Abstract
Ototoxicity is an often-underestimated sequela for cancer patients undergoing chemotherapy, with an incidence rate exceeding 50%, affecting approximately 4 million individuals worldwide each year. Despite the nearly 2,000 publications on chemotherapy-related ototoxicity in the past decade, the understanding of its prevalence, mechanisms, and preventative or therapeutic measures remains ambiguous and subject to debate. To date, only one drug, sodium thiosulfate, has gained FDA approval for treating ototoxicity in chemotherapy. However, its utilization is restricted. This review aims to offer clinicians and researchers a comprehensive perspective by thoroughly and carefully reviewing available data and current evidence. Chemotherapy-induced ototoxicity is characterized by four primary symptoms: hearing loss, tinnitus, vertigo, and dizziness, originating from both auditory and vestibular systems. Hearing loss is the predominant symptom. Amongst over 700 chemotherapeutic agents documented in various databases, only seven are reported to induce hearing loss. While the molecular mechanisms of the hearing loss caused by the two platinum-based drugs are extensively explored, the pathways behind the action of the other five drugs are primarily speculative, rooted in their therapeutic properties and side effects. Cisplatin attracts the majority of attention among these drugs, encompassing around two-thirds of the literature regarding ototoxicity in chemotherapy. Cisplatin ototoxicity chiefly manifests through the loss of outer hair cells, possibly resulting from damages directly by cisplatin uptake or secondary effects on the stria vascularis. Both direct and indirect influences contribute to cisplatin ototoxicity, while it is still debated which path is dominant or where the primary target of cisplatin is located. Candidates for hearing protection against cisplatin ototoxicity are also discussed, with novel strategies and methods showing promise on the horizon.
Keywords: Inner ear, cochlea, cisplatin, ototoxicity, hearing protection
Introduction
Antineoplastic or chemotherapeutic drugs for cancer treatment can also harm normal tissues and cells, leading to various adverse effects [1]. Ototoxicity is a notable sequala of cancer treatment, impacting hearing and balance with symptoms including hearing loss, vertigo, dizziness, and tinnitus [2-5]. The severity of these symptoms varies and is influenced by factors including the age of the patient, the specific chemotherapeutic agent used, dosage, and administration method [1,4,5]. Although ototoxicity in chemotherapy is not life-threatening, its consequences, such as communication difficulties, social isolation, depression, and fatigue, can significantly impair the quality of life [6,7]. Furthermore, hearing loss has been suggested as a significant modifiable risk factor for dementia [8]. In pediatric patients, hearing loss is even more devastating as it can delay the development of speech and language abilities, communication skills, and impede cognitive maturation [9].
To determine the ototoxic potential and mechanisms of chemotherapeutic drugs and shed light on emerging treatment compounds and approaches, we performed a comprehensive review of literature and databases on cancer treatment drugs. A PubMed search revealed nearly 2,000 publications on chemotherapy-associated ototoxicity in the last decade. Among these publications, two-thirds are related to platinum-based agents used in 10-20% of the chemotherapy regimens. However, the mechanisms underlying the ototoxicity of chemotherapeutic drugs remain unclear, and effective strategies to mitigate this side effect are still in development. This review discusses the tentative mechanisms for ototoxicity and advances in hearing protection during cancer treatment, highlighting promising drugs and methodologies recently developed. Our objective is to provide clinicians who treat cancer with a foundational understanding of ototoxicity in chemotherapy. We also offer researchers insight into in-depth mechanisms and potential innovative protective strategies against it. Currently, sodium thiosulfate (STS) is the only FDA-approved drug to mitigate hearing loss during cancer treatment in pediatric patients [10].
Chemotherapeutic drugs affecting the inner ear
To identify compounds/drugs with potentially damaging effects on the inner ear during cancer therapy, we searched the online databases from the websites of the National Cancer Institute (NCI) [2], Chemocare [3], and Beaumont [4] using the search terms hearing loss, tinnitus, dizziness, and vertigo. Out of the approximately 700 compounds used in cancer treatment, only seven have listed hearing loss as a side effect (Table 1) [2-4].
Table 1.
Ototoxic chemotherapeutic drugs and their incidence of symptoms
| Drug name | Cisplatin | Carboplatin | Vinblastine | Vincristine | Dasatinib | isotretinoin | Tretinoin | |
|---|---|---|---|---|---|---|---|---|
| Drug Class | Alkylating agent | Plant alkaloid | Vinca alkaloid | Tyrosine kinase inhibitor | Retinoid | Retinoid | ||
| Antineoplastic Mechanism | DNA binding and cross-linking | microtubule inhibitor | Src kinase inhibitor | microtubule inhibitor | ||||
| Protein cross-linking | ||||||||
| Ototoxic Mechanism | ROS overload, proinflammatory cytokine production | Possible synergy with other drugs | Unclear. Possibly associated with their antitumor activity | |||||
| Incidence rate | Hearing loss | 31% | 12% | 10-29% | No data | 0.1-1% | No data | 6% |
| Tinnitus | 31% | 12% | No data | No data | 1-10% | No data | No data | |
| Dizziness | No data | No data | Rare | No data | 1-10% | No data | 20% | |
| Vertigo | No data | No data | Rare | No data | 0.1-1% | No data | No data | |
Ninety-seven drugs reported dizziness as a side effect but not hearing loss or tinnitus (Table 2). While dizziness may originate from the inner ear, it can also have other origins, such as the central nervous system. Since the mechanisms underlying dizziness are multifactorial, and our primary focus is on hearing loss, drugs causing only dizziness but not any other symptoms of ototoxicity are not further explored in this review. Some other drugs, such as doxorubicin (showing ototoxic effects in animal studies [11], but clinical evidence is missing) and nitrogen mustard (strongly restricted use as a chemical weapon), are also excluded.
Table 2.
Chemotherapeutic drugs causing only dizziness but not other symptoms of ototoxicity
| Dizziness (incidence rate > 30%) | ||
|
| ||
| Generic/Other Name | Brand Name | Mechanism of Action |
|
| ||
| Axicabtagene Ciloleucel | Yescarta | chimeric antigen receptor T-cell immunotherapy agent, binds CD19 B-lymphocyte antigen |
| Entrectinib | Rozlytrek | protein-tyrosine kinase inhibitor, inhibits tropomyosin receptor tyrosine kinases - inhibits high affinity nerve growth factor receptor, BDNF/NT-3 growth factor receptors, NT-3 growth factor receptor, proto-oncogene tyrosine-protein kinase ROS, tyrosine-protein kinase JAK2 |
| Tisagenlecleucel | Kymriah | chimeric antigen receptor T-cell immunotherapy agent, binds CD19-expressing cells and promotes T-cell expansion, activation, target cell elimination |
| Larotrectinib | Vitrakvi | tropomyosin receptor kinase (TRK) inhibitor, inhibits TRKA, TRKB, TRKC preventing neurotrophin-Trk interaction and Trk activation inducing apoptosis and inhibition of cell growth |
|
| ||
| Dizziness (incidence rate 10-29%) | ||
|
| ||
| Generic/Other Name | Brand Name | Mechanism of Action |
|
| ||
| 13-cis-Retinoic Acid | Accutane, Isotretinoin | retinoid, acts on nuclear receptors RAR or RXR |
| 5-Azacitidine, Azacitidine | Vidaza, Onureg | antimetabolite and demethylating agent |
| Abemaciclib | Verzenio | cyclin-dependent kinase inhibitor (CDK4 and CDK6), arrests G1 to S phase |
| Brentuximab vedotin | Adcetris | CD30-direct antibody drug conjugate (monoclonal antibody that disrupts microtubules) |
| Ado-Trastuzumab Emtansin | Kadcyla | Anti-HER2 monoclonal antibody combined with microtubule inhibitor DM1 (maytansine derivative) |
| Anagrelide | Agrylin | phospholipase A2 inhibitor (prevents maturation of megakaryocytes) |
| Hydrocortisone, Hydrocortone Phosphate, Ala-Cort, Cortisone, Hydrocortisone Sodium Succinate, Hydrocortisone Sodium Phosphate | Solu-Cortef, Hydrocort Acetate, Lanacort | glucocorticosteroid |
| Aldesleukin, Interleukin-2, IL-2 | Proleukin | cytokine, increases production of T lymphocytes and NK cells and improves function of lymphokine-activated killer cells and tumor-infiltrating lymphocytes |
| Alemtuzumab | Campath | CD52 monoclonal antibody |
| All-Trans Retinoic Acid, Tretinoin | Vesanoid | Retinoid, acts on nuclear receptors RAR or RXR |
| Interferon Alfa, alpha interferon, IFN-alpha | Intron A, Roferon-A | cytokine and biologic response modifier |
| Altretamine, Hexamethylmelamine, HMM | Hexalen | alkylating agent, hydrazine and triazine |
| Amifostine | Ethyol | chemoprotective agent, deactivates harmful components of chemotherapy drugs; scavenger, binds free radicals produced by cisplatin or radiation therapy |
| Aminoglutethimide | Cytadren | adrenal cortex corticosteroid production inhibitor, decreases production of estrogens and androgens |
| Nilutamide | Nilandron, Anandron | antiandrogen, blocks androgen/testosterone receptors |
| Apalutamide | Erleada | antiandrogen, blocks androgen/testosterone receptors |
| Arabinosylcytosine, Cytarabine, Ara-C | Cytosar-U | antimetabolite, inhibits DNA polymerase beta; cross-linking/alkylation of DNA, blocks G1/S |
| Nelarabine | Arranon | antimetabolite, adenosine deaminase inhibitor, incorporates into and destabilizes DNA, inhibits DNA polymerase alpha catalytic subunit; S phase-specific arrest |
| Arsenic Trioxide | Trisenox | not well understood, DNA fragmentation in leukemia cells; also damages or degrades the fusion protein PML-RAR |
| Avapritinib | Ayvakit | tyrosine kinase inhibitor, small molecule inhibitor of platelet-derived growth factor receptor alpha (PDGFR-A), targets PDGFRA and PDGFRA D842 mutants as well as multiple KIT mutations |
| Bevacizumab | Avastin, Mvasi, Zirabrev | targets and inhibits VEGF preventing angiogenesis |
| Belinostat | Beleodaq | histone deacetylase inhibitor |
| Carmustine, BCNU | BiCNU, Gliadel wafer | alkylating agent, nitrosurea, cross-links DNA and RNA |
| Binimetinib | Mektovi | oral MEK inhibitor, targets MEK1 and MEK2 protein kinase; usually given with a BRAF kinase inhibitor |
| Blinatumomab | Blincyto | bispecific T-cell engager monoclonal antibody, induces T-cells to bind CD19 on surface of B-cell leukemia or lymphoma cells |
| Bortezomib | Velcade | proteasome inhibitor, inhibits 26S proteasome, inhibits proteasome subunit beta type-5 and type-1; cell arrest in G2-M phase; in multiple myeloma, works by blocking adhesion molecule activation |
| Bosutinib | Bosulif | kinase inhibitor, targets ABL and SRC kinases |
| Encorafenib | Braftovi | BRAF kinase inhibitor |
| Busulfan | Busulfex, Myleran | alkylating agent, alkylsulfonate |
| Cabozantinib | Cometriq, Cabometyx | oral receptor tyrosine kinase inhibitor (RET, MET, VEGF), blocks cell division pathways |
| Carfilzomib | Kyprolis | tetrapeptide epoxyketone proteasome inhibitor, irreversibly binds to N-terminal threonine-containing active sites of 20S proteasome |
| Crizotinib | Xalkori capsules | oral receptor tyrosine kinase inhibitor, inhibits ALK, hepatocyte growth factor receptor (HGFR, c-Met) and receptor d’origine natais (RON); blocks cell division pathway |
| Decitabine | Dacogen | antimetabolite and demethylating agent, restores function of tumor suppressor genes and cytotoxic effect on rapidly dividing cells |
| Daratumumab and Hyaluronidase | Darzalex Faspro | CD38 monoclonal antibody (present on myeloma cells), IgG1k human monoclonal antibody binds CD38 and induces apoptosis through mediated cross linking and immune mediated tumor cell lysis through complement dependent cytotoxicity, antibody mediated cytotoxicity, and antibody dependent cellular phagocytosis; hyaluronidase helps with absorption into blood |
| Daunorubicin and Cytarabine (Liposomal) | Vyxeos | Daunorubicin is an anthracycline, intercalates between DNA base pairs inhibiting DNA synthesis and DNA-dependent RNA synthesis; Cytarabine is an antimetabolite (S phase); liposome helps with drug distribution and lengthens time of effect of the drug allowing for extended treatment effect |
| Glasdegib | Daurismo | hedgehog pathway inhibitor, inhibits increases in tumor size and decrease the amount of CD45+/CD33+ cells in the bone marrow; binds and inhibits Smoothened (SMO) receptor |
| Dexamethasone, Dexamethasone Sodium Phosphate, Dexamethasone Acetate | Decadron, Dexasone, Diodex, Hexadrol, Maxidex | glucocorticosteroid |
| Prednisolone | Delta-Cortef, Orapred, Pediapred, Prelone | glucocorticosteroid |
| Prednisone | Deltasone, Liquid Pred, Meticorten, Orasone | glucocorticosteroid |
| Denileukin Diftitox | Ontak | biologic response modifier agent, a fusion protein (combination of diphtheria toxin and IL-2), selectively delivers the cell-killing activity of diphtheria toxin to targeted cells; binds to lymphoma cells that express high affinity IL-2 receptor (IL-2 part of fusion protein binds cell surface) and halts protein synthesis |
| Dexrazoxane | Zinecard | chemoprotectant agent, binds free radicals formed by doxorubicin; extravasation antidote, binds chemotherapy drug that leaked from vein preventing damage to surrounding tissue |
| Methylprednisolone | Duralone, Medrol, Medralone, M-Prednisol, Solu-Medrol | glucocorticosteroid |
| Eculizumab | Soliris | monoclonal antibody, binds C5 complement protein preventing formation of MAC, prevents hemolysis and stabilizes hemoglobin |
| Eltrombopag | Promacta | colony stimulating factor, thrombopoietic agent, growth factor that stimulates platelet production by binding to and activating the thrombopoietin (TPO) receptor; a thrombopoietin nonpeptide agonist |
| Tagraxofusp-erzs | Elzonris | biologic response modulator and cytokine, combination of recombinant human IL-3 and truncated diphtheria toxin, binds CD123 (alpha chain of IL-3 receptor) and delivers diphtheria toxin to cells, blocks protein synthesis; binds ADP-ribosylation factor-like protein 2 |
| Fam-trastuzumab deruxtecan-nxki | Enhertu | anti-HER2 monoclonal antibody (anti-HER2 IgG1) combined with a topoisomerase I inhibitor; antibody attached to chemotherapy, allows selective delivery into HER2 overexpressing cells, DNA damage |
| Enzalutamide | Xtandi | antiandrogen (second generation), blocks androgen/testosterone receptors |
| Toremifene | Fareston | anti-estrogen, estrogen receptor antagonist, blocks estrogen binding and uptake into cells |
| Gilteritinib | Xospata | protein-tyrosine kinase inhibitor, inhibits FLT3 receptor, serotonin receptors, TPKR UFO, and ALK tyrosine kinase receptor |
| Trastuzumab | Herceptin (Biosimilars: Herzuma, Kanjinti, Ogivri, Ontruzant) | HER2/neu receptor monoclonal antibody |
| Ibritumomab, Ibritumomab Tiuxetan | Zevalin | CD20 monoclonal antibody linked with Yttrium-90 (radioactive substance), directly delivers radiation to CD20+ cells |
| Ibrutinib | Imbruvica | binds to and inhibits the bruton’s tyrosine kinase (BTK) signaling molecule of the B-cell receptor signaling complex |
| Ponatinib | Iclusig | tyrosine kinase inhibitor |
| IL-11, Oprelvekin, Interleukin-11 | Neumega | biologic response modifier and cytokine, stimulates production, maturation and activation of platelets |
| Talimogene Laherparepvec, T-VEC | Imlygic | genetically modified weakened form of live HSV (oncolytic), replicates within tumors and produces GM-CSF to promote anti-tumor immune response |
| Interferon Alfa-2b (PEG Conjugate), PEG Interferon | PEG-Intron | biologic response modifier and cytokine, activates human type 1 interferon causing them to dimerize which activates JAK/STAT pathway |
| Ruxolitinib | Jakafi | oral receptor tyrosine kinase inhibitor, inhibits JAK1 and JAK2 |
| Pembrolizumab | Keytruda | highly selective humanized monoclonal IgG4 antibody directed against the PD-1 receptor on cell surface, prevents binding and activation of PD-L1 and PD-L2 which activates T-cell mediated immune response against tumor cells |
| Lenalidomide | Revlimid | immunomodulatory agent and antiangiogenic agent, inhibits protein cereblon and TNF ligand superfamily member 11, antagonizes cadherin-5, negative modulator of prostaglandin G/H synthase 2 |
| Lenvatinib | Lenvima | oral receptor tyrosine kinase inhibitor, inhibits VEGF, VEGFR, FGF, PDGFR alpha, KIT and RET |
| Lorlatinib | Lorbrena | reversible tyrosine kinase inhibitor, blocks abnormal ALK protein |
| Luspatercept | Reblozyl | recombinant fusion protein, hematopoiesis agent (contains modified form of the extracellular domain of human activin receptor), binds and inhibits transforming growth factor beta super family molecules increasing expression of blood cell precursors |
| Olaparib | Lynparza | poly (ADP-ribose) polymerase (PARP) enzyme inhibitor (PARP1, PARP2, PARP3), induces synthetic lethality in BRCA1/2 deficient tumor cells through formation of double-strand DNA breaks |
| Procarbazine | Matulane | alkylating agent, hydrazine and triazine |
| Midostaurin | Rydapt | tyrosine kinase inhibitor, inhibits FLT3 inhibiting leukemic cell production |
| Niraparib | Zejula | PARP inhibitor, highly selective for PARP1 and PARP2 resulting in DNA damage and apoptosis, induces cytotoxicity in tumor cell lines w/ and w/o BRCA1/2 deficiencies |
| Romiplostim | Nplate | biologic response modifier, colony stimulating factor, promotes platelet production via the thrombopoietin receptor |
| Pertuzumab | Perjeta | HER2 monoclonal antibody (binds different area of HER2 protein than trastuzumab) |
| Pomalidomide | Pomalyst | thalidomide analogue, inhibits protein cereblon, TNF and prostaglandin G/H synthase 2 |
| Sipuleucel-T | Provenge | autologous cellular immunotherapy, selectively targets prostatic acid phosphatase (PAP), a PSA |
| Rucaparib | Rubraca | PARP inhibitor (PARP1, PARP2, PARP3), increases formation of PARP-DNA complexes; cytotoxicity in BRCA1/2 deficient tumor cell lines and other DNA repair genes |
| Sacituzumab Govitecan-hziy | Trodelvy | trop-2-directed antibody-drug conjugate combined with a topoisomerase I inhibitor (SN-38) attached by a linker, binds trop-2-expressing cancer cells and is internalized, SN-38 is released in cancer cell by breaking the linker |
| Sunitinib, SU11248 | Sutent | receptor protein-tyrosine kinase inhibitor, inhibits VEGF |
| Talazoparib | Talzenna | PARP inhibitor (PARP1, PARP2), strong catalytic inhibition and a PARP-trapping potential |
| Temozolomide | Temodar | alkylating agent, hydrazine and triazine (similar to dacarbazine, acts as a pro-drug) |
| Vorinostat | Zolinza | not fully understood, histone deacetylase inhibitor, inhibits HDAC2, HDAC2, HDAC3, HDAC6 |
| Zoledronic Acid | Zometa | bisphosphonate, decreases osteoclast actions on bone |
Platinum-based drugs. The two platinum-based alkylating agents, cisplatin (also referred to as cis-diamminedichloroplatinum (II), CDDP, and platinol) and carboplatin (paraplatin), are the most commonly reported compounds that cause hearing loss. According to the National Cancer Institute (NCI), cisplatin or similar platinum-based drugs are used in 10-20% of cancer chemotherapy regimens [12]. The most common indications include testicular, ovarian, cervical, bladder, and head and neck cancers. Multiple well-documented significant sequelae are nausea, vomiting, liver damage, kidney failure, hearing loss, tinnitus, and vertigo [13-17]. The prevalence of hearing loss during chemotherapy with cisplatin can be as high as 60-80% [14,18]. Based on the NCI estimated cancer patients of 2018, cisplatin may cause approximately 100-300 thousand new cases of ototoxicity annually in the United States. Oxaliplatin, a third-generation platinum-based chemotherapy drug, has not been considered ototoxic based on a clinical trial with 18 patients [19]. However, case reports showed that the drug might cause hearing loss [20-23].
Vinblastine (velban, alkaban-AQ) and Vincristine are plant alkaloids listed as ototoxic drugs, albeit with little evidence in the literature. As a microtubule inhibitor, vinblastine-induced hearing loss was observed in two case reports of patients with Hodgkin’s lymphoma who underwent combined chemotherapy, including doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) [24,25]. In one published case, vinblastine was given in a combination treatment with vincristine. It has been discussed that the observed cochlear damage [24] originated from vinblastine. However, it is also possible that the damage might have been caused by vincristine, a commonly used vinca alkaloid similar to vinblastine [26-28].
Dasatinib (Sprycel), a tyrosine kinase inhibitor targeting multiple cancer cells, is also reported to cause hearing loss in case reports but the incidence is rare (0.1-1%). Instead, more frequent side effects of dasatinib are tinnitus and dizziness.
Isotretinoin (cis-retinoic acid) and tretinoin (all-trans retinoic acid) are retinoids that can slow down the growth of cancer cells. Occasional case reports indicate that both can induce hearing loss [29,30], while detailed studies on isotretinoin’s and tretinoin’s ototoxicity are still missing. Furthermore, isotretinoin is mainly used to treat severe acne. In two clinic studies with cohorts of acne patients, isotretinoin treatment leads to transient hearing improvement instead [31,32]. These controversial results suggest their influence on the auditory system, while more studies are needed to determine the mechanisms.
Mechanisms of hearing loss in chemotherapy
The working theory for the mechanism of platinum-based drugs is related to the generation of reactive oxygen species (ROS) and DNA damage. However, their ototoxicity is mainly associated with excessive ROS generation [33,34], while DNA damage [35,36] likely plays a minor role. However, the mechanisms of other ototoxic chemotherapeutic drugs listed in Table 1 remain largely unclear because the studies are rare. Some studies have speculated that it is associated with their antitumor activity [24]. Due to the availability of literature, this review focuses mainly on the ototoxicity of platinum compounds, particularly cisplatin.
Inner ear structures targeted by cisplatin
Structural and functional features of the cochlea
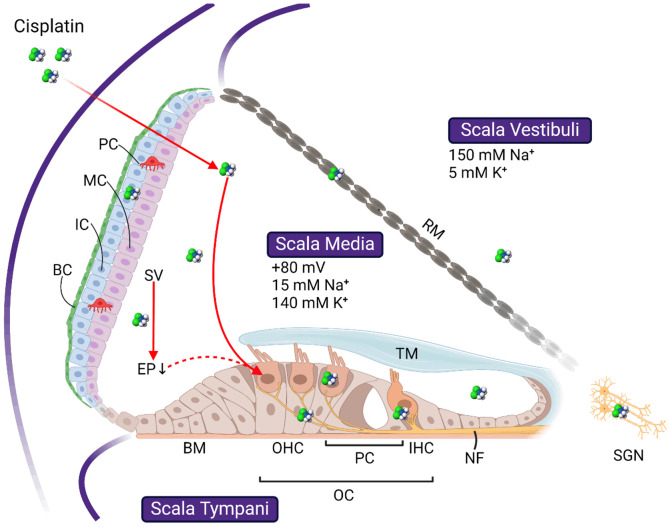
The cochlea comprises three fluid-filled tubes, scala tympani, scala media, and scala vestibuli (Figure 1). Scala vestibuli and scala media are separated by Reissner’s membrane, while scala media and scala tympani by the basilar membrane and reticular lamina. The organ of Corti is located on the basilar membrane. It contains two types of hair cells: the inner hair cells (IHCs) and outer hair cells (OHCs). IHCs and OHCs have hair bundles, or stereocilia, with ion channels responsible for transforming sound-induced vibrations of soft tissue structures of the inner ear into action potentials. The channels are named mechano-electrical transduction (MET) channels, of which the core domain is recently identified as transmembrane channel-like protein 1 [37]. Opening of MET channels in OHCs results in a depolarizing current enhancing the sound-induced vibrations of soft tissue structures by changing the stiffness and length of the OHCs. In IHCs, the opening of the MET channels releases neurotransmitters and generates action potentials. The MET current is primarily carried by calcium and potassium ions [38]. Please refer to [39-41] for recent reviews on the MET channels.
Figure 1.
Schematic image showing the basic cochlear structures and the targets of cisplatin ototoxicity. Cochlear duct forms three scalae, scala vestibuli, scala media, and scala tympani, separated by the basilar membrane (BM) and Reissner’s membrane (RM), respectively. Cells affected by cisplatin include the outer hair cells (OHC) and inner hair cells (IHC) in the organ of Corti (OC), basal cells (BC), intermedial cells (IC), and marginal cells (MC) in the stria vascularis (SV), and the spiral ganglion neurons (SGN) in the modiolus. Whether OHC loss is initiated directly through cisplatin uptake or indirectly through the drop of endocochlear potential (EP) following SV damage is still debating. Refer to the text for more details. TM: tectorial membrane; RM: Reissner’s membrane. All images are created with BioRender.com.
The driving force for the MET current is the voltage difference between the endocochlear potential (EP) of about 80 mV [42,43] and the resting potential of the hair cells. Intracochlear ion homeostasis, combined with the selective permeability for different ions across the boundaries of the scalae and active ion transport across stria vascularis, generates the EP. The perilymphatic ion concentration, high in sodium (~150 mM) and low in potassium (~5 mM), is similar to the ion concentration of the extracellular fluids. This differs from the endolymphatic ion concentration, low in sodium (~15 mM) and high in potassium (~140 mM), which is comparable with the ion composition of the cytoplasm [44]. The ion homeostasis of the endolymph is primarily controlled by the stria vascularis (SV), a structure lining the lateral wall of the scala media. The marginal cells in the SV regulate the potassium concentration [45], and the intermediate cells form tight junctions with basal cells to separate the endolymph from the surrounding perilymph (Figure 1, for recent reviews, see [46,47]).
This blood-labyrinthine barrier (BLB) is similar to the blood-brain barrier (BBB) in terms of cellular and molecular basis [48], which is only permeable to some small molecules (up to 500 KDa) [49]. Unfortunately, this permeability to the BLB includes most ototoxic drugs, while it excludes most otoprotective agents proven effective in vitro [50,51]. Furthermore, once inside the cochlea, the outflow of the ototoxic drugs is also difficult.
Path to hearing loss in chemotherapy
Cisplatin ototoxicity leads to structural changes, including shrinkage and inflammation of SV, loss of OHCs and IHCs, morphological changes of the stereocilia bundles, IHC synaptopathy, changes of supporting cells, and loss of spiral ganglion neurons (SGNs) (for reviews, see [15,52-56]). Recent cellular and molecular biology studies also revealed additional cells and structures affected by cisplatin ototoxicity, such as the spiral ligament [57,58], spiral limbus, spiral modiolar veins and lacunae [15,59], and pericytes in the SV [49,60]. Functional changes include decreased EP, threshold elevation of distortion product otoacoustic emissions (DPOAEs), auditory brainstem responses (ABRs), and compound action potentials (CAPs) [61-63]. DPOAE magnitudes and ABR wave-I amplitudes are also decreased. These changes are closely related to the cellular processes, including ROS generation, inflammation, and apoptosis [64]. Meanwhile, the inflammatory and apoptotic mechanisms of cisplatin ototoxicity are highly interconnected, which leads to a vicious cycle of inflammation, ROS production, nuclear and mitochondrial DNA damage, ER stress, and cell death, which will be discussed later.
Following cisplatin therapy, OHC loss is usually the most prominent and severe cochlear damage [46] and is likely the cause of permanent hearing loss. How OHCs are affected by cisplatin remains unclear (Figure 1). While early studies suggest direct damage of the OHCs following the uptake of cisplatin via different ion channels [65] or transporters [66,67], conclusive experimental evidence of whether platinum exists in the hair cells is still missing. OHC loss might also originate from changes in the EP resulting from compromised SV function [46,68]. This view is further supported by the following findings: (1) The highest platinum accumulation is found in the SV [20,68,69]. Solid evidence comes from Cunningham’s group, which showed that platinum mainly accumulates in SV after chemotherapy, using the inductively coupled plasma mass spectrometry visualization technique [68]. (2) The immediate decrease of the EP after cisplatin treatment suggests the involvement of the stria vascularis in cisplatin ototoxicity [46]. (3) Platinum-DNA adduct was observed in SV marginal cells as early as 8 hours after cisplatin treatment, while ROS accumulation was not shown even after 48 hours [69]. This finding suggests that ROS accumulation might be a secondary effect of SV damage. (4) A recent study shows in cell culture that the pericytes in SV, which are also critical for EP, are the targets of cisplatin and may account for BLB breakdown in CIHL [60].
Cisplatin uptake by cochlear cells
The first step for cisplatin ototoxicity is its uptake by cochlear cells, which may occur, like in other tissues, through the organic cation transporter 2 (OCT2) [67], copper transporter 1 (CTR1) [66,70], and LDL receptor-related protein 2 (LRP2) [71]. The high expression of CTR1 and OCT2 in IHCs, OHCs, spiral ganglion neurons (SGN), and SV [66] supports this view (for review, see [56,66,72]). The interaction and uptake of cisplatin via mechano-transducer (MET) channels of the hair cells have also been studied [65,73]. One early study shows that cisplatin blocks MET channels in chicken cochlear hair cells as an acute effect [73]. However, the paper has not demonstrated whether cisplatin could pass the MET channels. A study on the zebrafish lateral line organ shows that functionally intact MET channels are required for the toxicity of cisplatin to the hair cells. Hair cell death is prevented when the MET channels are non-functional through chemical blockage or mutation [65]. Interestingly, the same publication also suggests that the roles of OCT2 and CTR1 for cisplatin uptake are insignificant in these cells [65]. A more recent study on murine cochlear hair cell explants, however, shows that both MET channel and OCT are involved in the uptake of fluorescent dye-conjugated cisplatin [74]. In addition to transporters and MET channels, cisplatin may also enter cells through passive diffusion [75], as shown in the digestive system of rats [76] and a cochlear-derived cell line OC-k3 [77]. In the in vitro studies on OC-k3 cells, cisplatin enters the cells via first-order kinetics without saturation before the induction of cell death. Once inside the cell, cisplatin undergoes an aquation reaction and hydrolyzes as water ligands displace chloride ligands [75]. The ability of this activated and positively charged aqua-cisplatin compound to passively diffuse back across the plasma membrane is significantly decreased. The drug is then trapped intracellularly, leading to unimpeded damage [72,78,79].
ROS generation and its central role in cisplatin ototoxicity
Platinum has a high affinity for sulfur ligands [80]. Once it enters the cochlea, the highly reactive aquated form of cisplatin binds to both DNA and proteins [79], which triggers a series of signaling pathways (Figure 2). Cisplatin may induce NADPH oxidase 3 (NOX3) directly [81] and/or via cisplatin activated transient receptor potential cation channel subfamily V member 1 (TRPV1) channel, followed by calcium influx [82]. Cisplatin-activated NOX3 leads to the generation of ROS, specifically superoxide (O2 -.) [81]. O2 -. can induce mitochondrial translocation of the B-cell lymphoma 2 gene (Bcl2) associated X (Bax), leading to mitochondrial-dependent apoptosis (further discussed below) [72,83]. Another study demonstrates that NOX3-dependent ROS generation, rather than cisplatin itself, activates TRPV1 channels [84] (for review, see [72,85]). Nevertheless, both NOX3 induction and TRPV1 activation by cisplatin can result in ROS generation and calcium influx. In addition, reducing TRPV1 expression [84] and knockout of NOX3 [82] both attenuate cisplatin-induced hearing loss (CIHL), during which both SGNs and OHCs are protected [82]. ROS is also a potent inducer of NOX3, which is over 50 times more abundant in the inner ear compared to other tissues [81] (for review, see [79]). It leads to a vicious cycle of ROS-induced TRPV1 and NOX3 activation, causing further calcium influx, ROS production, and TRPV1 and NOX3 activation [84].
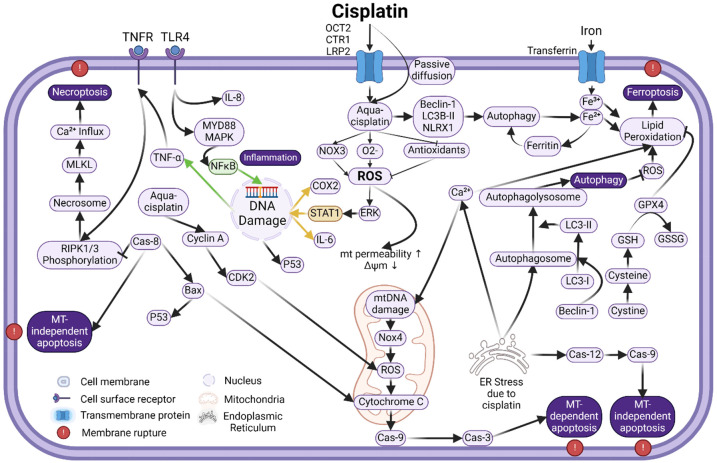
Figure 2.
Schematic image showing intracellular signaling pathways associated with cisplatin ototoxicity in the cochlea. Different cell death pathways, including apoptosis (either mitochondria dependent or independent) and non-apoptotic cell death pathways are involved and interconnected. Refer to the text for details.
ROS is detoxified by an intracellular antioxidant defense system composed of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH.Px), and glutathione reductase (GR) [13,56,72]. This antioxidant defense system can be rapidly overwhelmed by cisplatin [80,86]. The reactive platinum moiety of aqua-cisplatin reacts with intracellular oxygen molecules, generating O2 -. [56]. SOD converts O2 -. to hydrogen peroxide (H2O2), which CAT further catalyzes to produce water and oxygen [56,72]. In this process, GSH.Px converts glutathione from the reduced form (GSH) to the oxidized form (GSSG) during the conversion of H2O2 to water. GR catalyzes the conversion of GSSG back to GSH using NADPH as a cofactor [56,72]. Cisplatin can covalently bind to the thiol group of these antioxidant enzymes, leading to their inactivation [56]. This reactive cisplatin compound also directly binds to GSH, leading to its excretion or conversion back to GSSG [55]. NADPH depletion downregulates GR antioxidant activity, which further decreases GSH levels. Depletion of GSH downregulates GSH.Px activity [56]. Most experimental evidence shows that antioxidant enzymes are reduced by cisplatin, some to the extent of 50-70% [55,86,87] (for reviews, see [56,72,88,89]). However, other studies indicate that SOD and CAT activities can be increased instead [55,79,90]. Together, antioxidant depletion and ROS overload trigger a series of interconnected cell death pathways involving DNA damage, lipid peroxidation, protein oxidation and enzyme inactivation, ion channel expression changes, endoplasmic reticulum (ER) stress, and inflammation [56,59,72,83]. The cascade of ROS signaling involves processes in different compartments of the cells, including the cytoplasm, nucleus, and mitochondria (Figure 2). Different cell death processes are involved, including mitochondrial-dependent and mitochondrial-independent apoptosis, necroptosis, autophagy, ferroptosis, etc. (Figure 2).
Mitochondrial-dependent apoptosis associated with cisplatin ototoxicity
Mitochondrial-dependent apoptosis is characterized by releasing pro-apoptotic factors from the mitochondria to cytosol. This process can be triggered by, and is associated with, different factors and pathways during cisplatin treatment, including lipid peroxidation, DNA damage, signal transducer and activator of transcription 1 (STAT1), p53 activation, Bcl2/Bax activation, cyclin/cyclin-dependent kinase (CDK) activation, Cytochrome C, Caspases, etc. In the cochlea, mitochondrial dysfunction and apoptosis due to ROS overload and disruption of intracellular redox homeostasis result in the loss of OHCs and other cells [91,92] (for review, see [13]) (Figure 2). Most of the factors and pathways related to mitochondrial-dependent apoptosis have been studied. They are shown to be involved in cisplatin ototoxicity. First, cisplatin can integrate into DNA in the nucleus via adduct formation [13,16,69,93], leading to cross-linking and damage [93]. DNA damage activates p53, initiating the intrinsic mitochondrial apoptosis pathway [94]. P53 increases the expression of the pro-apoptotic molecule Bax [78], which is translocated to the mitochondria, where it permeates the outer mitochondrial membrane. Mitochondrial membrane permeabilization leads to the loss of the mitochondrial membrane potential, mitochondrial ROS (mtROS) production, and release of Cytochrome C and mtROS from the mitochondria into the cytoplasm [95,96]. Second, both NOX3-dependent ROS production [57,79,85,88,97] and ROS-mediated activation of extracellular signal-regulated kinases 1 (ERK1) [59,64] activate STAT1 signaling. STAT1 triggers p53 activation, leading to the above-described mitochondrial translocation of Bax and downstream mitochondrial-dependent apoptosis in the cochlea [57,85]. STAT1 activity also links the apoptotic and inflammatory pathways involved in cisplatin ototoxicity [64], which will be discussed later. Third, multiple factors, including cisplatin itself, ROS, lipid peroxidation, calcium influx, and CDKs, can act directly on mitochondria, causing an increase in its permeabilization and deterioration of its function. Cisplatin can also form mitochondrial DNA (mtDNA) adducts [98-101], which is likely the main factor in cisplatin-induced ototoxicity [15]. The platination of mtDNA leads to mtROS production and accumulation, and in turn, the damage of mtDNA, proteins, and lipids within the mitochondrial membrane. Cyclin A is another critical player in mtROS production, which can be upregulated by cisplatin and activates CDK2 kinase, consequently facilitating mtROS production [102]. Fourth, cytochrome C release and the activation of caspase-9 and caspase-3 mark the final stage of mitochondrial-dependent apoptosis, which is also observed in the cochlea [72] (Figure 2). As an essential component of the mitochondrial electron transport chain, cytochrome C is one of the apoptotic protease-activating factors, which activate caspase 9. Caspase 9 activation subsequently activates caspase 3, causing the fragmentation of chromosomal DNA through the cleavage of its substrates [103].
Mitochondrial-independent apoptosis pathways in cisplatin ototoxicity
Apoptosis can also be induced in the cytoplasm through ROS overload, ER stress [104], inflammation, and lipid peroxidation [79].
ER stress
ER stress results from oxidative damage, intracellular calcium imbalance, and protein damage [83]. It is involved in cisplatin ototoxicity through caspase-12 activation, located on the ER plasma membrane, and auto-cleaves in response to ER stress. Caspase-12 then activates caspase-9, which activates caspase-3, leading to apoptosis in a mitochondrial-independent manner [97,98,105]. This ER-specific apoptosis pathway is also closely linked to the mitochondrial-dependent path due to the simultaneous activation of C/EBP homologous protein (CHOP) [104]. CHOP plays an important role in ER stress-induced apoptosis while regulating Bcl2 family expression. Decreased Bcl2 enables increased Bax activity, leading to the release of apoptotic active substances from mitochondria to the cytoplasm [106], i.e., mitochondrial-dependent apoptosis [79,104] (Figure 2).
Inflammation
Inflammation is the body’s defense mechanism in response to harmful stimuli, such as damaged cells, which also plays a vital role in inducing apoptosis. Inflammatory signaling pathways, most commonly the nuclear-factor kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and JAK-STAT pathways, are also involved in cisplatin ototoxicity. NF-κB is a transcription factor mediating inflammatory responses by regulating the expression of various pro-inflammatory genes, such as tumor necrosis factor-α (TNF-α). In cisplatin ototoxicity, NF-κB can be activated directly by ROS [107] or toll-like receptor 4 (TLR4) [108], leading to its translocation to the nucleus and production of inflammatory cytokines [80,92]. TLR4 is a transmembrane protein that plays a fundamental role in pathogen recognition and activation of innate immunity. TLR4 can also be activated by cisplatin, which triggers the activation of proinflammatory cytokines such as interleukin-6 (IL-6), interleukin-8 (IL-8), TNF-α, and NF-κB [108]. Translocation of NF-κB to the nucleus, mediated by TNF-α and IL-6, further induces the de novo synthesis of TNF-α, IL-6, IL-1β, and inducible nitric oxide synthase (iNOS), and activates caspase-3 in a mitochondrial-independent manner [78,79]. Nevertheless, NF-κB translocation can occur much earlier (1-2 hours) than maximal ROS generation (1 day), suggesting an early involvement of the inflammatory pathways or even an upstream event of ROS formation [15,59,79,109,110]. NF-κB [111] (and also STAT1 [85]) induces an increased expression of iNOS, which produces nitric oxide (NO) (for reviews, see [78,79]). NO reacts with O2 -. to form peroxynitrite (ONOO-), a highly reactive oxidizing molecule that damages proteins [112]. Peroxynitrite induces protein peroxidation via nitration of tyrosine residues (nitrotyrosine) [112,113], which alters protein configuration and function (for reviews, see [13,15,72]). In addition, expression of TNF-α also further activate NF-κB [80,92] as well as the extrinsic apoptosis pathway by binding to TNF receptors (TNFR). It leads to caspase-8 activation, which in turn activates caspase-3, leading to mitochondrial-independent apoptosis (Figure 2) [71,93], which is also known as the death receptor pathway [42] or extrinsic apoptosis [94].
Other than the NF-κB pathways, early phosphorylation of two well-characterized MAPK families, ERKs and the c-Jun N-terminal kinases (JNKs), is activated by cisplatin treatment in two cochlear-derived cell lines - House Ear Institute-Organ of Corti 1 (HEI-OC1) cells [109] and OC-k3 cells [77] (Figure 1). Early activation of JNK may play a minor role in cisplatin-induced ototoxicity [109] or may assist in DNA repair in response to cisplatin-DNA adducts [114]. The activation of MAPK/ERK also facilitates secretion of the pre-existing TNF-α, IL-1β, and IL-6 [77] (Figure 2), which in turn activates the translocation of NF-κB to the nucleus [109]. Nevertheless, most of the studies mentioned above are performed on cell cultures, although the role of the MAPK family in cisplatin toxicity is well documented [115]. It is also worth noting that most of the research on the molecular mechanism of cisplatin ototoxicity included in this review has been conducted using in vitro cell cultures or ex vivo cochlear explants. A recent study has highlighted that the molecular pathways implicated in cisplatin ototoxicity may differ between the in vitro/ex vivo and the in vivo settings [116].
JAK-STAT pathway activation allows the transfer of signals from the receptors to the nucleus. It thus involves a repertoire of processes, such as apoptosis and tissue repair, through a cytokine-membrane receptor-JAK-STAT cascade [117]. In CIHL, the JAK-STAT pathway regulates cell death and inflammatory responses by activating the expression of inflammatory cytokines such as cyclooxygenase-2 (Cox-2) and TNF-α. Suppression of the JAK-STAT pathway with an adenosine A1 receptor (A1AR) agonist decreases cisplatin-induced apoptosis of OHCs [118]. Among STAT proteins (STAT1-6), STAT1 is likely the most relevant to CIHL because it is known to directly induce apoptosis and p53-mediated apoptosis [64,85]. In rat models, STAT1 signaling is linked with ROS, causing cisplatin-induced cochlear cell apoptosis [85]. Pre-treatment with STAT1 siRNA (48 hours before) [85] or oral uptake of an inhibitor of STAT1 signaling (45 minutes before) [64] both protect against CIHL. STAT3 and STAT6 are also important players in CIHL [119]. STAT6 works through inflammatory cytokines IL-4 and IL-13, and knockout of STAT6 protects against CIHL [119].
Lipid peroxidation
Downstream effects of O2 -. can also cause lipid peroxidation. Catalyzed by SOD, O2 -. is converted to hydrogen peroxide, which is catalyzed by iron to form hydroxyl free radicals [15]. These highly reactive ROS react with polyunsaturated fatty acids in cellular membranes, producing the highly toxic aldehydes, 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) [72]. Antioxidant enzyme depletion has been linked to increased levels of MDA, indicating high lipid peroxidation [80]. This inverse relationship between glutathione and MDA activity (low glutathione levels and high MDA levels) has been shown in vivo using cisplatin-treated rats [86]. Lipid peroxidation, especially the production of 4-HNE, induces calcium influx into the cells [15,88]. ROS further enhances calcium influx. An early study found that O2 -., but not H2O2, increases the intracellular calcium concentration via transmembrane influx in OHCs of guinea pig cochleae. Researchers suggested that O2 -. stimulates voltage-sensitive calcium channels. However, they did not rule out the possibility of increased calcium permeability caused by lipid peroxidation [120]. According to a review article, ROS can open the ER calcium channel, ryanodine receptor, L-type, T-type, and the TRPV1 plasma membrane calcium channels [79]. A study by Yoshida et al. found that intracellular NO and H2O2 open TRPV1 channels and facilitate calcium influx [121]. In an earlier study, inhibition of T-type calcium channels did not inhibit ROS generation. Still, it significantly inhibited lipid peroxidation, mitochondrial membrane permeabilization, and cytochrome c release in HEI-OC1 cells and rat organ of Corti explants treated with cisplatin [96]. The findings suggest that calcium influx via T-type calcium channels occurs downstream of ROS and plays a major role in intrinsic apoptosis of cochlear cells under cisplatin-induced stress conditions. Nevertheless, increased cytosolic calcium causes mitochondrial membrane permeabilization and loss of membrane potential, initiating the intrinsic mitochondrial-dependent apoptosis pathway [122].
Other cell death pathways
Necroptosis
In addition to activation of the extrinsic apoptosis pathways, TNF-α induces cell death via necroptosis pathway activation in cisplatin-induced ototoxicity [116,123] (for reviews, see [59,124]). Studies indicate a dose-dependent effect on cisplatin-induced cell death using HEI-OC1 cells [103] and OC-k3 cells [77]. Specifically, apoptosis occurs at lower doses, while necroptosis occurs at higher doses. As opposed to the organized breakdown of cells during apoptotic cell death, necroptosis results from cellular and organelle membrane permeation, releasing intracellular substances and exacerbating inflammation [122]. Activation of caspase-8 inactivates receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3, which induce activation of the extrinsic mitochondrial-independent apoptosis pathway as described above. Inactivation of caspase-8 leads to RIPK3 activation, shifting death receptor-mediated cell death from apoptotic to necroptotic pathways [124]. In necroptosis, TNF-α binds its receptor 1 (TNFR1) and, without caspase-8, leads to the formation of the RIPK1/RIPK3 complex, also known as necrosome [123,124]. Downstream effects of necrosomes involve the activation of Mixed Lineage Kinase Domain-Like Pseudokinase (MLKL), which induces calcium influx via Transient Receptor Potential Cation channel, subfamily M, member 7 (TRPM7) channels. It creates pores in the plasma membrane, leading to leakage of substances, cell lysis, and cell death [125].
Autophagy
As a protective mechanism of cells, autophagy removes specific cellular structures or components, such as damaged organelles. Under stress conditions such as cisplatin treatment, autophagy can also lead to cell death [126]. The interest in the involvement of autophagy in CIHL has increased rapidly in recent years. Increased expression of three autophagic mediators has been observed in CIHL, including Beclin-1 (the initial autophagy promoter), microtubule-associated protein light chain 3 II (LC3-II), and mitochondrial-bound [127] nucleotide-binding domain and leucine-rich-repeat-containing family member X1 (NLRX1) [59]. Autophagy signaling involves specific factors and interconnections with other cell death pathways. First, intracellular organelle or protein damage leads to the activation of AMP-activated protein kinase, which inhibits the mammalian target of rapamycin (mTOR), leading to autophagy [128] and to the activation of phosphoinositide 3-kinase (PI3K), inducing Beclin-1 formation of the phagosome [129]. Second, beclin-1 activates the conversion of unlipidated LC3-I to lipidated LC3-II, which is required for complete autophagosome formation and autophagosome-lysosome fusion [126,129]. Third, overexpression of NLRX1 correlates with the accumulation of autophagosomes and acceleration of autophagic cell death [83,126]. In addition, NLRX1 overexpression also accelerates mitochondrial-dependent apoptosis in cisplatin treatment, evidenced by increased Bax, caspase-3, and ROS levels [127]. The interactions between autophagy and apoptosis pathways are also shown in mitochondrial autophagy or mitophagy. Activation of mitophagy can negatively regulate cisplatin-induced apoptosis in hair cells and SGNs [130] and vice versa [131], suggesting a protective role of mitophagy in CIHL. Nevertheless, questions still exist about whether autophagy plays a protective role or induces cell death in CIHL, and the arguments are far from settled (for reviews, see [59,132,133]).
Ferroptosis
Ferroptosis is another form of non-apoptotic programmed cell death, which requires irons generated by processes such as lipid peroxidation and autophagy in response to stress [134,135]. Hallmarks of ferroptosis, such as lipid peroxidation and impaired antioxidant capacity, have been observed in cochleae in mice after cisplatin treatment, suggesting its potential roles in CIHL [135]. A recent study showed that transferrin 1, a marker of ferroptosis, is increased in OHCs but not IHCs, SV, or supporting cells after cisplatin treatment. RNA sequencing completed in the same study showed that the expression of the ferroptosis-related gene is upregulated in CIHL [136]. Suppressing ferroptosis using ferrostatin-1 reduces CIHL by protecting cochlear hair cells [135,137,138] while facilitating ferroptosis by blocking lipid repair function leads to an exacerbated breakdown of mitochondrial membrane potential in cultured HEI-OC1 cells. Ferrostatin-1 also protects hearing against CIHL in mice and rescues OHCs in a knockout mouse model lacking a key regulator for ferroptosis [136]. However, ferroptosis is highly dependent on mitochondrial function and interconnected with other signaling pathways, including lipid peroxidation and autophagy. Further studies are needed to address the potential interference of ferroptosis with the antitumor effects in chemotherapy involving cisplatin. More discussion regarding the roles of ferroptosis in CIHL and the underlying mechanism can be found in some recent studies [139,140] and reviews [89].
Hearing protection in chemotherapy
Current studies on hearing protection in chemotherapy are mainly focused on cisplatin ototoxicity. Among the four ototoxic chemotherapeutic drugs listed in Table 1, vinblastine and dasatinib have rarely been studied in hearing protection, while the studies on carboplatin are almost all associated with cisplatin [141,142]. Since the damage to the cochlea is largely irreversible and regeneration has not been successful yet, intervention before and during chemotherapy to protect hearing is critical. However, preventing cisplatin ototoxicity using drug therapies is facing severe challenges, including interference with the therapeutic effects of cisplatin, bioavailability, and side effects. Concerns regarding interference with the antitumor effects of cisplatin lie in the fact that interventions with antioxidants that reduce cisplatin ototoxicity may also affect the outcomes of the cancer therapy by deactivating cisplatin [78,143,144] and by protecting tumor cells [145] (for review, see [78,146]). Challenges to bioavailability include the permeability of otoprotective drugs through BLB [50,51] and cell membranes. Side effects are manifested by the toxicity of hearing protective drugs to other tissues. An example is amifostine [147], for which high drug doses are required for treatment because of the drug’s impermeability of the BLB in vivo.
Drug delivery strategies
Local drug administration
The challenges mentioned above can be addressed through trans-tympanic delivery [148], which has proven to be successful for some candidate drugs for hearing protection. Local drug administration through trans-tympanic injection has been developed in early studies to treat inner ear disorders such as Ménière’s disease and sudden hearing loss using steroids (for review, see [149]). It is also adopted to treat cisplatin ototoxicity with antioxidants to avoid interfering with the antitumor effects [143,148,150,151]. Depending on the site, two types of local drug administration are usually used: intratympanic (middle ear and round window) and intra-cochlear/labyrinthine (perilymph). Intra-tympanic injection, sometimes combined with a small tube through tympanic annulus for multiple doses, allows localized and high-dose treatment, as shown in some clinical trials treating cisplatin ototoxicity [152]. Intra-cochlear/labyrinthine delivery further renders the benefits of passing through the BLB, sustained treatment, and (semi-) dose control when combined with chronic implantation of an osmotic pump [153]. Local administration is shown to be effective for many drugs without interfering with the antitumor effect of cisplatin [154-156]. Concerns for local drug administration include potential pain during myringotomy and damage to the tympanic membrane, middle ear structures, and round window. Intra-cochlear/labyrinthine injection may even cause permanent damage to the inner ear, which may prevent its potential application in clinics. A more detailed introduction of the local delivery techniques and their application in cisplatin ototoxicity [79] can be found in research papers and reviews [157-161].
Other drug administration
Some other strategies have been developed to increase the efficiency of hearing protection and reduce the side effects and risk of counteracting the antitumor effect of cisplatin. Hydrogel has been used for intra-tympanic drug delivery to improve drug sustainability on the round window by reducing drainage through the eustachian tube [162-164]. However, subsequent conductive hearing loss posed by the residue on the round window and middle ear ossicles might be a concern. Nanoparticles, with a diameter of less than 1 μm, are another strategy to increase the efficacy of local drug delivery for treating cisplatin ototoxicity [165]. A few groups have studied the influence of particle size and various transportation vehicles [166]. The paper by Yu et al. details various nanoparticles and hydrogel preparations for improved drug delivery to the inner ear and cochlea manipulations [79]. As stated in the paper, inadequate cellular uptake of nanoparticles limits its use. However, it can be reduced with various endogenous (pH, redox, and enzymes) and exogenous (heat, ultrasound, light, and magnetic field) measures. Delayed or staggered drug delivery is also used to reduce the possibility of interference with the antitumor effect of cisplatin. Nevertheless, showing accurate drug concentration in the cochlea will help to determine the mechanism and effective doses of the drugs. In this regard, proof of the existence of the drugs in the cochlea by measuring the concentration from the extraction of cochlear fluids is a gold standard for candidates claiming hearing protection [167-169].
Antioxidants as traditional candidates for hearing protection against cisplatin ototoxicity
Since ROS plays a central role in cisplatin ototoxicity, various exogenous antioxidants that work as free radical scavengers have been tested for hearing protection in chemotherapy in early studies [51,170-172]. A common feature of antioxidants is the presence of a thiol, which can act as a substrate for redox reactions. Some antioxidants have been tested in clinical practice, such as N-acetyl-L-cysteine (NAC) [172], D-methionine [173,174], STS [155,163], and amifostine [147,175] (for reviews, see [13,72,78,142,147,176]). A list of antioxidants tested for their hearing protective effects against cisplatin ototoxicity is presented in Table 3. Some well-known and promising candidates and their roles are shown in Figure 3 and described below.
Table 3.
Antioxidant drug candidates for protection against CIHL
| Drug | Main mechanism of oto-protection | Experiment model | Drug dose | Route | Outcome |
|---|---|---|---|---|---|
| STS (sodium thiosulfate) | By forming complexes with platinum & antioxidant activity (PMID: 25994788) | Phase III clinical trial on hepatoblastoma in children (n = 109) (PMID: 29924955) | Cisplatin (80 mg/m2) per dose over 6 doses | IV | Reduction in hearing loss grade 1-4 by 48%. |
| Branded as Nithiodote & PedMark | STS (20 g/m2) 6 hours after each cisplatin treatment | IV | |||
| Phase III Clinical trial on children (n = 104) with cancers (PMID: 27914822) | Cisplatin (totaling 200 mg/m2) | IV | Reduction in hearing loss grade 1-4 (OR = 0.31; 95% CI 0.13-0.73; P = 0.0036). | ||
| STS (16 g/m2) 6 hours after each cisplatin treatment | IV | ||||
| NAC (N-acetyl-L-cysteine) | Antioxidant with a thiol donating hydrogen (PMID: 29429900) | Rat cochlea cell culture treated with NAC (PMID: 35346799) | Cisplatin (50 uM) treatment to cell cultures in the background of NAC at 37°C for 48 hours | Cell culture | Hair cell loss prevented, seen through Ab staining. |
| Branded as Acetadote, Fluimucil & Mucomyst | NAC (20 mM) | ||||
| Rat treated with NAC (PMID: 15219317) | Cisplatin (6 mg/kg) | IA | Hearing protected at 4 kHz (~10 dB), 8 kHz (~20 dB), 12 kHz (~22 dB), 16 kHz (~18 dB) by change in threshold. | ||
| NAC (400 mg/kg) 15 minutes before cisplatin | IV | ||||
| Phase I clinical trial on children (n = 52) with cancers (PMID: 37134194) | Cisplatin (totaling 200 mg/m2) | IV | Reduction in the risk of SIOP ≥ 2 hearing loss post-chemotherapy (OR = 0.13, CI 0.021-0.847, P = 0.033). | ||
| NAC (6 g) 4 hours after each cisplatin treatment | IV | ||||
| Double blind clinical trial on cancer patients (n = 114) (PMID:29993216) | Cisplatin (not stated) | Not stated | No significant changes in auditory thresholds. | ||
| 0.4-0.8 ml NAC (10%) | Intratympanic | ||||
| D-methionine (D-met) | D-methionine can donate a cysteine which can act as an antioxidant (PMID: 16366723) | Mice treated with D-methionine (PMID: 8951454) | Cisplatin (16 mg/kg) | IP | Hearing protected at 1, 4, 8, 14 kHz. |
| D-met (300 mg/kg) | IP | ||||
| Chinchilla treated with D-met (PMID: 12087338) | Cisplatin (125 µg) | Intracochlear | Hearing protected at 8, 16 kHz. | ||
| D-met (4 µg) 30 minutes before cisplatin treatment | Intracochlear | ||||
| Rats with MTLn3 breast cancer cells (PMID: 11405249) | Cisplatin (5 mg/kg) per dose for 3 doses with 72 hours interval | IP | Hearing protected at 1, 2, 4, 8, 16, 18 kHz. OHC protected. | ||
| L-met (300 mg/kg) 30 minutes before each cisplatin injection | IP | ||||
| Phase II clinical trial in cancer patients (n = 27) (PMID: 34622731) | Cisplatin totaling (264 mg/m2) | IV | Hearing protected in the left ear only at 11.2 kHz by a mean difference of 22.97 dB. | ||
| D-met (100 mg/kg) oral dose 1 hour before cisplatin injection | Oral | ||||
| Amifostine (WR-2771) | Antioxidant activity through WR-1065 (PMID: 11201306) | Scid mice model of human ovarian cancer with implanted tumor cells (PMID: 9389935) | Paclitaxel (27 mg/kg) over 5 doses | IP | Amifostine improved animal survival. In cell cultures, amifostine protected normal cells from paclitaxel, while cytotoxicity was increased in malignant cells. |
| Branded as Ethyol | Amifostine (200 mg/kg) over 5 doses | IP | |||
| Hamsters treated with amifostine (PMID: 15185124) | Cisplatin (15 mg/kg) over 5 doses | IP | Protected hearing at 8 kHz (~15 db), 16 kHz (~20 db), 20 kHz (~18 db) by ABR threshold shift. | ||
| Amifostine (40 mg/kg) over 5 doses 30 minutes before cisplatin injection | IP | ||||
| Non-randomized clinical study with cancer patients (n = 62) (PMID: 18669462) | Cisplatin (300 mg/m2) over 4 doses | IV | Amifostine caused ~22% reduction in the probability of requiring hearing aid. Amifostine related adverse reaction in 19% patients. | ||
| Amifostine (600 mg/m2) 3 hours before and immediately before cisplatin injection | IV | ||||
| A randomized clinical study (n = 25) investigating medulloblastoma in children (PMID: 15999362) | Cisplatin (40 mg/m2) per day for 5 days | IV | Amifostine did not offer otoprotection against cisplatin combined with etoposide and bleomycin. | ||
| Amifostine (825 mg/m2) per day for 5 days 30 minutes before cisplatin | IV | ||||
| Etoposide (100 mg/m2) per day for 5 days | IV | ||||
| Bleomycin (15 IU/m2) per day for 5 days | IV | ||||
| Taurourso-deoxycholic acid (TUDCA) | HO1 and SOD2 mediated antioxidant activity (PMID: 32061715) | Rats treated with TUDCA (PMID: 32061715) | Cisplatin (5 mg/kg) per day for 3 days | IP | Protected hearing at 4, 8, 16, 32, 40 kHz. |
| TUDCA (100 mg/mL) 1 hour before cisplatin treatment | IP | ||||
| TUDCA | HO1 and SOD2 mediated antioxidant activity of TUDCA (PMID: 32061715) | Rats treated with TUDCA (PMID: 33631298) | Cisplatin (4.6 mg/kg) per day for 3 days | IP | Protected hearing at 8, 16, 24, 32, 40 kHz. |
| TUDCA (500 mg/kg) 1 day before cisplatin treatment | Subcutaneous | ||||
| Ebselen | Antioxidant activity acting as a GPx mimic | Mice treated with ebselen (PMID: 19286452) | Cisplatin (16 mg/kg) | IP | Protected hearing at 4, 8, 16, 32 kHz. |
| Ebselen (16 mg/kg) | IP | ||||
| Rat treated with ebselen (PMID: 21804453) | Cisplatin (14 mg/kg) | IV | Did not protect hearing. | ||
| Ebselen (12 mg/kg) 1 hour before cisplatin treatment | IP | ||||
| Rat treated with ebselen and allopurinol (PMID: 15721563) | Cisplatin (16 mg/kg) | IP | IP ebselen + allopurinol protected hearing at 8, 16, 24 kHz. | ||
| Ebselen (8 mg/kg) 1 hour before cisplatin treatment | IP/oral gavage | ||||
| Allopurinol (8 mg/kg) 1 hour before cisplatin treatment | IP/oral gavage |
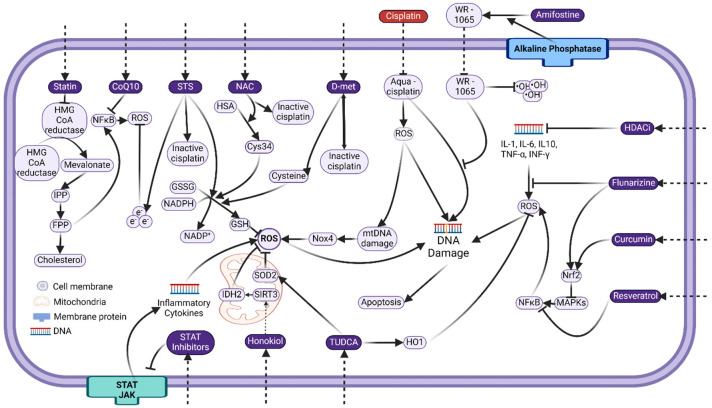
Figure 3.
Schematic image showing key targeting cellular factors and signaling steps for preventative strategies against cisplatin ototoxicity. Cisplatin induces DNA damage and ROS generation. Multiple processes and cytokines also contribute to ROS generation along the apoptosis and other (such as inflammation) signaling pathways. Proposed agents (in purple) target different sites in these processes. Refer to the text for details and abbreviations.
STS
STS has been traditionally used to treat metal poisoning. It can form strong complexes with metal ions, including platinum, thus deactivating cisplatin. Its antioxidant property comes from a reduced sulfur residue. The hearing protective effect of STS has been proven in both animal studies [172,177] and clinical trials [178,179]. Local delivery through intra-cochlear injection [148,152] or delayed administration [177] is required to treat cisplatin ototoxicity. Although the effect of local delivery is controversial [148,152], delayed administration of STS by 4-8 hours is proven to be protective to hearing [178,179], while it does not interfere with the therapeutic effect of cisplatin [180]. In these clinical studies, the incidence of cisplatin ototoxicity decreased by about half, and no significant difference in overall or event-free survival was observed between the treated group and the cisplatin-alone group [178,179]. In 2022, the FDA approved STS for protective treatment against cisplatin ototoxicity in pediatric patients with localized, non-metastatic solid tumors. It is the first and the only approved drug to treat cisplatin ototoxicity in clinics.
NAC
NAC is a precursor to GSH, a ubiquitous thiol-containing antioxidant and a widely used cytoprotectant with a promising hearing protective effect [181-184]. As a weak free radical scavenger, NAC may protect against cisplatin ototoxicity by induction of endogenous GSH and blocking apoptosis through the caspase signaling pathway [181,185]. Pre-treatment of NAC (400 mg/kg) improves cisplatin-induced hearing threshold shift in rats [186], although the results are slightly inconsistent with an organo-culture study [185]. Nevertheless, since NAC also protects against cisplatin cytotoxicity [181], a local or staggered administration is required for its otoprotection [186,187]. In clinical studies using intra-tympanic injection, NAC failed to prevent hearing loss during cisplatin treatment, although it was suggested for some patients [154,184]. In a recent phase I clinical study with children and adolescents newly diagnosed with nonmetastatic tumors delayed NAC application through intravenous (i.v.) injection (4 hours after cisplatin injection, peak concentration of NAC up to 450 mg/kg) was effective for preventing CIHL without adverse events [188]. These studies indicate that NAC might be another promising drug for treating cisplatin ototoxicity in clinics.
D-methionine
D-methionine is the enantiomeric counterpart of L-methionine, a naturally occurring L-alpha-amino acid. The antioxidant property of methionine is due to methionine being oxidized into methionine sulfoxide, which inhibits ROS. It also deactivates cisplatin by forming an inactive complex with it [78]. D-methionine is one of the earliest antioxidants tested in the protection against CIHL [189] and has proven to be effective both in animal studies [152,189,190] and in humans [174]. Since it is known that D-methionine interferes with the therapeutic effects of cisplatin, local, delayed, or staggered delivery should be considered in clinical trials. The in-human study, completed in India in 2022, used oral uptake of D-methionine (100 mg/kg) one hour before cisplatin treatment. The study did not show adverse events significantly different from the placebo group [174]. However, the study analyzed data from only 27 of the 50 enrolled participants. Furthermore, the study did not gather data on whether D-methionine affected cisplatin cytotoxicity in tumors. Overall, more careful examinations of the research and further larger scale clinical trials might be necessary to assess the influence of D-methionine on the antitumor efficacy of cisplatin.
Amifostine
Amifostine is used in chemotherapy and radiotherapy as a cytoprotective adjuvant for reducing renal toxicity [191]. Alkaline phosphatase can dephosphorylate it into an active free thiol metabolite that functions as an antioxidant [192]. Its cytoprotective action is believed to be achieved through scavenging free radicals, stabilizing DNA, and upregulating p53 [176], thereby selectively protecting normal tissues due to their high alkaline phosphatase levels [193]. Moreover, amifostine does not affect the antitumor activity of platinum drugs [192]. In animal research, multiple high doses of amifostine, given before cisplatin, protect hearing in hamsters (40 mg/kg/day) [194] and guinea pigs (100 mg/kg/day) [195]. However, lower doses (18 mg/kg/day) fail to achieve this effect [196]. Clinical studies on amifostine’s ability to protect against cisplatin ototoxicity have yielded mixed results. For instance, one study with 97 pediatric patients with medulloblastoma indicates a reduced need for hearing aids following amifostine treatment (600 mg/m2) [147]. In contrast, another study with 11 patients shows no protective effect (1000 mg/m2) [197]. A follow-up clinical trial with 379 patients reveals that amifostine protects from cisplatin ototoxicity only in average-risk but not in high-risk medulloblastoma patients [198]. In another study with 25 cases of pediatric germ cell tumor, amifostine treatment (825 mg/m2) does not confer otoprotection compared to historical controls [175]. These varying outcomes may be attributed to differences in treatment regimens, sample sizes, age groups, and cancer types across studies. As a note, two retrospective evaluations of the efficacy of amifostine in preventing cisplatin ototoxicity have been either inconclusive [199] or insignificant [200], possibly due to similar complexities. While high doses of amifostine are crucial for the treatment, they are associated with adverse events, including neurotoxicity (in animal studies) [194], hypocalcemia, hypotension, and nausea and vomiting (in clinical settings) [147]. Complex administration protocols, careful medical attention, and sustained monitoring of vital signs are required to ensure patient safety during amifostine treatment [201]. A more detailed review can be found in [78].
Coenzyme Q10
Coenzyme Q10 is a crucial enzyme for electron transport in the mitochondrial respiratory chain. It also works as an anti-inflammatory and antioxidant agent in dietary supplements [202]. Co-application of coenzyme Q10 and multivitamins was tested for protection against CO in both an animal model [203] and a pilot case-control clinical trial [204]. In the animal study, Q10 terclatrate (500 mg/kg) and vitamin supplements (vitamin E and B12) given before cisplatin (4.6 mg/kg/day for three days) treatment protected against CIHL in rats. In the pilot study on cancer patients treated with a coenzyme Q10 plus dietary multivitamin, the incidence of hearing disorders and tinnitus induced by cisplatin was reduced significantly. A significant difference in threshold shift only occurred at 8 kHz [204]. However, a fully powered clinical study has not been completed, even six years after the pre-clinical animal study.
Candidates/approaches targeting specific pathways/factors
Candidates targeting more specific cell signaling pathways in cisplatin ototoxicity are also often proposed and tested for their hearing protective effects. An early attempt is dexamethasone, a glucocorticosteroid that can downregulate proinflammatory cytokines, inhibit apoptosis, and upregulate antioxidant enzymes [78]. While animal studies yielded promising results [150,151,162], dexamethasone failed in most clinical trials [154,156], although attempts were made using nanoparticles [205] or poloxamer hydrogels [162] to increase local drug concentration in the cochlea. A detailed review regarding the protective effect of dexamethasone against cisplatin ototoxicity can be found in [78], with no further discussion in this review. As the understanding of cisplatin ototoxicity signaling pathways grows, more specific targets on factors/cytokines in metabolic [203,204], inflammatory [206,207], or apoptosis signaling pathways [102,208,209] have been identified with promising results. Some novel candidates that have been tested are listed in Table 4, and their potential mechanism is shown in Figure 3. These candidates include neurotrophins [210], hormones [154], molecules involved in endogenous metabolism [204], modulators of cell signaling pathways [102], etc.
Table 4.
Non-antioxidant drug candidates for protection against CIHL
| Drug | Main mechanism of oto-protection | Experiment model | Drug dose | Route | Outcome |
|---|---|---|---|---|---|
| Dexa-methasone | Inhibiting inflammatory cytokines (PMID: 9486420) | Mice treated with dexamethasone (PMC2720789) | Cisplatin (14 mg/kg) treatment after the first dexamethasone injection | IP | Protected hearing at 16 kHz. |
| Branded as DexPak & Decadron | Dexamethasone (24 mg/ml) per day over 5 days | Intratympanic | |||
| Guinea pigs treated with dexamethasone (PMID: 21521888) | Cisplatin (12 mg/kg) | IP | Not protective. | ||
| Dexamethasone (6 mg) treatment on the day before and on the day of cisplatin treatment | Intratympanic | ||||
| Clinical trial on cancer patients (n = 26) on dexamethasone (PMID: 24618499) | Cisplatin (total 400 mg) | IP | Protected hearing at 4-8 kHz. | ||
| Dexamethasone (~8.5 mg) | Intratympanic | ||||
| Coenzyme Q10 | Anti-inflammatory dietary supplements (PMID: 35326965) | Rats treated with Coenzyme Q10 and vitamins (PMID: 27632426) | Cisplatin (4.6 mg/kg) per day for 3 days | IP | Protected hearing at 2, 4, 8, 16, 32 kHz. |
| Branded as ubiquinone & CoQ10 | Q10 terclatrate (500 mg/kg) | Oral | |||
| Acuval 400 (100 mg/kg) | Oral | ||||
| Clinical trial on cancer patients (n = 26) being treated with Coenzyme Q10 and vitamins (PMID: 28239674) | Cisplatin (100 mg/m2) every 21 days | IV | Protected hearing at 8 kHz. | ||
| Acuval Audio (1.8 g) per day starting 7 days before the first cisplatin treatment | Oral | ||||
| Statins | Inhibiting inflammatory cytokines (PMID: 35125240). | Mice treated with lovastatin (PMID: 32062294) | Cisplatin (3 mg/kg) per day for 4 days, followed by 10 days of recovery. 3 cycles (total 36 mg/kg) | IP | Protected hearing at 20, 40 kHz. |
| Branded as Lipitor & Mevacor | Lovastatin (40 mg/kg) daily from 3 days before cisplatin treatment | Oral gavage | |||
| Clinical trial on head and neck cancer patients (n = 277) on atorvastatin (PMID: 33393488) | Cisplatin (~80 mg/m2) every 3 weeks. Median cumulative cisplatin was 200 mg/m2. | Not stated | Protected hearing at 4, 6, 8, 12.5 kHz. | ||
| Atorvastatin (10~80 mg) | Oral | ||||
| HDACi | Epigenetically downregulating genes linked to apoptosis (PMID: 23558232) | Guinea pigs treated with sodium butyrate (PMID: 16467722) | Cisplatin (14 mg/kg) | IP | Protected hearing at 3.5~20 kHz, statistical significance not shown. |
| Branded as Buphenyl & Avmacol | Sodium butyrate (1.2 mg/kg) per day for 7 days before and 5 days after cisplatin treatment | IP | |||
| Rats treated with sulforaphane (PMID: 34344210) | Cisplatin (7 mg/kg) twice a day for 7 days | IP | Protected hearing at 4, 8, 16, 24, 32 kHz. OHC partially protected. | ||
| SFN (30 mg/kg) per day for 7 days | IP | ||||
| Mice treated with RG108 (PMID: 35530135) | Cisplatin (30 umol/L) | IP | Protected hearing at 8, 16, 24, 32 kHz. OHC protected seen through IHC. | ||
| RG108 (100 umol/L) 2 hours before cisplatin treatment | IP | ||||
| STAT inhibitors | Anti-inflammatory and anti-apoptosis | Rats treated with STAT1 siRNA (PMID: 21776018) | Cisplatin (11 mg/kg) | IP | Protected hearing at 8, 16, 32 kHz. OHC protected seen through electron microscopy. |
| Branded as Nifuroxazide etc | STAT1 siRNA (0.g µg) 48 hours before cisplatin treatment | Intratympanic | |||
| Rats treated with STAT1 inhibitor EGCG (PMID: 28703809) | Cisplatin (11 mg/kg) | IP | Protected hearing at 8, 16, 32 kHz. OHC protected. | ||
| EGCG (100 mg/kg) 45 minutes before cisplatin + 3 more post-cisplatin treatments | Oral | ||||
| STAT6 -/- mice (PMID: 21321603) | Cisplatin (4 mg/kg) per day for 4 days | IP | Hearing protection at 4, 16, and 32 kHz. | ||
| Cytokine inhibitors | Inhibiting inflammatory cytokines | Mice treated with flunarizine (PMID: 18584244) | Cisplatin (4 mg/kg) | IP | Reduction of cochlear TNF-a and IL-1b.No ABR recording. |
| Branded as Basiliximab, daclizumab | Sibelium (143 ug/kg) | Oral | |||
| Rats treated with etanercept (PMID: 28730299) | Cisplatin (16 mg/kg) | IP | Protected hearing at 4.5, 6, 8, 10 kHz on day 3. | ||
| Etanercept (6 mg/kg) 24 hours before cisplatin treatment | IP | ||||
| Honokiol | SIRT3-mediated antioxidant activity (PMID: 21172655) | Mice treated with honokiol (PMID: 33415008) | Cisplatin (20 mg/kg) | IP | Protected hearing at 4-32 kHz. Cisplatin cytotoxicity not affected. |
| Branded as Honobsolute | Honokiol (20 mg/kg) treatment 1 hour before cisplatin treatment |
Epigenetic modulators
The significance of epigenetic modulation in inner ear development, damage, and protection was noticed over a decade ago [211-213], while it has garnered more attention only in recent years [214-216]. Histone deacetylases (HDACs) and their inhibitors (HDACis) regulate transcription and control cell cycle through modulation of histone acetylation. HDACis, such as sodium butyrate [212] and sulforaphane [215], are protective against cisplatin ototoxicity in animal models (guinea pigs and rats). The mechanism might be related to the pro-survival pathway activation, as shown in the study on HDAC inhibition against kanamycin and furosemide-induced hearing loss [214]. In addition, both sodium butyrate [217] and sulforaphane [218] can induce the expression of an antioxidant-responsive gene, Nrf2, which may also account for their hearing protective effects [219]. HDACs have antitumor activity and are well tolerated. They are ideal candidates for treating cisplatin ototoxicity. However, their mechanisms of action are not fully understood, and explanations rely on indirect evidence only. More studies regarding their specificity are needed before testing them in clinical trials.
Other promising epigenetic modifications have been reported recently. They are still conducted in cell cultures and animals. A recent study showed that a DNA methyltransferase inhibitor, RG108, alleviates CIHL in a mouse model [216]. Inhibition of DNA methylation by RG108 upregulates BCL-2 and downregulates mitochondrial-dependent apoptosis pathway factors BAX and BCL2 Associated Agonist of Cell Death (BAD) in HEI-OC1 cell cultures. Another histone methyltransferase inhibitor, BIX01294, has been shown to protect against CIHL in a mouse model established through the combination of i.p. furosemide and subcutaneous cisplatin delivery [220]. The mechanism, verified primarily on cell cultures, is associated with activating the autophagy pathway through the Forkhead box G1 gene, a critical regulator for morphogenesis of the mammalian inner ear during development. Nevertheless, further studies to verify these results and mechanisms are necessary, and their interference with the antitumor effects of cisplatin and other adverse effects are still unclear.
Statins
Statins are widely used as anti-inflammatory agents to control lipid peroxidation. They are proposed to protect hearing against various insults (noise [221], drug [222], and age [223]. For a review, see [224]). Statins are also used in an ongoing clinical trial against sudden hearing loss (ClinicalTrials.gov ID: NCT04826237). Two recent studies showed that statins protect against CIHL in animals (lovastatin [206]) and humans (atorvastatin [207]). The non-randomized, retrospective clinical study executed by Cunningham’s group showed that atorvastatin (with unknown doses) decreased the incidence of CIHL by 53% without affecting the three-year survival rates in head and neck cancer patients [207]. The same group will perform a randomized phase III clinical study (NCT04915183) on atorvastatin (20 mg) against CIHL. In this clinical trial, 186 patients with squamous cell carcinoma of the head and neck will be involved. The study aims to assess hearing using audiograms before and 2-4 months after cisplatin treatment and compare outcomes between atorvastatin users and controls. CTCAE criteria will be used for data analysis, and reduced bias will be expected. Although it might not be a major concern, sequelae of statins include muscle pain, liver damage, increased blood sugar, and memory loss.
Protein kinase inhibitors
Protein kinase phosphorylation cascades regulate multiple signal transduction pathways, including inflammation, apoptosis, and proliferation. Cytokine inhibitors can reduce cytokine synthesis and concentration and interfere with the interaction between cytokines and their receptors. Certain kinase inhibitors have been studied for their potential to protect against CIHL. Flunarizine, known initially as a calcium channel blocker, could reduce cisplatin-induced inflammatory cytokines (TNF-α, IL-1β, NF-κB seen through IHC) in mouse cochleae [110]. The reduction of cytokines was through the activation of Nrf2/HO-1 signaling. However, no results on ABR threshold changes were shown in the paper. Multiple inhibitors for CDK2 (kenpaullone and two unrevealed compounds) protect hearing against CIHL and noise-induced hearing loss [102,208,225], suggesting that CDK2 is an important target for hearing protection. Dabrafenib, a BRAF (a member of Raf family kinases) inhibitor, is also protective against CIHL and noise-induced hearing loss when combined with the CDK2 inhibitor AZD5438 [209]. However, the influence of cisplatin on the antitumor effect must be verified in tumor-bearing mice before its potential application in clinical practice.
Polyphenols
Polyphenols are a diverse group of naturally occurring compounds abundant in plants. Many polyphenols are natural antioxidants and anti-inflammatory agents that protect against various stress conditions such as UV light radiation and microbial infections. The roles of polyphenols in hearing protection against different insults are recognized, and the mechanisms studied most recently (for review, see [47,226]). Epicatechin protected hearing in rats against CIHL by inhibiting ERK signaling in an early study [227,228]. However, the protective effect of epicatechin needs further verification with cochlear whole-mount samples and frequency-specific ABR data. Resveratrol, a polyphenol abundant in fruits and red wine, was also shown to protect against CIHL in guinea pigs [229] and rats [230] at 10 and 0.1-0.5 mg/kg/day, respectively. In contrast, high doses (50, 10, and 1 mg/kg/day) were toxic to the cochlea in rats [230,231]. The hearing protection mechanism is associated with activating the anti-oxidative response and reducing inflammatory responses mediated by NF-kB, IL6, and IL1β [231]. Curcumin, a bright yellow pigment extracted from the plant turmeric, is also shown to protect against CIHL in rats at 200 mg/kg [232,233]. The mechanism is associated with decreased lipid peroxidation, potentially through the Nrf2 pathway [232]. Although arguably [234], curcumin is reactive, unstable, and non-bioavailable, which has shown to be unsuccessful in any clinical trial [235]. In addition, the interaction with the antitumor function of cisplatin is yet to be investigated. Luteolin is a flavone derived from celery, green pepper, and chamomile with anti-inflammatory, antioxidant, and anticarcinogenic properties. In a recent study, luteolin showed promising protection against CIHL by inhibiting ferroptosis [136]. The authors comprehensively studied the involvement of Gpx4, the key regulator for ferroptosis in CIHL. Luteolin protection through alleviation of ferroptosis was proven in cell cultures, cochlear explants cultures, and in vivo Gpx4 knockout mice levels [136]. Honokiol, a lignan extracted from the plant Magnolia, is a multifunctional small polyphenol with both antitumor effects that synergize with cisplatin [236-238] and protective effect to various tissues and organs against oxidative stress, including the brain [239-241], heart [242-244], kidney [245], liver [246], etc. In a recent study from our lab, honokiol has shown strong hearing protective effects against cisplatin ototoxicity in tumor-bearing mice undergoing chemotherapy [247]. Honokiol also increases animal survival without interfering with cisplatin’s antitumor effects. The mechanism is associated with activating sirtuin 3 (SIRT3), a member of the sirtuin NAD+-dependent deacetylase family, critical regulators of the intrinsic anti-ROS systems.
Sirtuins comprise a highly conserved NAD+-dependent deacetylase family, all key regulators of the intrinsic anti-ROS systems [248-251]. Seven members (SIRT1-7) of the sirtuin family are expressed in the cytoplasm (SIRT1&2), mitochondria (SIRT3-5), and the nucleus (SIRT1, 2, 6, 7). The involvement of sirtuins (mostly SIRT3) in hearing protection has been shown in noise-induced [252,253], drug-induced [254], and age-related hearing loss [255,256]. Activation of sirtuins (mostly SIRT1) by polyphenols has also been reported in resveratrol [257], curcumin [258], and epicatechin [259]. Sirtuins might represent novel targets for hearing protection against cisplatin ototoxicity, and polyphenols are promising candidates. For example, honokiol does not interfere with the therapeutic effects of cisplatin, as shown in our study [247] and other publications [237,238,260,261]. It can pass the BBB [262,263] and diffuse across plasma and mitochondrial membranes in vivo [243,263]. Although bioavailability is still a concern for most polyphenols because of their insolubility and instability, different ways for assisting the delivery of polyphenols, such as liposomal [264-266], amorphous solid dispersion [267,268], and biodegradable microsphere [269], have made significant progress in recent years.
Summary and outlook
This paper sought to comprehensively review the current understanding and research on ototoxicity in chemotherapy (particularly with cisplatin) and explore strategies and compounds for hearing protection. Using symptoms including hearing loss, tinnitus, vertigo, and dizziness, we identified potential ototoxic drugs, among which four are specifically causing hearing loss. While the ototoxic mechanisms of cisplatin are extensively studied, other agents like isotretinoin, vinblastine, and dasatinib remain less understood.
The primary target of cisplatin in the cochlea is still debatable, although OHC loss is the most commonly observed change in cisplatin ototoxicity. Damage of the SV might be the leading cause, which affects the EP and may result in cisplatin influx into the scala media. These changes induce a series cascade of cellular processes, eventually leading to cell death. ROS generation is the predominant factor in this process, and its subsequent signaling pathways are discussed.
The review emphasizes the urgent need for effective otoprotective strategies that can mitigate the adverse effects of chemotherapy on hearing without compromising its anticancer efficacy. Various antioxidants, such as STS, NAC, and D-methionine, have been explored for their potential to counteract the ROS-mediated ototoxicity of cisplatin. STS, in particular, has gained FDA approval for reducing hearing loss in pediatric cancer patients receiving cisplatin. However, the challenge of ensuring that these agents do not interfere with the chemotherapeutic efficacy of cisplatin remains a critical consideration in their clinical application.
Emerging research has focused on more specific molecular targets and pathways involved in cisplatin-induced hearing loss. The review highlights the exploration of various compounds, including statins, protein kinase inhibitors, and polyphenols like epicatechin, resveratrol, curcumin, luteolin, and honokiol, for their protective effects against CIHL. These agents, often possessing antioxidant and anti-inflammatory properties, offer new avenues for otoprotection, with some showing promise in preclinical and clinical studies. Particularly, honokiol has been noted for its synergistic antitumor effects with cisplatin and its ability to protect against ototoxicity, potentially through activating the sirtuin pathway.
The review also discusses innovative drug delivery methods, such as local administration via trans-tympanic injection and advanced formulations like nanoparticles and hydrogels, to enhance the efficacy of otoprotective agents and minimize their systemic side effects. These strategies aim to improve the bioavailability of protective agents in the cochlea and reduce the risk of interfering with the systemic anticancer activity of chemotherapeutic drugs.
In conclusion, while significant advances have been made in understanding the mechanisms of chemotherapy-induced ototoxicity and identifying potential otoprotective strategies, further research is needed to develop safe and effective treatments that can be integrated into standard oncology care. The balance between preserving hearing and maintaining the antineoplastic effectiveness of chemotherapy presents a complex challenge that future studies must address to improve the quality of life for cancer patients undergoing treatment.
Acknowledgements
This work is supported by grants from the NIH R01-DC019434, Hearing Health Foundation, and American Hearing Research Foundation awarded to XT.
Disclosure of conflict of interest
None.
Abbreviations
- ABR
Auditory Brainstem Response
- ABVD
Doxorubicin, Bleomycin, Vinblastine, Dacarbazine
- AMPK
AMP-activated Protein Kinase
- ARHL
Age-Related Hearing Loss
- Bax
Bcl-2-Associated X Protein
- Bcl2
B-cell Lymphoma 2
- BBB
Blood-Brain Barrier
- BLB
Blood-Labyrinthine Barrier
- CAP
Compound Action Potential
- CAT
Catalase
- CDK
Cyclin-Dependent Kinase
- CHOP
C/EBP Homologous Protein
- CIHL
Cisplatin-Induced Hearing Loss
- CTR1
Copper Transporter 1
- DPOAE
Distortion product Oto-Acoustic Emission
- EP
Endocochlear Potential
- ER
Endoplasmic Reticulum
- FDA
Food and Drug Administration
- Gpx4
Glutathione Peroxidase 4
- GR
Glutathione Reductase
- GSH.Px
Glutathione Peroxidase
- HDAC
Histone Deacetylase
- HDACi
Histone Deacetylase Inhibitor
- HEI-OC1
House Ear Institute-Organ of Corti 1
- IG
Immunoglobin
- IHCs
Inner Hair Cells
- IL
Interleukin
- iNOS
Inducible Nitric Oxide Synthase
- i.p.
Intraperitoneal
- i.v.
Intravenous
- JAK-STAT
Janus Kinase-Signal Transducer and Activator of Transcription
- LC3-II
Microtubule-associated Protein 1A/1B-light Chain 3
- LRP2
LDL Receptor-related Protein 2
- MAPK
Mitogen-Activated Protein Kinase
- MET
Mechano-Electrical Transduction
- MLKL
Mixed Lineage Kinase Domain-Like Pseudokinase
- mtDNA
mitochondrial DNA
- mTOR
Mammalian Target of Rapamycin
- mtROS
mitochondrial ROS
- NAC
N-acetyl-L-cysteine
- NCI
National Cancer Institute
- NF-κB
Nuclear Factor Kappa-light-chain-enhancer of activated B cells
- NLRX1
Nucleotide-binding Domain and Leucine-rich-repeat-containing Family Member X1
- NOX3
NADPH Oxidase 3
- Nrf2
Nuclear Factor Erythroid 2-Related Factor 2
- O2 -.
Superoxide
- OCT2
Organic Cation Transporter 2
- OHCs
Outer Hair Cells
- ONOO-
Peroxynitrite
- RIPK
Receptor-Interacting Protein Kinase
- ROS
Reactive Oxygen Species
- SGN
Spiral Ganglion Neuron
- SIRT
Sirtuin
- SOD
Superoxide Dismutase
- STAT1
Signal Transducer and Activator of Transcription 1
- STS
Sodium Thiosulfate
- SV
Stria Vascularis
- TNF-α
Tumor Necrosis Factor Alpha
- TRPV1
Transient Receptor Potential Vanilloid 1
References
- 1.NCI. Side effects of cancer treatment - National Cancer Institute. n.d.; https://www.cancer.gov/about-cancer/treatment/side-effects.
- 2.NCI. Cancer Drugs - National Cancer Institute. n.d.; https://www.cancer.gov/about-cancer/treatment/drugs.
- 3.Chemocare. Chemotherapy Drugs and Drugs often Used During Chemotherapy. n.d.; https://chemocare.com/chemotherapy/drug-info/default.aspx.
- 4.Beaumont. Chemotherapy Drugs and Side Effects. 2022. https://www.beaumont.org/treatments/chemotherapy-drugs-side-effects.
- 5.ACS. Chemotherapy Side Effects. 2022. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html.
- 6.Tavanai E, Khalili ME, Shahidipour Z, Jalaie S, Ghahraman MA, Rouhbakhsh N, Rahimi V. Hearing handicaps, communication difficulties and depression in the older adults: a comparison of hearing aid users and non-users. Eur Arch Otorhinolaryngol. 2023;280:5229–5240. doi: 10.1007/s00405-023-08012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil JD, Alrashid MA, Eltabbakh A, Fredericks S. The association between stress, emotional states, and tinnitus: a mini-review. Front Aging Neurosci. 2023;15:1131979. doi: 10.3389/fnagi.2023.1131979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewster KK, Deal JA, Lin FR, Rutherford BR. Considering hearing loss as a modifiable risk factor for dementia. Expert Rev Neurother. 2022;22:805–813. doi: 10.1080/14737175.2022.2128769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minasian LM, Frazier AL, Sung L, O’Mara A, Kelaghan J, Chang KW, Krailo M, Pollock BH, Reaman G, Freyer DR. Prevention of cisplatin-induced hearing loss in children: informing the design of future clinical trials. Cancer Med. 2018;7:2951–2959. doi: 10.1002/cam4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon S. Sodium thiosulfate: pediatric first approval. Paediatr Drugs. 2023;25:239–244. doi: 10.1007/s40272-022-00550-x. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Zhang ZJ, Tokuriki M, Shibamori Y, Yamamoto T, Noda I, Ohtsubo T, Saito H. Doxorubicin ototoxicity is induced in mice by combination treatment with cyclosporin A. Acta Otolaryngol. 2001;121:787–793. doi: 10.1080/00016480152602212. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Savage SR, Calinawan AP, Lin C, Zhang B, Wang P, Starr TK, Birrer MJ, Paulovich AG. A highly annotated database of genes associated with platinum resistance in cancer. Oncogene. 2021;40:6395–6405. doi: 10.1038/s41388-021-02055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177–186. doi: 10.1620/tjem.219.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 2012;295:1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15:364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- 16.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti Panici P, Greggi S, Scambia G, Baiocchi G, Lomonaco M, Conti G, Mancuso S. Efficacy and toxicity of very high-dose cisplatin in advanced ovarian carcinoma: 4-year survival analysis and neurological follow-up. Int J Gynecol Cancer. 1993;3:44–53. doi: 10.1046/j.1525-1438.1993.03010044.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuce S, Seker MM, Koc S, Uysal IO, Kacan T, Dogan M, Dogan M, Babacan NA, Kilickap S. Oxaliplatin and ototoxicity: is it really safe for hearing? Turk J Med Sci. 2014;44:586–589. doi: 10.3906/sag-1304-76. [DOI] [PubMed] [Google Scholar]
- 20.Gersten BK, Fitzgerald TS, Fernandez KA, Cunningham LL. Ototoxicity and platinum uptake following cyclic administration of platinum-based chemotherapeutic agents. J Assoc Res Otolaryngol. 2020;21:303–321. doi: 10.1007/s10162-020-00759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SY, Wasif N, Garcon MC, Rodriguez G, Saif MW. Ototoxicity associated with oxaliplatin in a patient with pancreatic cancer. JOP. 2013;14:676–679. doi: 10.6092/1590-8577/1629. [DOI] [PubMed] [Google Scholar]
- 22.Kim SB, Kim SY, Kim KH, Kim TN. Oxaliplatin-induced sudden hearing loss in a patient with pancreatic cancer. Korean J Gastroenterol. 2020;76:261–264. doi: 10.4166/kjg.2020.121. [DOI] [PubMed] [Google Scholar]
- 23.Guvenc MG, Dizdar D, Dizdar SK, Okutur SK, Demir G. Sudden hearing loss due to oxaliplatin use in a patient with colon cancer. J Chemother. 2016;28:341–342. doi: 10.1179/1973947815Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 24.Moss PE, Hickman S, Harrison BR. Ototoxicity associated with vinblastine. Ann Pharmacother. 1999;33:423–425. doi: 10.1345/aph.18288. [DOI] [PubMed] [Google Scholar]
- 25.Nirban RK, Kapoor A, Narayan S, Maharia S, Singhal MK, Kumar HS. Ototoxicity following Vinblastine chemotherapy in a patient of Hodgkin’s Lymphoma. Indian J Otology. 2015;21:229–230. [Google Scholar]
- 26.Lugassy G, Shapira A. Sensorineural hearing loss associated with vincristine treatment. Blut. 1990;61:320–321. doi: 10.1007/BF01732887. [DOI] [PubMed] [Google Scholar]
- 27.Aydogdu I, Ozturan O, Kuku I, Kaya E, Sevinc A, Yildiz R. Bilateral transient hearing loss associated with vincristine therapy: case report. J Chemother. 2000;12:530–532. doi: 10.1179/joc.2000.12.6.530. [DOI] [PubMed] [Google Scholar]
- 28.Kalcioglu MT, Kuku I, Kaya E, Oncel S, Aydogdu I. Bilateral hearing loss during vincristine therapy: a case report. J Chemother. 2003;15:290–292. doi: 10.1179/joc.2003.15.3.290. [DOI] [PubMed] [Google Scholar]
- 29.Rosende L, Verea-Hernando MM, de Andres A, Pineyro-Molina F, Barja J, Castro-Castro S, Fonseca E. Hypoacusia in a patient treated by isotretinoin. Case Rep Med. 2011;2011:789143. doi: 10.1155/2011/789143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Lee JH, Lee JM. All-trans retinoic acid-induced ototoxicity during chemotherapy in pediatric acute promyelocytic leukemia. Children (Basel) 2021;8:27. doi: 10.3390/children8010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akdag M, Akkurt ZM, Gul A, Ucmak D, Yilmaz B, Sengul E, Topcu I. The effects of oral isotretinoin (13-Cis retinoic acid) on the inner ear: a prospective clinical study. Clin Invest Med. 2014;37:E102–E107. doi: 10.25011/cim.v37i2.21092. [DOI] [PubMed] [Google Scholar]
- 32.Karabulut H, Karadag AS, Acar B, Dagli M, Karabulut I, Ozmen E, Babademez MA, Karasen RM. The effect of oral isotretinoin (13-cis retinoic acid) on hearing systems in patients with acne vulgaris: a prospective study. Int J Dermatol. 2011;50:1139–1143. doi: 10.1111/j.1365-4632.2011.04959.x. [DOI] [PubMed] [Google Scholar]
- 33.Bragado P, Armesilla A, Silva A, Porras A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007;12:1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 34.Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, Kim HJ, Jeong H, Kim HM, Hwang D, Kim HS, Choi S. Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS One. 2015;10:e0135083. doi: 10.1371/journal.pone.0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto AL, Lippard SJ. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780:167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 36.Chvalova K, Brabec V, Kasparkova J. Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007;35:1812–1821. doi: 10.1093/nar/gkm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan B, Akyuz N, Liu XP, Asai Y, Nist-Lund C, Kurima K, Derfler BH, Gyorgy B, Limapichat W, Walujkar S, Wimalasena LN, Sotomayor M, Corey DP, Holt JR. TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron. 2018;99:736–753. e736. doi: 10.1016/j.neuron.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci. 2006;26:10992–11000. doi: 10.1523/JNEUROSCI.2188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abeytunge S, Gianoli F, Hudspeth AJ, Kozlov AS. Rapid mechanical stimulation of inner-ear hair cells by photonic pressure. Elife. 2021;10:e65930. doi: 10.7554/eLife.65930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caprara GA, Peng AW. Mechanotransduction in mammalian sensory hair cells. Mol Cell Neurosci. 2022;120:103706. doi: 10.1016/j.mcn.2022.103706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ó Maoiléidigh D, Ricci AJ. A bundle of mechanisms: inner-ear hair-cell mechanotransduction. Trends Neurosci. 2019;42:221–236. doi: 10.1016/j.tins.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 43.Wangemann P, Liu J, Marcus DC. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res. 1995;84:19–29. doi: 10.1016/0378-5955(95)00009-s. [DOI] [PubMed] [Google Scholar]
- 44.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH, Chuva de Sousa Lopes SM. Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev Neurobiol. 2015;75:1219–1240. doi: 10.1002/dneu.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prayuenyong P, Baguley DM, Kros CJ, Steyger PS. Preferential cochleotoxicity of cisplatin. Front Neurosci. 2021;15:695268. doi: 10.3389/fnins.2021.695268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan WJT, Vlajkovic SM. Molecular characteristics of cisplatin-induced ototoxicity and therapeutic interventions. Int J Mol Sci. 2023;24:16545. doi: 10.3390/ijms242216545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med. 2019;11:eaao0935. doi: 10.1126/scitranslmed.aao0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review) Hear Res. 2016;338:52–63. doi: 10.1016/j.heares.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fetoni AR, Troiani D, Eramo SL, Rolesi R, Paludetti Troiani G. Efficacy of different routes of administration for Coenzyme Q10 formulation in noise-induced hearing loss: systemic versus transtympanic modality. Acta Otolaryngol. 2012;132:391–399. doi: 10.3109/00016489.2011.652307. [DOI] [PubMed] [Google Scholar]
- 51.Howell SB, Taetle R. Effect of sodium thiosulfate on cis-dichlorodiammineplatinum(II) toxicity and antitumor activity in L1210 leukemia. Cancer Treat Rep. 1980;64:611–616. [PubMed] [Google Scholar]
- 52.Paken J, Govender CD, Pillay M, Sewram V. A review of cisplatin-associated ototoxicity. Semin Hear. 2019;40:108–121. doi: 10.1055/s-0039-1684041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAlpine D, Johnstone BM. The ototoxic mechanism of cisplatin. Hear Res. 1990;47:191–203. doi: 10.1016/0378-5955(90)90151-e. [DOI] [PubMed] [Google Scholar]
- 55.Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 1995;76:386–394. doi: 10.1111/j.1600-0773.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 56.Sheth S, Mukherjea D, Rybak LP, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci. 2017;11:338. doi: 10.3389/fncel.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237:219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CH, Lee DH, Lee SM, Kim ASY. Otoprotective effects of Zingerone on cisplatin-induced ototoxicity. Int J Mol Sci. 2020;21:3503. doi: 10.3390/ijms21103503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gentilin E, Simoni E, Candito M, Cazzador D, Astolfi L. Cisplatin-induced ototoxicity: updates on molecular targets. Trends Mol Med. 2019;25:1123–1132. doi: 10.1016/j.molmed.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Anfuso CD, Cosentino A, Agafonova A, Zappala A, Giurdanella G, Trovato Salinaro A, Calabrese V, Lupo G. Pericytes of stria vascularis are targets of cisplatin-induced ototoxicity: new insights into the molecular mechanisms involved in blood-labyrinth barrier breakdown. Int J Mol Sci. 2022;23:15790. doi: 10.3390/ijms232415790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sluyter S, Klis SF, de Groot JC, Smoorenburg GF. Alterations in the stria vascularis in relation to cisplatin ototoxicity and recovery. Hear Res. 2003;185:49–56. doi: 10.1016/s0378-5955(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 62.Freitas MR, Silva VC, Brito GA, Carvalho Junior JV, Gomes Junior RM, Ribeiro Rde A. Distortion-product otoacoustic emissions and auditory brainstem responses sensitivity assessment in cisplatin-induced ototoxicity in rats. Braz J Otorhinolaryngol. 2009;75:476–484. doi: 10.1016/S1808-8694(15)30483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Bielefeld EC, Mellott JG, Wang W, Mafi AM, Yamoah EN, Bao J. Early physiological and cellular indicators of cisplatin-induced ototoxicity. J Assoc Res Otolaryngol. 2021;22:107–126. doi: 10.1007/s10162-020-00782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borse V, Al Aameri RFH, Sheehan K, Sheth S, Kaur T, Mukherjea D, Tupal S, Lowy M, Ghosh S, Dhukhwa A, Bhatta P, Rybak LP, Ramkumar V. Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis. 2017;8:e2921. doi: 10.1038/cddis.2017.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci. 2013;33:4405–4414. doi: 10.1523/JNEUROSCI.3940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L, Cunningham LL. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8:1654. doi: 10.1038/s41467-017-01837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas JP, Lautermann J, Liedert B, Seiler F, Thomale J. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol Pharmacol. 2006;70:23–29. doi: 10.1124/mol.106.022244. [DOI] [PubMed] [Google Scholar]
- 70.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riedemann L, Lanvers C, Deuster D, Peters U, Boos J, Jurgens H, am Zehnhoff-Dinnesen A. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 72.Tang Q, Wang X, Jin H, Mi Y, Liu L, Dong M, Chen Y, Zou Z. Cisplatin-induced ototoxicity: updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm. 2021;163:60–71. doi: 10.1016/j.ejpb.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Kimitsuki T, Nakagawa T, Hisashi K, Komune S, Komiyama S. Cisplatin blocks mechano-electric transducer current in chick cochlear hair cells. Hear Res. 1993;71:64–68. doi: 10.1016/0378-5955(93)90021-r. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Liu C, Kaefer S, Youssef M, Zhao B. The mechanotransduction channel and organic cation transporter are critical for cisplatin ototoxicity in murine hair cells. Front Mol Neurosci. 2022;15:835448. doi: 10.3389/fnmol.2022.835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Ruijven MW, de Groot JC, Smoorenburg GF. Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. A quantitative histological study. Hear Res. 2004;197:44–54. doi: 10.1016/j.heares.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 76.Binks SP, Dobrota M. Kinetics and mechanism of uptake of platinum-based pharmaceuticals by the rat small intestine. Biochem Pharmacol. 1990;40:1329–1336. doi: 10.1016/0006-2952(90)90400-f. [DOI] [PubMed] [Google Scholar]
- 77.Previati M, Lanzoni I, Astolfi L, Fagioli F, Vecchiati G, Pagnoni A, Martini A, Capitani S. Cisplatin cytotoxicity in organ of Corti-derived immortalized cells. J Cell Biochem. 2007;101:1185–1197. doi: 10.1002/jcb.21239. [DOI] [PubMed] [Google Scholar]
- 78.Hazlitt RA, Min J, Zuo J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J Med Chem. 2018;61:5512–5524. doi: 10.1021/acs.jmedchem.7b01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu D, Gu J, Chen Y, Kang W, Wang X, Wu H. Current strategies to combat cisplatin-induced ototoxicity. Front Pharmacol. 2020;11:999. doi: 10.3389/fphar.2020.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am J Otol. 2000;21:513–520. [PubMed] [Google Scholar]
- 81.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 82.Mukherjea D, Jajoo S, Sheehan K, Kaur T, Sheth S, Bunch J, Perro C, Rybak LP, Ramkumar V. NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss. Antioxid Redox Signal. 2011;14:999–1010. doi: 10.1089/ars.2010.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu J, Ye J, Kong W, Zhang S, Zheng Y. Programmed cell death pathways in hearing loss: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2020;53:e12915. doi: 10.1111/cpr.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, Ramkumar V. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28:13056–13065. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011;2:e180. doi: 10.1038/cddis.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999;109:1740–1744. doi: 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Lautermann J, Crann SA, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res. 1997;114:75–82. doi: 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 88.Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-associated ototoxicity: a review for the health professional. J Toxicol. 2016;2016:1809394. doi: 10.1155/2016/1809394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramkumar V, Mukherjea D, Dhukhwa A, Rybak LP. Oxidative stress and inflammation caused by cisplatin ototoxicity. Antioxidants (Basel) 2021;10:1919. doi: 10.3390/antiox10121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Berrocal JR, Nevado J, Ramirez-Camacho R, Sanz R, Gonzalez-Garcia JA, Sanchez-Rodriguez C, Cantos B, Espana P, Verdaguer JM, Trinidad Cabezas A. The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. Br J Pharmacol. 2007;152:1012–1020. doi: 10.1038/sj.bjp.0707405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed Res Int. 2015;2015:617207. doi: 10.1155/2015/617207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong AC, Ryan AF. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci. 2015;7:58. doi: 10.3389/fnagi.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romano A, Capozza MA, Mastrangelo S, Maurizi P, Triarico S, Rolesi R, Attina G, Fetoni AR, Ruggiero A. Assessment and management of platinum-related ototoxicity in children treated for cancer. Cancers (Basel) 2020;12:1266. doi: 10.3390/cancers12051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benkafadar N, Menardo J, Bourien J, Nouvian R, Francois F, Decaudin D, Maiorano D, Puel JL, Wang J. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol Med. 2017;9:7–26. doi: 10.15252/emmm.201606230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci. 2010;30:3933–3946. doi: 10.1523/JNEUROSCI.6054-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.So HS, Park C, Kim HJ, Lee JH, Park SY, Lee JH, Lee ZW, Kim HM, Kalinec F, Lim DJ, Park R. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hear Res. 2005;204:127–139. doi: 10.1016/j.heares.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Monroe JD, Johnston AM, Smith ME. The monofunctional platinum(II) compounds, phenanthriplatin and pyriplatin, modulate apoptosis signaling pathways in HEI-OC1 auditory hybridoma cells. Neurotoxicology. 2020;79:104–109. doi: 10.1016/j.neuro.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee HS, Kim YR, Lee IK, Kim UK, Baek JI, Lee KY. KL1333, a derivative of beta-lapachone, protects against cisplatin-induced ototoxicity in mouse cochlear cultures. Biomed Pharmacother. 2020;126:110068. doi: 10.1016/j.biopha.2020.110068. [DOI] [PubMed] [Google Scholar]
- 99.Murata T, Hibasami H, Maekawa S, Tagawa T, Nakashima K. Preferential binding of cisplatin to mitochondrial DNA and suppression of ATP generation in human malignant melanoma cells. Biochem Int. 1990;20:949–955. [PubMed] [Google Scholar]
- 100.Olivero OA, Semino C, Kassim A, Lopez-Larraza DM, Poirier MC. Preferential binding of cisplatin to mitochondrial DNA of Chinese hamster ovary cells. Mutat Res. 1995;346:221–230. doi: 10.1016/0165-7992(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 101.Yang Z, Schumaker LM, Egorin MJ, Zuhowski EG, Guo Z, Cullen KJ. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res. 2006;12:5817–5825. doi: 10.1158/1078-0432.CCR-06-1037. [DOI] [PubMed] [Google Scholar]
- 102.Teitz T, Fang J, Goktug AN, Bonga JD, Diao S, Hazlitt RA, Iconaru L, Morfouace M, Currier D, Zhou Y, Umans RA, Taylor MR, Cheng C, Min J, Freeman B, Peng J, Roussel MF, Kriwacki R, Guy RK, Chen T, Zuo J. CDK2 inhibitors as candidate therapeutics for cisplatin- and noise-induced hearing loss. J Exp Med. 2018;215:1187–1203. doi: 10.1084/jem.20172246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- 104.Zong S, Liu T, Wan F, Chen P, Luo P, Xiao H. Endoplasmic reticulum stress is involved in cochlear cell apoptosis in a cisplatin-induced ototoxicity rat model. Audiol Neurootol. 2017;22:160–168. doi: 10.1159/000480346. [DOI] [PubMed] [Google Scholar]
- 105.Kishino A, Hayashi K, Maeda M, Jike T, Hidai C, Nomura Y, Oshima T. Caspase-8 regulates endoplasmic reticulum stress-induced necroptosis independent of the apoptosis pathway in auditory cells. Int J Mol Sci. 2019;20:5896. doi: 10.3390/ijms20235896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu H, Tian M, Ding C, Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chung WH, Boo SH, Chung MK, Lee HS, Cho YS, Hong SH. Proapoptotic effects of NF-kappaB on cisplatin-induced cell death in auditory cell line. Acta Otolaryngol. 2008;128:1063–1070. doi: 10.1080/00016480701881811. [DOI] [PubMed] [Google Scholar]
- 108.Babolmorad G, Latif A, Domingo IK, Pollock NM, Delyea C, Rieger AM, Allison WT, Bhavsar AP. Toll-like receptor 4 is activated by platinum and contributes to cisplatin-induced ototoxicity. EMBO Rep. 2021;22:e51280. doi: 10.15252/embr.202051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY, Moon SK, Lim DJ, Park R. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.So H, Kim H, Kim Y, Kim E, Pae HO, Chung HT, Kim HJ, Kwon KB, Lee KM, Lee HY, Moon SK, Park R. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol. 2008;9:290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, Yagi T. Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 2002;22:4081–4085. [PubMed] [Google Scholar]
- 112.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JE, Nakagawa T, Kita T, Kim TS, Iguchi F, Endo T, Shiga A, Lee SH, Ito J. Mechanisms of apoptosis induced by cisplatin in marginal cells in mouse stria vascularis. ORL J Otorhinolaryngol Relat Spec. 2004;66:111–118. doi: 10.1159/000079329. [DOI] [PubMed] [Google Scholar]
- 114.Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- 115.Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med. 2018;16:96. doi: 10.1186/s12967-018-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruhl D, Du TT, Wagner EL, Choi JH, Li S, Reed R, Kim K, Freeman M, Hashisaki G, Lukens JR, Shin JB. Necroptosis and apoptosis contribute to cisplatin and aminoglycoside ototoxicity. J Neurosci. 2019;39:2951–2964. doi: 10.1523/JNEUROSCI.1384-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27:1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaur T, Borse V, Sheth S, Sheehan K, Ghosh S, Tupal S, Jajoo S, Mukherjea D, Rybak LP, Ramkumar V. Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J Neurosci. 2016;36:3962–3977. doi: 10.1523/JNEUROSCI.3111-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim HJ, Oh GS, Lee JH, Lyu AR, Ji HM, Lee SH, Song J, Park SJ, You YO, Sul JD, Park C, Chung SY, Moon SK, Lim DJ, So HS, Park R. Cisplatin ototoxicity involves cytokines and STAT6 signaling network. Cell Res. 2011;21:944–956. doi: 10.1038/cr.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ikeda K, Sunose H, Takasaka T. Effects of free radicals on the intracellular calcium concentration in the isolated outer hair cell of the guinea pig cochlea. Acta Otolaryngol. 1993;113:137–141. doi: 10.3109/00016489309135781. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 122.Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129–137. doi: 10.1016/j.heares.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi MJ, Kang H, Lee YY, Choo OS, Jang JH, Park SH, Moon JS, Choi SJ, Choung YH. Cisplatin-induced ototoxicity in rats is driven by RIP3-dependent necroptosis. Cells. 2019;8:409. doi: 10.3390/cells8050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin H, Yang Q, Cao Z, Li H, Yu Z, Zhang G, Sun G, Man R, Wang H, Li J. Activation of NLRX1-mediated autophagy accelerates the ototoxic potential of cisplatin in auditory cells. Toxicol Appl Pharmacol. 2018;343:16–28. doi: 10.1016/j.taap.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 127.Yin H, Sun G, Yang Q, Chen C, Qi Q, Wang H, Li J. NLRX1 accelerates cisplatin-induced ototoxity in HEI-OC1 cells via promoting generation of ROS and activation of JNK signaling pathway. Sci Rep. 2017;7:44311. doi: 10.1038/srep44311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang B, Xiao H. Rapamycin alleviates cisplatin-induced ototoxicity in vivo. Biochem Biophys Res Commun. 2014;448:443–447. doi: 10.1016/j.bbrc.2014.04.123. [DOI] [PubMed] [Google Scholar]
- 129.Youn CK, Kim J, Park JH, Do NY, Cho SI. Role of autophagy in cisplatin-induced ototoxicity. Int J Pediatr Otorhinolaryngol. 2015;79:1814–1819. doi: 10.1016/j.ijporl.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 130.Yang Q, Sun G, Yin H, Li H, Cao Z, Wang J, Zhou M, Wang H, Li J. PINK1 protects auditory hair cells and spiral ganglion neurons from cisplatin-induced ototoxicity via inducing autophagy and inhibiting JNK signaling pathway. Free Radic Biol Med. 2018;120:342–355. doi: 10.1016/j.freeradbiomed.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 131.Cho SI, Jo ER, Song H. Mitophagy impairment aggravates cisplatin-induced ototoxicity. Biomed Res Int. 2021;2021:5590973. doi: 10.1155/2021/5590973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Zhang T, Song Q, Gao D, Li Y, Jie H, Huang P, Zheng G, Yang J, He J. Cisplatin ototoxicity mechanism and antagonistic intervention strategy: a scope review. Front Cell Neurosci. 2023;17:1197051. doi: 10.3389/fncel.2023.1197051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Zhou Y, Wang D, Wang Y, Zhou Z, Ma X, Liu X, Dong Y. Cisplatin-induced ototoxicity: from signaling network to therapeutic targets. Biomed Pharmacother. 2023;157:114045. doi: 10.1016/j.biopha.2022.114045. [DOI] [PubMed] [Google Scholar]
- 134.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jian B, Pang J, Xiong H, Zhang W, Zhan T, Su Z, Lin H, Zhang H, He W, Zheng Y. Autophagy-dependent ferroptosis contributes to cisplatin-induced hearing loss. Toxicol Lett. 2021;350:249–260. doi: 10.1016/j.toxlet.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 136.Liu Z, Zhang H, Hong G, Bi X, Hu J, Zhang T, An Y, Guo N, Dong F, Xiao Y, Li W, Zhao X, Chu B, Guo S, Zhang X, Chai R, Fu X. Inhibition of Gpx4-mediated ferroptosis alleviates cisplatin-induced hearing loss in C57BL/6 mice. Mol Ther. 2024;32:1387–1406. doi: 10.1016/j.ymthe.2024.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu B, Liu Y, Chen X, Zhao J, Han J, Dong H, Zheng Q, Nie G. Ferrostatin-1 protects auditory hair cells from cisplatin-induced ototoxicity in vitro and in vivo. Biochem Biophys Res Commun. 2020;533:1442–1448. doi: 10.1016/j.bbrc.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 138.Mei H, Zhao L, Li W, Zheng Z, Tang D, Lu X, He Y. Inhibition of ferroptosis protects House Ear Institute-Organ of Corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. J Cell Mol Med. 2020;24:12065–12081. doi: 10.1111/jcmm.15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang W, Ma P, Gao W, Lu P, Ding X, Chen J, Yuan H, Lu L. Nrf2 knockout affected the ferroptosis signaling pathway against cisplatin-induced hair cell-like HEI-OC1 cell death. Oxid Med Cell Longev. 2022;2022:2210733. doi: 10.1155/2022/2210733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cho S, Hong SJ, Kang SH, Park Y, Kim SK. Alpha-lipoic acid attenuates apoptosis and ferroptosis in cisplatin-induced ototoxicity via the reduction of intracellular lipid droplets. Int J Mol Sci. 2022;23:10981. doi: 10.3390/ijms231810981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neuwelt EA, Brummett RE, Doolittle ND, Muldoon LL, Kroll RA, Pagel MA, Dojan R, Church V, Remsen LG, Bubalo JS. First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J Pharmacol Exp Ther. 1998;286:77–84. [PubMed] [Google Scholar]
- 142.Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput K, Sullivan M, Neuwelt EA. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Videhult P, Laurell G, Wallin I, Ehrsson H. Kinetics of Cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp Biol Med (Maywood) 2006;231:1638–1645. doi: 10.1177/153537020623101009. [DOI] [PubMed] [Google Scholar]
- 144.Sooriyaarachchi M, George GN, Pickering IJ, Narendran A, Gailer J. Tuning the metabolism of the anticancer drug cisplatin with chemoprotective agents to improve its safety and efficacy. Metallomics. 2016;8:1170–1176. doi: 10.1039/c6mt00183a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herr I, Ucur E, Herzer K, Okouoyo S, Ridder R, Krammer PH, von Knebel Doeberitz M, Debatin KM. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 2003;63:3112–3120. [PubMed] [Google Scholar]
- 146.Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17:3591–3604. doi: 10.2174/092986710792927895. [DOI] [PubMed] [Google Scholar]
- 147.Fouladi M, Chintagumpala M, Ashley D, Kellie S, Gururangan S, Hassall T, Gronewold L, Stewart CF, Wallace D, Broniscer A, Hale GA, Kasow KA, Merchant TE, Morris B, Krasin M, Kun LE, Boyett JM, Gajjar A. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J. Clin. Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang J, Lloyd Faulconbridge RV, Fetoni A, Guitton MJ, Pujol R, Puel JL. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology. 2003;45:380–393. doi: 10.1016/s0028-3908(03)00194-1. [DOI] [PubMed] [Google Scholar]
- 149.Bear ZW, Mikulec AA. Intratympanic steroid therapy for treatment of idiopathic sudden sensorineural hearing loss. Mo Med. 2014;111:352–356. [PMC free article] [PubMed] [Google Scholar]
- 150.Hill GW, Morest DK, Parham K. Cisplatin-induced ototoxicity: effect of intratympanic dexamethasone injections. Otol Neurotol. 2008;29:1005–1011. doi: 10.1097/MAO.0b013e31818599d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Calli C, Pinar E, Oncel S, Alper Bagriyanik H, Umut Sakarya E. Recovery of hearing in Cisplatin-induced ototoxicity in the Guinea pig with intratympanic dexamethasone. Indian J Otolaryngol Head Neck Surg. 2012;64:46–50. doi: 10.1007/s12070-011-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wimmer C, Mees K, Stumpf P, Welsch U, Reichel O, Suckfull M. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol Neurotol. 2004;25:33–40. doi: 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 153.Wolters FL, Klis SF, Hamers FP, de Groot JC, Smoorenburg GF. Perilymphatic application of alpha-melanocyte stimulating hormone ameliorates hearing loss caused by systemic administration of cisplatin. Hear Res. 2004;189:31–40. doi: 10.1016/S0378-5955(03)00396-4. [DOI] [PubMed] [Google Scholar]
- 154.Sarafraz Z, Ahmadi A, Daneshi A. Transtympanic injections of n-acetylcysteine and dexamethasone for prevention of cisplatin-induced ototoxicity: double blind randomized clinical trial. Int Tinnitus J. 2018;22:40–45. doi: 10.5935/0946-5448.20180007. [DOI] [PubMed] [Google Scholar]
- 155.Meyer F, Rolland V, Bairati I, Guitton M, Fortin A, Côté M. Randomized controlled trial to test the efficacy of trans-tympanic injections of a sodium thiosulfate gel to prevent cisplatin-induced ototoxicity. J. Clin. Oncol. 2017;35:e17560. doi: 10.1186/s40463-019-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marshak T, Steiner M, Kaminer M, Levy L, Shupak A. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: a randomized controlled study. Otolaryngol Head Neck Surg. 2014;150:983–990. doi: 10.1177/0194599814524894. [DOI] [PubMed] [Google Scholar]
- 157.McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 2010;31:156–165. doi: 10.1097/AUD.0b013e3181c351f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Borenstein JT. Intracochlear drug delivery systems. Expert Opin Drug Deliv. 2011;8:1161–1174. doi: 10.1517/17425247.2011.588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rivera T, Sanz L, Camarero G, Varela-Nieto I. Drug delivery to the inner ear: strategies and their therapeutic implications for sensorineural hearing loss. Curr Drug Deliv. 2012;9:231–242. doi: 10.2174/156720112800389098. [DOI] [PubMed] [Google Scholar]
- 160.Peppi M, Marie A, Belline C, Borenstein JT. Intracochlear drug delivery systems: a novel approach whose time has come. Expert Opin Drug Deliv. 2018;15:319–324. doi: 10.1080/17425247.2018.1444026. [DOI] [PubMed] [Google Scholar]
- 161.Liu SS, Yang R. Inner ear drug delivery for sensorineural hearing loss: current challenges and opportunities. Front Neurosci. 2022;16:867453. doi: 10.3389/fnins.2022.867453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fernandez R, Harrop-Jones A, Wang X, Dellamary L, LeBel C, Piu F. The sustained-exposure dexamethasone formulation OTO-104 offers effective protection against cisplatin-induced hearing loss. Audiol Neurootol. 2016;21:22–29. doi: 10.1159/000441833. [DOI] [PubMed] [Google Scholar]
- 163.Rolland V, Meyer F, Guitton MJ, Bussières R, Philippon D, Bairati I, Leclerc M, Côté M. A randomized controlled trial to test the efficacy of trans-tympanic injections of a sodium thiosulfate gel to prevent cisplatin-induced ototoxicity in patients with head and neck cancer. J Otolaryngol Head Neck Surg. 2019;48:4. doi: 10.1186/s40463-019-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naples JG, Ruckenstein MJ, Singh J, Cox BC, Li D. Intratympanic diltiazem-chitosan hydrogel as an otoprotectant against cisplatin-induced ototoxicity in a mouse model. Otol Neurotol. 2020;41:115–122. doi: 10.1097/MAO.0000000000002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martin-Saldana S, Palao-Suay R, Trinidad A, Aguilar MR, Ramirez-Camacho R, San Roman J. Otoprotective properties of 6alpha-methylprednisolone-loaded nanoparticles against cisplatin: in vitro and in vivo correlation. Nanomedicine. 2016;12:965–976. doi: 10.1016/j.nano.2015.12.367. [DOI] [PubMed] [Google Scholar]
- 166.Cai H, Liang Z, Huang W, Wen L, Chen G. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int J Pharm. 2017;532:55–65. doi: 10.1016/j.ijpharm.2017.08.084. [DOI] [PubMed] [Google Scholar]
- 167.Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14:350–360. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: influence of drug properties on intratympanic applications. Hear Res. 2018;368:28–40. doi: 10.1016/j.heares.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003;11:328–333. doi: 10.1097/00020840-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 171.Treskes M, Nijtmans LG, Fichtinger-Schepman AM, van der Vijgh WJ. Effects of the modulating agent WR2721 and its main metabolites on the formation and stability of cisplatin-DNA adducts in vitro in comparison to the effects of thiosulphate and diethyldithiocarbamate. Biochem Pharmacol. 1992;43:1013–1019. doi: 10.1016/0006-2952(92)90607-k. [DOI] [PubMed] [Google Scholar]
- 172.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 173.Campbell K, Claussen A, Meech R, Verhulst S, Fox D, Hughes L. D-methionine (D-met) significantly rescues noise-induced hearing loss: timing studies. Hear Res. 2011;282:138–144. doi: 10.1016/j.heares.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 174.Campbell KC, Rehemtulla A, Sunkara P, Hamstra D, Buhnerkempe M, Ross B. Oral D-methionine protects against cisplatin-induced hearing loss in humans: phase 2 randomized clinical trial in India. Int J Audiol. 2022;61:621–631. doi: 10.1080/14992027.2021.1983215. [DOI] [PubMed] [Google Scholar]
- 175.Marina N, Chang KW, Malogolowkin M, London WB, Frazier AL, Womer RB, Rescorla F, Billmire DF, Davis MM, Perlman EJ, Giller R, Lauer SJ, Olson TA Children’s Oncology Group. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a children’s oncology Group study. Cancer. 2005;104:841–847. doi: 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- 176.Mukherjea D, Dhukhwa A, Sapra A, Bhandari P, Woolford K, Franke J, Ramkumar V, Rybak L. Strategies to reduce the risk of platinum containing antineoplastic drug-induced ototoxicity. Expert Opin Drug Metab Toxicol. 2020;16:965–982. doi: 10.1080/17425255.2020.1806235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, Egorin MJ, Neuwelt EA. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- 178.Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, Laithier V, Ronghe M, Dall’Igna P, Hiyama E, Brichard B, Skeen J, Mateos ME, Capra M, Rangaswami AA, Ansari M, Rechnitzer C, Veal GJ, Covezzoli A, Brugieres L, Perilongo G, Czauderna P, Morland B, Neuwelt EA. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378:2376–2385. doi: 10.1056/NEJMoa1801109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Freyer DR, Chen L, Krailo MD, Knight K, Villaluna D, Bliss B, Pollock BH, Ramdas J, Lange B, Van Hoff D, VanSoelen ML, Wiernikowski J, Neuwelt EA, Sung L. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:63–74. doi: 10.1016/S1470-2045(16)30625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Harned TM, Kalous O, Neuwelt A, Loera J, Ji L, Iovine P, Sposto R, Neuwelt EA, Reynolds CP. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res. 2008;14:533–540. doi: 10.1158/1078-0432.CCR-06-2289. [DOI] [PubMed] [Google Scholar]
- 181.Wu YJ, Muldoon LL, Neuwelt EA. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther. 2005;312:424–431. doi: 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- 182.van den Berg JH, Beijnen JH, Balm AJ, Schellens JH. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev. 2006;32:390–397. doi: 10.1016/j.ctrv.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 183.Ezerina D, Takano Y, Hanaoka K, Urano Y, Dick TP. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H(2)S and sulfane sulfur production. Cell Chem Biol. 2018;25:447–459. e444. doi: 10.1016/j.chembiol.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yoo J, Hamilton SJ, Angel D, Fung K, Franklin J, Parnes LS, Lewis D, Venkatesan V, Winquist E. Cisplatin otoprotection using transtympanic L-N-acetylcysteine: a pilot randomized study in head and neck cancer patients. Laryngoscope. 2014;124:E87–94. doi: 10.1002/lary.24360. [DOI] [PubMed] [Google Scholar]
- 185.Wang W, Chen E, Ding X, Lu P, Chen J, Ma P, Lu L. N-acetylcysteine protect inner hair cells from cisplatin by alleviated celluar oxidative stress and apoptosis. Toxicol In Vitro. 2022;81:105354. doi: 10.1016/j.tiv.2022.105354. [DOI] [PubMed] [Google Scholar]
- 186.Thomas Dickey D, Muldoon LL, Kraemer DF, Neuwelt EA. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear Res. 2004;193:25–30. doi: 10.1016/j.heares.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 187.Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol. 2004;25:910–915. doi: 10.1097/00129492-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 188.Orgel E, Knight KR, Chi YY, Malvar J, Rushing T, Mena V, Eisenberg LS, Rassekh SR, Ross CJD, Scott EN, Neely M, Neuwelt EA, Muldoon LL, Freyer DR. Intravenous N-acetylcysteine to prevent cisplatin-induced hearing loss in children: a nonrandomized controlled phase I trial. Clin Cancer Res. 2023;29:2410–2418. doi: 10.1158/1078-0432.CCR-23-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 190.Korver KD, Rybak LP, Whitworth C, Campbell KM. Round window application of D-methionine provides complete cisplatin otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- 191.Rybak LP, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear. 2019;40:197–204. doi: 10.1055/s-0039-1684048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Alberts DS, Speicher LA, Krutzsch M, Wymer J, Capizzi RL, Conlon J, Barrett A, Aickin M. WR-1065, the active metabolite of amifostine (Ethyol), does not inhibit the cytotoxic effects of a broad range of standard anticancer drugs against human ovarian and breast cancer cells. Eur J Cancer. 1996;32A(Suppl 4):S17–20. doi: 10.1016/s0959-8049(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 193.Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs. 2002;13:181–209. doi: 10.1097/00001813-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 194.Church MW, Blakley BW, Burgio DL, Gupta AK. WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol. 2004;5:227–237. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hyppolito MA, de Oliveira AA, Lessa RM, Rossato M. Amifostine otoprotection to cisplatin ototoxicity: a guinea pig study using otoacoustic emission distortion products (DPOEA) and scanning electron microscopy. Braz J Otorhinolaryngol. 2005;71:268–273. doi: 10.1016/S1808-8694(15)31322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kaltenbach JA, Church MW, Blakley BW, McCaslin DL, Burgio DL. Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol Head Neck Surg. 1997;117:493–500. doi: 10.1016/S0194-59989770020-2. [DOI] [PubMed] [Google Scholar]
- 197.Fisher MJ, Lange BJ, Needle MN, Janss AJ, Shu HK, Adamson PC, Phillips PC. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr Blood Cancer. 2004;43:780–784. doi: 10.1002/pbc.20132. [DOI] [PubMed] [Google Scholar]
- 198.Gurney JG, Bass JK, Onar-Thomas A, Huang J, Chintagumpala M, Bouffet E, Hassall T, Gururangan S, Heath JA, Kellie S, Cohn R, Fisher MJ, Panandiker AP, Merchant TE, Srinivasan A, Wetmore C, Qaddoumi I, Stewart CF, Armstrong GT, Broniscer A, Gajjar A. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro Oncol. 2014;16:848–855. doi: 10.1093/neuonc/not241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.van As JW, van den Berg H, van Dalen EC. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst Rev. 2014:CD009219. doi: 10.1002/14651858.CD009219.pub3. [DOI] [PubMed] [Google Scholar]
- 200.Duval M, Daniel SJ. Meta-analysis of the efficacy of amifostine in the prevention of cisplatin ototoxicity. J Otolaryngol Head Neck Surg. 2012;41:309–315. [PubMed] [Google Scholar]
- 201.Freyer DR, Sung L, Reaman GH. Prevention of hearing loss in children receiving cisplatin chemotherapy. J. Clin. Oncol. 2009;27:317–318. doi: 10.1200/JCO.2008.20.1160. author reply 318-319. [DOI] [PubMed] [Google Scholar]
- 202.Sifuentes-Franco S, Sanchez-Macias DC, Carrillo-Ibarra S, Rivera-Valdes JJ, Zuniga LY, Sanchez-Lopez VA. Antioxidant and anti-inflammatory effects of coenzyme Q10 supplementation on infectious diseases. Healthcare (Basel) 2022;10:487. doi: 10.3390/healthcare10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Astolfi L, Simoni E, Valente F, Ghiselli S, Hatzopoulos S, Chicca M, Martini A. Coenzyme Q10 plus multivitamin treatment prevents cisplatin ototoxicity in rats. PLoS One. 2016;11:e0162106. doi: 10.1371/journal.pone.0162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scasso F, Sprio AE, Canobbio L, Scanarotti C, Manini G, Berta GN, Bassi AM. Dietary supplementation of coenzyme Q10 plus multivitamins to hamper the ROS mediated cisplatin ototoxicity in humans: a pilot study. Heliyon. 2017;3:e00251. doi: 10.1016/j.heliyon.2017.e00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Martin-Saldana S, Palao-Suay R, Aguilar MR, Ramirez-Camacho R, San Roman J. Polymeric nanoparticles loaded with dexamethasone or alpha-tocopheryl succinate to prevent cisplatin-induced ototoxicity. Acta Biomater. 2017;53:199–210. doi: 10.1016/j.actbio.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 206.Fernandez K, Spielbauer KK, Rusheen A, Wang L, Baker TG, Eyles S, Cunningham LL. Lovastatin protects against cisplatin-induced hearing loss in mice. Hear Res. 2020;389:107905. doi: 10.1016/j.heares.2020.107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fernandez KA, Allen P, Campbell M, Page B, Townes T, Li CM, Cheng H, Garrett J, Mulquin M, Clements A, Mulford D, Ortiz C, Brewer C, Dubno JR, Newlands S, Schmitt NC, Cunningham LL. Atorvastatin is associated with reduced cisplatin-induced hearing loss. J Clin Invest. 2021;131:e142616. doi: 10.1172/JCI142616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hazlitt RA, Teitz T, Bonga JD, Fang J, Diao S, Iconaru L, Yang L, Goktug AN, Currier DG, Chen T, Rankovic Z, Min J, Zuo J. Development of second-generation CDK2 inhibitors for the prevention of cisplatin-induced hearing loss. J Med Chem. 2018;61:7700–7709. doi: 10.1021/acs.jmedchem.8b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ingersoll MA, Malloy EA, Caster LE, Holland EM, Xu Z, Zallocchi M, Currier D, Liu H, He DZZ, Min J, Chen T, Zuo J, Teitz T. BRAF inhibition protects against hearing loss in mice. Sci Adv. 2020;6:eabd0561. doi: 10.1126/sciadv.abd0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- 211.Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. J Neurochem. 2009;108:1226–1236. doi: 10.1111/j.1471-4159.2009.05871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Drottar M, Liberman MC, Ratan RR, Roberson DW. The histone deacetylase inhibitor sodium butyrate protects against cisplatin-induced hearing loss in guinea pigs. Laryngoscope. 2006;116:292–296. doi: 10.1097/01.mlg.0000197630.85208.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Layman WS, Zuo J. Epigenetic regulation in the inner ear and its potential roles in development, protection, and regeneration. Front Cell Neurosci. 2014;8:446. doi: 10.3389/fncel.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Layman WS, Williams DM, Dearman JA, Sauceda MA, Zuo J. Histone deacetylase inhibition protects hearing against acute ototoxicity by activating the Nf-kappaB pathway. Cell Death Discov. 2015;1:15012. doi: 10.1038/cddiscovery.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang J, Tian KY, Fang Y, Chang HM, Han YN, Chen FQ. Sulforaphane attenuates cisplatin-induced hearing loss by inhibiting histone deacetylase expression. Int J Immunopathol Pharmacol. 2021;35:20587384211034086. doi: 10.1177/20587384211034086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.He Y, Zheng Z, Liu C, Li W, Zhao L, Nie G, Li H. Inhibiting DNA methylation alleviates cisplatin-induced hearing loss by decreasing oxidative stress-induced mitochondria-dependent apoptosis via the LRP1-PI3K/AKT pathway. Acta Pharm Sin B. 2022;12:1305–1321. doi: 10.1016/j.apsb.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang B, Zhu X, Kim Y, Li J, Huang S, Saleem S, Li RC, Xu Y, Dore S, Cao W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2012;52:928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kubo E, Chhunchha B, Singh P, Sasaki H, Singh DP. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130. doi: 10.1038/s41598-017-14520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang W, Xiong H, Pang J, Su Z, Lai L, Lin H, Jian B, He W, Yang H, Zheng Y. Nrf2 activation protects auditory hair cells from cisplatin-induced ototoxicity independent on mitochondrial ROS production. Toxicol Lett. 2020;331:1–10. doi: 10.1016/j.toxlet.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 220.Mu YR, Zou SY, Li M, Ding YY, Huang X, He ZH, Kong WJ. Role and mechanism of FOXG1-related epigenetic modifications in cisplatin-induced hair cell damage. Front Mol Neurosci. 2023;16:1064579. doi: 10.3389/fnmol.2023.1064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Park JS, Kim SW, Park K, Choung YH, Jou I, Park SM. Pravastatin attenuates noise-induced cochlear injury in mice. Neuroscience. 2012;208:123–132. doi: 10.1016/j.neuroscience.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 222.Brand Y, Setz C, Levano S, Listyo A, Chavez E, Pak K, Sung M, Radojevic V, Ryan AF, Bodmer D. Simvastatin protects auditory hair cells from gentamicin-induced toxicity and activates Akt signaling in vitro. BMC Neurosci. 2011;12:114. doi: 10.1186/1471-2202-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Syka J, Ouda L, Nachtigal P, Solichova D, Semecky V. Atorvastatin slows down the deterioration of inner ear function with age in mice. Neurosci Lett. 2007;411:112–116. doi: 10.1016/j.neulet.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 224.Whitlon DS. Statins and hearing. Hear Res. 2022;425:108453. doi: 10.1016/j.heares.2022.108453. [DOI] [PubMed] [Google Scholar]
- 225.Hati S, Zallocchi M, Hazlitt R, Li Y, Vijayakumar S, Min J, Rankovic Z, Lovas S, Zuo J. AZD5438-PROTAC: A selective CDK2 degrader that protects against cisplatin- and noise-induced hearing loss. Eur J Med Chem. 2021;226:113849. doi: 10.1016/j.ejmech.2021.113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Osakabe N, Modafferi S, Ontario ML, Rampulla F, Zimbone V, Migliore MR, Fritsch T, Abdelhameed AS, Maiolino L, Lupo G, Anfuso CD, Genovese E, Monzani D, Wenzel U, Calabrese EJ, Vabulas RM, Calabrese V. Polyphenols in inner ear neurobiology, health and disease: from bench to clinics. Medicina (Kaunas) 2023;59:2045. doi: 10.3390/medicina59112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee JS, Kang SU, Hwang HS, Pyun JH, Choung YH, Kim CH. Epicatechin protects the auditory organ by attenuating cisplatin-induced ototoxicity through inhibition of ERK. Toxicol Lett. 2010;199:308–316. doi: 10.1016/j.toxlet.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 228.Kim CH, Kang SU, Pyun J, Lee MH, Hwang HS, Lee H. Epicatechin protects auditory cells against cisplatin-induced death. Apoptosis. 2008;13:1184–1194. doi: 10.1007/s10495-008-0242-5. [DOI] [PubMed] [Google Scholar]
- 229.Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, Sirvanci S, Yuksel M, Uneri C, Tutkun A. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol. 2012;76:404–408. doi: 10.1016/j.ijporl.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 230.Olgun Y, Kirkim G, Kolatan E, Kiray M, Bagriyanik A, Olgun A, Kizmazoglu DC, Ellidokuz H, Serbetcioglu B, Altun Z, Aktas S, Yilmaz O, Guneri EA. Friend or foe? Effect of oral resveratrol on cisplatin ototoxicity. Laryngoscope. 2014;124:760–766. doi: 10.1002/lary.24323. [DOI] [PubMed] [Google Scholar]
- 231.Lee CH, Kim KW, Lee SM, Kim SY. Dose-dependent effects of resveratrol on cisplatin-induced hearing loss. Int J Mol Sci. 2020;22:13. doi: 10.3390/ijms22010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fetoni AR, Eramo SL, Paciello F, Rolesi R, Podda MV, Troiani D, Paludetti G. Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol Neurotol. 2014;35:e169–177. doi: 10.1097/MAO.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 233.Soyalic H, Gevrek F, Koc S, Avcu M, Metin M, Aladag I. Intraperitoneal curcumin and vitamin E combination for the treatment of cisplatin-induced ototoxicity in rats. Int J Pediatr Otorhinolaryngol. 2016;89:173–178. doi: 10.1016/j.ijporl.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 234.Bahadori F, Demiray M. A realistic view on “the essential medicinal chemistry of curcumin”. ACS Med Chem Lett. 2017;8:893–896. doi: 10.1021/acsmedchemlett.7b00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11:1139–1148. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu Y, Chen L, He X, Fan L, Yang G, Chen X, Lin X, Du L, Li Z, Ye H, Mao Y, Zhao X, Wei Y. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int J Gynecol Cancer. 2008;18:652–659. doi: 10.1111/j.1525-1438.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 238.Cheng N, Xia T, Han Y, He QJ, Zhao R, Ma JR. Synergistic antitumor effects of liposomal honokiol combined with cisplatin in colon cancer models. Oncol Lett. 2011;2:957–962. doi: 10.3892/ol.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maruyama Y, Kuribara H, Morita M, Yuzurihara M, Weintraub ST. Identification of magnolol and honokiol as anxiolytic agents in extracts of saiboku-to, an oriental herbal medicine. J Nat Prod. 1998;61:135–138. doi: 10.1021/np9702446. [DOI] [PubMed] [Google Scholar]
- 240.Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–166. doi: 10.1016/j.brainres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 241.Lin YR, Chen HH, Ko CH, Chan MH. Differential inhibitory effects of honokiol and magnolol on excitatory amino acid-evoked cation signals and NMDA-induced seizures. Neuropharmacology. 2005;49:542–550. doi: 10.1016/j.neuropharm.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 242.Lo YC, Teng CM, Chen CF, Chen CC, Hong CY. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol. 1994;47:549–553. doi: 10.1016/0006-2952(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 243.Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP, Gius D, Gupta MP. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tsai SK, Huang SS, Hong CY. Myocardial protective effect of honokiol: an active component in Magnolia officinalis. Planta Med. 1996;62:503–506. doi: 10.1055/s-2006-957957. [DOI] [PubMed] [Google Scholar]
- 245.Chiang CK, Sheu ML, Lin YW, Wu CT, Yang CC, Chen MW, Hung KY, Wu KD, Liu SH. Honokiol ameliorates renal fibrosis by inhibiting extracellular matrix and pro-inflammatory factors in vivo and in vitro. Br J Pharmacol. 2011;163:586–597. doi: 10.1111/j.1476-5381.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chiu JH, Ho CT, Wei YH, Lui WY, Hong CY. In vitro and in vivo protective effect of honokiol on rat liver from peroxidative injury. Life Sci. 1997;61:1961–1971. doi: 10.1016/s0024-3205(97)00836-9. [DOI] [PubMed] [Google Scholar]
- 247.Tan X, Zhou Y, Agarwal A, Lim M, Xu Y, Zhu Y, O’Brien J, Tran E, Zheng J, Gius D, Richter CP. Systemic application of honokiol prevents cisplatin ototoxicity without compromising its antitumor effect. Am J Cancer Res. 2020;10:4416–4434. [PMC free article] [PubMed] [Google Scholar]
- 248.Hall JA, Dominy JE, Lee Y, Puigserver P. The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest. 2013;123:973–979. doi: 10.1172/JCI64094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. 2011;5:485–496. doi: 10.1186/1479-7364-5-5-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Altern Med Rev. 2010;15:245–263. [PubMed] [Google Scholar]
- 251.Kelly GS. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 2. Altern Med Rev. 2010;15:313–328. [PubMed] [Google Scholar]
- 252.Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Patel S, Shah L, Dang N, Tan X, Almudevar A, White PM. SIRT3 promotes auditory function in young adult FVB/nJ mice but is dispensable for hearing recovery after noise exposure. PLoS One. 2020;15:e0235491. doi: 10.1371/journal.pone.0235491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Quan Y, Xia L, Shao J, Yin S, Cheng CY, Xia W, Gao WQ. Adjudin protects rodent cochlear hair cells against gentamicin ototoxicity via the SIRT3-ROS pathway. Sci Rep. 2015;5:8181. doi: 10.1038/srep08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev. 2010;131:480–486. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pang J, Xiong H, Ou Y, Yang H, Xu Y, Chen S, Lai L, Ye Y, Su Z, Lin H, Huang Q, Xu X, Zheng Y. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol Aging. 2019;80:127–137. doi: 10.1016/j.neurobiolaging.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 258.Zendedel E, Butler AE, Atkin SL, Sahebkar A. Impact of curcumin on sirtuins: a review. J Cell Biochem. 2018;119:10291–10300. doi: 10.1002/jcb.27371. [DOI] [PubMed] [Google Scholar]
- 259.de Boer VC, de Goffau MC, Arts IC, Hollman PC, Keijer J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech Ageing Dev. 2006;127:618–627. doi: 10.1016/j.mad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 260.Huang KJ, Kuo CH, Chen SH, Lin CY, Lee YR. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J Cell Mol Med. 2018;22:1894–1908. doi: 10.1111/jcmm.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jiang QQ, Fan LY, Yang GL, Guo WH, Hou WL, Chen LJ, Wei YQ. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer. 2008;8:242. doi: 10.1186/1471-2407-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lin JW, Chen JT, Hong CY, Lin YL, Wang KT, Yao CJ, Lai GM, Chen RM. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro Oncol. 2012;14:302–314. doi: 10.1093/neuonc/nor217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang X, Duan X, Yang G, Zhang X, Deng L, Zheng H, Deng C, Wen J, Wang N, Peng C, Zhao X, Wei Y, Chen L. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS One. 2011;6:e18490. doi: 10.1371/journal.pone.0018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Enaru B, Socaci S, Farcas A, Socaciu C, Danciu C, Stanila A, Diaconeasa Z. Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals (Basel) 2021;14:946. doi: 10.3390/ph14100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Li S, Li L, Chen J, Fan Y, Wang C, Du Y, Guo C, Chen F, Li W. Liposomal honokiol inhibits glioblastoma growth through regulating macrophage polarization. Ann Transl Med. 2021;9:1644. doi: 10.21037/atm-21-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Editorial O. Erratum to liposomal honokiol inhibits glioblastoma growth through regulating macrophage polarization. Ann Transl Med. 2023;11:402. doi: 10.21037/atm-2023-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mai NNS, Otsuka Y, Kawano Y, Hanawa T. Preparation and characterization of solid dispersions composed of curcumin, hydroxypropyl cellulose and/or sodium dodecyl sulfate by grinding with vibrational ball milling. Pharmaceuticals (Basel) 2020;13:383. doi: 10.3390/ph13110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ha ES, Choi DH, Baek IH, Park H, Kim MS. Enhanced oral bioavailability of resveratrol by using neutralized eudragit e solid dispersion prepared via spray drying. Antioxidants (Basel) 2021;10:90. doi: 10.3390/antiox10010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wu D, Zhou J, Creyer MN, Yim W, Chen Z, Messersmith PB, Jokerst JV. Phenolic-enabled nanotechnology: versatile particle engineering for biomedicine. Chem Soc Rev. 2021;50:4432–4483. doi: 10.1039/d0cs00908c. [DOI] [PMC free article] [PubMed] [Google Scholar]