Abstract
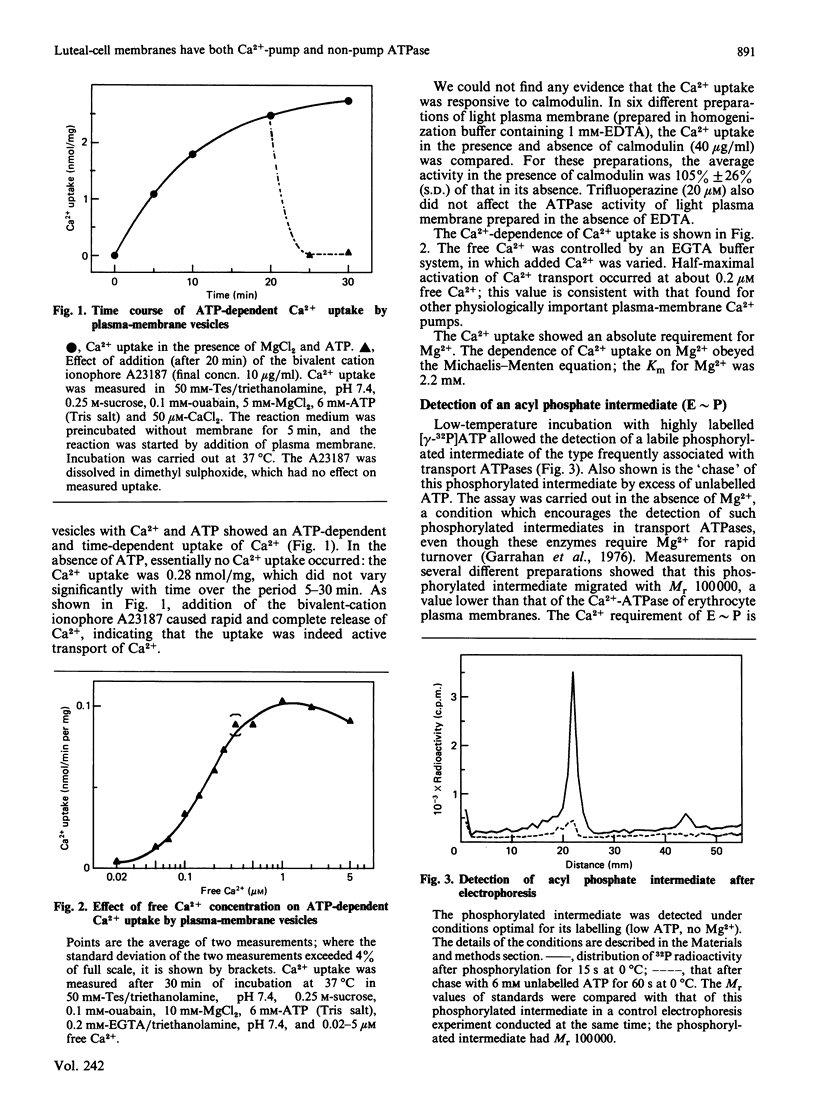
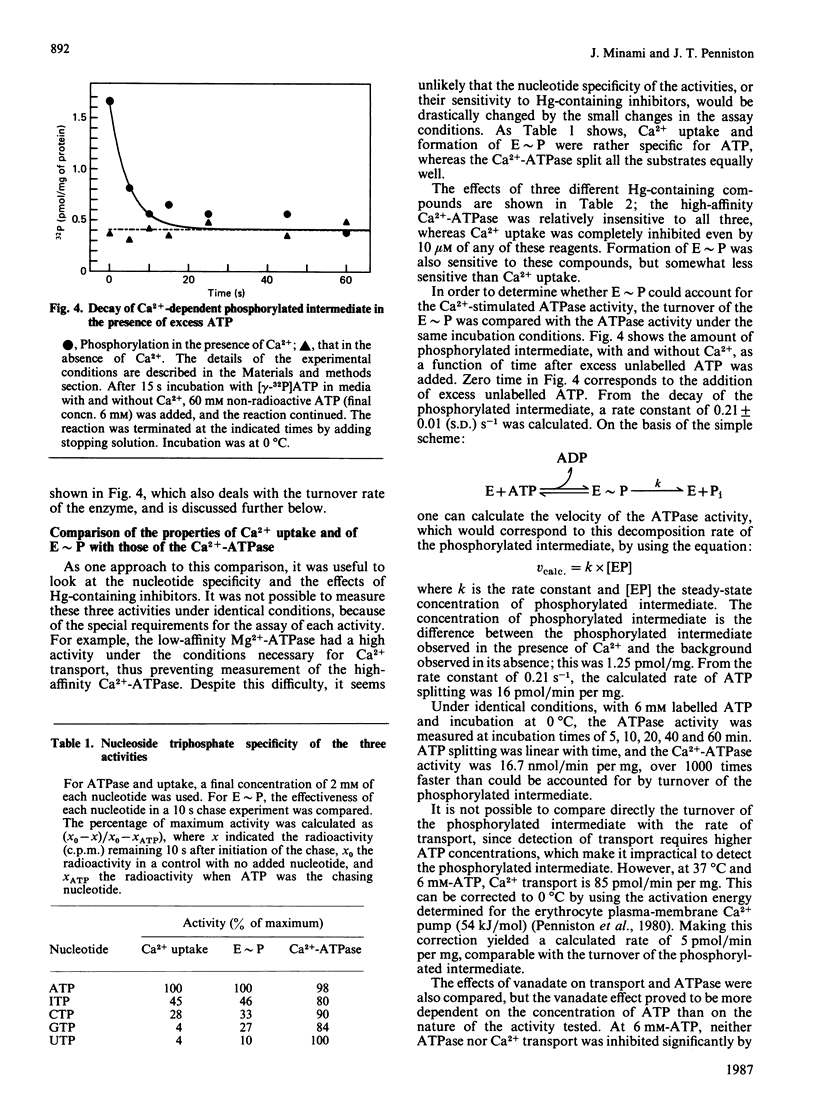
Plasma-membrane vesicles from rat corpus luteum showed an ATP-dependent uptake of Ca2+. Ca2+ was accumulated with a K1/2 (concn. giving half-maximal activity) of 0.2 microM and was released by the bivalent-cation ionophore A23187. A Ca2+-dependent phosphorylated intermediate (Mr 100,000) was detected which showed a low decomposition rate, consistent with it being the phosphorylated intermediate of the transport ATPase responsible for Ca2+ uptake. The Ca2+ uptake and the phosphorylated intermediate (E approximately P) displayed several properties that were different from those of the high-affinity Ca2+-ATPase previously observed in these membranes. Both Ca2+ uptake and E approximately P discriminated against ribonucleoside triphosphates other than ATP, whereas the ATPase split all the ribonucleoside triphosphates equally. Both Ca2+ uptake and E approximately P were sensitive to three different Hg-containing inhibitors, whereas the ATPase was inhibited much less. Ca2+ uptake required added Mg2+ (Km = 2.2 mM), whereas the ATPase required no added Mg2+. The maximum rate of Ca2+ uptake was about 400-fold less than that of ATP splitting; under different conditions, the decomposition rate of E approximately P was 1,000 times too slow to account for the ATPase activity observed. All of these features suggested that Ca2+ uptake was due to an enzyme of low activity, whose ATPase activity was not detected in the presence of the higher-specific-activity Ca2+-dependent ATPase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansah T. A., Molla A., Katz S. Ca2+-ATPase activity in pancreatic acinar plasma membranes. Regulation by calmodulin and acidic phospholipids. J Biol Chem. 1984 Nov 10;259(21):13442–13450. [PubMed] [Google Scholar]
- Bramley T. A., Ryan R. J. Interactions of gonadotropins with corpus luteum membranes. VIII. The different properties of rat luteal cell light and heavy membranes cannot be explained by fractionation of inside-out and outside-out plasma-membrane vesicles. Mol Cell Endocrinol. 1980 Jul;19(1):21–31. doi: 10.1016/0303-7207(80)90027-1. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Chan K. M., Junger K. D. Calcium transport and phosphorylated intermediate of (Ca2+ + Mg2+)-ATPase in plasma membranes of rat liver. J Biol Chem. 1983 Apr 10;258(7):4404–4410. [PubMed] [Google Scholar]
- De Jonge H. R., Ghijsen W. E., Van Os C. H. Phosphorylated intermediates of Ca2+ -ATPase and alkaline phosphatase in plasma membranes from rat duodenal epithelium. Biochim Biophys Acta. 1981 Sep 21;647(1):140–149. doi: 10.1016/0005-2736(81)90302-3. [DOI] [PubMed] [Google Scholar]
- De Schutter G., Wuytack F., Verbist J., Casteels R. Tissue levels and purification by affinity chromatography of the calmodulin-stimulated Ca2+ -transport ATPase in pig antrum smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):1–10. doi: 10.1016/0005-2736(84)90544-3. [DOI] [PubMed] [Google Scholar]
- De Smedt H., Parys J. B., Borghgraef R., Wuytack F. Calmodulin stimulation of renal (Ca2+ + Mg2+)-ATPase. FEBS Lett. 1981 Aug 17;131(1):60–62. doi: 10.1016/0014-5793(81)80887-3. [DOI] [PubMed] [Google Scholar]
- De Smedt H., Parys J. B., Wuytack F., Borghgraef R. Calcium-induced phosphorylations and [125I]calmodulin binding in renal membrane preparations. Biochim Biophys Acta. 1984 Sep 19;776(1):122–132. doi: 10.1016/0005-2736(84)90258-x. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., LINDBERG O. Determination of organic phosphorus compounds by phosphate analysis. Methods Biochem Anal. 1956;3:1–22. doi: 10.1002/9780470110195.ch1. [DOI] [PubMed] [Google Scholar]
- Enyedi A., Sarkadi B., Földes-Papp Z., Monostory S., Gárdos G. Demonstration of two distinct calcium pumps in human platelet membrane vesicles. J Biol Chem. 1986 Jul 15;261(20):9558–9563. [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F., Alonso G. L. The interaction of magnesium ions with the calcium pump of sarcoplasmic reticulum. Biochim Biophys Acta. 1976 Sep 21;448(1):121–132. doi: 10.1016/0005-2736(76)90081-x. [DOI] [PubMed] [Google Scholar]
- Hakim G., Itano T., Verma A. K., Penniston J. T. Purification of the Ca2+-and Mg2+-requiring ATPase from rat brain synaptic plasma membrane. Biochem J. 1982 Nov 1;207(2):225–231. doi: 10.1042/bj2070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K., Field J. B. Ca2+-stimulated, Mg2+-dependent ATPase in bovine thyroid plasma membranes. Biochim Biophys Acta. 1982 Feb 23;685(2):225–229. doi: 10.1016/0005-2736(82)90104-3. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Kostka P., Grover A. K., Law J. S., Daniel E. E. Calmodulin stimulation of plasmalemmal Ca2+-pump of canine aortic smooth muscle. Blood Vessels. 1986;23(1):22–33. doi: 10.1159/000158622. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lew P. D., Stossel T. P. Calcium transport by macrophage plasma membranes. J Biol Chem. 1980 Jun 25;255(12):5841–5846. [PubMed] [Google Scholar]
- Lichtman A. H., Segel G. B., Lichtman M. A. Calcium transport and calcium-ATPase activity in human lymphocyte plasma membrane vesicles. J Biol Chem. 1981 Jun 25;256(12):6148–6154. [PubMed] [Google Scholar]
- Lin S. H., Fain J. N. Purification of (Ca2+-Mg2+)-ATPase from rat liver plasma membranes. J Biol Chem. 1984 Mar 10;259(5):3016–3020. [PubMed] [Google Scholar]
- Lin S. H. Novel ATP-dependent calcium transport component from rat liver plasma membranes. The transporter and the previously reported (Ca2+-Mg2+)-ATPase are different proteins. J Biol Chem. 1985 Jul 5;260(13):7850–7856. [PubMed] [Google Scholar]
- Lin S. H. The rat liver plasma membrane high affinity (Ca2+-Mg2+)-ATPase is not a calcium pump. Comparison with ATP-dependent calcium transporter. J Biol Chem. 1985 Sep 15;260(20):10976–10980. [PubMed] [Google Scholar]
- Lotersztajn S., Hanoune J., Pecker F. A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence of an endogenous protein activator distinct from calmodulin. J Biol Chem. 1981 Nov 10;256(21):11209–11215. [PubMed] [Google Scholar]
- Michalak M., Famulski K., Carafoli E. The Ca2+-pumping ATPase in skeletal muscle sarcolemma. Calmodulin dependence, regulation by cAMP-dependent phosphorylation, and purification. J Biol Chem. 1984 Dec 25;259(24):15540–15547. [PubMed] [Google Scholar]
- Nellans H. N., Popovitch J. E. Calmodulin-regulated, ATP-driven calcium transport by basolateral membranes of rat small intestine. J Biol Chem. 1981 Oct 10;256(19):9932–9936. [PubMed] [Google Scholar]
- Ochs D. L., Reed P. W. ATP-dependent calcium transport in plasma membrane vesicles from neutrophil leukocytes. J Biol Chem. 1983 Aug 25;258(16):10116–10122. [PubMed] [Google Scholar]
- Ochs D. L., Reed P. W. Ca2+-stimulated, Mg2+-dependent ATPase activity in neutrophil plasma membrane vesicles. Coupling to Ca2+ transport. J Biol Chem. 1984 Jan 10;259(1):102–106. [PubMed] [Google Scholar]
- Papazian D. M., Rahamimoff H., Goldin S. M. Partial purification and functional identification of a calmodulin-activated, adenosine 5'-triphosphate-dependent calcium pump from synaptic plasma membranes. J Neurosci. 1984 Aug;4(8):1933–1943. doi: 10.1523/JNEUROSCI.04-08-01933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T., Graf E., Itano T. Calmodulin regulation of the Ca2+ pump of erythrocyte membranes. Ann N Y Acad Sci. 1980;356:245–257. doi: 10.1111/j.1749-6632.1980.tb29615.x. [DOI] [PubMed] [Google Scholar]
- Penniston J. T. Substrate specificity of the erythrocyte Ca2+-ATPase. Biochim Biophys Acta. 1982 Jun 28;688(3):735–739. doi: 10.1016/0005-2736(82)90286-3. [DOI] [PubMed] [Google Scholar]
- Sarkadi B. Active calcium transport in human red cells. Biochim Biophys Acta. 1980 Sep 30;604(2):159–190. doi: 10.1016/0005-2736(80)90573-8. [DOI] [PubMed] [Google Scholar]
- Shen V., Kohler G., Peck W. A. A high affinity, calmodulin-responsive (Ca2+ + Mg2+)-ATPase in isolated bone cells. Biochim Biophys Acta. 1983 Jan 19;727(2):230–238. doi: 10.1016/0005-2736(83)90408-x. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Penniston J. T. A high affinity Ca2+-stimulated and Mg2+-dependent ATPase in rat corpus luteum plasma membrane fractions. J Biol Chem. 1981 Feb 10;256(3):1269–1275. [PubMed] [Google Scholar]
- Wetzker R., Böhmer F. D., Klinger R., Müller E., Hegewald H., Scheven M., Grosse R. Purification and characterization of the Ca2+-ATPase of plasma membranes from Ehrlich ascites mammary carcinoma cells. Biochim Biophys Acta. 1986 Jan 16;854(1):117–123. doi: 10.1016/0005-2736(86)90071-4. [DOI] [PubMed] [Google Scholar]


