrett A, Lewis A, Hill WD, Browning D, McGee-Lawrence ME, Cai H, Liu K, Barrett JT, Gewirtz DA, Thangaraju M, Schoenlein PV. Upregulation of the EGFR/MEK1/MAPK1/2 signaling axis as a mechanism of resistance to antiestrogen-induced BimEL dependent apoptosis in ER(+) breast cancer cells. Int J Oncol. 2023;62:20. doi: 10.3892/ijo.2022.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russo GC, Crawford AJ, Clark D, Cui J, Carney R, Karl MN, Su B, Starich B, Lih TS, Kamat P, Zhang Q, Nair PR, Wu PH, Lee MH, Leong HS, Zhang H, Rebecca VW, Wirtz D. E-cadherin interacts with EGFR resulting in hyper-activation of ERK in multiple models of breast cancer. Oncogene. 2024;43:1445–1462. doi: 10.1038/s41388-024-03007-2. [DOI] [PubMed] [Google Scholar]
- 75.Pellecchia S, Franchini M, Viscido G, Arnese R, Gambardella G. Single cell lineage tracing reveals clonal dynamics of anti-EGFR therapy resistance in triple negative breast cancer. Genome Med. 2024;16:55. doi: 10.1186/s13073-024-01327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganesan K, Xu C, Wu J, Du B, Liu Q, Sui Y, Song C, Zhang J, Tang H, Chen J. Ononin inhibits triple-negative breast cancer lung metastasis by targeting the EGFR-mediated PI3K/Akt/mTOR pathway. Sci China Life Sci. 2024;67:1849–1866. doi: 10.1007/s11427-023-2499-2. [DOI] [PubMed] [Google Scholar]
- 77.Cheung A, Chenoweth AM, Johansson A, Laddach R, Guppy N, Trendell J, Esapa B, Mavousian A, Navarro-Llinas B, Haider S, Romero-Clavijo P, Hoffmann RM, Andriollo P, Rahman KM, Jackson P, Tsoka S, Irshad S, Roxanis I, Grigoriadis A, Thurston DE, Lord CJ, Tutt ANJ, Karagiannis SN. Anti-EGFR antibody-drug conjugate carrying an inhibitor targeting cdk restricts triple-negative breast cancer growth. Clin Cancer Res. 2024;30:3298–3315. doi: 10.1158/1078-0432.CCR-23-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forte L, Turdo F, Ghirelli C, Aiello P, Casalini P, Iorio MV, D’Ippolito E, Gasparini P, Agresti R, Belmonte B, Sozzi G, Sfondrini L, Tagliabue E, Campiglio M, Bianchi F. The PDGFRβ/ERK1/2 pathway regulates CDCP1 expression in triple-negative breast cancer. BMC Cancer. 2018;18:586. doi: 10.1186/s12885-018-4500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen L, Qi H, Zhang L, Li H, Shao J, Chen H, Zhong M, Shi X, Ye T, Li Q. Effects of FGFR gene polymorphisms on response and toxicity of cyclophosphamide-epirubicin-docetaxel-based chemotherapy in breast cancer patients. BMC Cancer. 2018;18:1038. doi: 10.1186/s12885-018-4951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camorani S, Hill BS, Collina F, Gargiulo S, Napolitano M, Cantile M, Di Bonito M, Botti G, Fedele M, Zannetti A, Cerchia L. Targeted imaging and inhibition of triple-negative breast cancer metastases by a PDGFRβ aptamer. Theranostics. 2018;8:5178–5199. doi: 10.7150/thno.27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh SB, Ross K, Isakoff SJ, Melkonjan N, He L, Matissek KJ, Schultz A, Mayer EL, Traina TA, Carey LA, Rugo HS, Liu MC, Stearns V, Langenbucher A, Saladi SV, Ramaswamy S, Lawrence MS, Ellisen LW. RASAL2 confers collateral MEK/EGFR dependency in chemoresistant triple-negative breast cancer. Clin Cancer Res. 2021;27:4883–4897. doi: 10.1158/1078-0432.CCR-21-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DiGiacomo JW, Godet I, Trautmann-Rodriguez M, Gilkes DM. Extracellular matrix-bound FGF2 mediates estrogen receptor signaling and therapeutic response in breast cancer. Mol Cancer Res. 2021;19:136–149. doi: 10.1158/1541-7786.MCR-20-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hassan RM, Ali IH, El Kerdawy AM, Abo-Elfadl MT, Ghannam IAY. Novel benzenesulfonamides as dual VEGFR2/FGFR1 inhibitors targeting breast cancer: design, synthesis, anticancer activity and in silico studies. Bioorg Chem. 2024;152:107728. doi: 10.1016/j.bioorg.2024.107728. [DOI] [PubMed] [Google Scholar]
- 84.Diep CH, Spartz A, Truong TH, Dwyer AR, El-Ashry D, Lange CA. Progesterone receptor signaling promotes cancer associated fibroblast mediated tumorigenicity in ER+ breast cancer. Endocrinology. 2024;165:bqae092. doi: 10.1210/endocr/bqae092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belli S, Esposito D, Ascione CM, Messina F, Napolitano F, Servetto A, De Angelis C, Bianco R, Formisano L. EGFR and HER2 hyper-activation mediates resistance to endocrine therapy and CDK4/6 inhibitors in ER+ breast cancer. Cancer Lett. 2024;593:216968. doi: 10.1016/j.canlet.2024.216968. [DOI] [PubMed] [Google Scholar]
- 86.Rajput PK, Varghese JF, Srivastava AK, Kumar U, Yadav UCS. Visfatin-induced upregulation of lipogenesis via EGFR/AKT/GSK3β pathway promotes breast cancer cell growth. Cell Signal. 2023;107:110686. doi: 10.1016/j.cellsig.2023.110686. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Zhang MZ, Zhang SJ, Sun X, Zhou C, Li J, Liu J, Feng J, Lu SY, Pei-Jun L, Wang JC. HIF-1α inhibitor YC-1 suppresses triple-negative breast cancer growth and angiogenesis by targeting PlGF/VEGFR1-induced macrophage polarization. Biomed Pharmacother. 2023;161:114423. doi: 10.1016/j.biopha.2023.114423. [DOI] [PubMed] [Google Scholar]
- 88.Hao XS, Feng PP, Zhang YY, Wang FZ, Wang GL, Fei HR. Scutebarbatine A induces ROS-mediated DNA damage and apoptosis in breast cancer cells by modulating MAPK and EGFR/Akt signaling pathway. Chem Biol Interact. 2023;378:110487. doi: 10.1016/j.cbi.2023.110487. [DOI] [PubMed] [Google Scholar]
- 89.Guo CH, Wang SY, Chung CH, Shih MY, Li WC, Chen PC, Lee SY, Hsia S. Selenium modulates AR/IGF-1R/EGFR and TROP2 signaling pathways and improves anticancer efficacy in murine mammary carcinoma 4T1. J Nutr Biochem. 2023;120:109417. doi: 10.1016/j.jnutbio.2023.109417. [DOI] [PubMed] [Google Scholar]
- 90.Zhou L, Li H, Sun T, Wen X, Niu C, Li M, Li W, Hoffman AR, Hu JF, Cui J. HULC targets the IGF1R-PI3K-AKT axis in trans to promote breast cancer metastasis and cisplatin resistance. Cancer Lett. 2022;548:215861. doi: 10.1016/j.canlet.2022.215861. [DOI] [PubMed] [Google Scholar]
- 91.Nafie MS, Boraei ATA. Exploration of novel VEGFR2 tyrosine kinase inhibitors via design and synthesis of new alkylated indolyl-triazole Schiff bases for targeting breast cancer. Bioorg Chem. 2022;122:105708. doi: 10.1016/j.bioorg.2022.105708. [DOI] [PubMed] [Google Scholar]
- 92.Lee HJ, Seo AN, Kim EJ, Jang MH, Kim YJ, Kim JH, Kim SW, Ryu HS, Park IA, Im SA, Gong G, Jung KH, Kim HJ, Park SY. Prognostic and predictive values of EGFR overexpression and EGFR copy number alteration in HER2-positive breast cancer. Br J Cancer. 2015;112:103–111. doi: 10.1038/bjc.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park HS, Jang MH, Kim EJ, Kim HJ, Lee HJ, Kim YJ, Kim JH, Kang E, Kim SW, Kim IA, Park SY. High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod Pathol. 2014;27:1212–1222. doi: 10.1038/modpathol.2013.251. [DOI] [PubMed] [Google Scholar]
- 94.Cicek E, Circir A, Oyken M, Akbulut Caliskan O, Dioken DN, Guntekin Ergun S, Cetin-Atalay R, Sapmaz A, Ovaa H, Sahin O, Erson-Bensan AE. EGF-SNX3-EGFR axis drives tumor progression and metastasis in triple-negative breast cancers. Oncogene. 2022;41:220–232. doi: 10.1038/s41388-021-02086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ 2nd. Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther. 2009;122:1–8. doi: 10.1016/j.pharmthera.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macdonald-Obermann JL, Pike LJ. Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J Biol Chem. 2014;289:26178–26188. doi: 10.1074/jbc.M114.586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weinberg F, Peckys DB, de Jonge N. EGFR expression in HER2-driven breast cancer cells. Int J Mol Sci. 2020;21:9008. doi: 10.3390/ijms21239008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Semba T, Manyam GC, Wang J, Shao S, Bertucci F, Finetti P, Krishnamurthy S, Phi LTH, Pearson T, Van Laere SJ, Burks JK, Cohen EN, Reuben JM, Yang F, Min H, Navin N, Trinh VN, Iwase T, Batra H, Shen Y, Zhang X, Tripathy D, Ueno NT. EGFR is a master switch between immunosuppressive and immunoactive tumor microenvironment in inflammatory breast cancer. Sci Adv. 2022;8:eabn7983. doi: 10.1126/sciadv.abn7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3’-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59:5475–5478. [PubMed] [Google Scholar]
- 101.Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017–8023. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 102.Zheng Z, Shao N, Weng H, Li W, Zhang J, Zhang L, Yang L, Ye S. Correlation between epidermal growth factor receptor and tumor stem cell markers CD44/CD24 and their relationship with prognosis in breast invasive ductal carcinoma. Med Oncol. 2015;32:275. doi: 10.1007/s12032-014-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky SH, Birnbaum D, Viens P, Wicha MS. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Laere SJ, Van der Auwera I, Van den Eynden GG, van Dam P, Van Marck EA, Vermeulen PB, Dirix LY. NF-kappaB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Br J Cancer. 2007;97:659–669. doi: 10.1038/sj.bjc.6603906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Reyes ME, Zhang D, Funakoshi Y, Trape AP, Gong Y, Kogawa T, Eckhardt BL, Masuda H, Pirman DA Jr, Yang P, Reuben JM, Woodward WA, Bartholomeusz C, Hortobagyi GN, Tripathy D, Ueno NT. EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer. Oncotarget. 2017;8:67904–67917. doi: 10.18632/oncotarget.18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian M, Schiemann WP. TGF-β stimulation of EMT programs elicits non-genomic ER-α activity and anti-estrogen resistance in breast cancer cells. J Cancer Metastasis Treat. 2017;3:150–160. doi: 10.20517/2394-4722.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeo SK, Wen J, Chen S, Guan JL. Autophagy differentially regulates distinct breast cancer stem-like cells in murine models via EGFR/Stat3 and Tgfβ/Smad signaling. Cancer Res. 2016;76:3397–3410. doi: 10.1158/0008-5472.CAN-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holdman XB, Welte T, Rajapakshe K, Pond A, Coarfa C, Mo Q, Huang S, Hilsenbeck SG, Edwards DP, Zhang X, Rosen JM. Upregulation of EGFR signaling is correlated with tumor stroma remodeling and tumor recurrence in FGFR1-driven breast cancer. Breast Cancer Res. 2015;17:141. doi: 10.1186/s13058-015-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 111.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 113.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 114.Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 115.Srabovic N, Mujagic Z, Mujanovic-Mustedanagic J, Softic A, Muminovic Z, Rifatbegovic A, Begic L. Vascular endothelial growth factor receptor-1 expression in breast cancer and its correlation to vascular endothelial growth factor a. Int J Breast Cancer. 2013;2013:746749. doi: 10.1155/2013/746749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kosaka Y, Kataoka A, Yamaguchi H, Ueo H, Akiyoshi S, Sengoku N, Kuranami M, Ohno S, Watanabe M, Mimori K, Mori M. Vascular endothelial growth factor receptor-1 mRNA overexpression in peripheral blood as a useful prognostic marker in breast cancer. Breast Cancer Res. 2012;14:R140. doi: 10.1186/bcr3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kapahi R, Guleria K, Sambyal V, Manjari M, Sudan M, Uppal MS, Singh NR. Association of VEGF and VEGFR1 polymorphisms with breast cancer risk in North Indians. Tumour Biol. 2015;36:4223–4234. doi: 10.1007/s13277-015-3059-1. [DOI] [PubMed] [Google Scholar]
- 118.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18:84. doi: 10.1186/s13058-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Incio J, Tam J, Rahbari NN, Suboj P, McManus DT, Chin SM, Vardam TD, Batista A, Babykutty S, Jung K, Khachatryan A, Hato T, Ligibel JA, Krop IE, Puchner SB, Schlett CL, Hoffmman U, Ancukiewicz M, Shibuya M, Carmeliet P, Soares R, Duda DG, Jain RK, Fukumura D. PlGF/VEGFR-1 signaling promotes macrophage polarization and accelerated tumor progression in obesity. Clin Cancer Res. 2016;22:2993–3004. doi: 10.1158/1078-0432.CCR-15-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen XW, Yu TJ, Zhang J, Li Y, Chen HL, Yang GF, Yu W, Liu YZ, Liu XX, Duan CF, Tang HL, Qiu M, Wang CL, Zheng H, Yue J, Guo AM, Yang J. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene. 2017;36:5045–5057. doi: 10.1038/onc.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo S, Colbert LS, Fuller M, Zhang Y, Gonzalez-Perez RR. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim Biophys Acta. 2010;1806:108–121. doi: 10.1016/j.bbcan.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pfister NT, Fomin V, Regunath K, Zhou JY, Zhou W, Silwal-Pandit L, Freed-Pastor WA, Laptenko O, Neo SP, Bargonetti J, Hoque M, Tian B, Gunaratne J, Engebraaten O, Manley JL, Børresen-Dale AL, Neilsen PM, Prives C. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulate VEGFR2 in breast cancer cells. Genes Dev. 2015;29:1298–1315. doi: 10.1101/gad.263202.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jahangiri A, Nguyen A, Chandra A, Sidorov MK, Yagnik G, Rick J, Han SW, Chen W, Flanigan PM, Schneidman-Duhovny D, Mascharak S, De Lay M, Imber B, Park CC, Matsumoto K, Lu K, Bergers G, Sali A, Weiss WA, Aghi MK. Cross-activating c-Met/β1 integrin complex drives metastasis and invasive resistance in cancer. Proc Natl Acad Sci U S A. 2017;114:E8685–E8694. doi: 10.1073/pnas.1701821114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 126.Tutunea-Fatan E, Majumder M, Xin X, Lala PK. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer. 2015;14:35. doi: 10.1186/s12943-015-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, Jindal S, Schedin P. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest. 2014;124:3901–3912. doi: 10.1172/JCI73777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen WS, Cao Z, Sugaya S, Lopez MJ, Sendra VG, Laver N, Leffler H, Nilsson UJ, Fu J, Song J, Xia L, Hamrah P, Panjwani N. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat Commun. 2016;7:11302. doi: 10.1038/ncomms11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Strell C, Folkvaljon D, Holmberg E, Schiza A, Thurfjell V, Karlsson P, Bergh J, Bremer T, Akslen LA, Wärnberg F, Östman A. High PDGFRb expression predicts resistance to radiotherapy in DCIS within the SweDCIS randomized trial. Clin Cancer Res. 2021;27:3469–3477. doi: 10.1158/1078-0432.CCR-20-4300. [DOI] [PubMed] [Google Scholar]
- 131.Thies KA, Hammer AM, Hildreth BE 3rd, Steck SA, Spehar JM, Kladney RD, Geisler JA, Das M, Russell LO, Bey JF 4th, Bolyard CM, Pilarski R, Trimboli AJ, Cuitiño MC, Koivisto CS, Stover DG, Schoenfield L, Otero J, Godbout JP, Chakravarti A, Ringel MD, Ramaswamy B, Li Z, Kaur B, Leone G, Ostrowski MC, Sizemore ST, Sizemore GM. Stromal platelet-derived growth factor receptor-β signaling promotes breast cancer metastasis in the brain. Cancer Res. 2021;81:606–618. doi: 10.1158/0008-5472.CAN-19-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal. 2013;11:97. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005;7:R788–795. doi: 10.1186/bcr1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhardwaj B, Klassen J, Cossette N, Sterns E, Tuck A, Deeley R, Sengupta S, Elliott B. Localization of platelet-derived growth factor beta receptor expression in the periepithelial stroma of human breast carcinoma. Clin Cancer Res. 1996;2:773–782. [PubMed] [Google Scholar]
- 135.Paulsson J, Sjöblom T, Micke P, Pontén F, Landberg G, Heldin CH, Bergh J, Brennan DJ, Jirström K, Ostman A. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am J Pathol. 2009;175:334–341. doi: 10.2353/ajpath.2009.081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu CP, Lusvarghi S, Wang JC, Hsiao SH, Huang YH, Hung TH, Ambudkar SV. Avapritinib: a selective inhibitor of KIT and PDGFRα that reverses ABCB1 and ABCG2-mediated multidrug resistance in cancer cell lines. Mol Pharm. 2019;16:3040–3052. doi: 10.1021/acs.molpharmaceut.9b00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jansson S, Aaltonen K, Bendahl PO, Falck AK, Karlsson M, Pietras K, Rydén L. The PDGF pathway in breast cancer is linked to tumour aggressiveness, triple-negative subtype and early recurrence. Breast Cancer Res Treat. 2018;169:231–241. doi: 10.1007/s10549-018-4664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jitariu AA, Raica M, Cîmpean AM, Suciu SC. The role of PDGF-B/PDGFR-BETA axis in the normal development and carcinogenesis of the breast. Crit Rev Oncol Hematol. 2018;131:46–52. doi: 10.1016/j.critrevonc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 139.Pinto MP, Dye WW, Jacobsen BM, Horwitz KB. Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Cancer. 2014;14:735. doi: 10.1186/1471-2407-14-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.D’Ippolito E, Plantamura I, Bongiovanni L, Casalini P, Baroni S, Piovan C, Orlandi R, Gualeni AV, Gloghini A, Rossini A, Cresta S, Tessari A, De Braud F, Di Leva G, Tripodo C, Iorio MV. miR-9 and miR-200 regulate PDGFRβ-mediated endothelial differentiation of tumor cells in triple-negative breast cancer. Cancer Res. 2016;76:5562–5572. doi: 10.1158/0008-5472.CAN-16-0140. [DOI] [PubMed] [Google Scholar]
- 141.Akhand SS, Chen H, Purdy SC, Liu Z, Anderson JC, Willey CD, Wendt MK. Fibroblast growth factor receptor facilitates recurrence of minimal residual disease following trastuzumab emtansine therapy. NPJ Breast Cancer. 2021;7:5. doi: 10.1038/s41523-020-00213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 143.Cheng Q, Ma Z, Shi Y, Parris AB, Kong L, Yang X. FGFR1 overexpression induces cancer cell stemness and enhanced Akt/Erk-ER signaling to promote palbociclib resistance in luminal A breast cancer cells. Cells. 2021;10:3008. doi: 10.3390/cells10113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tenhagen M, van Diest PJ, Ivanova IA, van der Wall E, van der Groep P. Fibroblast growth factor receptors in breast cancer: expression, downstream effects, and possible drug targets. Endocr Relat Cancer. 2012;19:R115–129. doi: 10.1530/ERC-12-0060. [DOI] [PubMed] [Google Scholar]
- 145.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 146.Courjal F, Cuny M, Simony-Lafontaine J, Louason G, Speiser P, Zeillinger R, Rodriguez C, Theillet C. Mapping of DNA amplifications at 15 chromosomal localizations in 1875 breast tumors: definition of phenotypic groups. Cancer Res. 1997;57:4360–4367. [PubMed] [Google Scholar]
- 147.Brunello E, Brunelli M, Bogina G, Caliò A, Manfrin E, Nottegar A, Vergine M, Molino A, Bria E, Massari F, Tortora G, Cingarlini S, Pedron S, Chilosi M, Zamboni G, Miller K, Martignoni G, Bonetti F. FGFR-1 amplification in metastatic lymph-nodal and haematogenous lobular breast carcinoma. J Exp Clin Cancer Res. 2012;31:103. doi: 10.1186/1756-9966-31-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover RN, Thomas G, Chanock SJ. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Formisano L, Stauffer KM, Young CD, Bhola NE, Guerrero-Zotano AL, Jansen VM, Estrada MM, Hutchinson KE, Giltnane JM, Schwarz LJ, Lu Y, Balko JM, Deas O, Cairo S, Judde JG, Mayer IA, Sanders M, Dugger TC, Bianco R, Stricker T, Arteaga CL. Correction: association of FGFR1 with ERα maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER(+) breast cancer. Clin Cancer Res. 2019;25:1433. doi: 10.1158/1078-0432.CCR-18-4268. [DOI] [PubMed] [Google Scholar]
- 150.Marian C, Ochs-Balcom HM, Nie J, Kallakury BV, Ambrosone CB, Trevisan M, Edge S, Shields PG, Freudenheim JL. FGFR2 intronic SNPs and breast cancer risk: associations with tumor characteristics and interactions with exogenous exposures and other known breast cancer risk factors. Int J Cancer. 2011;129:702–712. doi: 10.1002/ijc.25686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cerliani JP, Guillardoy T, Giulianelli S, Vaque JP, Gutkind JS, Vanzulli SI, Martins R, Zeitlin E, Lamb CA, Lanari C. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer Res. 2011;71:3720–3731. doi: 10.1158/0008-5472.CAN-10-3074. [DOI] [PubMed] [Google Scholar]
- 152.Cerliani JP, Vanzulli SI, Piñero CP, Bottino MC, Sahores A, Nuñez M, Varchetta R, Martins R, Zeitlin E, Hewitt SM, Molinolo AA, Lanari C, Lamb CA. Associated expressions of FGFR-2 and FGFR-3: from mouse mammary gland physiology to human breast cancer. Breast Cancer Res Treat. 2012;133:997–1008. doi: 10.1007/s10549-011-1883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnston CL, Cox HC, Gomm JJ, Coombes RC. Fibroblast growth factor receptors (FGFRs) localize in different cellular compartments. A splice variant of FGFR-3 localizes to the nucleus. J Biol Chem. 1995;270:30643–30650. doi: 10.1074/jbc.270.51.30643. [DOI] [PubMed] [Google Scholar]
- 154.Koziczak M, Hynes NE. Cooperation between fibroblast growth factor receptor-4 and ErbB2 in regulation of cyclin D1 translation. J Biol Chem. 2004;279:50004–50011. doi: 10.1074/jbc.M404252200. [DOI] [PubMed] [Google Scholar]
- 155.Brown WS, Akhand SS, Wendt MK. FGFR signaling maintains a drug persistent cell population following epithelial-mesenchymal transition. Oncotarget. 2016;7:83424–83436. doi: 10.18632/oncotarget.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim SY, Toretsky JA, Scher D, Helman LJ. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 158.Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;22:6589–6597. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- 159.Krassas GE, Pontikides N, Kaltsas T, Dumas A, Frystyk J, Chen JW, Flyvbjerg A. Free and total insulin-like growth factor (IGF)-I, -II, and IGF binding protein-1, -2, and -3 serum levels in patients with active thyroid eye disease. J Clin Endocrinol Metab. 2003;88:132–135. doi: 10.1210/jc.2002-021349. [DOI] [PubMed] [Google Scholar]
- 160.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 161.Duggan C, Wang CY, Neuhouser ML, Xiao L, Smith AW, Reding KW, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R, McTiernan A. Associations of insulin-like growth factor and insulin-like growth factor binding protein-3 with mortality in women with breast cancer. Int J Cancer. 2013;132:1191–1200. doi: 10.1002/ijc.27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Y, Min J, Deng X, Feng T, Hu H, Guo X, Cheng Y, Xie B, Yang Y, Chen CC, Guo RT, Dong C, Zhou HB. Discovery of novel covalent selective estrogen receptor degraders against endocrine-resistant breast cancer. Acta Pharm Sin B. 2023;13:4963–4982. doi: 10.1016/j.apsb.2023.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liang Y, Liu J, Ge J, Shi Q, Zhang G, Wan A, Luo T, Tian H, Fan L, Wang S, Chen L, Tang P, Zhu K, Jiang J, Bian X, Zhang Y, Qi X. Safety and efficacy of anlotinib combined with taxane and lobaplatin in neoadjuvant treatment of clinical stage II/III triple-negative breast cancer in China (the neoALTAL trial): a single-arm, phase 2 trial. EClinicalMedicine. 2024;71:102585. doi: 10.1016/j.eclinm.2024.102585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krzyscik MA, Porębska N, Opaliński Ł, Otlewski J. Targeting HER2 and FGFR-positive cancer cells with a bispecific cytotoxic conjugate combining anti-HER2 Affibody and FGF2. Int J Biol Macromol. 2024;254:127657. doi: 10.1016/j.ijbiomac.2023.127657. [DOI] [PubMed] [Google Scholar]
- 165.Wang K, Yu Y, Wang W, Jiang Y, Li Y, Jiang X, Qiao Y, Chen L, Zhao X, Liu J, Yang A, Li J, Zhang R. Targeting the E3 ligase NEDD4 as a novel therapeutic strategy for IGF1 signal pathway-driven gastric cancer. Oncogene. 2023;42:1072–1087. doi: 10.1038/s41388-023-02619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saridogan T, Akcakanat A, Zhao M, Evans KW, Yuca E, Scott S, Kirby BP, Zheng X, Ha MJ, Chen H, Ng PKS, DiPeri TP, Mills GB, Rodon Ahnert J, Damodaran S, Meric-Bernstam F. Efficacy of futibatinib, an irreversible fibroblast growth factor receptor inhibitor, in FGFR-altered breast cancer. Sci Rep. 2023;13:20223. doi: 10.1038/s41598-023-46586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rajoria B, Zhang X, Yee D. IGF-1 stimulates glycolytic ATP production in MCF-7L cells. Int J Mol Sci. 2023;24:10209. doi: 10.3390/ijms241210209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.He Q, Kong L, Shi W, Ma D, Liu K, Yang S, Xin Q, Jiang C, Wu J. Ezetimibe inhibits triple-negative breast cancer proliferation and promotes cell cycle arrest by targeting the PDGFR/AKT pathway. Heliyon. 2023;9:e21343. doi: 10.1016/j.heliyon.2023.e21343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duan Z, Li Z, Wang Z, Chen C, Luo Y. Chimeric antigen receptor macrophages activated through TLR4 or IFN-γ receptors suppress breast cancer growth by targeting VEGFR2. Cancer Immunol Immunother. 2023;72:3243–3257. doi: 10.1007/s00262-023-03490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Baammi S, El Allali A, Daoud R. Potent VEGFR-2 inhibitors for resistant breast cancer: a comprehensive 3D-QSAR, ADMET, molecular docking and MMPBSA calculation on triazolopyrazine derivatives. Front Mol Biosci. 2023;10:1288652. doi: 10.3389/fmolb.2023.1288652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu X, Seraia E, Hatch SB, Wan X, Ebner DV, Aroldi F, Jiang Y, Ryan AJ, Bogenrieder T, Weyer-Czernilofsky U, Rieunier G, Macaulay VM. CHK1 inhibition exacerbates replication stress induced by IGF blockade. Oncogene. 2022;41:476–488. doi: 10.1038/s41388-021-02080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wester L, Venneker S, Hazenoot M, Pont C, Koedoot E, Timmermans AM, Martens JWM, Jansen MPHM, Kockx CEM, van IJcken WFJ, Meerman JHN, Zhang Y, van de Water B. A kinase inhibitor screen reveals MEK1/2 as a novel therapeutic target to antagonize IGF1R-mediated antiestrogen resistance in ERα-positive luminal breast cancer. Biochem Pharmacol. 2022;204:115233. doi: 10.1016/j.bcp.2022.115233. [DOI] [PubMed] [Google Scholar]
- 173.Chen L, Jiang YZ, Wu SY, Wu J, Di GH, Liu GY, Yu KD, Fan L, Li JJ, Hou YF, Hu Z, Chen CM, Huang XY, Cao AY, Hu X, Zhao S, Ma XY, Xu Y, Sun XJ, Chai WJ, Guo X, Chen X, Xu Y, Zhu XY, Zou JJ, Yang WT, Wang ZH, Shao ZM. Famitinib with camrelizumab and nab-paclitaxel for advanced immunomodulatory triple-negative breast cancer (FUTURE-C-Plus): an open-label, single-arm, phase II trial. Clin Cancer Res. 2022;28:2807–2817. doi: 10.1158/1078-0432.CCR-21-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abdelmalek CM, Hu Z, Kronenberger T, Küblbeck J, Kinnen FJM, Hesse SS, Malik A, Kudolo M, Niess R, Gehringer M, Zender L, Witt-Enderby PA, Zlotos DP, Laufer SA. Gefitinib-tamoxifen hybrid ligands as potent agents against triple-negative breast cancer. J Med Chem. 2022;65:4616–4632. doi: 10.1021/acs.jmedchem.1c01646. [DOI] [PubMed] [Google Scholar]
- 175.Tian M, Chen K, Huang J, Chu D, Li J, Huang K, Ma C. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother Res. 2021;35:6389–6400. doi: 10.1002/ptr.7292. [DOI] [PubMed] [Google Scholar]
- 176.Shin SU, Cho HM, Das R, Gil-Henn H, Ramakrishnan S, Al Bayati A, Carroll SF, Zhang Y, Sankar AP, Elledge C, Pimentel A, Blonska M, Rosenblatt JD. Inhibition of vasculogenic mimicry and angiogenesis by an anti-EGFR IgG1-human endostatin-P125A fusion protein reduces triple negative breast cancer metastases. Cells. 2021;10:2904. doi: 10.3390/cells10112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ni H, Guo M, Zhang X, Jiang L, Tan S, Yuan J, Cui H, Min Y, Zhang J, Schlisio S, Ma C, Liao W, Nister M, Chen C, Li S, Li N. VEGFR2 inhibition hampers breast cancer cell proliferation via enhanced mitochondrial biogenesis. Cancer Biol Med. 2021;18:139–154. doi: 10.20892/j.issn.2095-3941.2020.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grünewald S, Politz O, Bender S, Héroult M, Lustig K, Thuss U, Kneip C, Kopitz C, Zopf D, Collin MP, Boemer U, Ince S, Ellinghaus P, Mumberg D, Hess-Stumpp H, Ziegelbauer K. Rogaratinib: a potent and selective pan-FGFR inhibitor with broad antitumor activity in FGFR-overexpressing preclinical cancer models. Int J Cancer. 2019;145:1346–1357. doi: 10.1002/ijc.32224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311–319. [PubMed] [Google Scholar]
- 180.Joo WD, Visintin I, Mor G. Targeted cancer therapy--are the days of systemic chemotherapy numbered? Maturitas. 2013;76:308–314. doi: 10.1016/j.maturitas.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sooro MA, Zhang N, Zhang P. Targeting EGFR-mediated autophagy as a potential strategy for cancer therapy. Int J Cancer. 2018;143:2116–2125. doi: 10.1002/ijc.31398. [DOI] [PubMed] [Google Scholar]
- 182.Yamaoka T, Ohba M, Ohmori T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J Mol Sci. 2017;18:2420. doi: 10.3390/ijms18112420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 185.Metro G, Finocchiaro G, Cappuzzo F. Anti-cancer therapy with EGFR inhibitors: factors of prognostic and predictive significance. Ann Oncol. 2006;17(Suppl 2):ii42–45. doi: 10.1093/annonc/mdj920. [DOI] [PubMed] [Google Scholar]
- 186.Johnston JB, Navaratnam S, Pitz MW, Maniate JM, Wiechec E, Baust H, Gingerich J, Skliris GP, Murphy LC, Los M. Targeting the EGFR pathway for cancer therapy. Curr Med Chem. 2006;13:3483–3492. doi: 10.2174/092986706779026174. [DOI] [PubMed] [Google Scholar]
- 187.Giaccone G, González-Larriba JL, van Oosterom AT, Alfonso R, Smit EF, Martens M, Peters GJ, van der Vijgh WJ, Smith R, Averbuch S, Fandi A. Combination therapy with gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, gemcitabine and cisplatin in patients with advanced solid tumors. Ann Oncol. 2004;15:831–838. doi: 10.1093/annonc/mdh188. [DOI] [PubMed] [Google Scholar]
- 188.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Cetuximab and panitumumab in KRAS wild-type colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2011;26:823–833. doi: 10.1007/s00384-011-1149-0. [DOI] [PubMed] [Google Scholar]
- 189.Rocha-Lima CM, Soares HP, Raez LE, Singal R. EGFR targeting of solid tumors. Cancer Control. 2007;14:295–304. doi: 10.1177/107327480701400313. [DOI] [PubMed] [Google Scholar]
- 190.Tomasello C, Baldessari C, Napolitano M, Orsi G, Grizzi G, Bertolini F, Barbieri F, Cascinu S. Resistance to EGFR inhibitors in non-small cell lung cancer: clinical management and future perspectives. Crit Rev Oncol Hematol. 2018;123:149–161. doi: 10.1016/j.critrevonc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 191.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 192.Díaz-Serrano A, Gella P, Jiménez E, Zugazagoitia J, Paz-Ares Rodríguez L. Targeting EGFR in lung cancer: current standards and developments. Drugs. 2018;78:893–911. doi: 10.1007/s40265-018-0916-4. [DOI] [PubMed] [Google Scholar]
- 193.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20:660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ilic I, Jankovic S, Ilic M. Bevacizumab combined with chemotherapy improves survival for patients with metastatic colorectal cancer: evidence from meta analysis. PLoS One. 2016;11:e0161912. doi: 10.1371/journal.pone.0161912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 196.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 197.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 198.Tabernero J, Takayuki Y, Cohn AL. Correction to Lancet Oncol 2015; 16: 499-508. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:e262. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 199.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Pérol M. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 200.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 201.Al-Sanea MM, Hamdi A, Mohamed AAB, El-Shafey HW, Moustafa M, Elgazar AA, Eldehna WM, Ur Rahman H, Parambi DGT, Elbargisy RM, Selim S, Bukhari SNA, Magdy Hendawy O, Tawfik SS. New benzothiazole hybrids as potential VEGFR-2 inhibitors: design, synthesis, anticancer evaluation, and in silico study. J Enzyme Inhib Med Chem. 2023;38:2166036. doi: 10.1080/14756366.2023.2166036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li Y, Liu Y, Zhang D, Chen J, Yang G, Tang P, Yang C, Liu J, Zhang J, Ouyang L. Discovery, synthesis, and evaluation of novel dual inhibitors of a vascular endothelial growth factor receptor and Poly(ADP-Ribose) polymerase for BRCA wild-type breast cancer therapy. J Med Chem. 2023;66:12069–12100. doi: 10.1021/acs.jmedchem.3c00640. [DOI] [PubMed] [Google Scholar]
- 203.Levêque D, Becker G, Bilger K, Natarajan-Amé S. Clinical pharmacokinetics and pharmacodynamics of dasatinib. Clin Pharmacokinet. 2020;59:849–856. doi: 10.1007/s40262-020-00872-4. [DOI] [PubMed] [Google Scholar]
- 204.Weiss J, Glode A, Messersmith WA, Diamond J. Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer. Expert Rev Anticancer Ther. 2019;19:673–679. doi: 10.1080/14737140.2019.1654378. [DOI] [PubMed] [Google Scholar]
- 205.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 206.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 207.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 208.Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC, Hershberger VS, Pauly-Evers M, Sadikhov S, Szczesny P, Schwab D, Nogoceke E, Osborne A, Weikert R, Fauser S. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 209.Wu Y, Yi Z, Li J, Wei Y, Feng R, Liu J, Huang J, Chen Y, Wang X, Sun J, Yin X, Li Y, Wan J, Zhang L, Huang J, Du H, Wang X, Li Q, Ren G, Li H. FGFR blockade boosts T cell infiltration into triple-negative breast cancer by regulating cancer-associated fibroblasts. Theranostics. 2022;12:4564–4580. doi: 10.7150/thno.68972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dey N, Williams C, Leyland-Jones B, De P. Mutation matters in precision medicine: a future to believe in. Cancer Treat Rev. 2017;55:136–149. doi: 10.1016/j.ctrv.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 211.Shao F, Sun H, Deng CX. Potential therapeutic targets of triple-negative breast cancer based on its intrinsic subtype. Oncotarget. 2017;8:73329–73344. doi: 10.18632/oncotarget.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.García-Becerra R, Santos N, Díaz L, Camacho J. Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance. Int J Mol Sci. 2012;14:108–145. doi: 10.3390/ijms14010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rugo HS, Vidula N, Ma C. Improving response to hormone therapy in breast cancer: new targets, new therapeutic options. Am Soc Clin Oncol Educ Book. 2016;35:e40–54. doi: 10.1200/EDBK_159198. [DOI] [PubMed] [Google Scholar]
- 214.Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, Révil C, Jones A. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J. Clin. Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 215.Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 216.Guarneri V, Generali DG, Frassoldati A, Artioli F, Boni C, Cavanna L, Tagliafico E, Maiorana A, Bottini A, Cagossi K, Bisagni G, Piacentini F, Ficarra G, Bettelli S, Roncaglia E, Nuzzo S, Swaby R, Ellis C, Holford C, Conte P. Double-blind, placebo-controlled, multicenter, randomized, phase IIb neoadjuvant study of letrozole-lapatinib in postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative, operable breast cancer. J. Clin. Oncol. 2014;32:1050–1057. doi: 10.1200/JCO.2013.51.4737. [DOI] [PubMed] [Google Scholar]
- 217.Kalinsky K, Accordino MK, Chiuzan C, Mundi PS, Sakach E, Sathe C, Ahn H, Trivedi MS, Novik Y, Tiersten A, Raptis G, Baer LN, Oh SY, Zelnak AB, Wisinski KB, Andreopoulou E, Gradishar WJ, Stringer-Reasor E, Reid SA, O’Dea A, O’Regan R, Crew KD, Hershman DL. Randomized phase II trial of endocrine therapy with or without ribociclib after progression on cyclin-dependent kinase 4/6 inhibition in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: MAINTAIN trial. J. Clin. Oncol. 2023;41:4004–4013. doi: 10.1200/JCO.22.02392. [DOI] [PubMed] [Google Scholar]
- 218.Zhang X, Zhang B, Liu J, Liu J, Li C, Dong W, Fang S, Li M, Song B, Tang B, Wang Z, Zhang Y. Mechanisms of Gefitinib-mediated reversal of tamoxifen resistance in MCF-7 breast cancer cells by inducing ERα re-expression. Sci Rep. 2015;5:7835. doi: 10.1038/srep07835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Morrison G, Fu X, Shea M, Nanda S, Giuliano M, Wang T, Klinowska T, Osborne CK, Rimawi MF, Schiff R. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res Treat. 2014;144:263–272. doi: 10.1007/s10549-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnston S, Basik M, Hegg R, Lausoontornsiri W, Grzeda L, Clemons M, Dreosti L, Mann H, Stuart M, Cristofanilli M. Inhibition of EGFR, HER2, and HER3 signaling with AZD8931 in combination with anastrozole as an anticancer approach: phase II randomized study in women with endocrine-therapy-naïve advanced breast cancer. Breast Cancer Res Treat. 2016;160:91–99. doi: 10.1007/s10549-016-3979-5. [DOI] [PubMed] [Google Scholar]
- 221.Formisano L, Stauffer KM, Young CD, Bhola NE, Guerrero-Zotano AL, Jansen VM, Estrada MM, Hutchinson KE, Giltnane JM, Schwarz LJ, Lu Y, Balko JM, Deas O, Cairo S, Judde JG, Mayer IA, Sanders M, Dugger TC, Bianco R, Stricker T, Arteaga CL. Association of FGFR1 with ERα maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER(+) breast cancer. Clin Cancer Res. 2017;23:6138–6150. doi: 10.1158/1078-0432.CCR-17-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Servetto A, Formisano L, Arteaga CL. FGFR signaling and endocrine resistance in breast cancer: challenges for the clinical development of FGFR inhibitors. Biochim Biophys Acta Rev Cancer. 2021;1876:188595. doi: 10.1016/j.bbcan.2021.188595. [DOI] [PMC free article] [PubMed] [Google Scholar]